Abstract
Atypical femoral fractures (AFFs) are a rare association of anti-resorptive therapy for osteoporosis. Limited evidence-based management guidelines on their optimal treatment exist, with observational studies suggesting a role for teriparatide (TPTD) in AFF healing. We report a case of a 65-year-old woman with postmenopausal osteoporosis who sustained an AFF following long-term bisphosphonate therapy, and who subsequently developed a new contralateral AFF after completion of TPTD therapy and initiation of strontium ranelate (SR) treatment. The sequence of events in this case report showed that TPTD and SR did not prevent the development of a new AFF, and questions the optimal treatment of these stress fractures.
Abbreviations: AFF, Atypical femoral fracture; TPTD, Teriparatide; DXA, Dual energy X-ray absorptiometry; BMD, Bone mineral density; SR, Strontium ranelate; PTH, Parathyroid hormone
Keywords: Osteoporosis, Antiresorptive, Anabolic, Atypical femoral fracture, Teriparatide, Strontium ranelate
Highlights
-
•
A patient sustained a bisphosphonate-associated atypical femoral fracture (AFF).
-
•
A contralateral AFF occurred despite sequential therapy with teriparatide and strontium ranelate.
-
•
The role of teriparatide in the treatment of AFF remains uncertain.
-
•
The use of strontium ranelate following an AFF is cautioned.
1. Introduction
Antiresorptive drugs are well-established treatments for the management of osteoporosis, but atypical femoral fractures (AFFs) have emerged as a rare long-term association, particularly following bisphosphonate therapy. Since the first reports of bisphosphonate-associated AFF in 2005 (Odvina et al., 2005), a limited literature has examined the optimal treatment of these fractures. Observational studies and case reports suggest a possible role for conservative management of incomplete AFF, with success in fracture healing with teriparatide (TPTD) and strontium ranelate (SR) also being reported.
In 2010, the American Society of Bone and Mineral Research commissioned an International Task Force report to clinically define these fractures and provide guidelines for their management (Shane et al., 2010). This report was revised in 2013 (Shane et al., 2014) and recommended that TPTD be “considered for those who appear not to heal on conservative therapy”. The role of anabolic therapy in the conservative management of incomplete AFF is queried by the following case report of a patient developing a new contralateral AFF after completion of TPTD, and during SR, therapy.
2. Case report
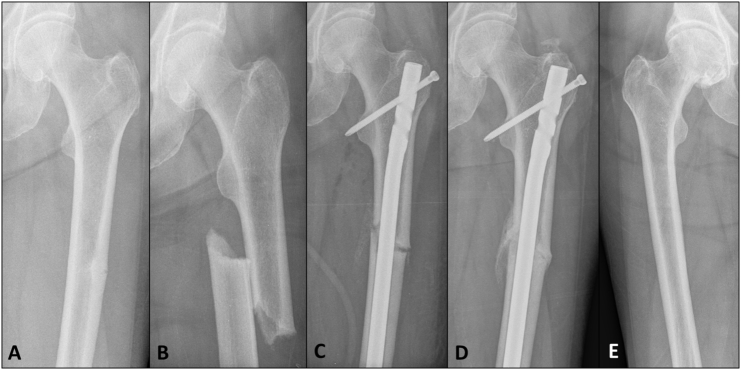
In 2010, a 61-year-old woman with postmenopausal osteoporosis presented to her treating physician with left thigh pain in the setting of eleven years of alendronate therapy. A plain x-ray revealed an incomplete stress fracture of the left femoral shaft consistent with a bisphosphonate-related atypical femoral fracture (AFF) (Fig. 1A).
Fig. 1.
2010 (A) Antero-posterior radiograph of left femur demonstrating an incomplete transverse mid-shaft lateral stress fracture consistent with an atypical femoral fracture. (B) Progression to a complete fracture following a minimal trauma fall. (C) Surgical fixation with an intra-medullary nail. (D) Radiographic healing present at 3 months post-fixation. (E) Radiograph of right femur confirms the absence of a contralateral incomplete AFF.
The patient's past history included multiple fragility fractures (left distal fibula, left fifth metatarsal fracture, T11 vertebral fracture); thyroidectomy for toxic multi-nodular goiter; surgically resected colon cancer in remission; depression; hypertension; and osteoarthritis. Severe postmenopausal osteoporosis was diagnosed in 1998 by dual-energy X-ray absorptiometry (DXA) scan, with bone mineral density (BMD) at the lumbar spine and left femoral neck of 0.595 g/cm2 (T-score − − 4.1) and 0.535 g/cm2 (T-score − 3.6), respectively (Fig. 2). Menopause occurred at the age of 47. Previous osteoporosis therapy included five years of hormone replacement therapy, eleven years of alendronate, and cholecalciferol supplementation. Prior attempts at bisphosphonate “drug holidays” were unsuccessful due to either a subsequent decline in bone density or a further fragility fracture (Fig. 2).
Fig. 2.
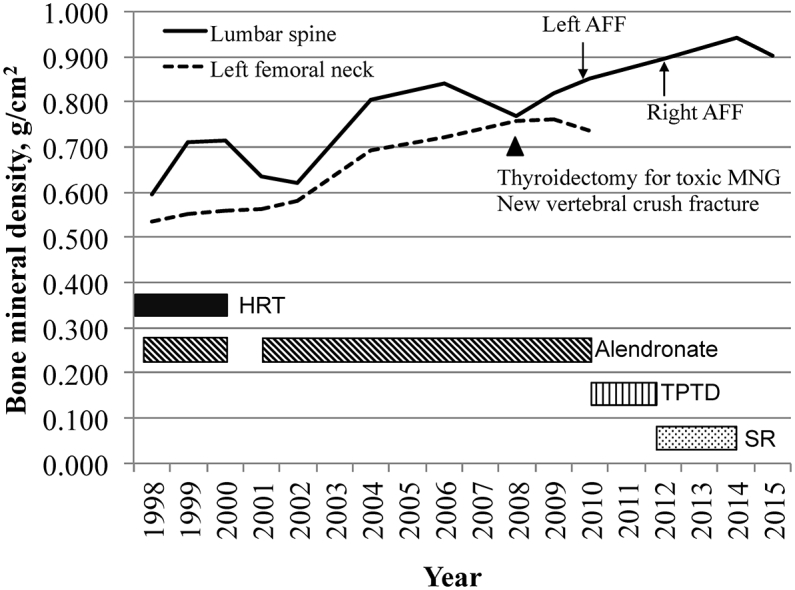
Trend in bone mineral density between 1998 and 2015, and correlation with osteoporosis therapy. HRT = hormone replacement therapy; AFF = Atypical femoral fracture; MNG = multi-nodular goiter; BMD = bone mineral density; TPTD = teriparatide; SR = Strontium ranelate.
Following the diagnosis of the AFF, alendronate was immediately discontinued, limited weight-bearing was advised, and the patient was referred for an early surgical opinion. Within one week, however, she sustained a complete fracture at the site following a fall (Fig. 1B). This required urgent surgical fixation with an intramedullary rod (Fig. 1C). Biochemical evaluation at the time of fracture revealed insufficient 25-hydroxy vitamin D levels [43, reference range (RR) 50–150 nmol/l], but otherwise normal levels of albumin-corrected calcium (2.42, RR 2.12–2.63 mmol/l); phosphate (1.16, RR 0.87–1.45 mmol/l); creatinine (88, RR 49–90 μmol/L); alkaline phosphatase (87, RR 50–140 U/l); and thyrotropin stimulating hormone (0.59, RR 0.35–4.94 μU/ml). Bone mineral density measurements, prior to surgical fixation, of the lumbar spine and left femoral neck were 0.851 g/cm2 (T-score − 2.7) and 0.736 g/cm2 (T-score − 2.0), respectively.
Post-operative recovery was uncomplicated, TPTD was commenced in the early post-operative period, and cholecalciferol supplementation was increased to 2000 IU per day. Radiographic evidence of fracture union was seen three months post-fixation (Fig. 1D) and radiographs of the right femur performed at that time did not reveal a contralateral stress fracture (Fig. 1E).
In 2012, the patient completed 18 months of TPTD therapy and was commenced on sequential strontium ranelate (SR) treatment. Bone density at the lumbar spine and right femoral neck had significantly increased, being 0.895 g/cm2 (T-score − 2.4) and 0.908 g/cm2 (T-score − 0.6), respectively. 25-Hydroxyvitamin D levels were sufficient on cholecalciferol supplementation (82 nmol/l) and a procollagen type 1 amino-terminal propeptide level (P1NP) was not suppressed (58, RR < 76 μg/l). Calcium, phosphate, creatinine and alkaline phosphate levels remained normal.
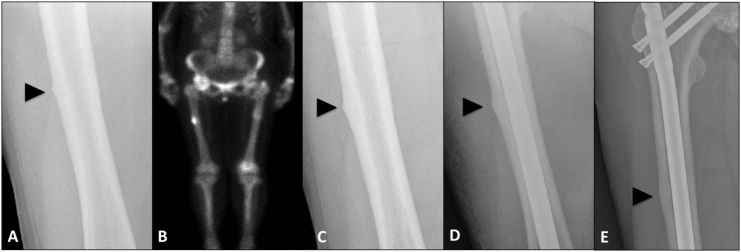
However, within four months of TPTD cessation and during SR therapy, the patient complained of new right-sided thigh pain. An initial radiograph demonstrated a possible right mid-shaft femoral cortical reaction (Fig. 3A) and a bone scan confirmed uptake in the right femur consistent with a new incomplete contralateral AFF (Fig. 3B). This was initially managed conservatively with limited weight-bearing, but serial radiographs confirmed AFF progression with development of a cortical lucency at the fracture site (Fig. 3C), and the fracture required surgical stabilization (Fig. 3D). Four months following fixation, near resolution of the stress fracture was seen radiographically (Fig. 3E). SR was continued for another 22 months following right femoral fixation, and ceased in 2014 due to concern about potential adverse cardiovascular effects. In 2015, a follow-up DXA scan revealed stable osteopenia at the lumbar spine (BMD 0.902 g/cm2, T-score − 2.3) and the patient remained symptom-free and functionally independent.
Fig. 3.
2012 (A) Anterio-posterior radiograph demonstrating cortical reaction at the mid-shaft of the right femur. (B) Bone scan showing uptake at the mid-shaft of the right femur consistent with a new contralateral stress fracture. (C) Radiological evidence of incomplete AFF progression with development of cortical linear lucency. (D) Prophylactic surgical fixation with an intramedullary nail. (E) Four months following fixation, radiographic evidence of near resolution of stress fracture healing.
3. Discussion
We report the case of a postmenopausal woman who sustained a left-sided AFF following long-term bisphosphonate therapy. Contrary to the reported success of anabolic therapy in the management of AFF (Carvalho et al., 2011, Gomberg et al., 2011, Huang et al., 2012, Tarazona-Santabalbina and Aguilella-Fernandez, 2013), following a course of TPTD treatment and initiation of SR, a new contralateral AFF developed. This report highlights the need for more systematic research into the efficacy of TPTD or SR in the routine management of AFF.
AFFs are considered rare associations of antiresorptive therapy, particularly following long-term bisphosphonate therapy. The incidence of AFF in bisphosphonate naïve populations is estimated to be 0.09 per 10,000 patient-years, but rises to 5.5 per 10,000 patient-years in bisphosphonate-users (Schilcher, Michaelsson, and Aspenberg, 2011). AFF is associated with a longer duration of bisphosphonate use (Dell et al., 2012) and, although the risk of further AFF is reduced by 70% within the first year of bisphosphonate therapy cessation (Schilcher et al., 2011), there still remains an ongoing risk for a contralateral AFF, which may be explained by the long skeletal half-life of bisphosphonates. Contralateral AFF can occur in up to 40% of cases, and may present up to four years following the index case (Lo et al., 2012). Following the index AFF, there may be increased weight-bearing on the contralateral unaffected limb, thus increasing the mechanical load and predisposing to a contralateral AFF.
Although the true pathogenesis of AFF remains largely unknown, its association with anti-resorptive therapy requires consideration. Bisphosphonate therapy suppresses bone remodeling, and may change the material quality of bone by (i) altering collagen maturity and cross-linking, (ii) allowing accumulation of advanced glycation end products, (ii) altering the hydroxyapatite crystalline structure, and (iv) forming fully mineralized and homogenous cortical bone (Ettinger, Burr, and Ritchie, 2013). These contribute to bone that is stiffer and brittle. Mechanical loading on brittle bone can lead to micro-crack development. Suppression of bone remodeling by bisphosphonates impedes intracortical repair of micro-cracks, and can result in micro-crack progression to a complete or displaced fracture.
Once an AFF is complete or displaced, urgent surgical fixation is clearly required. However, in the setting of an incomplete or early AFF, the evidence for management is less defined, with two options available: (a) early prophylactic fixation, or (b) conservative management with limited weight-bearing.
The natural history of incomplete AFF has been associated with low rates of spontaneous healing if managed conservatively, with up to 72% requiring surgical intervention due to progression to complete AFF, or due to clinical or radiological deterioration (Ha, Cho, Park, Kim, and Koo, 2010). The presence of cortical lucency, or the ‘dreaded black line’, is associated with higher rates of progression to complete or displaced fracture (Koh, Goh, Png, Kwek, and Howe, 2010). As demonstrated in our case, cortical lucency was seen on the initial radiographs of the left-sided AFF (Fig. 1A), and the patient progressed to a complete fracture within a week. In the conservative management of our patient's contralateral right-sided AFF, serial radiographs showed progression with the development of cortical lucency (Fig. 3C). Evidence suggests that cortical lucency may herald an impending complete fracture, and may be a reliable radiological indicator of those who need semi-urgent prophylactic fixation.
There has been much interest in the use of pharmacological agents to aid fracture union and healing time in patients with AFF, thus allowing a non-surgical management option with perhaps faster time to achieving mobility independence. TPTD is a recombinant portion of the amino-terminal 34 amino acids of human parathyroid hormone and is approved for use in the management of severe osteoporosis. Intermittent administration has pleiotropic effects which leads to an increase in osteoblast cell number and bone remodeling, resulting in a net increase in bone formation (Jilka, 2007).
TPTD has also been shown to accelerate fracture healing in a dose-dependent manner in animal studies, by increasing the rate of callus formation, new bone mineralization and mechanical strength (Barnes et al., 2008). Dobnig et al. (Dobnig et al., 2009) showed that treatment with TPTD attenuated micro-damage accumulation in iliac crest biopsies of human patients previously treated with long-term alendronate, suggesting a possible role in stress fracture repair. In studies examining fracture healing, a randomized control trial of TPTD 20 mcg daily demonstrated faster healing time for wrist fractures compared with placebo, but higher doses of TPTD did not show efficacy (Aspenberg et al., 2010). A non-randomized control trial using full-length parathyroid hormone [PTH (1–84)] found that it accelerated fracture healing and improved functional outcomes in elderly patients with osteoporotic pelvic fractures (Peichl, Holzer, Maier, and Holzer, 2011). Although these pre-clinical and clinical studies suggest that there is a potential role for TPTD in improving fracture outcomes, this evidence may not be generalizable to AFF.
Retrospective studies investigating TPTD therapy in the management of AFF, have shown that it may aid fracture healing of incomplete AFF without radiological cortical lucency (Saleh et al., 2012), and can reduce fracture healing time and rates of non-union in the post-operative setting (Miyakoshi et al., 2015). In the only published prospective study of the use of TPTD, Chiang et al. (Chiang et al., 2013) showed that in 14 cases of AFF, TPTD improved fracture healing rates and pain scores, and non-TPTD treated patients had poorer outcomes, either proceeding to surgical intervention, or had persistent pain and non-union during follow-up. They concluded that TPTD may assist in healing of AFF and restoring bone quality. In this case, although the use of TPTD was associated with rapid healing of the initial AFF, the development of a new contralateral AFF highlights that TPTD does not indefinitely protect against future AFF. This suggests that TPTD may be limited in its ability to reverse the skeletal effects of long term bisphosphonate therapy, and caution should apply when considering sequential therapies with anti-resorptive properties, including strontium ranelate.
To our knowledge, there has been no case reported of AFF occurring in association with SR treatment. In fact, it has been suggested in the literature that SR may improve AFF healing (Carvalho et al., 2011). SR is an osteoporosis therapy, which works primarily through the incorporation of strontium into bone and increasing BMD (Stepan, 2013). It may also have weak anti-resorptive properties, and given the proposed mechanism of AFF development, this case report raises the question as to whether SR should be avoided in those with a history of AFF. Recurrence of incomplete AFF with the re-introduction of anti-resorptive treatment following a course of TPTD therapy has already been reported in the literature (Ramchand, Chiang, Zebaze, and Seeman, 2016).
In one case report of sequential therapy with TPTD and SR in a patient with alendronate associated-AFF, the authors concluded that sequential treatment was successful and may be a rational treatment option in managing incomplete AFF (Lampropoulou-Adamidou et al., 2013). In this case, TPTD therapy was delayed for 11 months following fixation of a complete AFF, however, the patient sustained an incomplete contralateral AFF during treatment with TPTD and 22 months following the first AFF. The patient was continued on TPTD, then sequentially treated with SR and did not proceed to surgical fixation of the incomplete AFF. Although the long delay between the index AFF and development of the contralateral incomplete AFF is similar to our case, we did not find success with sequential treatment with strontium ranelate, as the fracture progression evident on serial radiographs necessitated surgery.
In summary, we present a case of a postmenopausal woman with osteoporosis who developed a new contralateral AFF despite 18 months of anabolic therapy with TPTD and during SR therapy. This case raises a few interesting points: (i) a contralateral AFF occurred following a long delay after bisphosphonate cessation, illustrating the potential long-term skeletal effects of bisphosphonates; (ii) TPTD may have a role in AFF healing, but may not be effective in reversing the bisphosphonate-associated changes in the skeleton and therefore may have limited effectiveness in preventing the development of a contralateral AFF (iii) the re-introduction of anti-resorptive therapies following AFF, including SR, may have been implicated in the development of a contralateral AFF.
We acknowledge that this single case report is limited in advising on AFF management. Further robust research, such as a well-designed prospective randomized control trial, is required to explore the role of TPTD in AFF management. Given the decline in the use of SR globally, further research into the effects of SR on AFF may be limited.
Although this case highlights the challenges clinicians face in the management of patients with osteoporosis, it is of equal importance that we acquire more understanding into AFF pathogenesis, its association with anti-resorptive therapies, and predisposing risk factors in order to prevent these fractures.
Disclosures
PE reports receiving research funding from Eli Lilly.
Acknowledgements
The authors wish to acknowledge Ms. Sue Pankridge for preparation of the figures.
References
- Aspenberg P., Genant H.K., Johansson T., Nino A.J., See K., Krohn K., Garcia-Hernandez P.A., Recknor C.P., Einhorn T.A., Dalsky G.P., Mitlak B.H., Fierlinger A., Lakshmanan M.C. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 2010;25(2):404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- Barnes G.L., Kakar S., Vora S., Morgan E.F., Gerstenfeld L.C., Einhorn T.A. Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J. Bone Joint Surg. Am. 2008;90(Suppl. 1):120–127. doi: 10.2106/JBJS.G.01443. [DOI] [PubMed] [Google Scholar]
- Carvalho N.N., Voss L.A., Almeida M.O., Salgado C.L., Bandeira F. Atypical femoral fractures during prolonged use of bisphosphonates: short-term responses to strontium ranelate and teriparatide. J. Clin. Endocrinol. Metab. 2011;96(9):2675–2680. doi: 10.1210/jc.2011-0593. [DOI] [PubMed] [Google Scholar]
- Chiang C.Y., Zebaze R.M., Ghasem-Zadeh A., Iuliano-Burns S., Hardidge A., Seeman E. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone. 2013;52(1):360–365. doi: 10.1016/j.bone.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Dell R.M., Adams A.L., Greene D.F., Funahashi T.T., Silverman S.L., Eisemon E.O., Zhou H., Burchette R.J., Ott S.M. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J. Bone Miner. Res. 2012;27(12):2544–2550. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- Dobnig H., Stepan J.J., Burr D.B., Li J., Michalska D., Sipos A., Petto H., Fahrleitner-Pammer A., Pavo I. Teriparatide reduces bone microdamage accumulation in postmenopausal women previously treated with alendronate. J. Bone Miner. Res. 2009;24(12):1998–2006. doi: 10.1359/jbmr.090527. [DOI] [PubMed] [Google Scholar]
- Ettinger B., Burr D.B., Ritchie R.O. Proposed pathogenesis for atypical femoral fractures: lessons from materials research. Bone. 2013;55(2):495–500. doi: 10.1016/j.bone.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Gomberg S.J., Wustrack R.L., Napoli N., Arnaud C.D., Black D.M. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J. Clin. Endocrinol. Metab. 2011;96(6):1627–1632. doi: 10.1210/jc.2010-2520. [DOI] [PubMed] [Google Scholar]
- Ha Y.C., Cho M.R., Park K.H., Kim S.Y., Koo K.H. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin. Orthop. Relat. Res. 2010;468(12):3393–3398. doi: 10.1007/s11999-010-1583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.T., Kang L., Huang P.J., Fu Y.C., Lin S.Y., Hsieh C.H., Chen J.C., Cheng Y.M., Chen C.H. Successful teriparatide treatment of atypical fracture after long-term use of alendronate without surgical procedure in a postmenopausal woman: a case report. Menopause. 2012;19(12):1360–1363. doi: 10.1097/gme.0b013e318260143d. [DOI] [PubMed] [Google Scholar]
- Jilka R.L. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J.S., Goh S.K., Png M.A., Kwek E.B., Howe T.S. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J. Orthop. Trauma. 2010;24(2):75–81. doi: 10.1097/BOT.0b013e3181b6499b. [DOI] [PubMed] [Google Scholar]
- Lampropoulou-Adamidou K., Tournis S., Balanika A., Antoniou I., Stathopoulos I.P., Baltas C., Triantafillopoulos I.K., Papaioannou N.A. Sequential treatment with teriparatide and strontium ranelate in a postmenopausal woman with atypical femoral fractures after long-term bisphosphonate administration. Hormones (Athens) 2013;12(4):591–597. doi: 10.14310/horm.2002.1448. [DOI] [PubMed] [Google Scholar]
- Lo J.C., Huang S.Y., Lee G.A., Khandelwal S., Provus J., Ettinger B., Gonzalez J.R., Hui R.L., Grimsrud C.D. Clinical correlates of atypical femoral fracture. Bone. 2012;51(1):181–184. doi: 10.1016/j.bone.2012.02.632. [DOI] [PubMed] [Google Scholar]
- Miyakoshi N., Aizawa T., Sasaki S., Ando S., Maekawa S., Aonuma H., Tsuchie H., Sasaki H., Kasukawa Y., Shimada Y. Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J. Bone Miner. Metab. 2015;33(5):553–559. doi: 10.1007/s00774-014-0617-3. [DOI] [PubMed] [Google Scholar]
- Odvina C.V., Zerwekh J.E., Rao D.S., Maalouf N., Gottschalk F.A., Pak C.Y. Severely suppressed bone turnover: a potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005;90(3):1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- Peichl P., Holzer L.A., Maier R., Holzer G. Parathyroid hormone 1–84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J. Bone Joint Surg. Am. 2011;93(17):1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- Ramchand S.K., Chiang C.Y., Zebaze R.M., Seeman E. Recurrence of bilateral atypical femoral fractures associated with the sequential use of teriparatide and denosumab: a case report. Osteoporos. Int. 2016;27(2):821–825. doi: 10.1007/s00198-015-3354-0. [DOI] [PubMed] [Google Scholar]
- Saleh A., Hegde V.V., Potty A.G., Schneider R., Cornell C.N., Lane J.M. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J. 2012;8(2):103–110. doi: 10.1007/s11420-012-9275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilcher J., Michaelsson K., Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N. Engl. J. Med. 2011;364(18):1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- Shane E., Burr D., Ebeling P.R., Abrahamsen B., Adler R.A., Brown T.D., Cheung A.M., Cosman F., Curtis J.R., Dell R., Dempster D., Einhorn T.A., Genant H.K., Geusens P., Klaushofer K., Koval K., Lane J.M., McKiernan F., McKinney R., Ng A., Nieves J., O'Keefe R., Papapoulos S., Sen H.T., van der Meulen M.C., Weinstein R.S., Whyte M. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010;25(11):2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- Shane E., Burr D., Abrahamsen B., Adler R.A., Brown T.D., Cheung A.M., Cosman F., Curtis J.R., Dell R., Dempster D.W., Ebeling P.R., Einhorn T.A., Genant H.K., Geusens P., Klaushofer K., Lane J.M., McKiernan F., McKinney R., Ng A., Nieves J., O'Keefe R., Papapoulos S., Howe T.S., van der Meulen M.C., Weinstein R.S., Whyte M.P. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- Stepan J.J. Strontium ranelate: in search for the mechanism of action. J. Bone Miner. Metab. 2013;31(6):606–612. doi: 10.1007/s00774-013-0494-1. [DOI] [PubMed] [Google Scholar]
- Tarazona-Santabalbina F.J., Aguilella-Fernandez L. Bisphosphonate long-term treatment related bilateral subtrochanteric femoral fracture. Can teriparatide be useful? Aging Clin. Exp. Res. 2013;25(5):605–609. doi: 10.1007/s40520-013-0137-3. [DOI] [PubMed] [Google Scholar]