Abstract
We propose that the extracellular matrix signals CD44, a hyaluronan receptor, to increase the responsiveness to mechanical stimulation. We report that intradermal injection of hyaluronidase induces mechanical hyperalgesia, that is inhibited by co-administration of a CD44 receptor antagonist, A5G27. The intradermal injection of low (LMWH) but not high (HMWH) molecular weight hyaluronan also induces mechanical hyperalgesia, an effect that was attenuated by the pretreatment with HMWH or A5G27. Pretreatment with HMWH also attenuated the hyperalgesia induced by hyaluronidase. Similarly, intradermal injection of A6, a CD44 receptor agonist, produced hyperalgesia that was inhibited by HMWH and A5G27. Inhibitors of protein kinase A and Src, but not protein kinase C, significantly attenuated the hyperalgesia induced by both A6 and LMWH. Finally, to determine if CD44 receptor signaling is involved in a preclinical model of inflammatory pain, we evaluated the effect of A5G27 and HMWH on the mechanical hyperalgesia associated with the inflammation induced by carrageenan. Both A5G27 and HMWH attenuated carrageenan-induced mechanical hyperalgesia. Thus, while LMWH acts at its cognate receptor, CD44, to induce mechanical hyperalgesia, HMWH acts at the same receptor as an antagonist. That the local administration of HMWH or A5G27 inhibits carrageenan-induced hyperalgesia supports the suggestion that carrageenan produces changes in the extracellular matrix that contributes to inflammatory pain. These studies define a clinically relevant role for signaling by the hyaluronan receptor, CD44, in increased responsiveness to mechanical stimulation.
Keywords: Extracellular Matrix, CD44, hyaluronan, nociceptor, hyperalgesia
There has been a rapid increase in our understanding of the role that the extracellular matrix (ECM) plays in diverse pathological states (Martignetti et al., 2001; Busch and Silver, 2007; Hynes, 2009; Lu et al., 2011; Bhattacharyya et al., 2014). While several of these clinical conditions are characterized by acute or chronic pain [e.g., inflamed tissues (Lee et al., 2013; Alkhatib et al., 2014) or nerve injury (Sugimoto et al., 2008; Tsuda et al., 2008; Yong and Guoping, 2009; Tsuda et al., 2013)], the role of the ECM in the associated pain syndromes remains poorly understood. We have previously shown that versican, a large chondroitin sulfate proteoglycan ECM molecule that labels the non-peptidergic, isolectin B4 staining (IB4+) population of nociceptors helps to determine the function of these nociceptors (Bogen et al., 2005; Bogen et al., 2015). However, how ECM molecules signal to nociceptors, to influence their function, remains to be elucidated.
The largest member of the hyalectan (hyaluronan- and lectin-binding proteoglycan) gene family of extracellular molecules, versican contains hyaluronan-binding tandem repeats and has diverse binding partners, important to its function, including other extracellular and cell surface molecules, such as hyaluronan (Yamagata et al., 1993; Bandtlow and Zimmermann, 2000; Karvinen et al., 2003; Matsumoto et al., 2003; Wu et al., 2005; Wight, 2008; Ween et al., 2011; Wight et al., 2014). In addition to being able to bind hyaluronan, versican also binds cell surface proteins such as CD44, a cognate hyaluronan receptor (Bajorath et al., 1998; Teriete et al., 2004). Hyaluronan and versican can function together to signal to CD44 (Yamagata et al., 1993; Karvinen et al., 2003; Wu et al., 2005). Importantly, the intra-articular injection of high molecular weight hyaluronan (HMWH) is used clinically in the treatment of osteoarthritis (Dougados et al., 1993; Altman and Moskowitz, 1998; Cohen et al., 2008; Triantaffilidou et al., 2013). While intra-articular hyaluronan does attenuate nociceptor sensitization in an animal model of osteoarthritis (Hashizume et al., 2010), it is generally considered that its therapeutic effect is mediated by its viscoelastic properties (Radin et al., 1970; Unsworth et al., 1975; Mabuchi et al., 1994; Elmorsy et al., 2014; Cowman et al., 2015). Recent evidence suggests that hyaluronan modulates nociceptor function by action on CD44 (Ghosh et al., 2011). To begin to unravel how ECM molecules signal to primary afferent nociceptors, we have evaluated the role of high and low molecular weight hyaluronan, and its cognate receptor, CD44, in nociceptor function and in an animal model of inflammatory pain in which changes in the ECM are well described (Dina et al., 2004; Li et al., 2012; Vieira et al., 2012; Vieira et al., 2013).
2. Experimental procedures
2.1. Animals
All experiments were performed on adult male Sprague Dawley rats (220–400 g; Charles River Laboratories). Animals were housed, 3 per cage, under a 12-hour light/dark cycle in a temperature- and humidity-controlled room in the animal care facility of the University of California, San Francisco. Food and water were available ad libitum. Nociceptive testing was performed between 10:00 am and 5:00 pm, for all experiments. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at The University of California, San Francisco, and adhered to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Every effort was made to minimize the number of animals used and their suffering.
2.2. Testing mechanical nociceptive threshold
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter® (Randall-Selitto paw-withdrawal test; Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat's hind paw, as previously described (Randall and Selitto, 1957; Taiwo and Levine, 1989; Taiwo et al., 1989). Nociceptive threshold was defined as the force in grams at which the rat withdrew its paw. Baseline paw-pressure threshold was defined as the mean of the 3 readings taken before a test agent was injected. Each paw was treated as an independent measure and each experiment performed on a different group of rats. Data are presented as the mean change from baseline nociceptive threshold.
3.3. Drugs
The following reagents were used in this study: hyaluronidase from Streptomyces hyalurolyticus, λ-carrageenan (inflammatory agent), and SU6656 (a selective Src family kinase inhibitor), from Sigma-Aldrich (St. Louis, MO); hyaluronic acid sodium salt from Streptococcus pyrogenes [high molecular weight hyaluronan (HMWH)], from Calbiochem (San Diego, CA); hyaluronic acid oligosaccharide dp6 [low molecular weight hyaluronan (LMWH)], from AMSBIO (Cambridge, MA), H-89 dihydrochloride [protein kinase A (PKA) inhibitor)], from Santa Cruz Biotechnology (Dallas, TX, USA); bisindolylmalemide 1 HCl [BIMM, protein kinase C (PKC) inhibitor)], from Calbiochem-Novabiochem (La Jolla, CA); and, the CD44 receptor-related peptides, A6, a CD44 agonist (Piotrowicz et al., 2011; Finlayson, 2015), and A5G27, a CD44 antagonist (Hibino et al., 2004; Pesarrodona et al., 2014), obtained from GenScript USA Inc (Piscataway, NJ).
Hyaluronidase was dissolved in 0.9% NaCl to the concentration of 1U/μl; aliquots containing 1 μg/μl of HMWH, LMWH, A6 or A5G27, dissolved in distilled water, were diluted in 0.9% NaCl to the concentration of 0.2 μg/μl; aliquots containing 1 μg/μl of H-89, BIMM or SU6656, dissolved in absolute dimethyl sulfoxide (DMSO), were diluted in 0.9% NaCl containing 10% DMSO to the concentration of 0.2 μg/μl. The injection volume of all drugs was 5 μl.
All drugs except carrageenan were administered intradermally on the dorsum of the hind paw using a 30-gauge beveled hypodermic needle attached to a microsyringe (Hamilton Company, Reno, NV) by a short length of polyethylene (PE-10) tubing. Because of its high viscosity, carrageenan was injected using a 27-gauge needle. The concentration of carrageenan (1%, in 0.9% NaCl) used to produce robust mechanical inflammatory hyperalgesia, observed already 30 minutes after injection, peaking at the 4th h, has been determined in previous studies (Aley et al., 2000; Dina et al., 2008). The administration of H-89, BIMM or SU6656 was preceded by a hypotonic shock to transiently facilitate enhanced cell permeability to these agents (2 μL of distilled water, separated by a bubble of air to avoid mixing in the same syringe) to get reagents inside the nerve terminal (Borle and Snowdowne, 1982; Burch and Axelrod, 1987).
2.4. Statistics
In all experiments, the dependent variable was paw-withdrawal threshold, expressed as percentage change from baseline. The average paw withdrawal threshold before the experiments was 125.5 ± 0.8 g (n = 150 paws). To compare the changes in the nociceptive threshold induced by the injection of hyaluronidase, LMWH, A6 or carrageenan in the control groups with the groups pretreated with inhibitors, repeated measures analysis of variance, followed by Bonferroni post-test, or Student's t-test, was used. The injection of the A5G27, H-89, SU6656 or B I MM alone did not cause change in the mechanical threshold (data not shown). GraphPad Prism 5.0 (GraphPad Software, Inc, San Diego, CA) was used for the graphics and to perform statistical analyses; P < 0.05 was considered statistically significant. Data are presented as mean ± standard error of the mean.
3. Results
3.1. Hyaluronidase and low molecular weight hyaluronan induce mechanical hyperalgesia
We have previously shown that components of the ECM, such as versican, play a role in nociceptor function (Bogen et al., 2009), suggesting an important functional interaction between ECM and the nociceptor. To begin to investigate the role of ECM in nociceptor function, we first evaluated if hyaluronidase, an enzyme that d egrades hyaluronan, a main component of ECM (Jiang et al., 2011), whose action has been associated to inflammatory diseases such as rheumatoid arthritis (Jones, 1950; Regan and Meyer, 1950) and periodontitis (Hershon, 1971), induces mechanical hyperalgesia. Intradermal injection of hyaluronidase (5U) on the dorsum of the hind paw induced intense mechanical hyperalgesia (Fig. 1A). Since hyaluronidase degrades hyaluronan, releasing fragments with different molecular weight from the ECM (Sherman and Back, 2008; Jiang et al., 2011; Preston and Sherman, 2011), we next investigated if the injection of low (LMWH) or high (HMWH) molecular weight hyaluronan would induce changes in the mechanical nociceptive threshold. We observed that HMWH did not significantly change the mechanical threshold, whereas the injection of LMWH produced robust mechanical hyperalgesia (Fig. 1B).
Figure 1. Time course for mechanical hyperalgesia induced by hyaluronidase (A) and hyaluronan (B).
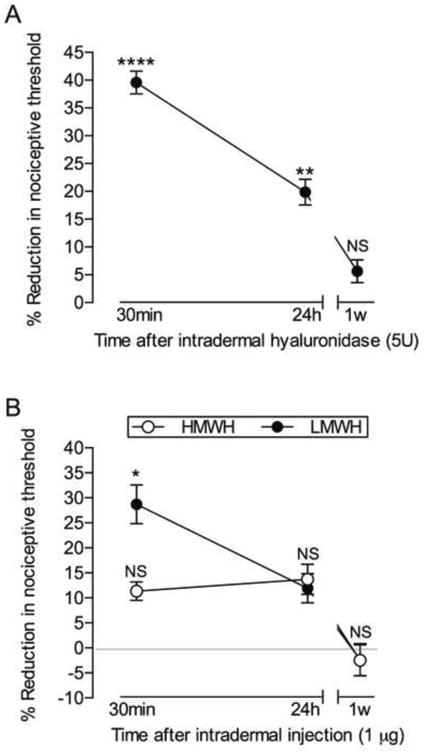
Panel A: Rats received an intradermal injection of hyaluronidase (5U) on the dorsum of the hind paw. Mechanical nociceptive thresholds were evaluated before and 30 min, 24 h and 1 week after injection, by the Randall-Selitto paw withdrawal test. The average baseline mechanical nociceptive threshold was 121.3 ± 1.3 grams. Marked mechanical hyperalgesia was observed when testing was performed 30 min after injection of hyaluronidase (**** p < 0.0001, when mechanical threshold is compared to pre-hyaluronidase level), and was still significant (** p = 0.0031) 24 h later. When evaluated after 1 week, the mechanical nociceptive threshold was no longer different (NS, p = 0.1145) from pre-hyaluronidase levels (one-way repeated measures ANOVA followed by Bonferroni's post hoc test); Panel B: Different groups of rats received intradermal injection of high (HMWH, 1 μg, open symbols) or low (LMWH, 1 μg, dark symbols) molecular weight hyaluronan on the dorsum of the hind paw. The mechanical thresholds were evaluated 30 min, 24 h and 1 week later. Average baseline mechanical nociceptive threshold was 116.3 ± 1.8 grams for HMWH group and 114.6 ± 3.2 for LMWH group. Two-way repeated measures ANOVA followed by Bonferroni's post hoc test showed no significant change (NS) in the mechanical nociceptive threshold after the injection of HMWH, when compared to pre-injection levels. However, significant hyperalgesia was observed at 30 min in the LMWH group (* p = 0.0092, when the mechanical thresholds before and 30 min after injection are compared). When both groups were evaluated again 24 h and one week later, the mechanical thresholds were not statistically different from the baseline levels. n = 6 paws (all groups)
Since HMWH has been shown to have analgesic properties in a rat model of osteoarthritis (Castro et al., 2007), we evaluated its effect against the hyperalgesia induced by hyaluronidase and LMWH. Pretreatment with HMWH (1 μg) significantly attenuated the hyperalgesia produced by both hyaluronidase and LMWH (Fig. 2).
Figure 2. Effect of high molecular weight hyaluronan (HMWH) on the mechanical hyperalgesia induced by hyaluronidase or low molecular weight hyaluronan (LMWH).
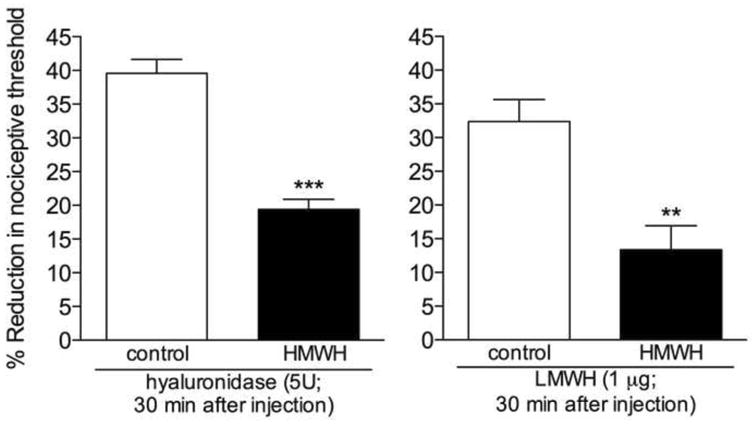
Different groups of rats received an intradermal injection of vehicle (control) or HMWH (1 μg) on the dorsum of the hind paw. 10 min later, hyaluronidase (5U, left panel) or LMWH (1 μg, right panel) was injected at the same site. Comparison of the mechanical thresholds before and 30 min after the injection of hyaluronidase or LMWH showed intense mechanical hyperalgesia in both groups. However, in the groups pretreated with HMWH it was significantly attenuated (left panel: t5 = 7.214, *** p = 0.0008; right panel: t5 = 4.130, ** p = 0.0091, when HMWH-treated and the control groups are compared), indicating an anti-hyperalgesic effect of the HMWH. (Student's t test ; n = 6 paws per group)
3.2. CD44 receptor-mediated hyperalgesia
Hyaluronan has been described to act as an agonist on the CD44 receptor (Bajorath et al., 1998; Teriete et al., 2004). Therefore the next series of experiments evaluated the involvement of the CD44 receptor in hyperalgesia induced by hyaluronidase or LMWH. Pretreatment with the CD44 antagonist A5G27 (1 μg) significantly attenuated the hyperalgesia induced by hyaluronidase or LMWH, indicating a role of this receptor in the increased nociceptor response to mechanical stimulation (Fig. 3).
Figure 3. Role of CD44 in the hyperalgesia induced by hyaluronidase or low molecular weight hyaluronan (LMWH).
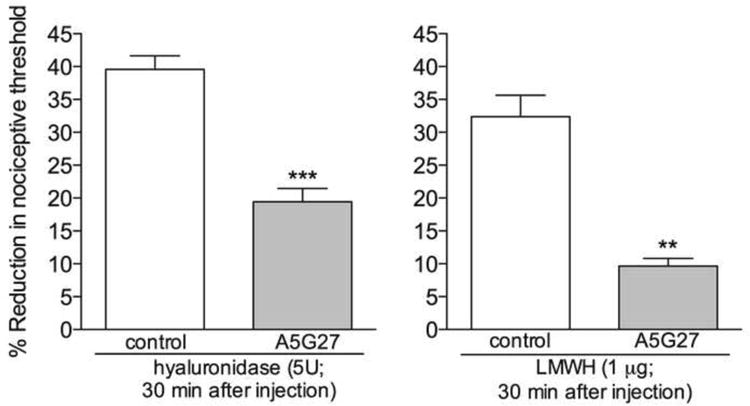
Rats received intradermal injection of vehicle (control) or the CD44 receptor antagonist A5G27 (1 μg) on the dorsum of the hind paw. 10 min later, hyaluronidase (5U, left panel) or LMWH (1 μg, right panel) was injected at the same site. Although mechanical hyperalgesia was observed 30 min after the injection of hyaluronidase or LMWH, in the groups that were pretreated with A5G27 it was significantly attenuated (left panel: t5 = 6.077, *** p = 0.0017; right panel: t5 = 6.644, ** p = 0.0012, when A5G27-treated and the control groups are compared), indicating a role of the CD44 receptor in the hyperalgesia induced by hyaluronidase and LMWH. (Student's t test ; n = 6 paws per group)
Next, we tested if activation of the CD44 receptor would induce changes in the mechanical threshold. Intradermal injection of the peptide A6 (1 μg), a CD44 agonist (Piotrowicz et al., 2011; Finlayson, 2015), induced hyperalgesia that was inhibited by both A5G27 and HMWH (Fig. 4).
Figure 4. CD44 agonist induces mechanical hyperalgesia that is attenuated by the CD44 antagonist A5G27, and high molecular weight hyaluronan (HMWH).
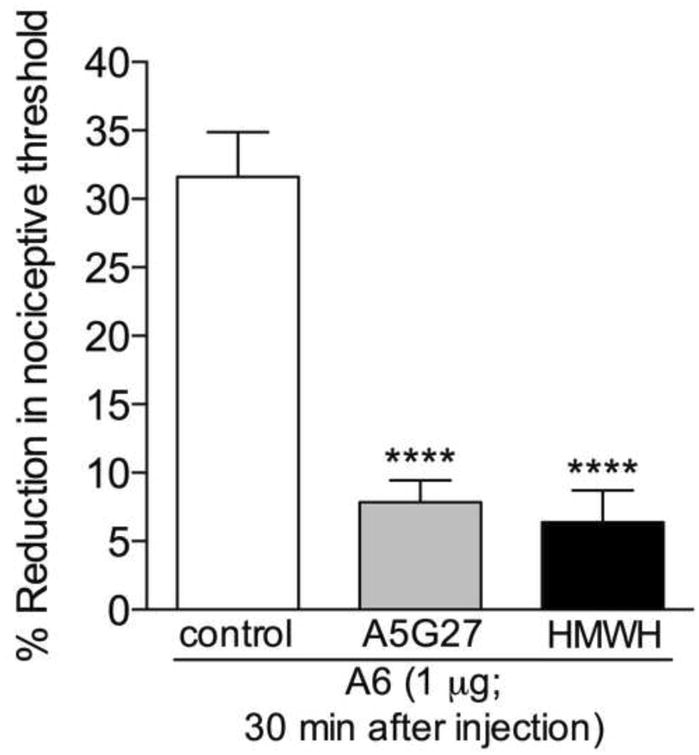
Rats received an intradermal injection of vehicle (control, blank bar), the CD44 receptor antagonist A5G27 (1 μg, gray bar) or HMWH (1 μg, black bar) on the dorsum of the hind paw. 10 minutes later, the CD44 agonist A6 (1 μg) was injected at the same site. Mechanical nociceptive thresholds were evaluated before and 30 min after A6. While in the control group we observed intense mechanical hyperalgesia, in the groups pretreated with A5G27 or HMWH it was significantly attenuated (**** p < 0.0001, when the A5G27- and the HMWH-treated groups are compared to the control group). (One-way ANOVA followed by Bonferroni's post hoc test; n = 6 paws per group)
3.3. Second messengers activated by the CD44 receptor
To investigate the second messengers downstream of the CD44 receptor that play a role in the hyperalgesia induced by its activation, we pretreated rats with H-89 or BIMM, inhibitors for second messengers involved in inflammatory hyperalgesia, PKA and PKC respectively (Gold et al., 1996; Gold et al., 1998; Khasar et al., 1998; Lynn and O'Shea, 1998; Aley and Levine, 1999; Khasar et al., 1999a; Khasar et al., 1999a; Aley et al., 2000; Sachs et al., 2009), 10 minutes before the injection of LMWH, shown to induce hyperalgesia by acting on the CD44 receptor (Fig. 3, left panel), or the CD44 receptor agonist A6. In addition, since Src kinases have been implicated in several models of nociceptor sensitization (Alessandri-Haber et al., 2005), we tested if the Src inhibitor SU6656 would also have an effect on hyperalgesia induced by both agents. Inhibitors of PKA and Src, but not of PKC, significantly attenuated the hyperalgesia induced by LMWH (Fig. 5A) and A6 (Fig. 5B), indicating the signaling pathway downstream of the CD44 that produces mechanical hyperalgesia.
Figure 5. PKA and Src are involved in the mechanical hyperalgesia induced by low molecular weight hyaluronan (LMWH) or a CD44 receptor agonist.
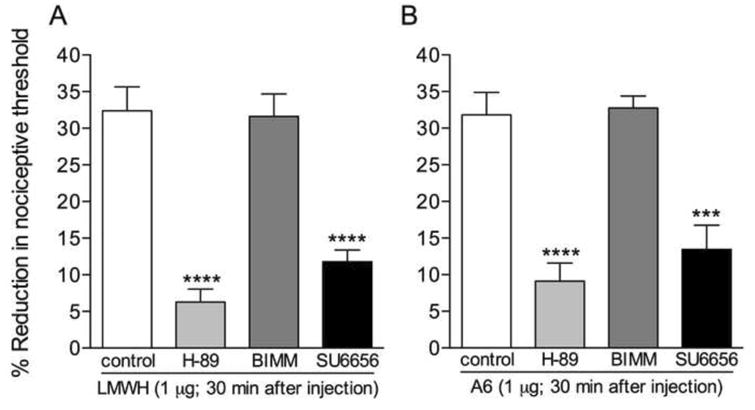
Different groups of rats received an intradermal injection of vehicle (control, blank bars), the PKA inhibitor H-89 (1 μg, light gray bars), the non-selective PKC inhibitor BIMM (1 μg, darker gray bars) or the Src inhibitor SU6656 (1 μg, black bars) on the dorsum of the hind paw. 10 minutes later, LMWH (1 μg, panel A) or the CD44 agonist A6 (1 μg, panel B) was injected at the same site. Mechanical nociceptive thresholds were evaluated before and 30 min after LMWH/A6. We observed significant hyperalgesia in the control and the BIMM-treated groups (both panels). However, in the groups pretreated with H-89 or SU6656 there was significant attenuation of mechanical hyperalgesia (**** p < 0.0001; *** p = 0.005, when the groups treated with H-89 or SU6656 are compared to the control groups), indicating a role of PKA and Src in the hyperalgesia induced by LMWH and A6. (One-way ANOVA followed by Bonferroni's post hoc test; n = 6 paws per group)
3.4 Role of ECM in a preclinical model of inflammatory pain
To investigate the involvement of the ECM in the hyperalgesia produced by inflammation, we used the preclinical model of inflammatory pain produced by intradermal injection of carrageenan (1%) on the dorsum of the rat hind paw (Aley et al., 2000). The CD44 antagonist A5G27 or HMWH, both of which attenuate the hyperalgesia induced by LMWH (Figs. 2 and 3), were injected 10 minutes before carrageenan. 4 h after carrageenan injection, the mechanical threshold was evaluated at the same site. While intense hyperalgesia was observed in the control group, its magnitude was significantly attenuated in the groups pretreated with A5G27 or HMWH, compatible with the suggestion that the hyperalgesia induced by carrageenan is, at least in part, produced by changes in the ECM (Fig. 6).
Figure 6. Role of CD44 receptor in carrageenan-induced mechanical hyperalgesia.
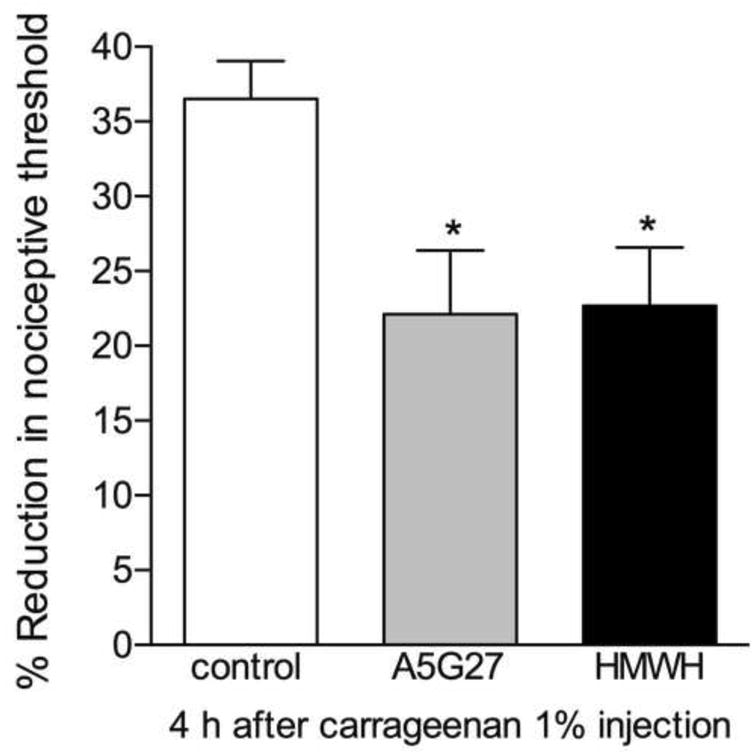
Rats received an intradermal injection of vehicle (control, blank bar) and, the CD44 receptor antagonist A5G27 (1 μg, gray bar) or HMWH (1 μg, black bar) on the dorsum of the hind paw. 10 minutes later, carrageenan (1%) was injected at the same site. Mechanical nociceptive thresholds were evaluated before and 4 h after the injection of carregeenan. Significant attenuation of the hyperalgesia induced by carrageenan was observed in the groups pretreated with A5G27 or HMWH (* p = 0.021, when compared to the control groups), indicating a role for the ECM in nociceptor sensitization produced by carrageenan. (One-way ANOVA followed by Bonferroni's post hoc test; n = 6 paws per group)
4. Discussion
There is increasing evidence stressing the important role of the ECM in the regulation/modulation of the inflammatory process (Toole, 2004; De Bock et al., 2015; Murai, 2015; Schwertfeger et al., 2015; Sawyer and Kyriakides, 2016). For example, versican, hyaluronan and other components of the ECM such as fibronectin or laminin, have been shown to interact with resident cells during inflammation, contributing to their proliferation and migration (Gee et al., 2004; Petrey and de la Motte, 2014; Andersson-Sjöland et al., 2015; Schwertfeger et al., 2015). Moreover, versican was demonstrated to participate in the mechanical hyperalgesia induced by monocyte chemoattractant protein 1 (MCP-1), an inflammatory mediator whose receptor is present on the nociceptor (Bogen et al., 2009), suggesting that ECM contributes to the development of inflammatory pain. In the present study we investigated the mechanism by which hyaluronan, a major component of the ECM, regulates mechanical nociceptive threshold.
Hyaluronan is used in the treatment of pain in patients with osteoarthritis. While its therapeutic action has been considered to be by the viscoelastic action of the high molecular weight hyaluronan (HMWH) in the joint, recent studies of nociceptor function in animal models of osteoarthritis suggest that some of the available compounds, chemically modified hyaluronan, attenuate nociceptor sensitization (Castro et al., 2007; Hashizume et al., 2010). Of note, low molecular weight hyaluronan (LMWH) was less effective at attenuating nociceptor sensitization in the osteoarthritic rat. These findings are in line with our results showing induction of hyperalgesia by hyaluronidase and LMWH, which was attenuated by the pretreatment with HMWH. Also, our experiments confirm distinct functions of different molecular weight forms of hyaluronan in the nervous system, as suggested by previous reports (Sherman and Back, 2008; Preston and Sherman, 2011; Preston et al., 2013). Since inflammation stimulates the secretion of hyauronidase, expressed in the nervous system (Al'Qteishat et al., 2006; Sloane et al., 2010) and by resident cells (Jiang et al., 2011), which degrades hyaluronan, generating products that can act at cell surface receptors to produce a wide range of effects (Sherman and Back, 2008; Jiang et al., 2011; Preston and Sherman, 2011), the hyperalgesia resulting from the hyaluronidase injection is likely the consequence of the production of LMWH, ultimately reflected as an effect in the nociceptor. Thus, we investigated the mechanism involved in this interaction between hyaluronan and the nociceptor terminal.
The CD44 receptor has been described as the main receptor that modulates cell-extracellular matrix interactions (Pesarrodona et al., 2014), and is considered as the cognate receptor for hyaluronan (Bajorath et al., 1998; Teriete et al., 2004; Dzwonek and Wilczynski, 2015). It is found in several cell types, throughout the nervous system, such as glial cells (Bignami and Dahl, 1986; Gorlewicz et al., 2009; McKenzie et al., 1982) and neurons (Ailane et al., 2013; Raber et al., 2014), and in the ECM (Dzwonek and Wilczynski, 2015; Finlayson, 2015; Multhaupt et al., 2016; Murai, 2015; Orian-Rousseau and Sleeman, 2014). Moreover, it has been shown to play a role in cell migration and activation during inflammation (Gee et al., 2004), and in neuronal development and plasticity (Kochlamazashvili et al., 2010; Wlodarczyk et al., 2011; Dzwonek and Wilczynski, 2015). Considering that it is also present on peripheral sensory neurons (Ghosh et al., 2011), the next step in our study was to test the hypothesis that hyaluronan affects nociceptor function by acting at the CD44 receptor.
To evaluate the role of the CD44 receptor in the mechanical hyperalgesia induced by hyaluronidase or LMWH we used the peptide A5G27, demonstrated to bind to the CD44 receptor and to block CD44 signaling (Hibino et al., 2004; Pesarrodona et al., 2014). Since A5G27 significantly attenuated the hyperalgesia induced by hyaluronidase and LMWH, we concluded that the interaction between the nociceptor and LMWH is CD44-mediated. Pretreatment with HMWH significantly attenuated the hyperalgesia induced by hyaluronidase and LMWH, supporting the suggestion that the HMWH effect was also due to an action at the CD44 receptor. Thus, both LMWH and HMWH act at the same receptor, the former as an agonist, and the latter as an antagonist. Since the compounds are administered intradermally in the skin of the rat hind paw, where other cells in addition to the terminals of the nociceptors are present, there is the possibility that the observed effects involve other cells at the site of the injections. Hence, although the CD44 receptor is expressed in dorsal root ganglion neurons (Ghosh et al., 2011), whether this is a direct or indirect signaling mechanism between the ECM and the nociceptor remains to be demonstrated.
To determine the signaling mechanisms by which CD44 regulates nociceptor function, we investigated if second messengers previously shown to play a role in different models of mechanical hyperalgesia (Gold et al., 1996; Gold et al., 1998; Khasar et al., 1998; Lynn and O'Shea, 1998; Aley and Levine, 1999; Khasar et al., 1999a; Khasar et al., 1999a; Aley et al., 2000; Alessandri-Haber et al., 2005; Sachs et al., 2009) were involved in the hyperalgesia produced by activation of CD44. All three intracellular messengers that we evaluated, PKA, PKC and Src, have also been associated to CD44 receptor signaling (Lee et al., 2008; Bourguignon et al., 2009; Bourguignon et al., 2010; Campo et al., 2010; Zhang et al., 2014). Although it has not been established how CD44 interacts with PKA, it has been shown that CD44 can directly activate PKC (Bourguignon et al., 2009; Campo et al., 2010), and members of the Src family kinases are considered crucial for CD44 signaling (Ponta et al., 2003; Skupien et al., 2014; Dzwonek and Wilczynski, 2015). To directly activate the CD44 receptor we used the peptide A6 (Piotrowicz et al., 2011; Finlayson, 2015); its administration produced robust hyperalgesia that was inhibited by both the CD44 antagonist A5G27 and HMWH, confirming the action of A6 on the CD44 receptor. We also evaluated the role of those second messengers in the hyperalgesia induced by LMWH, which is dependent on the CD44 receptor. Both the LMWH- and the A6-induced hyperalgesia were attenuated by inhibitors of PKA and Src, but not PKC, providing evidence for a pathway downstream of the CD44 receptor that produces hyperalgesia. In fact, the attenuation of the CD44-mediated hyperalgesia by either the PKA or the Src inhibitor is compatible with previous reports that indicate crosstalk between PKA and Src mediated signaling (Kawasaki et al., 2004; Obara et al., 2004; Belcher et al., 2005; Gui et al., 2006).
The relationship between changes in the ECM and the increase in the sensitivity of sensory neurons to stimulation has been investigated (Hucho and Levine, 2007; Jeske et al., 2009; Hu et al., 2010; Traverso, 2011; Kubo et al., 2012; Caires et al., 2015). Since the ECM can function as a storage depot for biologically active molecules, such as MCP-1 and tumor necrosis alpha (Edovitsky et al., 2006; Nasser, 2008; Goodall et al., 2014), and pathological conditions can release mediators that can contribute to changes in mechanical, or even thermal, sensitivity (Yamanaka et al., 2004; Li et al., 2012), the ECM can be considered to contribute to inflammatory pain, the integrity of the ECM playing a role in sensory neuron homeostasis (Li et al., 2012). Inflammatory pain is caused, at least in part, by the local release of a wide range of pro-inflammatory cytokines (Liou et al., 2011). Our results support the suggestion that the degradation of the ECM by the inflammatory process (Parish, 2006; Goodall et al., 2014) can activate specific receptors, such as the CD44 and affect nociceptor function. The attenuation of the carrageenan-induced mechanical hyperalgesia by the inhibitors A5G27 and HMWH brings additional information about the mechanisms involved in models of inflammatory pain.
5. Conclusions
In summary, our experiments demonstrate a role of hyaluronan in the modulation of nociceptor function. In addition to confirm a direct effect of the clinically used high molecular weight hyaluronan at the CD44 receptor, the results presented here contribute to our understanding of how the ECM may interact with the nociceptors, which might help in the design of strategies for the treatment of pain of inflammatory origin.
Highlights.
- Hyaluronan modulates nociceptor function by acting on CD44 receptors;
- Different forms of hyaluronan produce distinct effects on the nociceptor;
- Carrageenan-induced hyperalgesia is partially dependent on the extracellular matrix;
- Hyperalgesia produced by CD44 receptor activation is dependent on PKA and Src.
Acknowledgments
This study was funded by the National Institutes of Health (NIH), NS084545.
Abbreviations
- ECM
extracellular matrix
- HMWH
high molecular weight hyaluronan
- LMWH
low molecular weight hyaluronan
- PKA
protein kinase A
- PKC
protein kinase C
Footnotes
Author's contribution: LFF: designed research and performed experiments, analyzed the data and wrote the manuscript; DA: performed experiments; OB: designed research; JDL: designed research, wrote the manuscript. All authors read and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Luiz F. Ferrari, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 521 Parnassus Avenue, San Francisco, CA 94143, USA
Dioneia Araldi, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 521 Parnassus Avenue, San Francisco, CA 94143, USA.
Oliver Bogen, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 521 Parnassus Avenue, San Francisco, CA 94143, USA.
Jon D. Levine, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 521 Parnassus Avenue, San Francisco, CA 94143, USA
References
- Ailane S, Long P, Jenner P, Rose S. Expression of integrin and CD44 receptors recognising osteopontin in the normal and LPS-lesioned rat substantia nigra. Eur J Neurosci. 2013;38:2468–2476. doi: 10.1111/ejn.12231. [DOI] [PubMed] [Google Scholar]
- Al'Qteishat A, Gaffney J, Krupinski J, Rubio F, West D, Kumar S, Kumar P, Mitsios N, Slevin M. Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain. 2006;129:2158–2176. doi: 10.1093/brain/awl139. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib B, Rosenzweig DH, Krock E, Roughley PJ, Beckman L, Steffen T, Weber MH, Ouellet JA, Haglund L. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur Cell Mater. 2014;28:98–110. doi: 10.22203/ecm.v028a08. discussion 110. [DOI] [PubMed] [Google Scholar]
- Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol. 1998;25:2203–2212. [PubMed] [Google Scholar]
- Alvarez P, Green PG, Levine JD. Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain. 2014;155:1161–1167. doi: 10.1016/j.pain.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson-Sjöland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, Rydell-Törmänen K, Bjermer L, Malmström A, Karlsson JC, Westergren-Thorsson G. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology. 2015;25:243–251. doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajorath J, Greenfield B, Munro SB, Day AJ, Aruffo A. Identification of CD44 residues important for hyaluronan binding and delineation of the binding site. J Biol Chem. 1998;273:338–343. doi: 10.1074/jbc.273.1.338. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Tamaki Z, Wang W, Hinchcliff M, Hoover P, Getsios S, White ES, Varga J. FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci Transl Med. 2014;6:232ra50. doi: 10.1126/scitranslmed.3008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A, Dahl D. Brain-specific hyaluronate-binding protein. A product of white matter astrocytes. J Neurocytol. 1986;15:671–679. doi: 10.1007/BF01611865. [DOI] [PubMed] [Google Scholar]
- Bogen O, Bender O, Löwe J, Blenau W, Thevis B, Schröder W, Margolis RU, Levine JD, Hucho F. Neuronally produced versican V2 renders C-fiber nociceptors IB4 -positive. J Neurochem. 2015;134:147–155. doi: 10.1111/jnc.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Dreger M, Gillen C, Schröder W, Hucho F. Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. FEBS J. 2005;272:1090–1102. doi: 10.1111/j.1742-4658.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- Borle AB, Snowdowne KW. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982;217:252–254. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284:26533–26546. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Wong G, Earle C, Krueger K, Spevak CC. Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem. 2010;285:36721–36735. doi: 10.1074/jbc.M110.162305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch RM, Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987;84:6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Caires R, Luis E, Taberner FJ, Fernandez-Ballester G, Ferrer-Montiel A, Balazs EA, Gomis A, Belmonte C, de la Peña E. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat Commun. 2015;6:8095. doi: 10.1038/ncomms9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, Micali A, Nastasi G, Squadrito F, Altavilla D, Bitto A, Polito F, Rinaldi MG, Calatroni A, D'Ascola A, Campo S. High-molecular weight hyaluronan reduced renal PKC activation in genetically diabetic mice. Biochim Biophys Acta. 2010;1802:1118–1130. doi: 10.1016/j.bbadis.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Castro RR, Feitosa JP, da Cunha PL, da Rocha FA. Analgesic activity of a polysaccharide in experimental osteoarthritis in rats. Clin Rheumatol. 2007;26:1312–1319. doi: 10.1007/s10067-006-0506-9. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Altman RD, Hollstrom R, Hollstrom C, Sun C, Gipson B. Safety and efficacy of intra-articular sodium hyaluronate (Hyalgan) in a randomized, double-blind study for osteoarthritis of the ankle. Foot Ankle Int. 2008;29:657–663. doi: 10.3113/FAI.2008.0657. [DOI] [PubMed] [Google Scholar]
- Cowman MK, Schmidt TA, Raghavan P, Stecco A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Res. 2015;4:622. doi: 10.12688/f1000research.6885.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock M, Wang N, Decrock E, Bultynck G, Leybaert L. Intracellular Cleavage of the Cx43 C-Terminal Domain by Matrix-Metalloproteases: A Novel Contributor to Inflammation. Mediators Inflamm. 2015;2015:257471. doi: 10.1155/2015/257471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase Cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthritis Cartilage. 1993;1:97–103. doi: 10.1016/s1063-4584(05)80024-x. [DOI] [PubMed] [Google Scholar]
- Dzwonek J, Wilczynski GM. CD44: molecular interactions, signaling and functions in the nervous system. Front Cell Neurosci. 2015;9:175. doi: 10.3389/fncel.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edovitsky E, Lerner I, Zcharia E, Peretz T, Vlodavsky I, Elkin M. Role of endothelial heparanase in delayed-type hypersensitivity. Blood. 2006;107:3609–3616. doi: 10.1182/blood-2005-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmorsy S, Funakoshi T, Sasazawa F, Todoh M, Tadano S, Iwasaki N. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthritis Cartilage. 2014;22:121–127. doi: 10.1016/j.joca.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Finlayson M. Modulation of CD44 Activity by A6-Peptide. Front Immunol. 2015;6:135. doi: 10.3389/fimmu.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee K, Kryworuchko M, Kumar A. Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2004;52:13–26. [PubMed] [Google Scholar]
- Ghosh B, Li Y, Thayer SA. Inhibition of the plasma membrane Ca2+ pump by CD44 receptor activation of tyrosine kinases increases the action potential afterhyperpolarization in sensory neurons. J Neurosci. 2011;31:2361–2370. doi: 10.1523/JNEUROSCI.5764-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall KJ, Poon IK, Phipps S, Hulett MD. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One. 2014;9:e109596. doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlewicz A, Wlodarczyk J, Wilczek E, Gawlak M, Cabaj A, Majczynski H, Nestorowicz K, Herbik MA, Grieb P, Slawinska U, Kaczmarek L, Wilczynski GM. CD44 is expressed in non-myelinating Schwann cells of the adult rat, and may play a role in neurodegeneration-induced glial plasticity at the neuromuscular junction. Neurobiol Dis. 2009;34:245–258. doi: 10.1016/j.nbd.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem. 2006;281:14015–14025. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Koike N, Yoshida H, Suzuki M, Mihara M. High molecular weight hyaluronic acid relieved joint pain and prevented the progression of cartilage degeneration in a rabbit osteoarthritis model after onset of arthritis. Mod Rheumatol. 2010;20:432–438. doi: 10.1007/s10165-010-0299-1. [DOI] [PubMed] [Google Scholar]
- Hershon LE. Elaboration of hyaluronidase and chondroitin sulfatase by microorganisms inhabiting the gingival sulcus: evaluation of a screening method for periodontal disease. J Periodontol. 1971;42:34–36. doi: 10.1902/jop.1971.42.1.34. [DOI] [PubMed] [Google Scholar]
- Hibino S, Shibuya M, Engbring JA, Mochizuki M, Nomizu M, Kleinman HK. Identification of an active site on the laminin alpha5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. 2004;64:4810–4816. doi: 10.1158/0008-5472.CAN-04-0129. [DOI] [PubMed] [Google Scholar]
- Hu J, Chiang LY, Koch M, Lewin GR. Evidence for a protein tether involved in somatic touch. EMBO J. 2010;29:855–867. doi: 10.1038/emboj.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Henry MA, Milam SB. Fibronectin stimulates TRPV1 translocation in primary sensory neurons. J Neurochem. 2009;108:591–600. doi: 10.1111/j.1471-4159.2008.05779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ES. Hyaluronidase activity in the skin, rheumatic disease, and salicylates. Ann Rheum Dis. 1950;9:137–148. doi: 10.1136/ard.9.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvinen S, Kosma VM, Tammi MI, Tammi R. Hyaluronan, CD44 and versican in epidermal keratinocyte tumours. Br J Dermatol. 2003;148:86–94. doi: 10.1046/j.1365-2133.2003.05028.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999a;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999b;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- Kochlamazashvili G, Henneberger C, Bukalo O, Dvoretskova E, Senkov O, Lievens PM, Westenbroek R, Engel AK, Catterall WA, Rusakov DA, Schachner M, Dityatev A. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron. 2010;67:116–128. doi: 10.1016/j.neuron.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Katanosaka K, Mizumura K. Extracellular matrix proteoglycan plays a pivotal role in sensitization by low pH of mechanosensitive currents in nociceptive sensory neurones. J Physiol. 2012;590:2995–3007. doi: 10.1113/jphysiol.2012.229153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, van Wijnen AJ, Im HJ. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527:440–447. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Wang MJ, Sudhir PR, Chen JY. CD44 engagement promotes matrix-derived survival through the CD44-SRC-integrin axis in lipid rafts. Mol Cell Biol. 2008;28:5710–5723. doi: 10.1128/MCB.00186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang B, Gao T, Zhang X, Hao JX, Vlodavsky I, Wiesenfeld-Hallin Z, Xu XJ, Li JP. Heparanase overexpression reduces carrageenan-induced mechanical and cold hypersensitivity in mice. Neurosci Lett. 2012;511:4–7. doi: 10.1016/j.neulet.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Liou JT, Liu FC, Mao CC, Lai YS, Day YJ. Inflammation confers dual effects on nociceptive processing in chronic neuropathic pain model. Anesthesiology. 2011;114:660–672. doi: 10.1097/ALN.0b013e31820b8b1e. [DOI] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B, O'Shea NR. Inhibition of forskolin-induced sensitisation of frog skin nociceptors by the cyclic AMP-dependent protein kinase A antagonist H-89. Brain Res. 1998;780:360–362. doi: 10.1016/s0006-8993(97)01360-7. [DOI] [PubMed] [Google Scholar]
- Mabuchi K, Tsukamoto Y, Obara T, Yamaguchi T. The effect of additive hyaluronic acid on animal joints with experimentally reduced lubricating ability. J Biomed Mater Res. 1994;28:865–870. doi: 10.1002/jbm.820280805. [DOI] [PubMed] [Google Scholar]
- Martignetti JA, Aqeel AA, Sewairi WA, Boumah CE, Kambouris M, Mayouf SA, Sheth KV, Eid WA, Dowling O, Harris J, Glucksman MJ, Bahabri S, Meyer BF, Desnick RJ. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet. 2001;28:261–265. doi: 10.1038/90100. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, Watanabe H. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. 2003;278:41205–41212. doi: 10.1074/jbc.M305060200. [DOI] [PubMed] [Google Scholar]
- McKenzie JL, Dalchau R, Fabre JW. Biochemical characterisation and localization in brain of a human brain-leucocyte membrane glycoprotein recognised by a monoclonal antibody. J Neurochem. 1982;39:1461–1466. doi: 10.1111/j.1471-4159.1982.tb12592.x. [DOI] [PubMed] [Google Scholar]
- Multhaupt HA, Leitinger B, Gullberg D, Couchman JR. Extracellular matrix component signaling in cancer. Adv Drug Deliv Rev. 2016;97:28–40. doi: 10.1016/j.addr.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Murai T. Lipid Raft-Mediated Regulation of Hyaluronan-CD44 Interactions in Inflammation and Cancer. Front Immunol. 2015;6:420. doi: 10.3389/fimmu.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser NJ. Heparanase involvement in physiology and disease. Cell Mol Life Sci. 2008;65:1706–1715. doi: 10.1007/s00018-008-7584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara Y, Labudda K, Dillon TJ, Stork PJ. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci. 2004;117:6085–6094. doi: 10.1242/jcs.01527. [DOI] [PubMed] [Google Scholar]
- Orian-Rousseau V, Sleeman J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv Cancer Res. 2014;123:231–254. doi: 10.1016/B978-0-12-800092-2.00009-5. [DOI] [PubMed] [Google Scholar]
- Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- Pesarrodona M, Ferrer-Miralles N, Unzueta U, Gener P, Tatkiewicz W, Abasolo I, Ratera I, Veciana J, Schwartz S, Villaverde A, Vazquez E. Intracellular targeting of CD44+ cells with self-assembling, protein only nanoparticles. Int J Pharm. 2014;473:286–295. doi: 10.1016/j.ijpharm.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowicz RS, Damaj BB, Hachicha M, Incardona F, Howell SB, Finlayson M. A6 peptide activates CD44 adhesive activity, induces FAK and MEK phosphorylation, and inhibits the migration and metastasis of CD44-expressing cells. Mol Cancer Ther. 2011;10:2072–2082. doi: 10.1158/1535-7163.MCT-11-0351. [DOI] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J, Baggenstoss B, Weigel PH, Montine TJ, Back SA, Sherman LS. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73:266–280. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M, Sherman LS. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 2011;3:1165–1179. doi: 10.2741/218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Olsen RH, Su W, Foster S, Xing R, Acevedo SF, Sherman LS. CD44 is required for spatial memory retention and sensorimotor functions. Behav Brain Res. 2014;275:146–149. doi: 10.1016/j.bbr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin EL, Swann DA, Weisser PA. Separation of a hyaluronate-free lubricating fraction from synovial fluid. Nature. 1970;228:377–378. doi: 10.1038/228377a0. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- Regan C, Meyer K. Hyaluronic acid-hyaluronidase and the rheumatic diseases. Ann N Y Acad Sci. 1950;52:1108–1111. doi: 10.1111/j.1749-6632.1950.tb54012.x. [DOI] [PubMed] [Google Scholar]
- Sachs D, Villarreal C, Cunha F, Parada C, Ferreira S. The role of PKA and PKCepsilon pathways in prostaglandin E2-mediated hypernociception. Br J Pharmacol. 2009;156:826–834. doi: 10.1111/j.1476-5381.2008.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer AJ, Kyriakides TR. Matricellular proteins in drug delivery: Therapeutic targets, active agents, and therapeutic localization. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertfeger KL, Cowman MK, Telmer PG, Turley EA, McCarthy JB. Hyaluronan, Inflammation, and Breast Cancer Progression. Front Immunol. 2015;6:236. doi: 10.3389/fimmu.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Back SA. A ‘GAG’ reflex prevents repair of the damaged CNS. Trends Neurosci. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Skupien A, Konopka A, Trzaskoma P, Labus J, Gorlewicz A, Swiech L, Babraj M, Dolezyczek H, Figiel I, Ponimaskin E, Wlodarczyk J, Jaworski J, Wilczynski GM, Dzwonek J. CD44 regulates dendrite morphogenesis through Src tyrosine kinase-dependent positioning of the Golgi. J Cell Sci. 2014;127:5038–5051. doi: 10.1242/jcs.154542. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14:953–961. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res. 1989;487:148–151. doi: 10.1016/0006-8993(89)90950-5. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res. 1989;492:397–399. doi: 10.1016/0006-8993(89)90928-1. [DOI] [PubMed] [Google Scholar]
- Teriete P, Banerji S, Noble M, Blundell CD, Wright AJ, Pickford AR, Lowe E, Mahoney DJ, Tammi MI, Kahmann JD, Campbell ID, Day AJ, Jackson DG. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Mol Cell. 2004;13:483–496. doi: 10.1016/s1097-2765(04)00080-2. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Traverso S. Mechanical signalling in tissues and its possible role in nociception. Theor Biol Forum. 2011;104:75–84. [PubMed] [Google Scholar]
- Triantaffilidou K, Venetis G, Bika O. Efficacy of hyaluronic acid injections in patients with osteoarthritis of the temporomandibular joint. A comparative study. J Craniofac Surg. 2013;24:2006–2009. doi: 10.1097/SCS.0b013e3182a30566. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. P2X4 receptors and neuropathic pain. Front Cell Neurosci. 2013;7:191. doi: 10.3389/fncel.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Nasu-Tada K, Koizumi S, Yamamoto K, Ando J, Inoue K. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- Unsworth A, Dowson D, Wright V. Some new evidence on human joint lubrication. Ann Rheum Dis. 1975;34:277–285. doi: 10.1136/ard.34.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira CP, De Aro AA, Da Ré Guerra F, De Oliveira LP, De Almeida MS, Pimentel ER. Inflammatory process induced by carrageenan in adjacent tissue triggers the acute inflammation in deep digital flexor tendon of rats. Anat Rec (Hoboken) 2013;296:1187–1195. doi: 10.1002/ar.22729. [DOI] [PubMed] [Google Scholar]
- Vieira CP, de Aro AA, de Almeida MS, de Mello GC, Antunes E, Pimentel ER. Effects of acute inflammation induced in the rat paw on the deep digital flexor tendon. Connect Tissue Res. 2012;53:160–168. doi: 10.3109/03008207.2011.620189. [DOI] [PubMed] [Google Scholar]
- Ween MP, Oehler MK, Ricciardelli C. Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. Int J Mol Sci. 2011;12:1009–1029. doi: 10.3390/ijms12021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN. Arterial remodeling in vascular disease: a key role for hyaluronan and versican. Front Biosci. 2008;13:4933–4937. doi: 10.2741/3052. [DOI] [PubMed] [Google Scholar]
- Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–161. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk J, Mukhina I, Kaczmarek L, Dityatev A. Extracellular matrix molecules, their receptors, and secreted proteases in synaptic plasticity. Dev Neurobiol. 2011;71:1040–1053. doi: 10.1002/dneu.20958. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Saga S, Kato M, Bernfield M, Kimata K. Selective distributions of proteoglycans and their ligands in pericellular matrix of cultured fibroblasts. Implications for their roles in cell-substratum adhesion. J Cell Sci. 1993;106:55–65. doi: 10.1242/jcs.106.1.55. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Obata K, Fukuoka T, Dai Y, Kobayashi K, Tokunaga A, Noguchi K. Tissue plasminogen activator in primary afferents induces dorsal horn excitability and pain response after peripheral nerve injury. Eur J Neurosci. 2004;19:93–102. doi: 10.1046/j.1460-9568.2003.03080.x. [DOI] [PubMed] [Google Scholar]
- Yong N, Guoping C. The role and mechanism of the up-regulation of fibrinolytic activity in painful peripheral nerve injury. Neurochem Res. 2009;34:587–592. doi: 10.1007/s11064-008-9826-2. [DOI] [PubMed] [Google Scholar]
- Zhang P, Goodrich C, Fu C, Dong C. Melanoma upregulates ICAM-1 expression on endothelial cells through engagement of tumor CD44 with endothelial E-selectin and activation of a PKCα-p38-SP-1 pathway. FASEB J. 2014;28:4591–4609. doi: 10.1096/fj.11-202747. [DOI] [PMC free article] [PubMed] [Google Scholar]


