Abstract
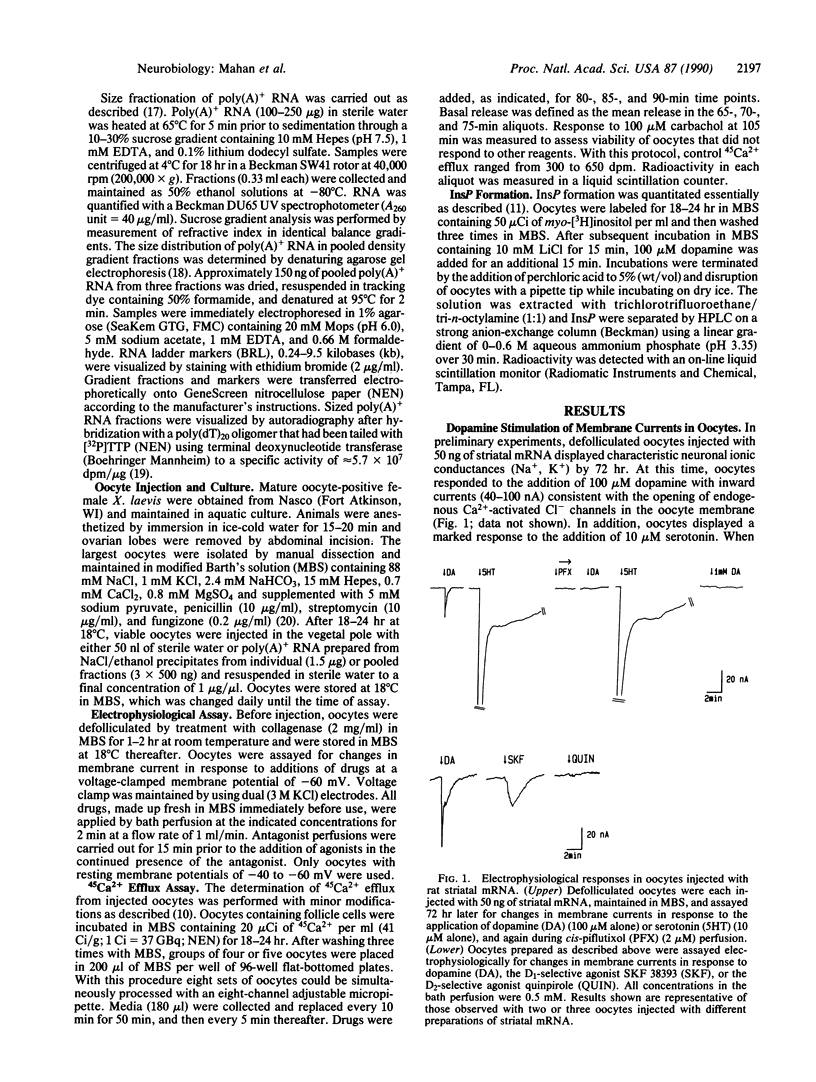
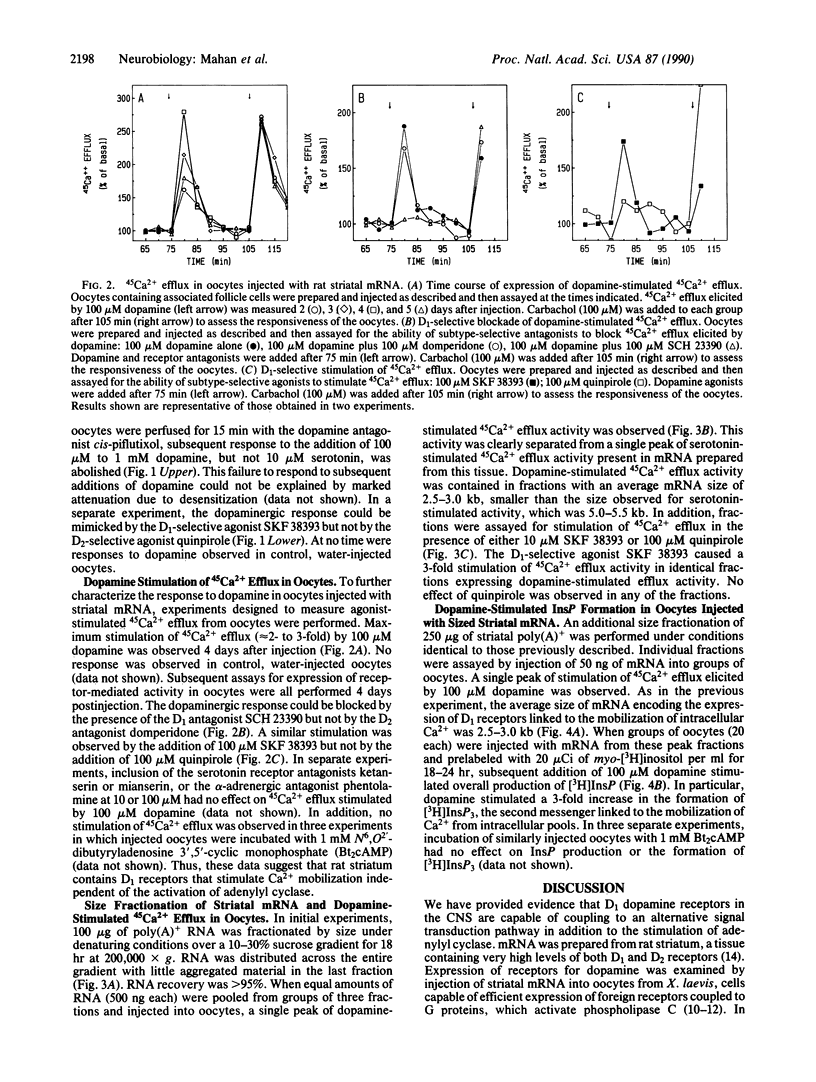
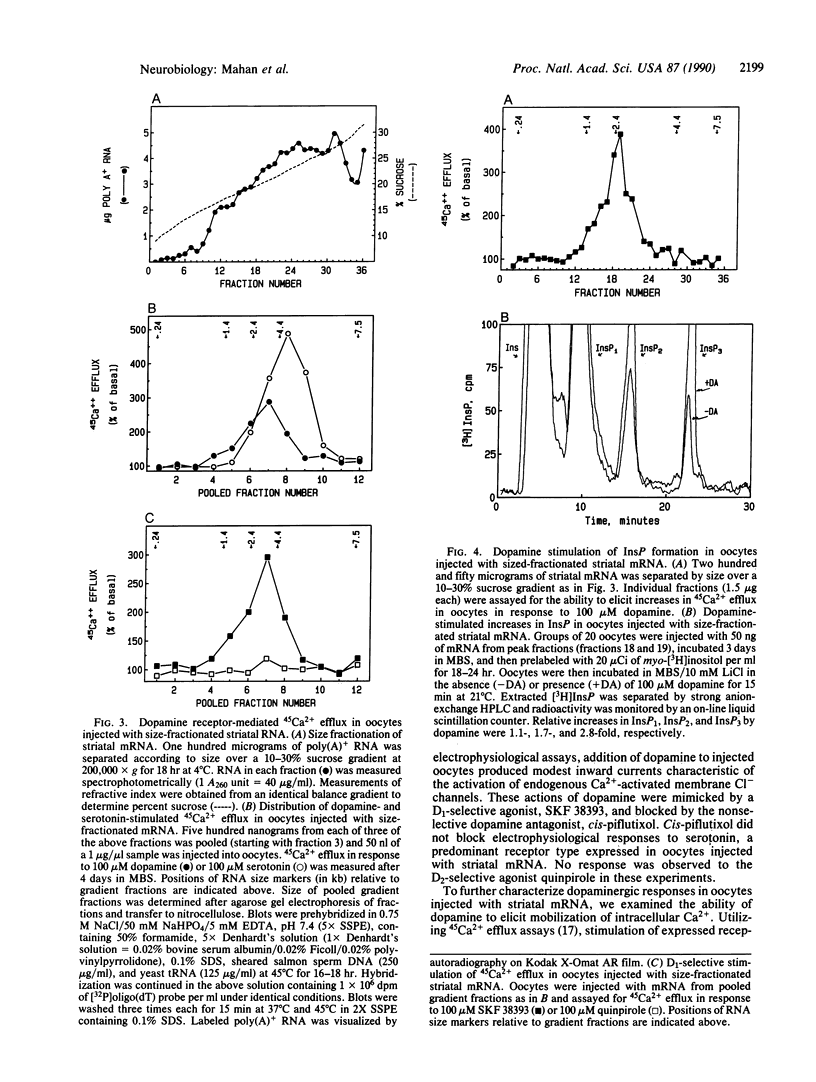
Expression of central nervous system receptors for dopamine was examined by injection of poly(A)+ RNA (mRNA) from rat striatum into oocytes from Xenopus laevis. Electrophysiological measurements in mRNA-injected oocytes indicated that addition of 100 microM dopamine induced an inward current (40-100 nA) that was consistent with the activation of endogenous Ca2(+)-dependent Cl- channels. This current was also elicited by addition of the selective D1 agonist SKF 38393 but not by the selective D2 agonist quinpirole. Prior addition of the dopaminergic antagonist cis-piflutixol completely abolished dopamine-induced currents but had no effect on currents produced by serotonin. Using 45Ca2+ efflux assays, addition of 100 microM dopamine to injected oocytes stimulated efflux 2- to 3-fold. This increase was mimicked by SKF 38393 and was blocked by the D1-selective antagonist (+)SCH 23390 but not by the D2-selective antagonist domperidone. No increase in 45Ca2+ efflux was seen with 100 microM quinpirole. Size fractionation of striatal mRNA yielded a single peak (2.5-3.0 kilobases) of D1 receptor-mediated 45Ca2+ efflux activity in injected oocytes. In addition, dopamine stimulation of oocytes injected with peak fractions and prelabeled with myo-[3H]inositol caused a 3-fold increase in [3H]inositol 1,4,5-triphosphate [( 3H]InsP3) formation. No effect on [3H]InsP3 production or 45Ca2+ efflux was observed, however, in injected oocytes incubated with 1 mM N6,O2'-dibutyryladenosine 3',5'-cyclic monophosphate. Thus, in addition to D1 receptors that stimulate adenylyl cyclase, rat striatum contains D1 receptors that can couple to InsP3 formation and mobilization of intracellular Ca2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G., Norman A. B., Hess E. J., Creese I. D2 dopamine receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in rat striatum. Neurosci Lett. 1985 Aug 30;59(2):177–182. doi: 10.1016/0304-3940(85)90196-x. [DOI] [PubMed] [Google Scholar]
- Battaglia G., Norman A. B., Hess E. J., Creese I. Forskolin potentiates the stimulation of rat striatal adenylate cyclase mediated by D-1 dopamine receptors, guanine nucleotides, and sodium fluoride. J Neurochem. 1986 Apr;46(4):1180–1185. doi: 10.1111/j.1471-4159.1986.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Bigornia L., Suozzo M., Ryan K. A., Napp D., Schneider A. S. Dopamine receptors on adrenal chromaffin cells modulate calcium uptake and catecholamine release. J Neurochem. 1988 Oct;51(4):999–1006. doi: 10.1111/j.1471-4159.1988.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Bonner T. I. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989 Apr;12(4):148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Boyson S. J., McGonigle P., Molinoff P. B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986 Nov;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Christensen A. V., Arnt J., Hyttel J., Larsen J. J., Svendsen O. Pharmacological effects of a specific dopamine D-1 antagonist SCH 23390 in comparison with neuroleptics. Life Sci. 1984 Apr 16;34(16):1529–1540. doi: 10.1016/0024-3205(84)90607-6. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., Schwinn D. A., Randall R. R., Lefkowitz R. J., Caron M. G., Kobilka B. K. Molecular cloning and expression of the cDNA for the hamster alpha 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I., Sibley D. R., Hamblin M. W., Leff S. E. The classification of dopamine receptors: relationship to radioligand binding. Annu Rev Neurosci. 1983;6:43–71. doi: 10.1146/annurev.ne.06.030183.000355. [DOI] [PubMed] [Google Scholar]
- De Keyser J., Dierckx R., Vanderheyden P., Ebinger G., Vauquelin G. D1 dopamine receptors in human putamen, frontal cortex and calf retina: differences in guanine nucleotide regulation of agonist binding and adenylate cyclase stimulation. Brain Res. 1988 Mar 8;443(1-2):77–84. doi: 10.1016/0006-8993(88)91600-9. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Blecher M., Jose P. A. Dopamine-1-mediated stimulation of phospholipase C activity in rat renal cortical membranes. J Biol Chem. 1989 May 25;264(15):8739–8745. [PubMed] [Google Scholar]
- Felder C. C., Jose P. A., Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989 Jan;248(1):171–175. [PubMed] [Google Scholar]
- Freedman J. E., Weight F. F. Single K+ channels activated by D2 dopamine receptors in acutely dissociated neurons from rat corpus striatum. Proc Natl Acad Sci U S A. 1988 May;85(10):3618–3622. doi: 10.1073/pnas.85.10.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M., Casey P. J., Gilman A. G. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989 Aug;3(10):2125–2131. [PubMed] [Google Scholar]
- Hartig P. R. Molecular biology of 5-HT receptors. Trends Pharmacol Sci. 1989 Feb;10(2):64–69. doi: 10.1016/0165-6147(89)90080-1. [DOI] [PubMed] [Google Scholar]
- Hoyer D. Molecular pharmacology and biology of 5-HT1C receptors. Trends Pharmacol Sci. 1988 Mar;9(3):89–94. doi: 10.1016/0165-6147(88)90174-5. [DOI] [PubMed] [Google Scholar]
- Jope R. S., Li X. Inhibition of inositol phospholipid synthesis and norepinephrine-stimulated hydrolysis in rat brain slices by excitatory amino acids. Biochem Pharmacol. 1989 Feb 15;38(4):589–596. doi: 10.1016/0006-2952(89)90203-7. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Kato K., Yamagishi S., Sugiyama H., Nomura Y. GTP-binding proteins Gi and Go transplanted onto Xenopus oocyte by rat brain messenger RNA. Brain Res. 1987 Dec;427(1):11–19. doi: 10.1016/0169-328x(87)90039-8. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Matsui H., Kobilka T. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J., Regan J. W. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987 Oct 30;238(4827):650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- Mailman R. B., Schulz D. W., Kilts C. D., Lewis M. H., Rollema H., Wyrick S. The multiplicity of the D1 dopamine receptor. Adv Exp Med Biol. 1986;204:53–72. doi: 10.1007/978-1-4684-5191-7_4. [DOI] [PubMed] [Google Scholar]
- McIntosh R. P., Catt K. J. Coupling of inositol phospholipid hydrolysis to peptide hormone receptors expressed from adrenal and pituitary mRNA in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9045–9048. doi: 10.1073/pnas.84.24.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Okayama H., Kawaichi M., Brownstein M., Lee F., Yokota T., Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- Regan J. W., Kobilka T. S., Yang-Feng T. L., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6301–6305. doi: 10.1073/pnas.85.17.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch T. P. The use of Xenopus oocytes to probe synaptic communication. Trends Neurosci. 1988 Jun;11(6):250–256. doi: 10.1016/0166-2236(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Smith J. C., Bell L., Richards B. M., Barnard E. A. The molecular cloning and characterisation of cDNA coding for the alpha subunit of the acetylcholine receptor. Nucleic Acids Res. 1982 Oct 11;10(19):5809–5822. doi: 10.1093/nar/10.19.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Vallar L., Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol Sci. 1989 Feb;10(2):74–77. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- Watling K. J., Dowling J. E. Dopaminergic mechanisms in the teleost retina. I. Dopamine-sensitive adenylate cyclase in homogenates of carp retina; effects of agonists, antagonists, and ergots. J Neurochem. 1981 Feb;36(2):559–568. doi: 10.1111/j.1471-4159.1981.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Williams J. A., McChesney D. J., Calayag M. C., Lingappa V. R., Logsdon C. D. Expression of receptors for cholecystokinin and other Ca2+-mobilizing hormones in Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4939–4943. doi: 10.1073/pnas.85.13.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]



