The recent Zika virus (ZIKV) epidemic in the Americas has been declared a global public health emergency because of its association with severe fetal brain injury during infection of pregnant women.1 ZIKV is a mosquito-transmitted flavivirus that historically caused a self-limited febrile syndrome characterized by rash, conjunctivitis, and arthralgia. However, infection in pregnant women has become a major concern because of a congenital ZIKV syndrome that includes a range of central nervous system anomalies such as marked cerebral atrophy, ventriculomegaly, intracerebral necrosis/calcifications, cerebellar hypoplasia, and ocular manifestations.2 The lack of an experimental animal model that closely resembles human pregnancy has hindered the ability to directly test the causal relationship between ZIKV infection and fetal brain injury3, although recent studies in mice demonstrate that ZIKV infection of pregnant dams can cause fetal brain injury and demise.4–6 Herein, we describe the development of a pigtail macaque model of ZIKV infection during pregnancy that results in fetal brain malformations and lesions. Periventricular lesions developed 10 days after ZIKV inoculation and evolved asymmetrically in the occipital-parietal lobes on serial MRI scans. At term, autopsy of the fetus revealed ZIKV RNA in the brain and significant cerebral white matter hypoplasia, periventricular white matter gliosis, axonal and ependymal injury and marked induction of activated caspase 3 in subependymal cells. Fetal brain injury occurred in the context of a clinically inapparent ZIKV infection in the pregnant dam, which mimics the majority of human cases. This pigtail macaque confirms the teratogenic potential of ZIKV and will enable testing of new therapeutics to prevent fetal brain injury.
We inoculated ZIKV (strain FSS13025, Cambodia 2010) subcutaneously at five separate locations on the forearms, each with 107 plaque-forming units (PFU) into a healthy pregnant pigtail macaque at 119 days gestation (~28 weeks human pregnancy) to test directly whether ZIKV causes fetal brain injury. The pregnant animal appeared healthy throughout the study and never developed signs of rash, conjunctivitis or fever; thus, this is a model of a clinically inapparent ZIKV infection in a nonhuman primate. Cesarean section was performed at 162 days gestation (~38 weeks human pregnancy), at which time the animal was not in labor. The fetus was imaged weekly by ultrasound for biometric measurements and to evaluate for possible fetal brain abnormalities. Fetal dates were determined initially by measurement of the crown rump at 45 days; ultrasound measurements of the biparietal diameter (BPD) at 101 days gestation supported the dating. Over time, a lag in growth of the BPD developed (Fig. 1A), which fell three standard deviations over six weeks following ZIKV inoculation. In contrast, femur length (Fig. 1B) remained 1 to 2 standard deviations above the mean for the duration of the study. Baseline imaging of the fetal head showed normal architecture of the cerebral hemispheres, ventricles and posterior fossa; over time, however, an area of linear heterogeneity developed adjacent to the lateral ventricle in the posterior right brain (Fig. 1C).
Figure 1. Fetal Biometry and Ultrasound of the Fetal Brain.
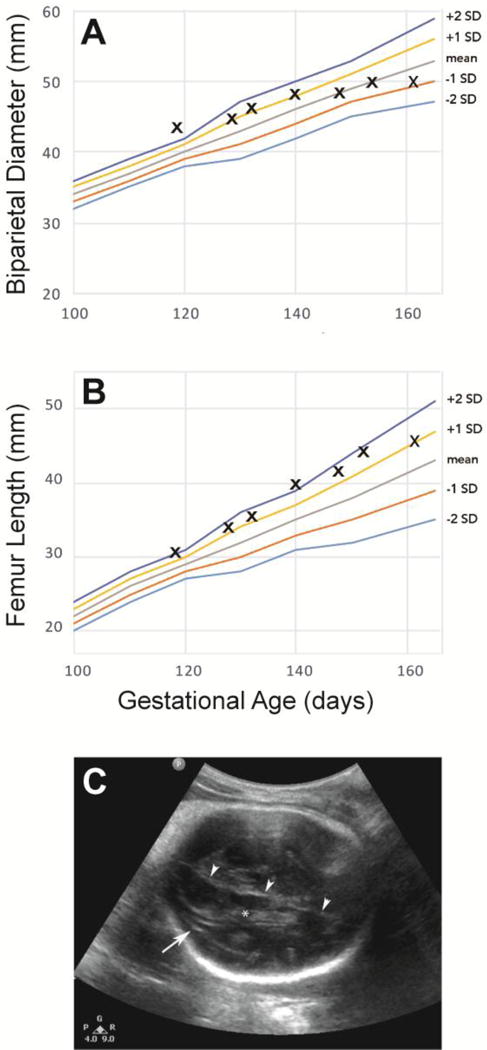
Fetal biometry demonstrates a lag in BPD growth (A) in contrast to continued growth of the femur length (B). An area of linear heterogeneity (arrow) developed in the fetal brain adjacent to the lateral ventricle in the posterior right brain (C). Note the choroid plexus in the lateral ventricle (*) and falx (arrowheads).
The first MRI of the fetal brain was performed 10 days following ZIKV inoculation and demonstrated a bilateral periventricular T2 hyperintense signal in the parietal-occipital regions surrounding the occipital horns of the lateral ventricles (Fig. 2, and Fig. S1–S3). This abnormality evolved over the course of the study and enlarged on the right; on the left, the T2 hyperintense focus became hypointense and was associated with volume loss and ventricular collapse by 162 days gestation. Magnetic resonance (MR) imaging with 3D isotropic resolution and motion correction with careful tissue segmentation (0.5 mm; Fig. S4) revealed that white matter volume did not change significantly over the last 3 weeks of the study (17–43 days post-inoculation), which correlated with the plateau in growth of the biparietal diameter on ultrasound (Table S1 and Fig. 1A). In contrast, the gray matter consistently increased in volume. The white matter surface area, shape and cortical folding continued to evolve (Table S1 and Fig. S5). At this developmental stage in this species, the posterior ventricular cerebrospinal fluid regions normally contract in size with development of the parietal and occipital lobes; after ZIKV inoculation, large posterior ventricular cerebrospinal fluid spaces persisted into late gestation. The surrounding posterior white matter also showed enhancement in T2 weighting, indicating increased water content. These combined fluid regions were manually delineated from the 3D images to provide a summary volume measure of the enhancing T2 weighted fluid abnormality (Table S1). There was no evidence of cortical malformation (e.g. polymicrogyria) discernable on MR imaging over the six weeks of this study. The brainstem and cerebellum also appeared grossly normal. MR spectroscopy demonstrated a marked reduction in N-acetyl aspartate (NAA), a metabolite associated with neuronal integrity, in the posterior (near the MR abnormality) versus the anterior brain (Fig. S6). The rapid development of an enhancing T2 weighted fluid abnormality suggests that fetal brain injury begins to occur quickly following ZIKV infection of the dam and may begin in the periventricular regions similar to other flaviviruses [e.g. Japanese Encephalitis Virus (JEV)].
Figure 2. Serial Fetal Brain MRI Imaging.
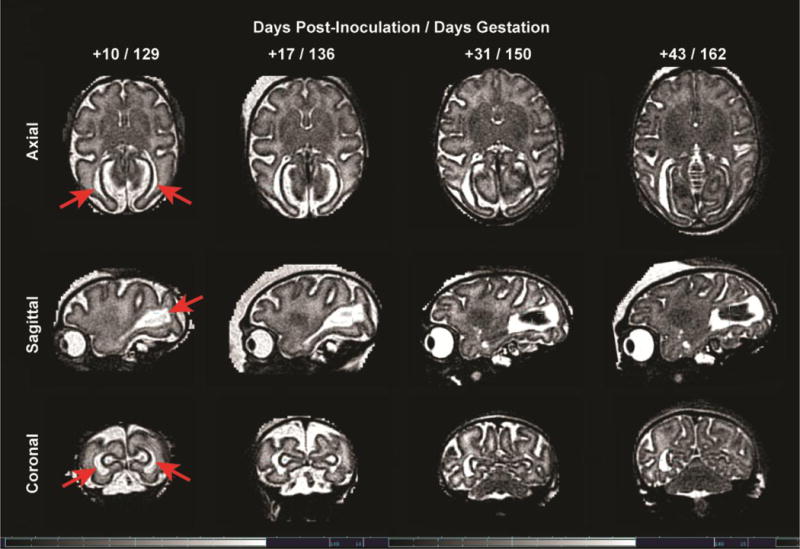
Serial MRI images of the fetal brain over time from 10 to 43 days post-inoculation (129 to 162 days gestation) demonstrate a bilateral periventricular T2 hyperintense foci that evolves over time with concomitant loss of volume in the posterior brain.
Macroscopic examination of the fetal brain at autopsy demonstrated marked deficiency of the white matter posteriorly in comparison to controls (Fig. 3). Histologically, white matter gliosis was present bilaterally with many apoptotic and mitotic figures (Fig. 3D and 3F). Immunohistochemistry revealed increased density of glial fibrillary acidic protein (GFAP)-positive reactive astrocytes, predominantly in the white matter (Fig. 3H) and increased CD163-positive microglial cells (data not shown). Flow cytometry of matched regions of the fetal and maternal brain also indicated an enrichment of astrocytes in the fetal brain (Table S2). Ovoid eosinophilic structures were seen in the gliotic white matter, consistent with axonal spheroids (Fig. 3F inset), a pathologic sign of axon injury. The occipital horn of the right lateral ventricle was mildly dilated and there was mild focal gliosis in the gray matter. A notable finding was the bilateral patchy loss of ventricular ependymal epithelium with underlying hypercellularity of the parenchyma (Fig. S7B) in the ZIKV-infected animal; there was an associated marked increase in activated caspase 3 immunoreactivity (Fig. S7D) with CD163-positive histiocytes adhered to the denuded ventricle surface (data not shown). In contrast, few caspase 3 immunoreactive cells were present in the periventricular zone of an uninfected fetal control of similar gestational age (Fig. S7C). Necrosis, hemorrhage, calcifications or definite viral cytopathy were absent. Minimal inflammation was observed in the brain parenchyma or meninges; CD20 and CD3-positive cells were rare (not shown). The fetal brainstem and other maternal/fetal organs were unremarkable. Histopathology of the placenta revealed only mild deciduitis, which was also present in some uninfected controls.
Figure 3. Neuropathology of the ZIKV Infected Fetus and Control.
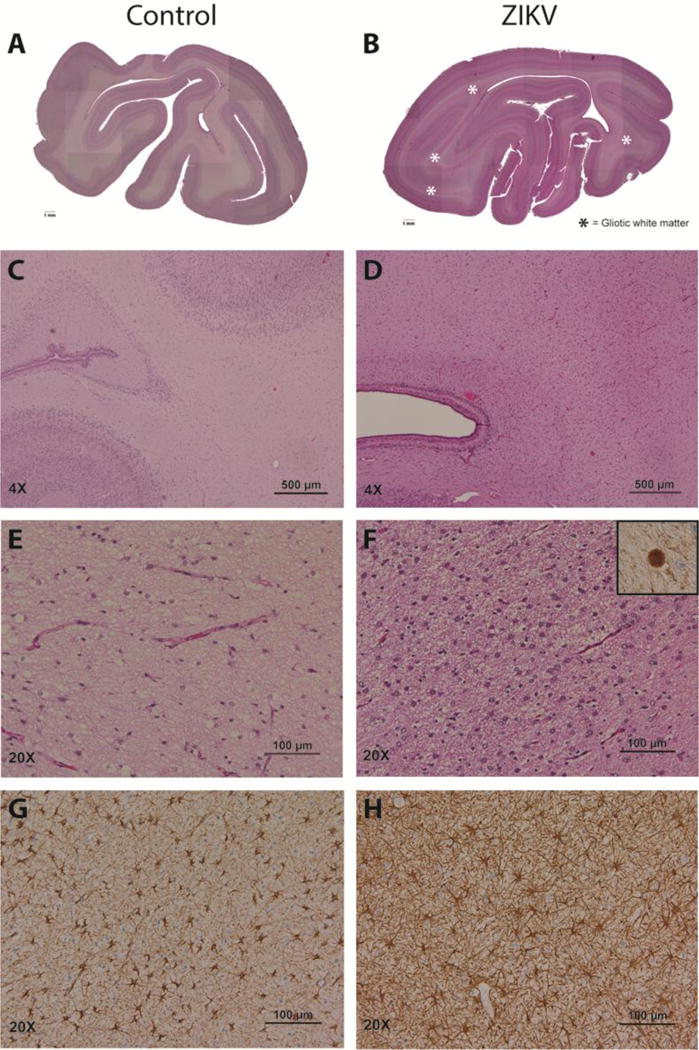
In contrast with sections from similar regions of a two-week younger control brain (A, C, E, G), the occipital cortex of the ZIKV-infected fetus (B, D, F, H) showed marked white matter hypoplasia and gliosis (pink bands indicated by asterisks). Gliosis in the deep white matter corresponded to abundant reactive astrocytes (D, F versus C, E) with increased GFAP immunoreactivity (H versus G). Axonal spheroids, a marker of severe axon injury, were seen only in the ZIKV fetus; the spheroids were confirmed to express neurofilament protein (F, inset).
Prior to inoculation, serology was negative for prior infection with ZIKV, Dengue virus (DENV), West Nile virus, or chikungunya virus (CHIKV). The dam (mother) became seropositive for ZIKV-specific IgG at 14 days post-inoculation and remained positive at all subsequent time points; the fetus was seropositive at birth (Table S3). At delivery, ZIKV RNA was detected in the fetal brain, eyes, and testes, as well as the maternal eyes, kidney and chorionic villi of the placenta (Fig. 4A and Table S4). Zika virus E protein was detected in the fetal brain by immunostaining (Fig. 4B). ZIKV RNA was not detected in the liver, adrenal glands, lungs, or digestive organs. Viral plaque assays on all tissues with detectable ZIKV RNA were negative for replicating virus suggesting that the level of infectious virus was too low for detection or that the infectious virus had been cleared.
Figure 4. ZIKV RNA and Proteins in Fetal Tissues.
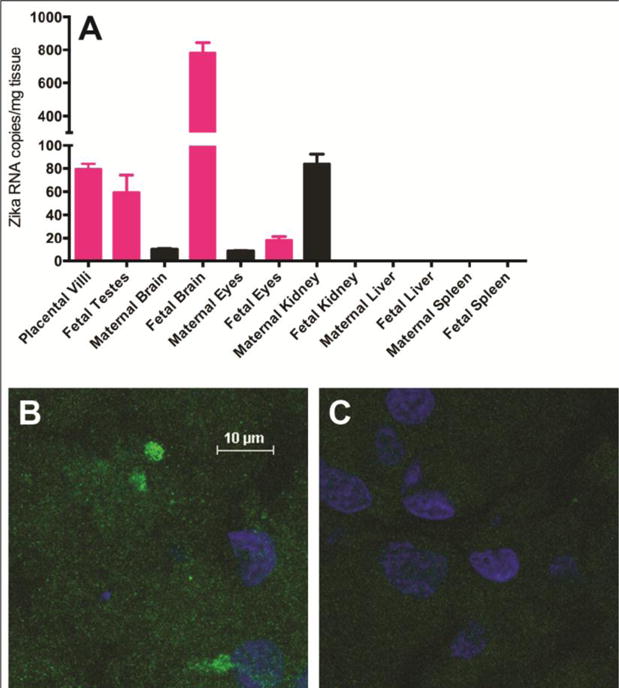
ZIKV RNA levels were measured by a Taqman qRT-PCR assay and calculated as mean viral copy number per mg of tissue using a standard curve (analyzed in triplicate; A). Error bars reflect standard deviation in technical replicates. Confocal fluorescent microscopy was performed on fetal brain sections stained with a primary antibody against the Zika virus E protein (B) and with secondary antibody alone (C). Nuclei were counterstained with DAPI (blue). All images are at the same scale.
Detection of ZIKV RNA in fetal and maternal tissues six weeks after viral challenge suggests that ZIKV may persistently replicate in some organs, which has implications for horizontal (sexual) and vertical (maternal-to-fetal) ZIKV transmission. The highest copy number of ZIKV RNA in our study was found in the fetal brain indicating that arrested growth of the fetal brain is likely a consequence of viral infection. Persistent ZIKV RNA in the fetal testes is consistent with reports of the virus in semen and male sexual transmission7 and recent studies in mice.8,9 Apart from the brain and eyes, the other maternal organ with persistent ZIKV RNA was the kidney, which correlates with presence of ZIKV RNA in urine much longer than observed in sera or peripheral blood mononuclear cells.10 The persistence of ZIKV in the placental chorionic villi represents a possible source of virus that may underlie persistent maternal viremia throughout pregnancy as described in a case report.11 These data indicate the permissive nature of these tissues for viral replication and possible persistence during infections in vivo.
Our study describes the first case of fetal brain injury in a nonhuman primate after maternal ZIKV infection. Arrested fetal brain growth, white matter injury, and detection of ZIKV RNA in multiple fetal organs (brain, eyes) are features consistent with a congenital ZIKV syndrome. Further, the fetal brain injury occurred in the context of a clinically silent infection, which likely mimics the majority of human cases. Thus, at least in animal models of vertical transmission, clinically apparent ZIKV infection of the mother is not required for the development of congenital malformations in the fetus. The distribution of white matter injury is predicted to result in visual field loss (injury to optic tracts) and neurodevelopmental delay.12 A similar, but more severe pattern of white matter injury with involvement of the occipital horns of the lateral ventricle also was seen in a Brazilian child with congenital ZIKV syndrome (Fig. S8). Although we did not observe many of the ultrasound findings associated with human congenital ZIKV syndrome including brain atrophy, we posit that this may be due to terminating the study 6 weeks post-inoculation or virus inoculation in the latter half of pregnancy.
Development of a nonhuman primate model with strong similarities to human pregnancy is critical for understanding the factors that contribute to fetal injury from ZIKV infection in pregnancy. Nonhuman primates are ideal for studying infection in pregnancy, because they most closely mimic human gestation in many key respects including placentation, gray/white matter ratios in brain, timing of neurodevelopment and placentation.13 ZIKV has been detected in several nonhuman primate species (e.g. rhesus, marmoset and howler monkeys)14–16, but it was unknown which species might manifest fetal brain injury similar to humans. The pigtail macaque is also known to be susceptible to multiple Flaviviridae family members (e.g., DENV, JEV, and hepatitis C virus), other viruses (e.g., CHIKV and Kaposi’s sarcoma associated herpesvirus), and other human pathogens (e.g., malaria, tuberculosis, and chlamydia), which makes them an excellent model of human infectious diseases.17–20 In summary, we demonstrate that a clinically inapparent maternal ZIKV infection during pregnancy has the capacity to result in fetal brain injury in a pregnant pigtail macaque. Further infection studies at different times in pregnancy are needed to corroborate and extend the observations and investigate mechanisms of fetal injury. Finally, the pigtail macaque model may have significant utility for testing novel vaccines and therapeutics to prevent vertical transmission and the congenital ZIKV syndrome.
Supplementary Material
Acknowledgments
We would like to acknowledge Jan Hamanishi for technical assistance with preparation of the figures and Dr. Lara Brandão (Neuroradiologist, - Clínica Felippe Mattoso, Grupo Fleury, Rio de Janeiro, Brazil) for providing images and clinical information from a child with congenital ZIKV syndrome. We thank Dr. Robert Tesh, (University of Texas Medical Branch at Galveston/World Reference Center for Emerging Viruses and Arboviruses) for providing viral seed stocks. We thank Dr. Ann Powers (Centers for Disease Control) for technical guidance for the qRT-PCR experiments and Connie Hughes for administrative assistance. We also thank Dr. Marie-Terese Little for technical editing.
This work was supported by funding from the University of Washington Department of Obstetrics & Gynecology and the National Institutes of Health, Grant # R01AI100989 to L.R and K. A. W, and AI104002, AI083019, and AI104002 to M.G Jr. and AI073755 to M.S.D. The NIH training grants T32 HD007233 (PI: Lisa Frenkel) and T32 AI07509 (PI: Lee Ann Campbell) supported E.B and J.V, respectively. This project was also supported by the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number P51OD010425.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions
KAW, JES, RPK, CS, MKD, MG Jr. and LR designed the study
KAW, JES, RPK, CS, EB, JV, AB, MKD, JT, SM, BA, JT, MAD, ECD, MRF, CG, TR, EF, MSD, LK, JO, GMG, WL, CE and LR performed the experiments
KAW, JES, RPK, CS, EB, JV, AB, MKD, JT, SM, BA, JT, MAD, ECD, MRF, CG, TR, GAG, SEJ, RFG, LK, DWWS, RFH, WBD, MG Jr. and LR analyzed the data
KAW, JES, RPK, CS, EB, JV, MKD, MAD, TR, MSD, DWWS, RFH, MG Jr. and LR drafted the manuscript
All authors reviewed the final draft of manuscript.
References
- 1.Gulland A. Zika virus is a global public health emergency, declares WHO. BMJ. 2016;352:i657. doi: 10.1136/bmj.i657. [DOI] [PubMed] [Google Scholar]
- 2.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects - Reviewing the Evidence for Causality. N Engl J Med. 2016 doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 4.Miner JJ, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cugola FR, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu KY, et al. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016;26:645–654. doi: 10.1038/cr.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso D, et al. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazear HM, et al. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016 doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi SL, et al. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016 doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driggers RW, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 2016 doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 12.Lennartsson F, Nilsson M, Flodmark O, Jacobson L. Damage to the immature optic radiation causes severe reduction of the retinal nerve fiber layer, resulting in predictable visual field defects. Invest Ophthalmol Vis Sci. 2014;55:8278–8288. doi: 10.1167/iovs.14-14913. [DOI] [PubMed] [Google Scholar]
- 13.Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG. 2011;118:136–144. doi: 10.1111/j.1471-0528.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 15.Favoretto S, et al. First detection of Zika virus in neotropical primates in Brazil: a possible new reservoir. bioRxiv. 2016 [Google Scholar]
- 16.Pan American Health Organization/World Health Organization. Zika Epidemiological Update, 4 April 2016. Washington, D.C.: 2016. [Google Scholar]
- 17.Bruce AG, et al. Next-generation sequence analysis of the genome of RFHVMn, the macaque homolog of Kaposi’s sarcoma (KS)-associated herpesvirus, from a KS-like tumor of a pig-tailed macaque. Journal of virology. 2013;87:13676–13693. doi: 10.1128/JVI.02331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakgoi K, et al. Dengue, Japanese encephalitis and Chikungunya virus antibody prevalence among captive monkey (Macaca nemestrina) colonies of Northern Thailand. Am J Primatol. 2014;76:97–102. doi: 10.1002/ajp.22213. [DOI] [PubMed] [Google Scholar]
- 19.Putaporntip C, et al. Ecology of malaria parasites infecting Southeast Asian macaques: evidence from cytochrome b sequences. Mol Ecol. 2010;19:3466–3476. doi: 10.1111/j.1365-294X.2010.04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sourisseau M, et al. Hepatic cells derived from induced pluripotent stem cells of pigtail macaques support hepatitis C virus infection. Gastroenterology. 2013;145:966–969 e967. doi: 10.1053/j.gastro.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


