Abstract
While inhibition of bone healing and increased rates of pseudarthrosis are known adverse outcomes associated with cigarette smoking, the underlying mechanisms by which this occurs are not well understood. Recent work has implicated the Aryl Hydrocarbon Receptor (Ahr) as one mediator of the anti-osteogenic effects of cigarette smoke (CS), which contains numerous toxic ligands for the Ahr. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin) is a high-affinity Ahr ligand frequently used to evaluate Ahr pathway activation. The purpose of this study was to elucidate the downstream mechanisms of dioxin action on bone regeneration and investigate Ahr antagonism as a potential therapeutic approach to mitigate the effects of dioxin on bone. Markers of osteogenic activity and differentiation were assessed in primary rat bone marrow stromal cells (BMSC) after exposure to dioxin, Ahr antagonists, or antagonist + dioxin. Four Ahr antagonists were evaluated: α-Naphthoflavone (ANF), resveratrol (Res), 3,3′-Diindolylmethane (DIM), and luteolin (Lut). Our results demonstrate that dioxin inhibited ALP activity, migratory capacity, and matrix mineralization, whereas co-treatment with each of the antagonists mitigated these effects. Dioxin also inhibited BMSC chemotaxis, while co-treatment with several antagonists partially rescued this effect. RNA and protein expression studies found that dioxin down-regulated numerous pro-osteogenic targets, whereas co-treatment with Ahr antagonists prevented these dioxin-induced expression changes to varying degrees. Our results suggest that dioxin adversely affects bone regeneration in a myriad of ways, many of which appear to be mediated by the Ahr. Our work suggests that the Ahr should be investigated as a therapeutic target to combat the adverse effects of CS on bone healing.
Keywords: Dioxin, TCDD, Aryl Hydrocarbon Receptor, Cigarette smoke, Bone regeneration
Highlights
-
•
Dioxin, a potent Ahr ligand, inhibits osteogenic differentiation of BMSC.
-
•
“Nutraceutical” Ahr antagonists found in red wine and broccoli protected against dioxin action.
-
•
Targets of dioxin action included Collagens, MMPs, Phex, CXCR4/CXCL12 axis.
-
•
The Ahr may in part mediate the adverse effects of cigarette smoke on osteogenic differentiation and bone healing.
1. Introduction
The impact of tobacco smoke on human health remains a critical problem worldwide. Cigarette smoke (CS) has a well-established role in the pathogenesis of numerous smoking-related disorders, including chronic obstructive pulmonary disease (COPD), cancer, and atherosclerosis (Middlekauff et al., 2014, Sasco et al., 2004). Although less frequently recognized, smoking also exacerbates musculoskeletal disease and presents serious challenges in the treatment of orthopaedic conditions (Porter and Hanley, 2001). In addition to promoting osteoporosis, degenerative disc disease, and wound complications, smoking drastically hinders osseointegration and bony union - deleterious outcomes that are associated with higher rates of revision procedures (Sloan et al., 2010, Schmitz et al., 1999). In spine surgery, smoking has been shown to negatively impact outcomes, with a pseudarthrosis rate nearly double that of non-smokers (26.5 vs. 14.2%) (Glassman et al., 2000). Although the adverse effects of smoking have been studied most extensively in spine research, similar effects are seen in other orthopaedic conditions as well, especially tibial fracture healing (Patel et al., 2013). Currently, surgeons are limited in their ability to treat these patients, and are left with the difficult choice of refusing surgical intervention or performing procedures with significantly increased risks.
Determining a singular mechanism by which CS inhibits bone growth is problematic, as smoke contains upwards of 4000 distinct chemical constituents (Hoffmann and Hoffmann, 1997, Castillo et al., 2005). However, several mechanisms are understood to be involved. Nicotine is a potent anti-inflammatory and immunosuppressive, and has been shown to have deleterious effects on fibroblasts, red blood cells, and macrophages (Zevin et al., 1998, Jorgensen et al., 1998, Leow and Maibach, 1998), in addition to diminishing blood flow to tissues by promoting vasoconstriction (Leow and Maibach, 1998, Bornmyr and Svensson, 1991). Interestingly, the overall impact of nicotine on bone formation is still uncertain, and may be concentration-dependent; high concentrations of nicotine have been shown to inhibit osteoblast proliferation, whereas low concentrations actually have a proliferative effect (Rothem et al., 2009, Daffner et al., 2015, Gotfredsen et al., 2009, Syversen et al., 1999). Numerous studies have proposed that reactive oxygen species and other pro-inflammatory constituents and metabolites are responsible for dysregulation of bone homeostasis, reduction in bone mineral density, and inhibition of fracture healing (Rothem et al., 2009, Syversen et al., 1999, Holzer et al., 2012).
Recent work has implicated the Aryl Hydrocarbon Receptor (Ahr) as a mediator of anti-osteogenic effects. The receptor binds an extensive array of exogenous ligands, such as natural plant flavonoids, polyphenolics, and indoles, as well as xenobiotic toxicants, such as polycyclic aromatic hydrocarbons (PAH, e.g. benzo[a]pyrene), halogenated aromatic hydrocarbons (HAH, e.g. dioxins), and polychlorinated biphenyls (PCBs). PAHs and similar compounds are formed during the incomplete combustion of organic matter, including tobacco (Leow and Maibach, 1998). 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin) is a halogenated aromatic hydrocarbon with incredibly high-affinity for the Ahr. As such, dioxin is a commonly used probe to investigate the role of the receptor on various biological systems and endpoints (Ryan et al., 2007). More recently, Ahr activation by dioxin has been shown to have significant adverse effects on bone (Singh et al., 2000). For example, downstream effects resulting from Ahr activation have been shown to inhibit osteoblast function and differentiation, resulting in reduced ossification (Jamsa et al., 2001, Naruse et al., 2002, Ryan et al., 2007).
In previous work, we found that chronic exposure to dioxin inhibits BMP-2-mediated bone regeneration and posterolateral (L4-L5) spine fusion in rats (Hsu et al., 2015). Cessation of exposure for a period of 4 half-lives facilitated a partial recovery of regenerative capacity. These pre-clinical findings further supported previous work that identified bone as a sensitive target for dioxin, and suggest a potential link between ligand-induced Ahr activation and the reduced healing rates seen in smokers after spinal arthrodesis. However, the mechanisms of dioxin action on the bone regenerative process are still unclear. With this study, we sought to clarify these mechanisms and identify a viable therapeutic strategy to mitigate these effects. Numerous Ahr antagonists of both synthetic and natural origin have shown the potential to protect against the adverse effects of dioxin and other exogenous Ahr ligands for various biological endpoints (Dong et al., 2010). We hypothesize here that the use of one or more of these compounds to limit Ahr activation could reduce the adverse effects of dioxin on osteogenic differentiation and bone healing.
2. Materials and Methods
2.1. BMSC isolation and culture
Bone marrow stromal cells (BMSC) were harvested from femurs and tibiae of six-week-old female Long-Evans rats purchased from Charles River Laboratories (Chicago, IL). Animals were euthanized under anesthesia in accordance with Institutional Animal Care and Use Committee (IACUC)-approved procedures, and animals were housed under controlled temperature (23 ± 1 °C) and relative humidity (50 to 60%). Isolated BMSC were incubated with standard media comprised of Dulbecco's Modified Eagle Medium (DMEM; Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 20 mM HEPES sodium salt, 50 μg/mL streptomycin, and 50 μg/mL gentamycin sulfate. After 3–5 days of incubation (at 80% confluence), cells were re-plated and grown in either standard media (SM) or osteogenic media (OM; comprised of standard media supplemented with 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 10 nM dexamethasone). Cells were treated with either vehicle control (dimethyl sulfoxide, DMSO; 0.1% final concentration) or the following: 10 nM dioxin, 50 μM nicotine, 0.5 μM α-Naphthoflavone (ANF), 4 μM resveratrol (Res), 10 μM 3,3′-Diindolylmethane (DIM), 0.2 μM luteolin (Lut), or dioxin + ANF, Res, DIM or Lut. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Treatment media was replaced twice per week at a minimum.
2.2. Alkaline Phosphatase activity
Alkaline Phosphatase (ALP) activity was quantitated using the SensoLyte pNPP Alkaline Phosphate Assay kit (Anaspec, Fremont, CA) and normalized to total protein. After supernatants were collected, enzymatic reactions were performed according to manufacturer's instructions. A minimum of three independent experiments were performed for quantitation of ALP activity as well as all other in vitro assays.
2.3. Matrix mineralization
BMSC were inoculated into 6-well plates at 1 × 104 cells per well. Cells were maintained in either standard or osteogenic growth conditions for 2 weeks, and were re-treated every 2–3 days. After 2 weeks, live cells were quantitated using an MTS assay (Promega, Madison, WI) for normalization purposes. Adherent cells were then washed twice with PBS, fixed with 4% paraformaldehyde, and stained with 2% Alizarin red solution. After collection of digital images, cells were de-stained with cetylpyridinium chloride, and A540 was quantified using a Cytation 3 spectrophotometer (BioTek Instruments, Winooski, VT).
2.4. Cell migration
The effect of dioxin on dermal wound closure was assessed using the CytoSelect Wound Healing Assay Kit (Cell Biolabs Inc., San Diego, CA). When BMSC cells reached confluence, the inserts were removed from the wells and washed twice with PBS. Cells were then incubated in standard media containing DMSO or dioxin for 15 h, after which time wells were stained according to the manufacturer's instructions. Representative digital images were collected at time points of 0, 8, 15, and 24 h with a light microscope in order to evaluate the rate of “wound” closure. The migration distance across each wound was quantified by a comparison of final and initial wound widths followed by calculation of the percent change.
2.5. Chemotaxis
Pre-treated cells were trypsinized and counted using a Countess automated cell counter (Invitrogen, Grand Island, NY). 2 × 105 cells were suspended in 100 μL of migration buffer (standard media containing 0.2% FBS/0.1% bovine serum albumin) and inoculated into the upper chambers of 24-well transwell inserts (8 μm pore size). The lower chambers were inoculated with 400 μL of migration buffer supplemented with one of the following: 200 ng/mL CXCL12, 200 ng/mL IL-8, 200 ng/mL CCL20, or 200 ng/mL BMP-2. Wells containing only migration buffer in both the upper and lower chambers were included as negative controls. Membranes were then fixed with 4% paraformaldehyde and stained with 0.05% crystal violet. After removing cells from upper side using cotton applicators, cells adhered to the underside of the membrane were visualized and counted under a microscope by three independent observers, and an average cell count was computed for each treatment group.
2.6. RNA isolation and gene expression
Quantitative real-time polymerase chain reaction (QPCR) was performed on BMSC treated under osteogenic conditions with either DMSO or dioxin. After pre-treatment, mRNA was isolated from BMSC and expression levels were quantified and normalized to Glyceraldehyde 3-phosphate Dehydrogenase (Gapdh). Primer set was synthesized by Integrated DNA Technologies (Coralville, IA), with sequences detailed in Table 1. cDNAs were synthesized using a qScript cDNA Synthesis Kit (Quanta Bioscience, Gaithersburg, MD), and QPCR reactions were prepared with IQ SYBR Green Supermix (BioRad, Hercules, CA). QPCR was performed in the Equipment Core Facility of the Simpson Querrey Institute at Northwestern University using the following program: 94 °C denaturation for 5 min; 40 repeated cycles of 94 °C, 45 s/55 °C, 1 min/68 °C for 1 min; 79 cycles at 55 °C for 30 s each for generation of melting curves. Expression levels from treatment groups were normalized to vehicle control in order to represent a relative fold difference.
Table 1.
Primer sets for qPCR.
| cDNA | Sequences 5″-3” | |
|---|---|---|
| ALP | Forward | TCG CCT ATC AGC TAA TGC AC |
| Reverse | GCC TTC TCA TCC AGT TCA TAT TCC | |
| BMP2 | Forward | AGC ATG TTT GGC CTG AAG CAG AGA |
| Reverse | TGA AAG TTC CTC GAT GGC TTC | |
| CXCL12 | Forward | CCG ATT CTT TGA GAG CCA TGT |
| Reverse | CAG ACT TGT CTG TTG TTG CTT | |
| CXCR4 | Forward | CGT TTG GTG CTC CGG TAG |
| Reverse | TCT CCA GAC CCT ACT TCT TCG | |
| COL1A1 | Forward | GCA TGG CCA AGA AGA CAT CC |
| Reverse | CCT CGG GTT TCC ACG TCT C | |
| COL2A1 | Forward | GAA CAA CCA GAT CGA GAG CA |
| Reverse | CCA GTA GTC TCC GCT CTT CC | |
| COL12A1 | Forward | ATG ATT GCC ACT GAT CCA GA |
| Reverse | AGG GCC CTT GAC ACT GTT AC | |
| DLX5 | Forward | AGG TGA GGA TGG TGA ATG GT |
| Reverse | CAG GGC GAG GTA CTG AGT CT | |
| MMP1 | Forward | CAT AGC TTC TTT GGC TTC CC |
| Reverse | AAC CTG GAT CCA TGG ACT GT | |
| MMP2 | Forward | AGG GCA CCT CCT ACA ACA GC |
| Reverse | CAG TGG ACA TAG CGG TCT CG | |
| MMP3 | Forward | TGA AGA TGA CAG GGA AGC TG |
| Reverse | ATT TGG GTG AAC CTG GAA AG | |
| MMP13 | Forward | AAG ATG TGG AGT GCC TGA TG |
| Reverse | AAG GCC TTC TCC ACT TCA GA | |
| OCN | Forward | TAT GGC ACC ACC GTT TAG GG |
| Reverse | CTG TGC CGT CCA TAC TTT CG | |
| OPN | Forward | CTG CCA GCA CAC AAG CAG AC |
| Reverse | TCT GTG GCA TCG GGA TAC TG | |
| OSX | Forward | ACT GGC TAG GTG GTG GTC AG |
| Reverse | GGT AGG GAG CTG GGT TAA GG | |
| PHEX | Forward | CTG CCA GAG AAC AAG TCC AA |
| Reverse | CTG TTC ATG GTG GAA TTT GC | |
| Rspo2 | Forward | TGT TTC TGC TAC ACG TTC CC |
| Reverse | CGC TGC TTT GAT GAA TGT CC | |
| Rspo3 | Forward | TTA GAA GCC AGC AAC CAT ACC |
| Reverse | CCG TGT TTC AGT CCC TCT TT | |
| RUNX2 | Forward | CAA ACA ACC ACA GAA CCA CAA G |
| Reverse | CTC AGA GCA CTC ACT GAC TC | |
| Gapdh | Forward | GTT CTA GAG AGA GCC GCA TC |
| Reverse | GTA ACC AGG CGT CCG ATA C |
2.7. Western blotting
Rapid immunoprecipitation assay buffer (RIPA buffer), blocking solutions, and protease inhibitors were purchased from GenDEPOT (Barker, TX). α-tubulin and RUNX2 antibodies were purchased from Cell Signaling Technology (Billerica, MA). Collagen Type 1A1 (COL1A1), Type 2A1 (COL2A1), and Type 12A1 (COL12A1) antibodies, as well as CXCR4, CCR6, and MMP13 antibodies were purchased from Abcam (Cambridge, MA). PHEX, MMP1, MMP2, and MMP3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CXCL12 antibody was purchased from EMD Millipore (Billerica, MA). Membranes were washed with PBST and incubated with horseradish peroxidase-conjugated secondary antibodies (Billerica, MA) for 1 h at room temperature. Signals were visualized by enhanced chemiluminescence (ECL) using Kodak film, and intensities were quantified using a computing densitometer program from Image Studio Lite (LI-COR, Lincoln, NE).
2.8. Statistical methods
The values given are mean ± standard deviation (SD). Data were analyzed for overall statistical significance using one-way ANOVA. Pairwise comparisons of means between treatment groups and control groups were assessed by performing post hoc Fisher's least significant difference (LSD) tests, with a significance threshold of p ≤ 0.05.
3. Results
3.1. Differential effects of dioxin and nicotine on osteogenic differentiation
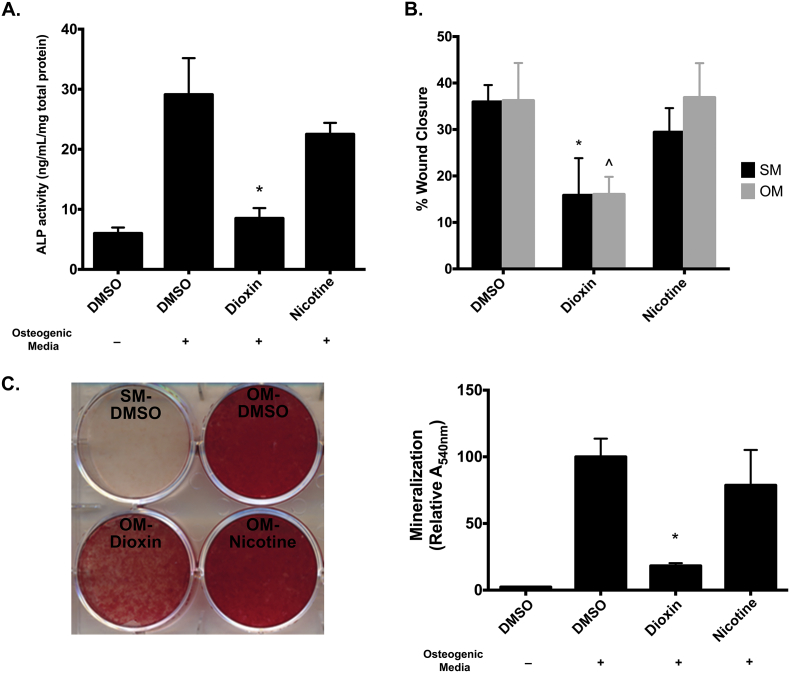
Because nicotine has been shown to have anti-osteogenic effects, we sought to compare the effects of dioxin with those of nicotine on BMSC differentiation. As expected, ALP activity was induced under OM conditions (29.1 vs. 6.0 ng/mL/mg total protein in SM conditions, p < 0.01; Fig. 1A). Dioxin treatment drastically inhibited ALP activity (8.5 ng/mL/mg) when compared to vehicle-treated cells grown in OM (p < 0.01), whereas nicotine had no significant effect (p = 0.15). In a scratch-wound assay for non-directional cell migration, dioxin pre-treatment impeded “wound” closure under both SM and OM conditions [35.9% and 36.2% in control- vs. 15.8% and 16.0% in dioxin-treated cells; SM (p < 0.01) and OM (p < 0.01), respectively; Fig. 1B]. Nicotine did not significantly inhibit wound closure in either SM (29.4%, p = 0.09) or OM (36.9%, p = 0.91) conditions. Similarly, dioxin treatment in OM decreased matrix mineralization (p < 0.001), whereas nicotine did not significantly alter mineral deposition (p = 0.20; Fig. 1C).
Fig. 1.
Differential effects of nicotine and dioxin. (A) ALP activity was assessed in BMSC grown in standard media (SM) or osteogenic media (OM). Dioxin- and nicotine-treated cells were cultured in OM. *p < 0.01, dioxin- vs. vehicle- and nicotine-treated cells. (B) BMSC migration capacity was assessed via wound-scratch assay. Significance is shown relative to both vehicle- and nicotine-treated cells grown in either SM (*p < 0.05) or OM (^p < 0.05). (C) Visualization and quantification of mineral deposition. Note that all dioxin-treated and nicotine-treated cells were grown in osteogenic media. Columns, means from at least three independent experiments *p < 0.01, dioxin-treated wells vs. all other groups.
3.2. Effects of Ahr activation and antagonism on osteogenic differentiation
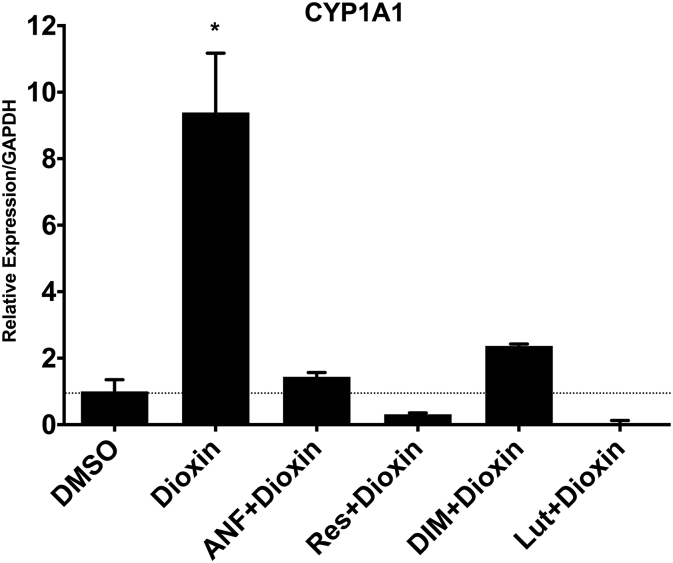
Cyp1a1 expression was quantified as a marker of dioxin exposure and Ahr activation. mRNA expression for the Ahr-dependent Cyp1a1 gene increased by 839% (1.0 vs. 9.39, p < 0.01) after treatment with dioxin relative to vehicle-treated cells (Fig. 2). This up-regulation of Cyp1a1 was abrogated when dioxin-treated cells were co-treated with Ahr antagonists (p < 0.05, all antagonists). Res and Lut showed the strongest inhibition of Cyp1a1 expression.
Fig. 2.
Cyp1a1 expression. Expression of Cyp1a1 mRNA after treatment with DMSO vehicle control, dioxin, or dioxin + Ahr antagonists. mRNA expression levels were normalized to vehicle-treated cells. Columns, means from at least three independent experiments *p < 0.05 = dioxin-treated vs. all other groups.
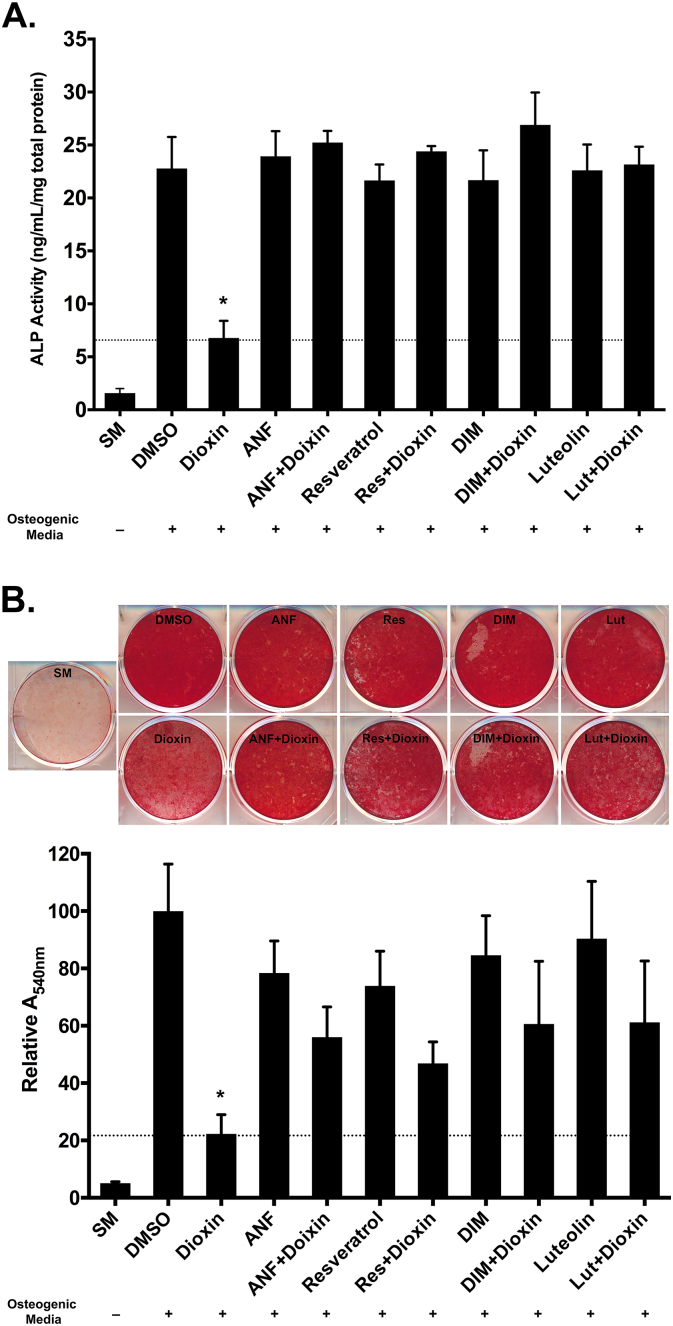
As early and late markers of osteogenic differentiation respectively, ALP activity and matrix mineralization were quantified after treatment with dioxin or dioxin + antagonists. Dioxin-treated BMSC showed significantly diminished ALP activity compared to vehicle-treated cells (6.78 vs. 22.8 ng/mL/mg total protein, respectively; p < 0.001; Fig. 3A). Co-treatment with Ahr antagonists completely rescued ALP activity (p < 0.001 for all antagonists relative to dioxin-treated). ALP levels in antagonist co-treated groups were similar to levels in vehicle-treated cells (p > 0.1, all co-treatments). Inhibition of matrix mineralization by dioxin was partially recoverable with antagonist co-treatment. BMSC co-treated with dioxin and antagonists deposited significantly more mineral relative to dioxin-only treated cells (p < 0.01, all antagonists; Fig. 3B).
Fig. 3.
(A) ALP activity. All dioxin-treated and antagonist-treated cells were cultured in osteogenic media. *p < 0.001 significance of ALP activity in dioxin-only treated cells relative to all other groups. (B) Matrix mineralization. Calcium deposition in the matrix of cells grown under standard or osteogenic conditions was visualized by Alizarin red staining, which was quantified using a cetylpyridinium chloride de-stain procedure. Note that all dioxin- and antagonist-treated cells were grown in osteogenic media. Columns, means from at least three independent experiments *p < 0.01, dioxin alone-treated wells vs. all other groups.
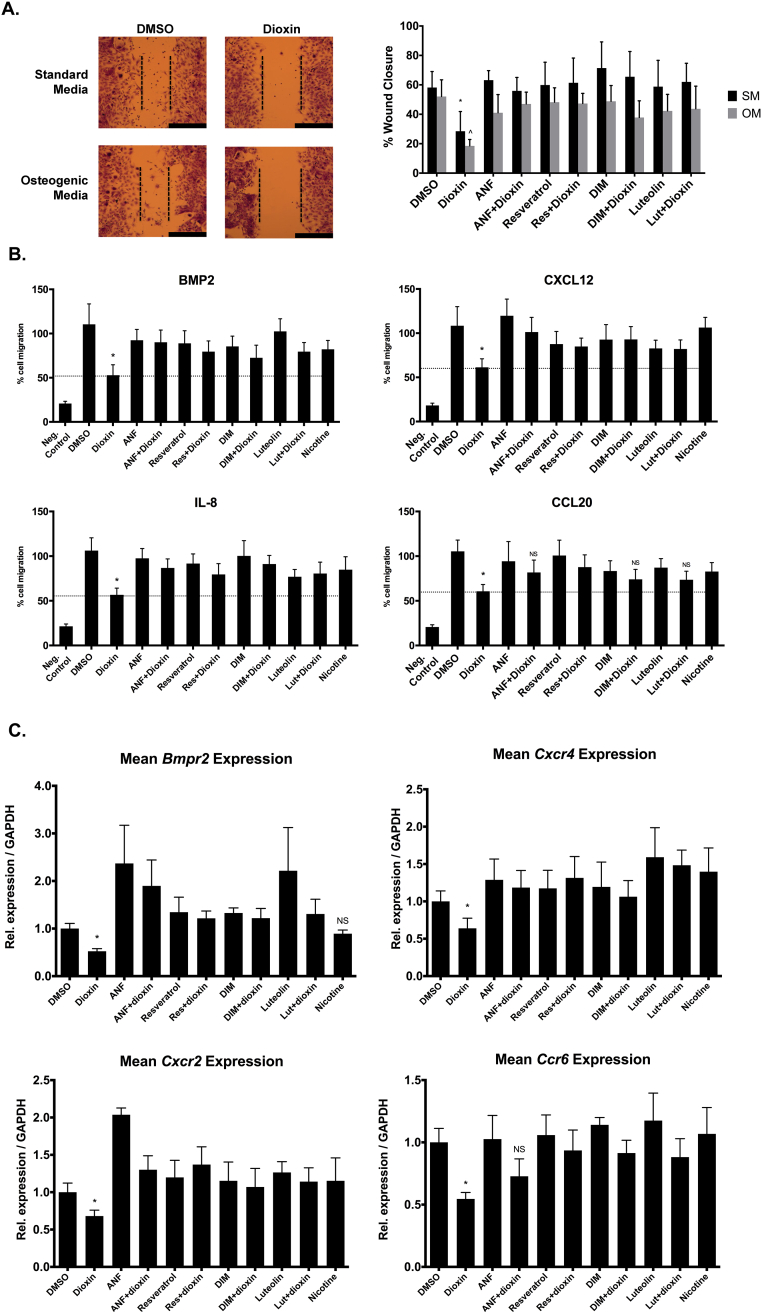
3.3. Effects of Ahr activity on BMSC migration and chemotaxis
BMSC migratory capacity and chemotactic potential play important roles in bone healing, where early migration to the site of injury is critical for the onset of the regenerative process. We utilized scratch-wound and transwell assays to evaluate the effect of dioxin on cell migration and chemotaxis, respectively. In vehicle-treated cells, wound closure at the 8-h time point was 58.2% and 52.1% in SM and OM, respectively. Dioxin treatment significantly suppressed cell migration (SM = 28.5%, p < 0.01; OM = 18.5%, p < 0.01), but this suppression was at least partially recoverable when co-treated with Ahr antagonists, under both SM and OM conditions (p < 0.01 dioxin alone vs. co-treatments, for both conditions; Fig. 4A). Directional migration assays were also performed in order to evaluate the effect of dioxin pre-treatment on migration towards various proteins to which BMSC are known to be chemoattractive. Dioxin treatment significantly inhibited BMSC chemotactic ability towards all four chemoattractants tested (BMP2, CXCL12, IL-8, and CCL20; p < 0.05; Fig. 4B). Co-treatment with each of the Ahr antagonists at least partially rescued cells from the dioxin-mediated inhibition of chemotaxis towards BMP2, CXCL12, and IL-8 such that chemotaxis rates were significantly increased relative to dioxin-only treated BMSC. Chemotaxis towards CCL20 was rescued after co-treatment with Res but not after co-treatment with ANF (p = 0.06), DIM (p = 0.20), or Lut (p = 0.22). In contrast, nicotine treatment did not affect BMSC chemotactic ability towards any of the chemoattractants. To investigate the mechanisms of chemotactic inhibition, mRNA levels of corresponding chemokine receptors were also evaluated. All four transcripts were significantly reduced after dioxin treatment: Bmpr2 (p < 0.01), CXCR4 (p < 0.01), Cxcr2 (p < 0.01), and CCR6 (p < 0.05; Fig. 4C). Co-treatment with each of the antagonist facilitated recovery of gene expression levels for CCR6 and Cxcr2, such that transcripts were significantly greater after co-treatment relative to dioxin only-treated BMSC (p < 0.05). Dioxin-mediated down-regulation of Bmpr2 was significantly decreased by ANF, DIM, and Lut (p < 0.05) but not Res (p = 0.48). Similarly, inhibition of CXCR4 expression was not recovered by co-treatment with ANF (p = 0.27) or DIM (p = 0.09).
Fig. 4.
(A) Cell migration wound assay. BMSC non-directional migration capacity was assessed via wound-scratch assays. Scale bar; 500um, *p < 0.01 dioxin-treated (SM) vs. all other SM treatment groups, ^p < 0.01 dioxin-treated (OM) vs. all other OM treatment groups. (B) Chemotaxis assay. Transwell assays were performed in order to evaluate the effect of dioxin on chemotactic ability, and the capacity of Ahr antagonists to rescue cells from these effects. Standard media was supplemented with one of the following chemoattractants: BMP-2, CXCL12, IL-8, or CCL20. *p < 0.05; significance of dioxin-treated cells vs. all other treatment groups. “NS” indicates the comparison relative to dioxin alone-treated cells is not statistically significant. (c) Gene expression of receptors for chemoattractant ligands presented in Fig. 3b. Columns, means from at least three independent experiments *p < 0.05, dioxin alone-treated wells vs. all other groups.
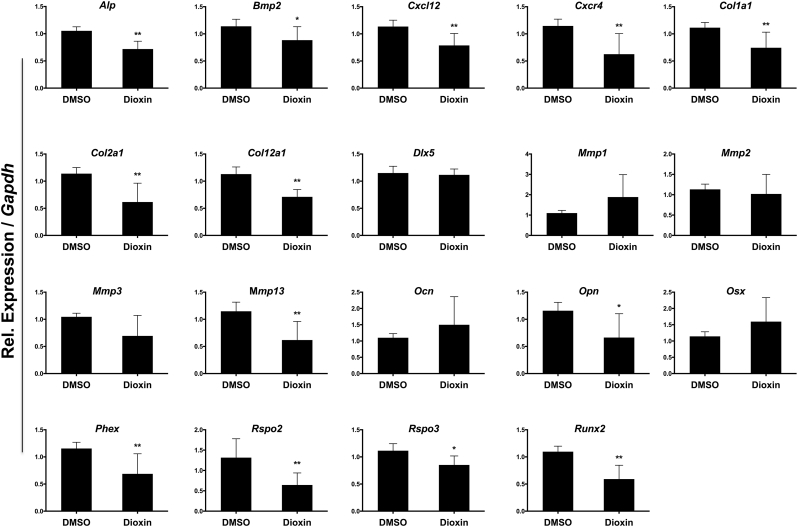
3.4. Dioxin modulates a wide array of pro-osteogenic gene and protein expression levels
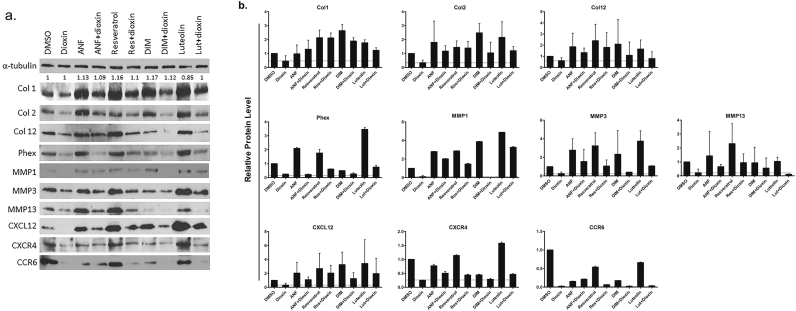
Thirteen of the 19 genes evaluated were significantly down-regulated after treatment with dioxin: ALP (36.0%; p < 0.01), BMP-2 (6.5%; p < 0.05), CXCL12 (21.3%; p < 0.01), CXCR4 (37.5%; p < 0.01), COL1A1 (25.6%; p < 0.01), COL2A1 (25.0%; p < 0.01), COL12A1 (26.3%; p < 0.01), MMP13 (34.5%; p < 0.01), OPN (8.3%; p < 0.05), PHEX (17.3%; p < 0.01), Rspo2 (35.7%; p < 0.01), Rspo3 (14.8%; p < 0.05), RUNX2 (33.0%; p < 0.01) (Fig. 5). MMP3 mRNA expression also trended downward, while levels of DLX5, MMP1, Mmp-2, OCN, and OSX were unchanged relative to vehicle controls. Western blotting showed that protein expression levels for COL2A1, COL12A1, PHEX, MMP3, MMP13, CXCL12, CXCR4, and Ccr6 were reduced in dioxin-treated cells relative to vehicle control (Fig. 6, lanes 1–2). This suppression appeared to be recoverable with antagonist co-treatment (lanes 4, 6, 8, and 10). In the case of treatment with ANF, Res, or Lut alone, expression levels were frequently higher than vehicle-treated cells (lanes 3, 5, and 9), whereas treatment with DIM had a more variable effect on protein expression (lane 7).
Fig. 5.
Osteogenesis-related gene expression after dioxin treatment. Expression of osteogenesis-related genes after treatment with 10 nM dioxin or DMSO vehicle-control in cells grown under osteogenic growth conditions. mRNA expression levels were normalized to control treated cells in osteogenic media. Columns, means from at least three independent experiments *p < 0.05; **p < 0.01 (relative to vehicle control).
Fig. 6.
Osteogenesis-related protein expression with antagonist co-treatment. (a) Expression of osteogenesis-related proteins after treatment with DMSO vehicle control, 10 nM dioxin, Ahr antagonist, or Ahr antagonist and dioxin in BMSC grown under osteogenic growth conditions. (b) Quantitation of protein expression by ImageJ. Columns, means from at least three independent experiments.
4. Discussion
Although prior studies suggest that a number of cigarette smoke (CS) constituents - including nicotine - may contribute to the inhibitory effects of smoking on bone healing, activation of the Aryl Hydrocarbon Receptor (Ahr) has only recently become a major focus of research (Jamsa et al., 2001, Naruse et al., 2002, Ryan et al., 2007). The Ahr plays an important role in xenobiotic metabolism, and previous studies have shown that pathway hyper-activation can have deleterious effects on bone biology (Singh et al., 2000, Jamsa et al., 2001, Naruse et al., 2002, Ryan et al., 2007). A proportionately minor constituent of CS, dioxin is a prototypical ligand frequently used to study Ahr involvement in biological activities, due to its extremely high affinity for the receptor and the widely-accepted belief that dioxin acts primarily through this mechanism (Hsu et al., 2015). Dioxin has previously been shown to inhibit some markers of osteoblastic differentiation in established murine cell lines (Singh et al., 2000, Ryan et al., 2007, Carpi et al., 2009). In an osteoblast differentiation model of rat MSC, dioxin modulated expression levels of proteins involved in bone growth, including structural proteins, molecular chaperones, heat-shock proteins, and calcium-binding proteins (Carpi et al., 2009). Moreover, dioxin was found to inhibit ALP activity in rat stem cells derived from the apical papilla (SCAPs) (Guo et al., 2015).
In this work, we found that the effects of nicotine on osteogenic differentiation were distinct from those of dioxin. (Fig. 1, Fig. 4). Previous work has shown that the effects of nicotine on osteoblasts are nuanced and may be concentration- and time-dependent (Rothem et al., 2009, Daffner et al., 2015, Marinucci et al., 2014). In this work, we utilized a moderate-to-low dose (Rothem et al., 2009) of nicotine (50 μM) and found that its effect on ALP activity, BMSC migration, and matrix mineralization were minor compared to those of dioxin. We found that treatment with nicotine over a 2-week period resulted in a roughly 25% decrease in ALP activity relative to vehicle control. Interestingly, these results appear to be consistent with the results of Rothem et al., which demonstrated that longer nicotine treatment for 72 h resulted in roughly a 20%–30% decrease in ALP expression (Rothem et al., 2009).
Our recent work showed that dioxin exposure inhibits spine fusion in the rat (Hsu et al., 2015). With the present study, we sought to elucidate the underlying mechanisms of dioxin-mediated effects on bone regeneration using rat primary BMSC, and to explore the capacity of Ahr antagonists as potential agents to mitigate these effects. We found that exposure to dioxin had numerous deleterious effects on pro-osteogenic markers and cellular functions, including ALP activity, cell migration, chemotaxis, matrix mineralization, and gene/protein expression. Moreover, co-treatment with known Ahr antagonists at least partially prevented the majority of these inhibitory effects. Our results validate the involvement and importance of the Ahr in the regenerative response after dioxin exposure, and provide a strong basis for further investigation into the use of Ahr antagonists as possible therapeutics to improve orthopaedic outcome in smokers.
A significant number of natural, synthetic, and endogenous Ahr ligands have been identified that have structural and chemical properties which are dramatically different from HAHs and PAHs. This is suggestive of a highly promiscuous Ahr binding pocket, and antagonists' binding properties are likely similarly diverse, along with their respective downstream effects (Denison and Nagy, 2003, Kwee, 2015). For example, α-Naphthoflavone (ANF) is a synthetic structural analog of flavone and a well-established Ahr antagonist. Upon binding, ANF elicits a conformational change in the receptor, altering the receptor's affinity for xenobiotic-responsive elements (XREs). This leads to a change in downstream gene expression levels and results in mixed agonist/antagonist effects (Wilhelmsson et al., 1994).
Resveratrol is an antifungal nutraceutical found in various spermatophyte plants, including grapes, peanuts, and eucalyptus (Dong et al., 2010). It can be found in red wine (Lyte and Bick, 1986). and is also widely available over-the-counter as a dietary supplement. Resveratrol is understood to have cardioprotective and potentially chemoprotective effects (Bertelli and Das, 2009). However unlike ANF, which has mixed agonist/antagonist activity, resveratrol is a pure Ahr antagonist. It has been shown to inhibit the effects of dioxin on pre-osteoblasts in vitro, restoring levels of ALP, Bone Scialoprotein (BSP), Type I Collagen, and Osteopontin (Singh et al., 2000).
3,3′-Diindolylmethane (DIM) is an acid-catalyzed breakdown product of indole-3-carbinol, a naturally-occurring compound found in cruciferous vegetables such as kale, brussels sprouts, broccoli, and cabbage. DIM has also been used safely for years as a health supplement and may have chemoprotective effects (Dong et al., 2010, Lyte and Bick, 1986). Studies involving human and animal models have shown that DIM elicits cellular effects contrasting those of dioxin, and may have strong anti-inflammatory properties (Dong et al., 2010, Lyte and Bick, 1986, Yao et al., 2013). Furthermore, in one study using mouse primary cardiac fibroblasts, DIM dramatically up-regulated the expression of factors that mediate the expression of antioxidant genes (Yao et al., 2013). Like DIM, luteolin is a naturally occurring phytochemical with chemoprotective properties. Previous studies using human and animal cell lines have shown that luteolin is effective at abrogating dioxin-induced Ahr-mediated cell responses (Zhang et al., 2003).
Gene and protein expression analyses found that dioxin exposure resulted in significant down-regulation of many pro-osteogenic targets (Fig. 5). OPN is a late-stage osteogenic marker and plays an important role in regulating biomineralization by serving as a bridge between HA and the extracellular matrix (ECM) in bone (Staines et al., 2012). PHEX is a zinc metalloendopeptidase that inhibits proteolytic cleavage of ASARM (acidic serine aspartate-rich MEPE-associated motif) peptides by binding to the ASARM motif of SIBLING proteins, such as OPN (Addison et al., 2008). The released ASARM substrate binds tightly to HA and inhibits mineralization (Addison et al., 2008). Previous studies have shown that SIBLING proteins become potent inhibitors of mineralization after cleavage of their ASARM or other post-translational modifications, and indeed OPN knockout mice exhibit increased bone mineral content and size (Staines et al., 2012, Addison et al., 2008). We therefore posit that the down-regulation of PHEX by dioxin may lead to reduced mineral deposition through the dysregulation of OPN activity. Moreover, OPN is a target of ALP; de-phosphorylation of OPN by ALP prevents much of the inhibitory effects of OPN on HA growth and matrix mineralization (Staines et al., 2012). We suspect that inhibition of ALP by dioxin in differentiating BMSC results in increased levels of phosphorylated OPN, contributing to reduce mineral deposition.
We found that expression levels of numerous proteins were reduced after dioxin treatment (PHEX, MMP3, CXCL12, CXCR4, and CCR6; Fig. 6); these effects were mitigated by antagonist co-treatment. Interestingly, ANF, resveratrol, and luteolin alone generally induced expression of these targets over vehicle control levels. However, the exception was DIM, which on its own, appeared to have little effect on protein expression. Nevertheless, this notion needs further investigation, since we did not see an appreciable increase in markers of differentiation—such as ALP activity or mineral deposition—after treatment with antagonists alone.
Dioxin markedly inhibited migratory capacity and chemotaxis (Fig. 4A–B), and the effects were partially recoverable by antagonist co-treatment. Cell migration was drastically decreased after exposure to dioxin under both standard and osteogenic conditions. We saw a similar, nearly 2-fold decline in chemotaxis towards all four chemoattractants tested (BMP2, CXCL12, CCL20, and IL-8). A likely contributor to these effects was the significant down-regulation of CXCL12 expression by dioxin. CXCL12 is a chemokine that plays a critical role in the initiation of osteoblast differentiation, as well as cell migration, and chemotaxis (Zhu et al., 2007). Previous studies have shown CXCL12 expression levels to be highest at sites of injury, where it actively recruits CXCR4-expressing mesenchymal stem cells in order to support tissue-specific repair or regeneration (Shi and Gronthos, 2003). We posit that the decreased expression of CXCL12 in cells exposed to dioxin contributes to the reduced cell motility observed in vitro, and could play an important role in the adverse effects of dioxin on bone regeneration in vivo. Dioxin-treated cells also expressed lower levels of CCR6, which encodes the receptor for CCL20. This chemokine has many roles, including cell migration and enhancement of MMP3 expression (Gilchrist and Stern, 2015, Honczarenko et al., 2006). Given these roles, the inhibitory effect of dioxin on CCR6 may directly impact BMSC migration and chemotaxis.
While our study focused on the anti-osteogenic effects specific to dioxin, many other CS constituents are also known Ahr ligands, including polychlorinated dibenzofurans (PCDFs), polychlorinated dibenzo-p-dioxins (PCDDs), coplanar polychlorinated biphenyls (Co-PCBs) and polycyclic aromatic hydrocarbons (PAHs) (Leow and Maibach, 1998, Kitamura and Kasai, 2007). As a result, these chemicals may have similar—and perhaps additive—inhibitory effects on osteogenesis. Moreover, there is wide variability in the stability and bioavailability of these compounds, which should be considered when evaluating their cumulative effects (Sloan et al., 2010).
Previous studies have focused on the isolated effects and mechanisms of specific chemical constituents present in CS; however, the cumulative effects of whole CS on Ahr activation and bone regeneration are not well understood. Our study notes the capacity of various Ahr antagonists to mitigate the adverse effects of dioxin on bone regeneration in vitro. While it is important to appreciate the effects of Ahr antagonism in relation to individual chemicals such as dioxin, a more clinically relevant question is how the multitude of Ahr ligands present in CS collectively impact bone regeneration, and how various Ahr antagonists can work to mitigate these effects. Future studies should focus on Ahr pathway involvement in the adverse effects of whole CS on bone regeneration and healing. Investigations into the protective effects of various Ahr antagonists after CS exposure could lay the groundwork for a viable therapeutic approach to reduce the negative impact of CS on bone. With surgeons limited in their ability to treat patients who smoke, and given the addictive nature of cigarettes and the low rates of compliance with surgeons' requests for smoking cessation, an effective measure that reduces risk and improves patient outcomes would be extremely beneficial for this problematic population.
Conflicts of interest
No authors have conflicts of interest relating to the contents of this manuscript.
Grant support
Funding for this study was provided by a grant from the Orthopaedic Research and Education Foundation (OREF) (Project number 60036756).
Authors' roles
Study design: EH, WH, and CY. Study conduct: CY, EH, and WH. Conducting experiments: CY, JW, DC, MS, RC, RF, AK, SM, CP, JY. Data analysis: JW, DC, MS, CY, EH. Data interpretation: EH, CY, JW, DC, MS, RC, WH. Drafting manuscript: CY, JW, DC, MS, RC, EH. Revising manuscript content: CY, JW, DC, RC, MS, WH, EH. Approving final version of manuscript: EH and WH. EH takes responsibility for the integrity of the data analysis.
Acknowledgements
This study was funded in part by a grant from the Orthopaedic Research and Education Foundation. QPCR, ALP, MTS, and Mineralization assays were performed using equipment in the Analytical BioNanoTechnology Equipment Core Facility of the Simpson Querrey Institute at Northwestern University. The U.S Army Research Office, the U.S. Army Medical Research and Materiel Command, and Northwestern University provided funding to develop this facility.
References
- Addison W.N., Nakano Y., Loisel T., Crine P., McKee M.D. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2008;23(10):1638–1649. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- Bertelli A.A., Das D.K. Grapes, wines, resveratrol, and heart health. J. Cardiovasc. Pharmacol. 2009;54(6):468–476. doi: 10.1097/FJC.0b013e3181bfaff3. [DOI] [PubMed] [Google Scholar]
- Bornmyr S., Svensson H. Thermography and laser-Doppler flowmetry for monitoring changes in finger skin blood flow upon cigarette smoking. Clin. Physiol. 1991;11(2):135–141. doi: 10.1111/j.1475-097x.1991.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Carpi D., Korkalainen M., Airoldi L. Dioxin-sensitive proteins in differentiating osteoblasts: effects on bone formation in vitro. Toxicol. Sci. 2009;108(2):330–343. doi: 10.1093/toxsci/kfp021. [DOI] [PubMed] [Google Scholar]
- Castillo R.C., Bosse M.J., MacKenzie E.J., Patterson B.M., Group LS Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J. Orthop. Trauma. 2005;19(3):151–157. doi: 10.1097/00005131-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Daffner S.D., Waugh S., Norman T.L., Mukherjee N., France J.C. Effect of serum nicotine level on posterior spinal fusion in an in vivo rabbit model. Spine J. 2015;15(6):1402–1408. doi: 10.1016/j.spinee.2015.02.041. [DOI] [PubMed] [Google Scholar]
- Denison M.S., Nagy S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Dong L., Xia S., Gao F., Zhang D., Chen J., Zhang J. 3,3′-Diindolylmethane attenuates experimental arthritis and osteoclastogenesis. Biochem. Pharmacol. 2010;79(5):715–721. doi: 10.1016/j.bcp.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Gilchrist A., Stern P. Chemokines and bone. Clin. Rev. Bone Miner. Metab. 2015;13(2):61–82. [Google Scholar]
- Glassman S.D., Anagnost S.C., Parker A., Burke D., Johnson J.R., Dimar J.R. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25(20):2608–2615. doi: 10.1097/00007632-200010150-00011. [DOI] [PubMed] [Google Scholar]
- Gotfredsen K., Lindh C.H., Berglundh T. Does longstanding nicotine exposure impair bone healing and osseointegration? An experimental study in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2009;91(2):918–923. doi: 10.1002/jbm.b.31475. [DOI] [PubMed] [Google Scholar]
- Guo H., Zhang L., Wei K. Exposure to a continuous low dose of tetrachlorodibenzo-p-dioxin impairs the development of the tooth root in lactational rats and alters the function of apical papilla-derived stem cells. Arch. Oral Biol. 2015;60(1):199–207. doi: 10.1016/j.archoralbio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann D., Hoffmann I. The changing cigarette, 1950–1995. J. Toxicol. Environ. Health. 1997;50(4):307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- Holzer N., Braun K.F., Ehnert S. Green tea protects human osteoblasts from cigarette smoke-induced injury: possible clinical implication. Langenbeck's archives of surgery/Deutsche Gesellschaft fur Chirurgie. 2012;397(3):467–474. doi: 10.1007/s00423-011-0882-8. [DOI] [PubMed] [Google Scholar]
- Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek A.M., Silberstein L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- Hsu E.L., Sonn K., Kannan A. Dioxin exposure impairs BMP-2-mediated spinal fusion in a rat arthrodesis model. J. Bone Joint Surg. Am. 2015;97(12):1003–1010. doi: 10.2106/JBJS.N.01311. [DOI] [PubMed] [Google Scholar]
- Jamsa T., Viluksela M., Tuomisto J.T., Tuomisto J., Tuukkanen J. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on bone in two rat strains with different aryl hydrocarbon receptor structures. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001;16(10):1812–1820. doi: 10.1359/jbmr.2001.16.10.1812. [DOI] [PubMed] [Google Scholar]
- Jorgensen L.N., Kallehave F., Christensen E., Siana J.E., Gottrup F. Less collagen production in smokers. Surgery. 1998;123(4):450–455. [PubMed] [Google Scholar]
- Kitamura M., Kasai A. Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett. 2007;252(2):184–194. doi: 10.1016/j.canlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Kwee J.K. Yin and yang of polyphenols in cancer prevention. A short review. Anti Cancer Agents Med. Chem. 2015 doi: 10.2174/1871520616666151116124549. [DOI] [PubMed] [Google Scholar]
- Leow Y.H., Maibach H.I. Cigarette smoking, cutaneous vasculature, and tissue oxygen. Clin. Dermatol. 1998;16(5):579–584. doi: 10.1016/s0738-081x(98)00042-x. [DOI] [PubMed] [Google Scholar]
- Lyte M., Bick P.H. Modulation of interleukin-1 production by macrophages following benzo(a)pyrene exposure. Int. J. Immunopharmacol. 1986;8(3):377–381. doi: 10.1016/0192-0561(86)90120-7. [DOI] [PubMed] [Google Scholar]
- Marinucci L., Bodo M., Balloni S., Locci P., Baroni T. Sub-toxic nicotine concentrations affect extracellular matrix and growth factor signaling gene expressions in human osteoblasts. J. Cell. Physiol. 2014;229(12):2038–2048. doi: 10.1002/jcp.24661. [DOI] [PubMed] [Google Scholar]
- Middlekauff H.R., Park J., Moheimani R.S. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J. Am. Coll. Cardiol. 2014;64(16):1740–1750. doi: 10.1016/j.jacc.2014.06.1201. [DOI] [PubMed] [Google Scholar]
- Naruse M., Ishihara Y., Miyagawa-Tomita S., Koyama A., Hagiwara H. 3-Methylcholanthrene, which binds to the arylhydrocarbon receptor, inhibits proliferation and differentiation of osteoblasts in vitro and ossification in vivo. Endocrinology. 2002;143(9):3575–3581. doi: 10.1210/en.2002-220003. [DOI] [PubMed] [Google Scholar]
- Patel R.A., Wilson R.F., Patel P.A., Palmer R.M. The effect of smoking on bone healing: a systematic review. Bone Joint Res. 2013;2(6):102–111. doi: 10.1302/2046-3758.26.2000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S.E., Hanley E.N., Jr. The musculoskeletal effects of smoking. J. Am. Acad. Orthop. Surg. 2001;9(1):9–17. doi: 10.5435/00124635-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Rothem D.E., Rothem L., Soudry M., Dahan A., Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J. Bone Miner. Metab. 2009;27(5):555–561. doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- Ryan E.P., Holz J.D., Mulcahey M., Sheu T.J., Gasiewicz T.A., Puzas J.E. Environmental toxicants may modulate osteoblast differentiation by a mechanism involving the aryl hydrocarbon receptor. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007;22(10):1571–1580. doi: 10.1359/jbmr.070615. [DOI] [PubMed] [Google Scholar]
- Sasco A.J., Secretan M.B., Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung cancer. 2004;45(Suppl. 2):S3–S9. doi: 10.1016/j.lungcan.2004.07.998. (Amsterdam, Netherlands) [DOI] [PubMed] [Google Scholar]
- Schmitz M.A., Finnegan M., Natarajan R., Champine J. Effect of smoking on tibial shaft fracture healing. Clin. Orthop. Relat. Res. 1999;365:184–200. doi: 10.1097/00003086-199908000-00024. [DOI] [PubMed] [Google Scholar]
- Shi S., Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Singh S.U., Casper R.F., Fritz P.C. Inhibition of dioxin effects on bone formation in vitro by a newly described aryl hydrocarbon receptor antagonist, resveratrol. J. Endocrinol. 2000;167(1):183–195. doi: 10.1677/joe.0.1670183. [DOI] [PubMed] [Google Scholar]
- Sloan A., Hussain I., Maqsood M., Eremin O., El-Sheemy M. The effects of smoking on fracture healing. The surgeon: journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2010;8(2):111–116. doi: 10.1016/j.surge.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Staines K.A., MacRae V.E., Farquharson C. The importance of the sibling family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 2012;214(3):241–255. doi: 10.1530/JOE-12-0143. [DOI] [PubMed] [Google Scholar]
- Syversen U., Nordsletten L., Falch J.A., Madsen J.E., Nilsen O.G., Waldum H.L. Effect of lifelong nicotine inhalation on bone mass and mechanical properties in female rat femurs. Calcif. Tissue Int. 1999;65(3):246–249. doi: 10.1007/s002239900692. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson A., Whitelaw M.L., Gustaffson J.A., Poellinger L. Agonistic and antagonistic effect of α-naphthoflavone on dioxin receptor function. J. Biol. Chem. 1994;269(29):19028–19033. [PubMed] [Google Scholar]
- Yao Z., Hu W., Yin S. 3,3′-Diindolymethane ameliorates adriamycin-induced cardiac fibrosis via activation of a BRCA1-dependent anti-oxidant pathway. Pharmacol. Res. 2013;70(1):139–146. doi: 10.1016/j.phrs.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Zevin S., Gourlay S.G., Benowitz N.L. Clinical pharmacology of nicotine. Clin. Dermatol. 1998;16(5):557–564. doi: 10.1016/s0738-081x(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Zhang S., Qin C., Safe S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environ. Health Perspect. 2003;111(16):1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Boachie-Adjei O., Rawlins B.A. A novel regulatory role for stromal-derived factor-1 signaling in bone morphogenic protein-2 osteogenic differentiation of mesenchymal C2C12 cells. J. Biol. Chem. 2007;282(26):18676–18685. doi: 10.1074/jbc.M610232200. [DOI] [PubMed] [Google Scholar]