Abstract
Objective
To identify whether there is a decline in sexual functioning related to the menopause transition or to hysterectomy.
Methods
In a cohort of 1,390 women aged 42–52, with intact uterus and at least one ovary, not using hormone therapy, and pre- or early perimenopausal at baseline, we fit piecewise linear growth curves to 5,798 repeated measurements (7 visits spanning 14.5 years) of a sexual functioning score (range, 5–25) as a function of time relative to date of final menstrual period (FMP) or hysterectomy.
Results
Mean sexual functioning at baseline in women with a dateable FMP was 18.0 (standard deviation, 3.4). There was no change in sexual function until 20 months before the FMP. From 20 months before until one year after the FMP, sexual function decreased by 0.35 annually (95% CI:−0.44, −0.26) and continued to decline more than one year after the FMP, but at a slower rate (−0.13 annually, 95% CI:−0.17, −0.10). The decline was smaller in African-Americans and larger in Japanese compared to whites. Vaginal dryness, lubricant use, depressive symptoms, or anxiety did not explain decline in sexual function. Women who had a hysterectomy prior to the FMP did not show decline in sexual function prior to hysterectomy, but scores declined afterwards (0.21 annually, 95% CI:−0.28, −0.14).
Conclusions
Decline in sexual function became apparent 20 months prior to FMP and slowed one year after FMP through 5 years afterwards. A decline in sexual function was observed immediately after hysterectomy and persisted for the 5 years of observation.
Keywords: menopause, final menstrual period, hysterectomy, oophorectomy, sexual functioning
Sexual functioning is an important component of women’s lives and has increasingly received public health, pharmaceutical, and medical attention.1 Over 75% of the middle-aged women in the Study of Women’s Health Across the Nation (SWAN) reported that sex was moderately to extremely important.2 Sexual functioning, however, declines with age,1,3–9 leading to much debate about the contribution of menopause to sexual activity and functioning.
The menopause transition is a gradual change from pre- to postmenopause; menopause is defined retrospectively after 12 months of amenorrhea has elapsed. Some evidence suggests that sexual functioning declines over the menopausal transition, yet whether this decline is due to menopause, aging, or other factors such as the availability of a partner,10–14 psychological function,1,10,11,15–18 and/or health10,12,14,15,19 remains inconclusive. Previous analyses have assessed sexual function changes in relation to menstrually-defined menopause transition categories, which classify stages of the menopause according to menstrual irregularity or number of months of amenorrhea. The present analyses seek to determine if sexual function declines at or around the final menstrual period (FMP), a more precise measure of the timing of menopause compared to menopause transition stages.
Research on menopause and sexual function typically focuses on women who have had a natural menopause and excludes women with hysterectomy.3,10,11,14,15,20–23 However, there is an entirely separate literature on the impact of hysterectomy on sexual functioning. Prospective studies often report that sexual functioning improves for most women following hysterectomy for benign indications.24,25,26 However, these studies typically compare pre-surgery sexual function, assessed within a few weeks prior to surgery, to subsequent symptom relief and may not consider the impact of a bilateral oophorectomy in conjunction with a hysterectomy, factors that may overstate benefits of hysterectomy. Research has not been conducted in longitudinal cohorts of women with extensive measurement of sexual function and relevant covariates well before and post-surgery to determine whether hysterectomy has an impact on sexual function separate from that of aging. Nor has research compared sexual functioning between naturally postmenopausal women and those who have had a hysterectomy. The present analyses use prospective data on sexual functioning to examine if and when the rate of change in sexual functioning occurs in relation to a hysterectomy performed prior to the FMP.
The primary objectives of this study, conducted in a multi-ethnic/racial cohort, are to: (1) identify whether there is a decline in sexual functioning around the time of the FMP for naturally menopausal women, and around the date of surgery for women who have undergone hysterectomy (with or without bilateral oophorectomy) before their FMP; (2) determine if the rate of decline in sexual functioning is related to age at FMP or hysterectomy (with or without bilateral oophorectomy), race/ethnicity, changes in health status, hormone therapy use, and partner status; and (3) evaluate whether a decline in sexual functioning around the FMP or hysterectomy (with or without bilateral oophorectomy), date can be explained by other changes that occur with menopause (vaginal dryness, vasomotor symptoms, depression, anxiety, or lubricant use).
METHODS
SWAN is a multi-racial/ethnic observational cohort study of the menopausal transition in 3,302 community-dwelling women at 7 sites across the United States.27 Details of enrollment, which began in 1996, have been previously reported.27 Baseline eligibility criteria included the following: age 42–52 years, intact uterus and at least one ovary, not currently using exogenous hormones affecting ovarian function, at least one menstrual period in the previous 3 months, and self-identification with a site’s designated racial/ethnic groups. Half of each site’s sample consisted of white women and the other half from one minority population (African-Americans, Japanese, Chinese, or Hispanic).
Standardized assessments were completed approximately annually in a clinic setting where trained interviewers administered physiological and self-report measures. Study forms were available in English, Cantonese, Japanese and Spanish with appropriate bilingual staff administering them. Each site received Institutional Review Board approval and all participants gave written informed consent.
Sexual Functioning Measure
Sexual outcome variables were measured annually at each study visit from baseline to visit 06 and biannually thereafter (at visits 08, 10, and 12) using a 20-item self-administered questionnaire designed to address sexual activity and function over the past 6 months in women with and without partners that was returned to staff in a sealed envelope. The questionnaire was derived from several sources,1,10,28,29 designed to cover sexual functioning domains of interest, and has been previously described.15,30 For the present analyses, we developed a single sexual functioning score based on the Female Sexual Functioning Index (FSFI).31 The score was developed using a separate dataset from the STRIDE study32 that contained both the FSFI and items from the SWAN questionnaire and matched five of the FSSI domains with those items from SWAN (sexual desire, emotional satisfaction, ability to climax, arousal, and pain). The resulting scale in STRIDE was highly correlated with the total FSFI score (rho=0.84). At SWAN visit 12, we administered the Short Form of the Personal Experiences Questionnaire (SPEQ)33 in addition to the SWAN questionnaire. Correlation between the SPEQ total score and our sexual functioning measure was 0.70, providing further validity of our measure. In the SWAN data, all responses were recorded on five-point Likert scales. We reverse-coded the item related to pain and then summed the five variables for a range of scores from 5–25 with higher numbers indicating better functioning (coefficient alpha =.70). The score was created only at the visits that the women reported being sexually active.
Primary Exposure Variables
The primary exposure was time (in months) before or after a woman’s FMP for women who had a natural menopause transition, and time (in months) before or after the date of surgery for women who had a hysterectomy (with or without bilateral oophorectomy), before natural menopause. FMP date was determined by annual, standardized interview and defined as the last menstrual bleeding date reported during the visit immediately before the first visit when the participant was classified as postmenopausal (12 months of amenorrhea). We excluded women who reported use of exogenous hormones one year before the FMP. For women who had a hysterectomy, we obtained the date of surgery from medical records, if available, or by participant self-report. Others have shown that self-reporting of hysterectomy status is relatively accurate.34 We included women who underwent a hysterectomy alone or with a unilateral or bilateral oophorectomy prior to a defined FMP. In SWAN, only a small number of women (n=16) had both ovaries removed without hysterectomy and they were excluded from these analyses.
Other Predictors
We examined the following variables, which have previously been related to sexual functioning,3,10,11,14,15,20–23 as covariates of sexual functioning trajectory: age at FMP or hysterectomy (in years), self-reported race/ethnicity (white, African-American, Chinese, Japanese, or Hispanic), partner status (married or partnered versus not married or partnered; time-varying, assessed at each visit), hormone therapy use, and overall health (time-varying, assessed at each visit). Overall health was self-assessed annually using a 5-level scale that was collapsed into 2 levels (excellent/very good/good vs. fair/poor).
To address our third objective, to examine mechanisms that might explain any change in sexual functioning around the time of the FMP, we examined the following time-varying factors that often co-occur with menopause: vasomotor symptoms (hot flashes and night sweats), vaginal dryness, lubricant use during intercourse, depression, and anxiety. The frequency of experiencing each symptom (hot flashes, night sweats, or vaginal dryness) in the past two weeks was reported at each study visit on a 5-level scale, collapsed into 3 levels for analysis (not at all, 1–5 days, and 6–14 days). Women who were sexually active were asked how often they had used lubricants in the past 6 months as part of the sexual function questionnaire. The Center for Epidemiologic Studies Depression Scale was administered annually to assess depressive symptoms.35 Anxiety, assessed annually, was the summed score of number of days in the past 2 weeks in which four symptoms (irritability or grouchiness, feeling tense or nervous, heart racing or pounding, fearful for no reason) were experienced.36 The total anxiety score was dichotomized with scores greater than or equal to four indicating high anxiety symptoms.36
Study Sample
Of the 3,302 women recruited into the SWAN cohort at baseline, 1,396 had known FMP date by visit 12 (they did not report use of exogenous hormones before or at the first visit classified as postmenopausal and thus the date of the FMP was not obscured by exogenous HT), and 305 women reported having undergone (1) hysterectomy only (n=65), (2) hysterectomy and unilateral oophorectomy (n=20), or (3) hysterectomy and bilateral oophorectomy (n=220). Women who reported bilateral oophorectomy without hysterectomy were excluded (n=16). At each visit, only women who completed the sexual function questionnaire and reported engaging in sexual activity with a partner (regardless of partner’s sex) were assigned a sexual function score. To be included in the analysis, a woman had to report sexual activity at one or more study visits. A total of 232 women in the natural menopause group and 79 women in the hysterectomy group were missing the sexual function score at all visits, leaving 1,164 women in the natural menopause group and 226 women in the hysterectomy group eligible to be included in the analysis of sexual functioning. Sexual functioning data were obtained from these women between one and seven times over the SWAN study; the majority of women (63%) provided data at four or more visits. There were a combined 4,932 measurements from the 1,164 women in the natural menopause group, and 866 measurements from the 226 women in the hysterectomy group.
Statistical Analysis
Separate but equivalent analyses were conducted in the women who went through a natural menopause and the women who had a hysterectomy before a natural menopause. In the former group, we estimated the trajectory of change in sexual function score over the menopause transition; in the latter group, we estimated the trajectory of sexual functioning before and after hysterectomy. To estimate these trajectories and to examine associations with factors that might affect the trajectory, we used a three-step process: (1) nonparametric locally-weighted scatterplot smoothing (LOESS) regression on time from FMP or date of hysterectomy to determine the functional form of the mean trajectory,37 (2) fitting a piecewise linear growth curve with different sets of inflection (or knots, where the slope changes) to determine optimal knot placement, and (3) mixed effects regression that fit a piecewise linear growth curve with fixed knots to estimate slopes in each segment and the associations of postulated factors with slopes.
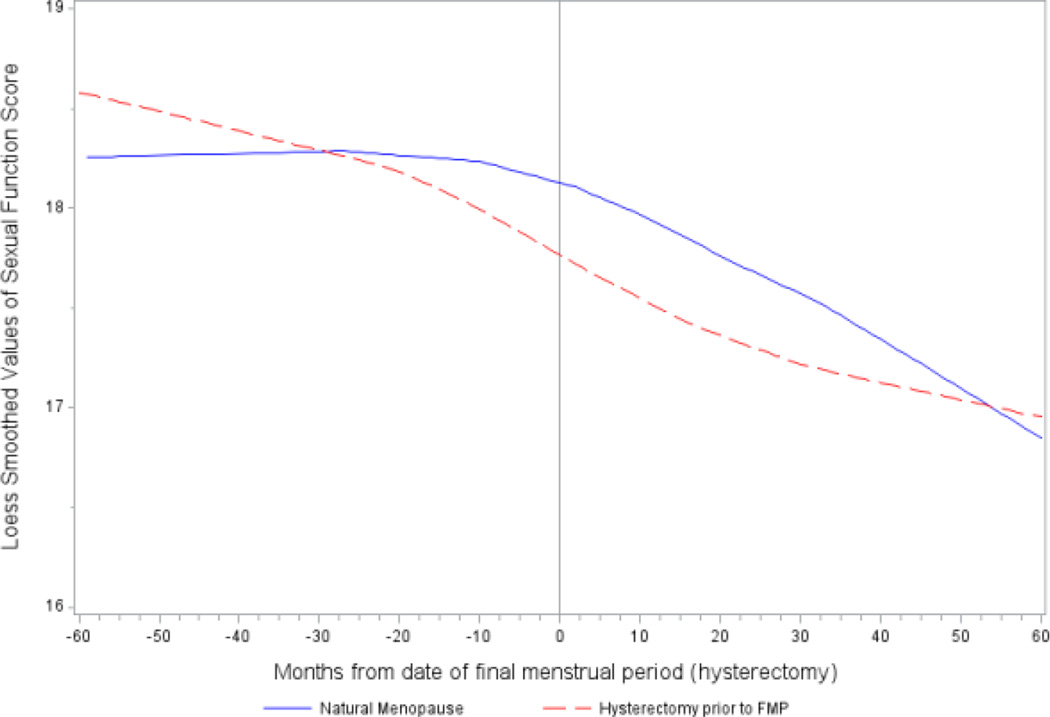
In step 1, we used nonparametric LOESS regression to fit a smooth curve to the repeated measures of sexual function score as a function of time from FMP or hysterectomy date. Our observations spanned from 13 years before to 15 years after the FMP. For this step only, we excluded observations more than 5 years before or after FMP (or date of hysterectomy) to avoid overfitting sparse data at the extremes. The LOESS plots revealed that the mean trajectory of sexual function score was piecewise linear in both groups of women, but with two inflections in the natural menopause women and only one inflection point in the hysterectomy women (Figure 1).
Figure 1.
LOESS plots of sexual function score in the natural and surgical menopause women and hysterectomy women in the 10-year period bracketing the date of the final menstrual period or hysterectomy.
In steps 2 and 3, we used mixed effects regression to fit piecewise linear models to the repeated measurements of sexual function score as function of time before or after the FMP or hysterectomy date, using linear splines with fixed knots. We included all available observations in steps 2 and 3, as mixed effects models are robust against the undue influence of outliers as well as to missing at random data, thus allowing the maximal use of all available data to estimate the large number of model parameters. Based on the results of the LOESS regression from step 1, we used a linear spline with two fixed knots and 3 time periods in the natural menopause group; time period 1 was the period from 13 years before to 20 months before the FMP, time period 2 was 20 months before to 1 year after the FMP, and time period 3 was from 1 year after to 15 years after the FMP. In the hysterectomy group, we use a 2-segment linear spline with one fixed knot, with time period 1 defined as the period from 13 years before to the date of the hysterectomy and time period 2 defined as from the date of hysterectomy to 15 years after. To account for within-woman correlation between repeated observations, we included person-specific random effects for the intercept and the slopes in each segment. Since the inflection points could not be precisely localized from the LOESS plots of step 1, in step 2, we tested appropriateness of knot locations by running null models with only random (woman-specific) effects for intercept and slopes, and no fixed effects, varying the locations of knots, and examining the residuals unexplained by the model. The residual within-woman variance in the natural menopause group was smallest when the two knots were placed at 20 months prior to the FMP date and 1 year after the FMP. In the hysterectomy group, it was smallest when the single knot was placed at the hysterectomy date.
In step 3, with the knots fixed at the optimal locations determined in step 2, we first added age at FMP (continuous), race/ethnicity, marital/partnership status (yes vs. no), and self-reported health (excellent/good vs. fair/poor) to the model, as fixed effects on the intercept/level and each of the time slopes, to assess how each of these covariates influenced the level and/or rate of change in each time period of the sexual function trajectory. We also adjusted for site and HT use subsequent to FMP or hysterectomy. In the model for the hysterectomy group, two additional covariates were included: bilateral oophorectomy status and menopause transition status prior to the hysterectomy. For time-varying covariates (such as marital status, health, and HT use), the value of the covariate at the current time was allowed to affect current level of sexual functioning, and the value of the covariate at the beginning of a segment (i.e. at study baseline for the first time period of the growth curve and at each of the knots for subsequent time periods) was allowed to affect the slope of that period. The estimated regression coefficients for a covariate’s effect on the slopes in the different segments of the sexual function trajectory were combined to obtain the total effect on cumulative change in sexual function over a 7-year period (from 2 years prior, to 5 years after) bracketing the FMP or hysterectomy date.
RESULTS
Sample Characteristics
At baseline, participants were 42–52 years of age with a mean age of 46. Approximately 45% were white. Baseline characteristics of the study participants, stratified by those who had a dateable FMP and those who had a hysterectomy (with or without bilateral oophorectomy), prior to their FMP, are presented in Table 1. Over the 14.5 years of follow-up, 16% (N=226) of the study sample underwent a hysterectomy before menopause. Of those, 157 (69.5%) had bilateral oophorectomies. Participants who had hysterectomy were younger (p=0.004), less likely to be Asian (p=0.004), more likely to report excellent or very good health (p=0.01), and more likely to have anxiety (p=0.001) compared to those with a datable FMP. The mean age at which women had a hysterectomy was lower than the mean age at which the FMP occurred in the remainder of the sample (p<.0001).
Table 1.
Characteristics of study sample at SWAN baseline
| Natural Menopause (N=1,164) |
Hysterectomy (N=226) |
|
|---|---|---|
| Age (years), median (mean ±SD) | 46.2 (46.3±2.6) | 45.4 (46.0±2.7) |
| Age at FMP, median (mean ±SD) | 52.1 (52.0±2.7) | 50.9 (51.6±4.9) |
| Race/ethnicity, n (%) | ||
| White | 520 (44.7) | 106 (46.9) |
| African-American | 332 (28.5) | 82 (36.3) |
| Hispanic | 70 (6.0) | 16 (7.1) |
| Chinese | 119 (10.2) | 12 (5.3) |
| Japanese | 123 (10.6) | 10 (4.4) |
| Study site, n (%) | ||
| Michigan | 193 (16.6) | 56 (24.8) |
| Boston | 176 (15.1) | 25 (11.1) |
| Chicago | 169 (14.5) | 32 (14.2) |
| UC Davis | 189 (16.2) | 18 (8.0) |
| UCLA | 186 (16.0) | 35 (15.5) |
| New Jersey | 106 (9.1) | 22 (9.7) |
| Pittsburgh | 145 (12.5) | 38 (16.8) |
| Menopause status, n (%) | ||
| Early perimenopause | 493 (42.8) | 104 (46.4) |
| Premenopause | 658 (57.2) | 120 (53.6) |
| How hard to pay for basics, n (%) | ||
| Very hard | 73 (6.3) | 16 (7.1) |
| Somewhat hard | 353 (30.5) | 66 (29.5) |
| Not hard | 730 (63.2) | 142 (63.4) |
| Education, n (%) | ||
| Less than HS or HS | 278 (24.2) | 43 (19.2) |
| Greater than HS/some college | 379 (32.9) | 85 (37.9) |
| College/post-college | 494 (42.9) | 96 (42.9) |
| Marital status, n (%) | ||
| Single/never married | 116 (10.1) | 24 (10.8) |
| Married | 871 (75.9) | 165 (74.0) |
| Separated/widowed/divorced | 160 (14.0) | 34 (15.2) |
| Self-assessed health, n (%) | ||
| Excellent/very good | 227 (19.6) | 63 (28.0) |
| Good | 767 (66.5) | 127 (56.4) |
| Fair/poor | 160 (13.9) | 35 (15.6) |
| VMS: at least 6/14 days, n (%) | 105 (9.1) | 30 (13.5) |
| Vaginal dryness past 2 weeks, n (%) | ||
| Not at all | 945 (81.6) | 175 (78.8) |
| 1–5 days | 164 (14.2) | 31 (14.0) |
| 6+ days | 49 (4.2) | 16 (7.2) |
| Lubricant use, n (%) | 237 (22.9) | 46 (23.6) |
| CES-D scale ≥16, n (%) | 240 (20.6) | 57 (25.2) |
| High anxiety, n (%) | 209 (18.1) | 59 (26.6) |
| Sexual function scorea (range:1–25), median (mean ±SD) |
18.0 (18.0±3.4) | 18.5 (18.3±3.3) |
FMP, Final menstrual period; VMS, Vasomotor symptoms; CES-D, Center for Epidemiological Studies Depression.
Data needed for creation of the sexual function score were not available until visit 03. Thus the baseline sexual function score was obtained at visit 03 for most of the sample and from visit 04 for those who did not provide data at visit 03.
Mean sexual function score at the baseline visit was 18.0 and standard deviation (SD) 3.4 in the natural menopause group, and 18.5 and 3.3 respectively, in the hysterectomy group (Table 1). By visit 12, mean sexual function score had fallen to 16.5 (SD 4.4) in the natural menopause women and 16.9 (SD 3.7) in the hysterectomy group. Change over time in other time-varying characteristics of the sample are shown in Supplemental tables 1–4.
Sexual Functioning Trajectories
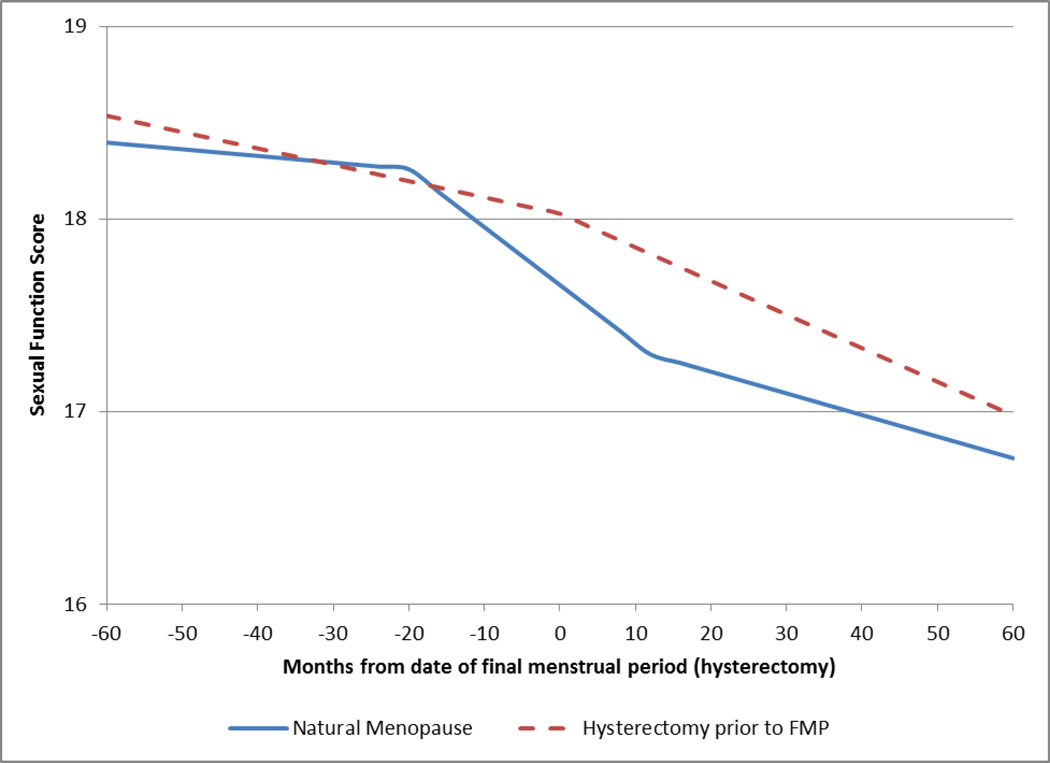
Figure 2 shows the unadjusted, model-predicted mean trajectory of sexual function scores as a function of time before and after the FMP in the sample with an observed FMP and in the sample that underwent a hysterectomy. Sexual function declined over time for both groups. Women with an observed FMP had a mean sexual function score at FMP date of 17.6 (SD=2.58). There was no significant change (M=−0.04 per year, SD=0.40, p=0.19) in sexual function score up to 20 months before the FMP (period 1). In the period of time 20 months before to one year after the FMP (period 2), sexual function score decreased by 0.35 per year (SD=0.39, 95% CI for mean:−0.44, −0.26, p<.0001). In the final time period, (period 3), sexual function continued to decline, but at a slower rate than that observed in the period 2 (M=−0.13 per year, SD=0.43, 95% CI for mean:−0.17, −0.10, p<.0001) (Figure 2, Table 2). Formal comparisons of the difference in slopes between these three time periods showed that the average rate of change in sexual function was significantly different between time periods 1 and 2 (p<.0001) and between time periods 2 and 3 (p<.0001). The mean cumulative change in sexual function score from two years before to five years after the natural FMP was −1.48 (95% CI=−1.70, −1.25, p<.0001). These results suggest that among women who experience a natural menopause, sexual function starts to decline at about 20 months before the FMP and that this decline slows, but does not cease, at about a year after FMP.
Figure 2.
Model-predicted mean trajectories of sexual function over the menopausal transition.
Table 2.
| Mean Sexual Function Score at FMP (95% CI) |
Mean Annualized Rate of Change in Sexual Function Score (95% CI) |
Mean Change in Sexual Function Score from 2 years before to 5 years after the FMP (95% CI) |
||
|---|---|---|---|---|
| Period 1: From 13 years before to 20 months before FMP |
Period 2: From 20 months before to 1 year after FMP |
Period 3: From 1 year after to 15 years after FMP |
||
| 17.6 (17.5, 17.8) |
−0.04 (−0.10, 0.02) |
−0.35 (−0.44, −0.26) |
−0.13 (−0.17, −0.10) |
−1.48 (−1.70, −1.25) |
FMP, final menstrual period
Results from a null, mixed effects model without covariates
4,932 observations are included in Table 2 analyses
Statistically significant results (p<.05) are shown in bold
P value for test of difference in slopes between segments 1 and 2: <.0001; P value for test of difference in slopes between segments 2 and 3: <.0001
The age at which a woman reached her FMP did not appear to be associated with either her sexual function at the time of the FMP or the cumulative 7-year change in sexual function (Table 3). African-American women experienced a smaller decline in sexual function and Japanese women a larger decline, compared to white women (the referent group). Chinese women (compared to white women) and women with fair/poor health (compared to women in good/excellent health) reported a lower level of sexual functioning at the time of their FMP but their total 7-year change was similar (Table 3).
Table 3.
Adjusted trajectory and associations of predictors with trajectories of sexual function in natural menopausea,b
| Sexual Function Score at FMP (95% CI) |
Annualized Rate of Change in Sexual Function Score (95% CI) | Mean Change in Sexual Function Score from 2 years before to 5 years after the FMP (95% CI) |
|||
|---|---|---|---|---|---|
| Period 1: From 13 years before to 20 months before FMP |
Period 2: From 20 months before to 1 year after FMP |
Period 3: From 1 year after to 15 years after FMP |
|||
|
Adjusted mean in referent groupc |
17.8 (17.0, 18.5) | −0.10 (−0.31, 0.11) | −0.50 (−0.74, −0.25) | −0.12 (−0.23, −0.02) | −1.86 (−2.47, −1.24) |
| Adjusted associations of predictors with trajectory parameters | |||||
| Time-invariant predictors | |||||
| Age at FMP (years) | −0.11 (−0.22, 0.002) | −0.04(−0.07, −0.01) | 0.03 (−0.01, 0.07) | −0.003 (−0.02, 0.01) | 0.05 (−0.05, 0.14) |
| Race/ethnicity | |||||
| African-American | −0.31 (−1.02, 0.40) | −0.03 (−0.22, 0.17) | 0.34 (0.07, 0.60) | 0.02 (−0.09, 0.14) | 0.98 (0.33, 1.63) |
| Chinese | −1.46 (−2.71, −0.22) | 0.18 (−0.12, 0.48) | −0.36 (−0.81, 0.08) | 0.16 (−0.03, 0.35) | −0.27 (−1.38, 0.84) |
| Japanese | −0.88 (−2.19, 0.44) | 0.16 (−0.18, 0.49) | −0.45 (−0.92, 0.01) | −0.09 (−0.28, 0.10) | −1.52 (−2.70, −0.34) |
| Hispanic | −0.76 (−5.73, 4.21) | −0.30 (−2.85, 2.25) | 0.31 (−0.81, 1.44) | −0.16 (−0.60, 0.28) | 0.09 (−2.81, 2.98) |
| Time-varying predictorsd | |||||
| Fair/poor health | −0.58 (−0.85, −0.30) | 0.19 (0.04, 0.33) | 0.16 (−0.11, 0.44) | −0.08 (−0.19, 0.03) | 0.19 (−0.62, 0.99) |
| Not married or partnered | 0.22 (−0.12, 0.56) | −0.22 (−0.35, −0.09) | 0.23 (0.003, 0.46) | 0.001 (−0.11, 0.11) | 0.55 (−0.10, 1.19) |
| Hormone use | −0.69 (−1.54, 0.16) | --- | --- | 0.90 (−0.68, 2.48) | --- |
4,255 observations are included in Table 3 analyses.
Statistically significant results (p<.05) are shown in bold
Referent group: 51.8 years old at FMP, white, in good or better overall health, married or partnered, not using hormones, and from Pittsburgh site. To obtain trajectory parameters (sexual function level at FMP, slopes in the 3 time segments, and cumulative 7-year change) in non-referent women, add means in referent women to the corresponding effect sizes for each of the predictors that differ between referent woman and non-referent woman. For example, 7-year change in a Japanese woman who is otherwise similar to the referent women is computed as follows: −1.86–1.52=−3.38.
For time-varying predictors, the association between change in the predictor over time and contemporaneous change in sexual function score is also given by the entries in the first column (score at FMP).
P value for test of difference in slopes between time periods 1 and 2 in referent group: 0.04; P value for test of difference in slopes between segments 2 and 3 in referent group: 0.02.
When potential mechanisms for the effect of natural menopause on sexual function were added to the model as predictors of both level (intercept) and slopes (Table 4) the sexual functioning trajectory (including level, slopes, and cumulative 7-year change) was not significantly affected; there remained a significant decrease in sexual function in time periods 2 and 3 in the referent group women. Relative to the referent group, defined as women without vaginal dryness, lubricant use, depression or anxiety, sexual function score at the FMP was 0.56 lower for those with vaginal dryness 1–5 days a week (95% CI=−0.74, −0.38, p<.0001) and 0.99 lower for those with vaginal dryness 6–7 days per week (95% CI=−1.25, −0.73, p<.0001), 0.31 higher for those who used lubricants (95% CI=0.11, 0.51, p=0.002), 0.30 lower for those with significant depressive symptoms (95% CI=−0.53, −0.07, p=0.02), and 0.30 lower for those with significant anxiety symptoms (95% CI=−0.52, −0.07, p=0.004). Despite their association with overall sexual functioning score, vasomotor symptoms, vaginal dryness, lubricant use and anxiety were not significantly associated with rate of change in sexual function for any of the time periods, suggesting that these variables did not explain the effect of FMP on change in sexual function.
Table 4.
| Sexual Function Score at FMP (95% CI) |
Annualized Rate of Change in Sexual Function Score (95% CI) | Mean Change in Sexual Function Score from 2 years before to 5 years after the FMP (95% CI) |
|||
|---|---|---|---|---|---|
| Period 1: From 13 years before to 20 months before FMP |
Period 2: From 20 months before to 1 year after FMP |
Period 3: From 1 year after to 15 years after FMP |
|||
|
Adjusted mean in referent groupc |
18.2 (17.5, 18.9) | −0.14 (−0.32, 0.04) | −0.34 (−0.58, −0.11) | −0.16 (−0.27, −0.04) | −1.59 (−2.20, −0.97) |
| Adjusted associations of predictors with trajectory parameters | |||||
| Time-invariant predictors | |||||
| Age at FMP (years) | −0.07 (−0.17, 0.04) | −0.03 (−0.06, −0.002) | 0.03 (−0.005, 0.07) | −0.006 (−0.02, 0.01) | 0.05 (−0.05, 0.14) |
| Race/ethnicity | |||||
| African-American | 0.09 (−0.63, 0.82) | −0.05 (−0.24, 0.14) | 0.31 (0.06, 0.56) | −0.01 (−0.12, 0.10) | 0.78 (0.15, 1.42) |
| Chinese | −1.48 (−2.68, 0.28) | 0.28 (0.01, 0.54) | −0.27 (−0.68, 0.15) | 0.18 (−0.01, 0.37) | 0.09 (−0.98, 1.16) |
| Japanese | −0.56 (−1.79, 0.67) | 0.37 (0.09, 0.65) | −0.62 (−1.04, −0.20) | −0.06 (−0.24, 0.12) | −1.76 (−2.86, −0.67) |
| Hispanic | −0.39 (−2.50, 1.72) | Not est | 0.60 (−0.52, 1.72) | −0.07 (−0.48, 0.35) | Not est |
| Time-varying predictorsd | |||||
| Fair/poor health | −0.43 (−0.70, 0.15) | 0.23 (0.07, 0.38) | 0.01 (−0.27, 0.29) | −0.002 (−0.12, 0.11) | 0.10 (−0.72, 0.91) |
| Not Married or partnered |
0.16 (−0.21, 0.53) | −0.21 (−0.36, −0.06) | 0.29 (0.05, 0.53) | −0.05 (−0.16, 0.07) | 0.52 (−0.15, 1.19) |
| Hormone use | −0.52 (−1.34, 0.29) | --- | --- | Not est | --- |
| Putative Mechanisms: Time-varyingd | |||||
| VMS at least 6 /14 days | −0.06 (−0.24, 0.13) | −0.16 (−0.35, 0.03) | 0.05 (−0.15, 0.25) | 0.02 (−0.06, 0.09) | 0.16 (−0.43, 0.75) |
| Vaginal dryness | |||||
| 1–5 days / week | −0.56 (−0.74, 0.38) | 0.01 (−0.13, 0.14) | 0.11 (−0.08, 0.30) | 0.07 (−0.01, 0.15) | 0.59 (−0.01, 1.18) |
| 6–7 days / week | −0.99 (−1.25, 0.73) | −0.23 (−0.50, 0.04) | 0.03 (−0.27, 0.33) | −0.04 (−0.15, 0.07) | −0.15 (−1.01, 0.72) |
| Lubricant use | 0.31 (0.11, 0.51) | −0.07 (−0.19, 0.05) | −0.04 (−0.21, 0.14) | −0.02 (−0.10, 0.06) | −0.20 (−0.72, 0.32) |
| Depression | −0.30 (−0.53, 0.07) | 0.02 (−0.13, 0.17) | 0.25 (0.01, 0.49) | −0.03 (−0.14, 0.08) | 0.55 (−0.19, 1.30) |
| Anxiety | −0.30 (−0.52, 0.07) | −0.06 (−0.21, 0.09) | −0.17 (−0.41, 0.06) | −0.02 (−0.13, 0.09) | −0.55 (−1.30, 0.20) |
3,391 observations are included in Table 4 analyses.
Statistically significant results (p<.05) are shown in bold
Referent group: 51.8 years old at FMP, white, in good or better overall health, married or partnered, not using hormones, VMS less than 6 days in past 2 weeks, no vaginal dryness, not using lubricant, no depression, no anxiety, and from Pittsburgh site.
Time-varying predictor: association between change in the predictor over time and contemporaneous change in sexual function score is also given by the entries in the first column (score at FMP).
P value for test of difference in slopes between segments 1 and 2 in referent group: 0.25; P value for test of difference in slopes between segments 2 and 3 in referent group: 0.20.
Women who had hysterectomy (with or without bilateral oophorectomy), had a mean sexual function score at the date of hysterectomy of 18.0 (SD=2.60). There was no statistically significant decline (M=−0.10 per year, SD=0.19, p=0.07) in sexual function before hysterectomy (Figure 2, Table 5). However, following hysterectomy there was a significant decline in mean sexual function score of 0.21 per year (SD=0.16, 95% CI for mean=−0.28, −0.14, p<.0001). The mean accumulated change in sexual function score from two years before to five years after hysterectomy was −1.25 (95% CI=−1.64, −0.86, p<.0001).
Table 5.
| Mean Sexual Function Score at hysterectomy (95% CI) |
Mean Annualized Rate of Change in Sexual Function Score (95% CI) |
Mean Change in Sexual Function Score from 2 years before to 5 years after hysterectomy (95% CI) |
|
|---|---|---|---|
| Period 1: From 13 years before to date of hysterectomy |
Period 2: From date of hysterectomy to 15 years after |
||
| 18.0 (17.5, 18.5) |
−0.10 (−0.21, 0.01) |
−0.21 (−0.28, −0.14) |
−1.25 (−1.64, −0.86) |
Results from a null, mixed effects model without covariates
865 observations are included in Table 5 analyses
Statistically significant results (p<.05) are shown in bold
P value for test of difference in slopes between segments 1 and 2: 0.15
Retention of both ovaries, age at the time of hysterectomy, race/ethnicity, health, marital status, HT use, and menopause transition stage prior to hysterectomy had no significant effect on trajectory of sexual functioning (Table 6). The referent category consisted of women with bilateral oophorectomy (because they were 70% of the surgical sample); the rate of decline in sexual function among those whose ovaries were retained did not appear to differ from those who underwent bilateral oophorectomy, but small sample size limits confidence in this lack of difference (Table 6). A significant decrease in sexual function score after hysterectomy (mean −0.25 per year in the referent group, 95% CI: −0.49, −0.003, p=0.04) also remained after the addition of potential explanatory factors to the adjusted model (Table 7). Relative to the referent group, sexual function score at hysterectomy was 0.92 lower for those with 1–5 days of vaginal dryness per week (95% CI=−1.40, −0.43, p=0.003) and 1.63 lower for those with vaginal dryness 6–7 days per week (95% CI=−2.28, −0.98, p<.0001). Experiencing vaginal dryness 6–7 days per week was also associated with a smaller decline in sexual function after hysterectomy. Vasomotor symptoms, anxiety, and depression were not significantly associated with rate of change in sexual function for either time segment.
Table 6.
Adjusted trajectory and associations of predictors with trajectories of sexual function in hysterectomy groupa,b
| Sexual Function Score at hysterectomy (95% CI) |
Annualized Rate of Change in Sexual Function Score (95% CI) | Mean Change in Sexual Function Score from 2 years before to 5 years after hysterectomy (95% CI) |
||
|---|---|---|---|---|
| Segment 1: From 13 years before to date of hysterectomy |
Segment 2: From date of hysterectomy to 15 years after |
|||
| Adjusted mean in referent groupc | 18.1 (16.8, 19.3) | −0.27 (−0.59, 0.05) | −0.25 (−0.50, −0.003) | −1.80 (−3.08, −0.51) |
| Adjusted associations of predictors with trajectory parameters | ||||
| Time-invariant predictors | ||||
| Age at FMP (years) | −0.08 (−0.21, 0.05) | −0.02 (−0.06, 0.02) | 0.001 (−0.02, 0.03) | −0.04 (−0.19, 0.10) |
| Race/ethnicity | ||||
| African-American | 1.18 (−0.16, 2.51) | 0.20 (−0.16, 0.57) | 0.13 (−0.07, 0.33) | 1.03 (−0.09, 2.16) |
| Chinese | 0.01 (−3.38, 3.39) | 0.15 (−0.65, 0.95) | −0.26 (−0.70, 0.19) | −0.98 (−3.51, 1.55) |
| Japanese | 0.53 (−2.20, 3.27) | 0.44 (−0.57, 1.44) | −0.57 (−0.99, −0.15) | −1.97 (−4.53, 0.60) |
| Hispanic | −1.81 (−5.71, 2.08) | −0.33 (−1.23, 0.57) | 0.52 (−0.22, 1.26) | 1.93 (−1.83, 5.68) |
| Time-varying predictorsd | ||||
| No bilateral oophorectomy | −0.08 (−0.97, 0.81) | --- | −0.12 (−0.32, 0.08) | --- |
| Menopause stage | ||||
| Late peri / Post | −0.02 (−0.97, 0.93) | 0.24 (−0.10, 0.58) | --- | --- |
| Fair/poor health | −0.47 (−1.10, 0.16) | 0.26 (−0.09, 0.60) | −0.16 (−0.39, 0.08) | −0.27 (−1.61, 1.07) |
| Not married or partnered | −0.03 (−0.75, 0.69) | −0.18 (−0.51, 0.15) | −0.04 (−0.24, 0.16) | −0.58 (−1.75, 0.59) |
| Hormone use | --- | --- | −0.07 (−0.25, 0.11) | --- |
794 observations are included in Table 6 analyses.
Statistically significant results (p<.05) are shown in bold
Referent group: Age at FMP = 51.8, white, in good or better overall health, not married or partnered, not using hormones, pre or e.peri at time of hysterectomy, who had bilateral oophorectomy, and from Pittsburgh site. To obtain trajectory parameters (sexual function level at FMP, slopes in the 3 segments, and cumulative 7-year change) in non-referent women, add means in referent women to the corresponding effect sizes for each of the predictors that differ between referent woman and non-referent woman. For example, the annualized slope in sexual function score after hysterectomy in a Japanese woman who is otherwise similar to the referent women is computed as follows: −0.25–0.57=−0.82.
For time-varying predictors, the association between change in the predictor over time and contemporaneous change in sexual function score is also given by the entries in the first column (score before hysterectomy).
P value for test of difference in slopes between segments 1 and 2 in referent group: 0.93
Table 7.
| Sexual Function Score at hysterectomy (95% CI) |
Annualized Rate of Change in Sexual Function Score (95% CI) | Mean Change in Sexual Function Score from 2 years before to 5 years after hysterectomy (95% CI) |
||
|---|---|---|---|---|
| Segment 1: From 13 years before to date of hysterectomy |
Segment 2: From date of hysterectomy to 15 years after |
|||
|
Adjusted mean in referent groupc |
18.6 (17.3, 19.8) | −0.25 (−0.61, 0.11) | −0.25 (−0.49, −0.003) | −1.74 (−3.06, −0.43) |
| Adjusted associations of predictors with trajectory parameters | ||||
| Time-invariant predictors | ||||
| Age at FMP (years) | −0.08 (−0.21, 0.06) | −0.01 (−0.05, 0.04) | −0.008 (−0.03, 0.02) | −0.05 (−0.20, 0.09) |
| Race/ethnicity | ||||
| African-American | 1.17 (−0.16, 2.51) | 0.19 (−0.24, 0.62) | 0.16 (−0.04, 0.36) | 1.18 (0.03, 2.36) |
| Chinese | −0.25 (−3.52, 3.02) | 0.43 (−0.44, 1.31) | −0.33 (−0.78, 0.11) | −0.79 (−3.44, 1.85) |
| Japanese | 0.31 (−2.32, 2.95) | 0.77 (−0.37, 1.92) | −0.41 (−0.80, −0.01) | −0.46 (−3.18, 2.27) |
| Hispanic | −1.27 (−5.13, 2.59) | −0.06 (−1.09, 0.98) | 0.40 (−0.30, 1.11) | 1.89 (−1.82, 5.60) |
| Time-varying predictorsd | ||||
| No bilateral oophorectomy | 0.25 (−0.67, 1.16) | --- | −0.19 (−0.38, 0.01) | --- |
| Late peri / postmenopause | 0.06 (−0.98, 1.09) | 0.29 (−0.11, 0.70) | --- | --- |
| Fair/poor health | −0.53 (−1.21, 0.15) | −0.08 (−0.59, 0.42) | −0.10 (−0.35, 0.14) | −0.69 (−2.31, 0.92) |
| Not married or partnered | −0.28 (−1.02, 0.47) | −0.26 (−0.61, 0.09) | −0.10 (−0.29, 0.10) | −1.00 (−2.16, 0.16) |
| Hormone use | --- | --- | −0.09 (−0.26, 0.10) | --- |
| Putative Mechanisms: Time-varyingd | ||||
| VMS at least 6 /14 days | −0.14 (−0.59, 0.30) | −0.42 (−0.85, 0.02) | −0.01 (−0.19, 0.16) | −0.89 (−2.12, 0.33) |
| Vaginal dryness | ||||
| 1–5 days / week | −0.92 (−1.40, 0.43) | 0.19 (−0.26, 0.64) | −0.11 (−0.30, 0.08) | −0.18 (−1.49, 1.13) |
| 6–7 days / week | −1.63 (−2.28, 0.98) | −0.20 (−0.73, 0.33) | 0.25 (0.03, 0.48) | 0.87 (−0.68, 2.43) |
| Depression | −0.53 (−1.07, 0.003) | 0.33 (−0.05, 0.72) | −0.05 (−0.28, 0.18) | 0.41 (−1.00, 1.82) |
| Anxiety | 0.15 (−0.36, 0.67) | 0.16 (−0.22, 0.54) | −0.01 (−0.21, 0.20) | 0.30 (−1.00, 1.59) |
761 observations included in Table 7 analyses.
Statistically significant results (p<.05) are shown in bold
Referent group: Age at FMP = 51.8, white, in good or better overall health, married or partnered, not using hormones, pre or early peri at time of hysterectomy, bilateral oophorectomy, VMS less than 6 days in past 2 weeks, no vaginal dryness, no depression, no anxiety, and from Pittsburgh site.
For time-varying predictors, the association between change in the predictor over time and contemporaneous change in sexual function score is also given by the entries in the first column (score before hysterectomy).
P value for test of difference in slopes between segments 1 and 2 in referent group: 0.98
DISCUSSION
This large, community-based study of middle-aged women who were sexually active during the menopausal transition examined longitudinal reports of sexual functioning over 14.5 years in two groups of women: those who experienced a natural transition and whose FMP date was known and those who underwent a hysterectomy (70% with bilateral oophorectomy) prior to their FMP. In the former group, we witnessed a decline in sexual functioning that began approximately 20 months prior to the FMP and slowed somewhat, but did not cease, at the one-year post FMP mark. Further, adjustment for possible confounders and/or mechanisms by which the menopause transition might influence sexual function (poorer health, vasomotor symptoms, vaginal dryness, depression, anxiety, or partner status) did not materially alter the estimated influence of the menopause transition on sexuality. Among women with a hysterectomy, onset of a decline in sexual function began immediately after surgery and the rate of decline persisted, unchanged, in the post-surgical period. This decline was not substantially altered by the addition of potential confounders and/or mechanisms and did not appear to be mitigated by ovarian retention.
Our results in the natural menopause sample agree with those from the only other published study of sexual functioning in relation to FMP,14 which also found that sexual desire began to decline prior to the FMP and that the decline was steeper in the period of time bracketing the FMP than it was further distant from the FMP. The previous study used both a different analytic approach and different assessment scale (a one-item question on sexual desire) than we did, but the similarities are striking: the steepest drop in sexual desire was from 3 years prior to FMP to 2 years after the FMP. Our study has the added advantages of including a longer post-FMP follow-up and a more diverse sample. We found important racial/ethnic differences in the decline in sexual function; African-American women experienced a smaller decline and Japanese women experienced a larger decline, compared to white women. For example a married Japanese women would have a decline of 3.35 (1.76 + 1.59, see table 4) which is comparable to one standard deviation of the sexual functioning score at baseline, and quite large. On the other hand, a married African-American women would have a decline of only 0.81 (1.59 – 0.78).
Our study supports a sizeable negative effect of menopause on sexual functioning in many women. The decline in sexual functioning scores average a half of a standard deviation over the 7 years bracketing the menopause transition; half a standard deviation is generally considered a meaningful change. To better interpret the change in total score, we looked at the mean change in each of the 5 items. Consistent with our previous work,15 the items that showed the most change in both the natural menopause and hysterectomy groups were increased pain and decreased desire; frequency of arousal and climaxing during sexual activity decreased less, and emotional satisfaction did not change at all in either group. In the case of pain, this might indicate a change from almost never to sometimes or almost always. Frequency of desire might change from “about once per week’ to “once or twice per month.”
To address whether the change in sexual function in relation to the FMP could be explained by factors that often co-occur with the FMP, we adjusted for VMS, vaginal dryness, the use of sexual lubricants, depression and anxiety--factors associated with both menopause and sexual function.15,38 Although all of these factors, except for VMS, were related to sexual function score at FMP, accounting for them did not impact sexual function score decline across the time segments, providing support that these factors did not explain the impact of menopause on sexual function decline.
Our study and that of Woods and colleagues,14 not only point to the influence of the menopause transition on sexuality, but underscore the manifestation of this a few years prior to the FMP and observed most strongly in the transmenopausal period (the few years bracketing the FMP).39 Further, the present analysis adds to the growing body of literature that attests to the influence of the transmenopause on biology and health; estradiol levels, bone density, bone turnover markers, integrated hip strength, and cardiovascular risk factors each show a remarkably similar pattern of onset of change prior to, with most notable changes bracketing, the FMP.39–43
Our results focusing on the decline in sexual functioning relative to FMP date are consistent with, but provide greater detail than, other research that has evaluated sexual functioning across menopausal stages. The time frame of 20 months prior to and through 1 year after FMP corresponds roughly to early and late perimenopause and 12 or more months following FMP would correspond to early postmenopause. Cross-sectional studies have shown greater pain30,44,45 and lower interest or desire among peri- or postmenopausal women10,20–22,44 compared to premenopausal women and longitudinal studies have shown that sexual dysfunction increases over the menopause transition.3,10,11,15,46 Previous longitudinal SWAN analyses reported a decrease in desire and increase in pain beginning in late perimenopause15 and the Melbourne study found a peak in sexual problems late in the transition.3 The longitudinal Penn Ovarian Aging11 reported that sexual dysfunction increased from pre to early perimenopause to postmenopause and that the transition from pre to late in the transition was particularly dramatic. None of these studies, however, has followed women as far into the postmenopausal years as SWAN. Our results suggest a continued, albeit slower, decline in sexual function after 20 months following the FMP.
Women who underwent hysterectomy (70% of whom also had bilateral oophorectomy) prior to their FMP reported a decline in sexual functioning following their hysterectomy, even after adjustment for covariates. Previous research has shown that although hysterectomy is generally associated with improved sexual function, concomitant oophorectomy with the resultant loss of estrogen may compromise sexual function.26,47–49 Although we did not find that ovarian retention lessened the post-operative drop in sexual function, this could be due to the small percentage of women who did not get a bilateral oophorectomy. In addition, due to the SWAN eligibility requirements of women being pre or early perimenopausal and with intact uterus at study entry, our study does not include women who had a hysterectomy at a younger age when the effect of loss of ovarian function may be even greater. Importantly, our study was able to compare change in sexual function among women who had their surgery prior to FMP with those who experienced a natural menopause transition. Although sexual function declined following surgery, sexual function scores were not different from those of women who experienced natural menopause. In fact, the scores over time are remarkably similar.
Limitations of these data should be mentioned. First, the sexual functioning questionnaire used was not one of the more newly developed, validated questionnaires such as the Short Personal Experiences Questionnaire (SPEQ)33 or Female Sexual Function Index (FSFI).31 However, the score was developed based on items that mapped to the FSFI and was further validated against the SPEQ. Second, some participants missed visits or were lost to follow-up and others did not answer the sexual functioning questionnaire at visits when they were not sexually active, resulting in missing data. However, the majority of the women (84%) with missing sexual functioning data were only missing data in interim visits; i.e. they provided sexual functioning data both before and after visits with missing data. Since the analytic methods used are relatively robust to missing data, and the multivariable models incorporated numerous factors strongly associated with the likelihood of data being missing, our findings are likely to not be affected by these missing data. It should be noted that 119 women in the sample (9.9%) stopped providing sexual functioning data although they continued to participate in SWAN. If some of these women stopped being sexually active for reasons related to the menopausal transition, our findings could represent underestimates of the average rates of decline in sexual functioning over the menopause transition. Third, the analysis does not account for changes in circulating sex steroid levels, psychosocial factors beyond depressive and anxiety symptoms, or partner factors (such as poor health or erectile dysfunction) that may help to explain our findings. Although endogenous estradiol and testosterone were related to sexual desire in the Seattle Midlife Women’s Health Study,14 only testosterone was related to sexual functioning in SWAN.50,51 Additionally, the hysterectomy group was predominantly women who also had bilateral oophorectomy and the mean decline rates in this group may have been driven by oophorectomy. The number of women who had hysterectomies with ovarian retention was too small (n=85) to identify a robust trajectory of sexual functioning in this group. Indication for hysterectomy was not collected in SWAN and could be a potentially important factor.
The present study has several strengths. The study includes a large community-based sample followed for a longer period of time than other studies, thus showing sexual functioning over a 10-year period before and after natural menopause or hysterectomy. The study uses a novel modeling approach that allows for a more precise understanding of the relation between onset and rates of change of sexual functioning surrounding the menopause transition. In addition, our multiethnic/racial sample allows for a better understanding of the impact of the menopause transition on different groups of women.
CONCLUSIONS
It is important for women and their health care providers to understand factors that may impact women’s experience of sexual function in relation to both the natural menopausal transition and to hysterectomy with or without ovarian conservation. Our study supports a meaningful decline in sexual functioning in relation to the menopause transition, most strongly apparent in the few years bracketing the final menstrual period. Women who underwent a hysterectomy (most with bilateral oophorectomy) prior to their final menstrual period, also had a decline in their sexual functioning. Although symptoms that accompany menopause--vaginal dryness, depressive symptoms, and anxiety-- were each independent predictors of greater sexual function decline, these symptoms did not explain the effect of menopause or surgery on sexual function. This study also underscores that sexual function decline varies in women with differing backgrounds: for example, in our sample, compared to the reference group of white married women undergoing natural menopause, married Japanese women’s drop in sexual functioning was twice as large.
Supplementary Material
Acknowledgments
Dr. Tepper previously received a research grant from Pfizer for a study of the use of novel anticoagulants among atrial fibrillation patients, which has ended.
National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Conflict of interest/financial disclosure: No other authors have any competing financial conflicts of interest.
Supplemental Table 1.docx
Supplemental Table 2.docx
Supplemental Table 3.docx
Supplemental Table 4.docx
References
- 1.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States - Prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 2.Cain VS, Johannes CB, Avis NE, et al. Sexual functioning and practices in a multi-ethnic study of midlife women: baseline results from SWAN. J Sex Res. 2003;40:266–276. doi: 10.1080/00224490309552191. [DOI] [PubMed] [Google Scholar]
- 3.Dennerstein L, Dudley EC, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril. 2001;76:456–460. doi: 10.1016/s0015-0282(01)01978-1. [DOI] [PubMed] [Google Scholar]
- 4.Gass MLS, Cochrane BB, Larson JC, et al. Patterns and predictors of sexual activity among women in the Hormone Therapy trials of the Women’s Health Initiative. Menopause. 2011;18:1160–1171. doi: 10.1097/gme.0b013e3182227ebd. [DOI] [PubMed] [Google Scholar]
- 5.Hallstrom T, Samuelsson S. Changes in women’s sexual desire in middle life: the longitudinal study of women in Gothenburg. Arch Sex Behav. 1990;19:259–268. doi: 10.1007/BF01541551. [DOI] [PubMed] [Google Scholar]
- 6.Hayes R, Dennerstein L. The impact of aging on sexual function and sexual dysfunction in women: a review of population-based studies. J Sex Med. 2005;2:317–330. doi: 10.1111/j.1743-6109.2005.20356.x. [DOI] [PubMed] [Google Scholar]
- 7.Kinsey AC, Pomeroy WB, Martin CW. Sexual behavior in the human female. Philadelphia, PA: WB Saunders; 1953. [Google Scholar]
- 8.Marsiglio W, Donnelly D. Sexual relations in later life: a national study of married persons. J Gerontol. 1991;46:S338–S344. doi: 10.1093/geronj/46.6.s338. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer E, Verwoerdt A, Davis GC. Sexual behavior in middle life. Am J Psychiatry. 1972;128:1262–1267. doi: 10.1176/ajp.128.10.1262. [DOI] [PubMed] [Google Scholar]
- 10.Avis NE, Stellato R, Crawford S, Johannes C, Longcope C. Is there an association between menopause status and sexual functioning? Menopause. 2000;7:297–309. doi: 10.1097/00042192-200007050-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gracia CR, Freeman EW, Sammel MD, Lin H, Mogul M. Hormones and sexuality during transition to menopause. Obstet Gynecol. 2007;109:831–840. doi: 10.1097/01.AOG.0000258781.15142.0d. [DOI] [PubMed] [Google Scholar]
- 12.Køster A, Garde K. Sexual desire and menopausal development. Maturitas. 1993;16:49–60. doi: 10.1016/0378-5122(93)90133-3. [DOI] [PubMed] [Google Scholar]
- 13.Leiblum S, Bachmann G, Kemmann E, Colburn D, Swartzman L. Vaginal atrophy in the postmenopausal woman. The importance of sexual activity and hormones. JAMA. 1983;249:2195–2198. [PubMed] [Google Scholar]
- 14.Woods NF, Mitchell ES, Smith-Di Julio K. Sexual desire during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Study. J Womens Health (Larchmt) 2010;19:209–218. doi: 10.1089/jwh.2009.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avis NE, Brockwell S, Randolph JF, Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009;16:442–452. doi: 10.1097/gme.0b013e3181948dd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bancroft J, Loftus J, Long JS. Distress about sex: a national survey of women in heterosexual relationships. Arch Intern Med. 2003;32:193–208. doi: 10.1023/a:1023420431760. [DOI] [PubMed] [Google Scholar]
- 17.Dennerstein L, Smith AMA, Morse CA. Sexuality and the menopause. J Psychosom Obstet Gynecol. 1994;15:59–66. doi: 10.3109/01674829409025630. [DOI] [PubMed] [Google Scholar]
- 18.Hawton K, Gath D, Day A. Sexual function in a community sample of middle-aged women with partners: Effects of age, marital, socioeconomic, psychiatric, gynecological, and menopausal factors. Arch Sex Behav. 1994;23:375–395. doi: 10.1007/BF01541404. [DOI] [PubMed] [Google Scholar]
- 19.Hunter MS. Somatic experience of the menopause: A prospective study. Psychosom Med. 1990;52:357–367. doi: 10.1097/00006842-199005000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Cawood EHH, Bancroft J. Steroid hormones, the menopause, sexuality and well-being of women. Psychol Med. 1996;26:925–936. doi: 10.1017/s0033291700035261. [DOI] [PubMed] [Google Scholar]
- 21.Hunter M, Battersby R, Whitehead M. Relationships between psychological symptoms, somatic complaints and menopausal status. Maturitas. 1986;8:217–228. doi: 10.1016/0378-5122(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 22.Hallstrom T. Sexuality in the climacteric. Clin Obstet Gynaecol. 1977;4:227–239. [PubMed] [Google Scholar]
- 23.Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174–180. doi: 10.1016/j.fertnstert.2005.01.119. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes JC, Kjerulff KH, Langenberg PW, Guzinski GM. Hysterectomy and sexual functioning. JAMA. 1999;282:1934–1941. doi: 10.1001/jama.282.20.1934. [DOI] [PubMed] [Google Scholar]
- 25.Carlson KJ, Miller BA, Fowler FJ., Jr The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83:556–565. doi: 10.1097/00006250-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Lonnèe-Hoffmann R, Pinas I. Effects of hysterectomy on sexual function. Curr Sex Health Rep. 2014;6:244–251. doi: 10.1007/s11930-014-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: A multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, JKelsey J, Marcus R, editors. Menopause: Biology and pathobiology. San Diego: Academic Press; 2000. [Google Scholar]
- 28.Abma J, Chandra A, Mosher W, Peterson L, Piccinino L. Fertility, family planning, and women’s health: New data from the 1995 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 1997:23. [PubMed] [Google Scholar]
- 29.The Women’s Health Initiative Study Group. The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 30.Avis NE, Zhao X, Johannes CB, Ory M, Brockwell S, Greendale GA. Correlates of sexual function among multi-ethnic middle-aged women: results from the Study of Women’s Health Across the Nation (SWAN) Menopause. 2005;12:385–398. doi: 10.1097/01.GME.0000151656.92317.A9. [DOI] [PubMed] [Google Scholar]
- 31.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 32.Hess R, Conroy MB, Ness R, et al. Association of lifestyle and relationship factors with sexual functioning of women during midlife. J Sex Med. 2009;6:1358–1368. doi: 10.1111/j.1743-6109.2009.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennerstein L, Anderson-Hunt M, Dudley E. Evaluation of a short scale to assess female sexual functioning. J Sex Marital Ther. 2002;28:389–397. doi: 10.1080/00926230290001510. [DOI] [PubMed] [Google Scholar]
- 34.Irwin KL, Wingo PA, Lee NC. Agreement with self-reported ovarian number following gynecologic surgery with medical record reports. J Clin Epidemiol. 1990;43:181–187. doi: 10.1016/0895-4356(90)90182-o. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D scale: A self-report depression scale for research in the eneral population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 36.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleveland WS, Devlin SJ. Locally weighted regression: An approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 38.Dennerstein L, Guthrie JR, Hayes RD, Derogatis LR, Lehert P. Sexual function, dysfunction, and sexual distress in a prospective, population-based sample of mid-aged, Australian-born women. J Sex Med. 2008;5:2291–2299. doi: 10.1111/j.1743-6109.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- 39.Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN) J Bone Miner Res. 2012;27:111–118. doi: 10.1002/jbmr.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishii S, Cauley JA, Greendale GA, et al. Trajectories of femoral neck strength in relation to the final menstrual period in a multi-ethnic cohort. Osteoporos Int. 2013;29:2471–2481. doi: 10.1007/s00198-013-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randolph JF, Jr, Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96:746–754. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sowers MR, Zheng H, Greendale GA, et al. Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab. 2013;98:2854–2863. doi: 10.1210/jc.2012-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennerstein L, Dudley EC, Hopper JL, Burger H. Sexuality, hormones and the menopausal transition. Maturitas. 1997;26:83–93. doi: 10.1016/s0378-5122(96)01093-6. [DOI] [PubMed] [Google Scholar]
- 45.Mishra GD, Kuh D. Sexual functioning throughout menopause: The perceptions of women in a British cohort. Menopause. 2006;13:880–890. doi: 10.1097/01.gme.0000228090.21196.bf. [DOI] [PubMed] [Google Scholar]
- 46.Osborn M, Hawton K, Gath D. Sexual dysfunction among middle aged women in the community. Br Med J. 1988;296:959–962. doi: 10.1136/bmj.296.6627.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erekson EA, Martin DK, Ratner ES. Oophorectomy: the debate between ovarian conservation and elective oophorectomy. Menopause. 2013;20:110–114. doi: 10.1097/gme.0b013e31825a27ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finch A, Metcalfe KA, Chiang JK, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011;121:163–168. doi: 10.1016/j.ygyno.2010.12.326. [DOI] [PubMed] [Google Scholar]
- 49.Hickey M, Ambekar M, Hammond I. Should the ovaries be removed or retained at the time of hysterectomy for benign disease? Hum Reprod Update. 2010;16:131–141. doi: 10.1093/humupd/dmp037. [DOI] [PubMed] [Google Scholar]
- 50.Randolph JF, Jr, Zheng H, Avis NE, Greendale GA, Harlow SD. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258–266. doi: 10.1210/jc.2014-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santoro N, Torrens J, Crawford S, et al. Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J Clin Endocrinol Metab. 2005;90:4836–4845. doi: 10.1210/jc.2004-2063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.