Abstract
Proteus mirabilis is a model organism for urease-producing uropathogens. These diverse bacteria cause infection stones in the urinary tract and form crystalline biofilms on indwelling urinary catheters, frequently leading to poly-microbial infection. Recent work has elucidated how P. mirabilis causes all of these disease states. Particularly exciting is the discovery that this bacterium forms large clusters in the bladder lumen that are sites for stone formation. These clusters, and other steps of infection, require two virulence factors in particular: urease and MR/P fimbriae. Highlighting the importance of MR/P fimbriae is the cotranscribed regulator, MrpJ, which globally controls virulence. Overall, P. mirabilis exhibits an extraordinary lifestyle, and further probing will answer exciting basic microbiological and clinically relevant questions.
Introduction to Proteus mirabilis
P. mirabilis is a gastrointestinal (GI) organism notorious for causing catheter-associated urinary tract infections (CAUTIs, see Glossary) and urinary stones. It is a model organism for urease+ bacteria, a phylogenetically diverse group of bacteria encoding the virulence factor urease, that often form resilient crystalline biofilms on catheters and potentially deadly stones in the urinary system. Recent studies have elucidated key steps in P. mirabilis's virulence lifestyle and the intracellular regulatory mechanisms that control these stages of infection. The combination of this new information with previous foundational studies has shed light on how this organism, and perhaps other urease+ bacteria, successfully initiates and establishes infection of the urinary system.
This review focuses on the recent advances in the P. mirabilis field in the context of CAUTIs and urinary stones. We first briefly describe these two disease states. Next, we summarize the P. mirabilis lifecycle from catheter colonization to the development of urinary stones. Then, we describe the role of urease and fimbriae during infection, as recent developments have further characterized the function and/or regulation of these two classes of virulence factors [1]. Lastly, we discuss polymicrobial infections as they, until recently, have been the subject of relatively few studies, yet may be important for understanding P. mirabilis-associated diseases. We propose that P. mirabilis and other urease+ bacteria are an understudied group of urinary pathogens, and that continued research into these organisms will provide insight into the treatment of potentially serious or deadly urinary tract diseases.
CAUTIs and Urinary Stones
CAUTIs are a predominant nosocomial infection within the US and are particularly prevalent in patients with long-term indwelling catheters [2,3]. CAUTIs associated with urease+ organisms are especially problematic as they can cause urinary stones and crystalline biofilms on catheters, which are notoriously difficult to treat. In recognition of their significant health and economic burden, CAUTIs are one of the top four healthcare-associated infections (HAIs) that the US is actively trying to eliminate, per the Center for Disease Control's (CDC's) National State and Healthcare-Associated Infections progress report.i Unfortunately, the incidence of CAUTI has not changed from years 2009 to 2014, unlike the other HAIs on this list, which saw decreases anywhere from 8 to 50%.i
Patients with an indwelling catheter are more likely to develop urinary stones [4,5]. Most are classified as infection stones, meaning that they have a bacterial etiology and often consist of struvite and/or apatite minerals [6]. Although the prevalence of infection stones has decreased over the past 30 years, the overall prognosis is still poor: the 20-year mortality rate for infection stones is 28% with nonsurgical management, and 7% with surgical intervention [4]. Obstructive pyelonephritis, or a kidney stone that has blocked the kidney ducts, is one of the more severe complications of urinary stones, requiring immediate treatment to avoid serious complications such as urosepsis and death [4,7]. Even with stone removal, residual stone fragments may remain in the urinary system, putting these patients at a 40–85% risk for recurrent stones, versus a 0–10% risk for patients who had complete stone removal [8].
Infection stones, particularly those composed of struvite, and crystalline biofilms are almost always caused by bacteria that produce the urea-hydrolyzing enzyme, urease [3,9]. Uropathogenic genera that encode urease include Proteus, Staphylococcus, Providencia, Ureaplasma, and less frequently, Pseudomonas and Klebsiella [9,10]. Infection stones and CAUTIs are often polymicrobial and may include these species as well as urease-negative bacteria, such as Escherichia coli, which potentially further complicates treatment of CAUTI-associated stones [11–15]. P. mirabilis in particular is a model organism used to study CAUTIs, infection stones, and now polymicrobial urinary tract infections (UTIs).
The Uropathogenic Lifestyle of P. mirabilis: From GI to UTI
Although P. mirabilis is an intestinal organism known to externally cross over to the urethra, it does not usually cause UTIs in patients with normal, unobstructed urinary tracts [16]. However, the presence of a chronic, indwelling catheter within the urethra and bladder permits less inhibited ascension of the bacteria up the catheter and into the urinary system (Figure 1) [17–19]. To do this, P. mirabilis may employ swarming motility, where the bacteria lengthen, become hyperflagellated, and move as a coordinated community across surfaces [18]. Swarming has been correlated with production of virulence factors, including urease and crystalline biofilm formation (discussed below) [20–22]. However, the contribution of swarming to UTI remains under debate, as swarming-defective mutants can still form crystalline biofilms, and differentiated swarmer cells are rarely observed during UTI [20,23]. Swarming motility will not be further discussed in this review, as it has been extensively covered elsewhere [21,22].
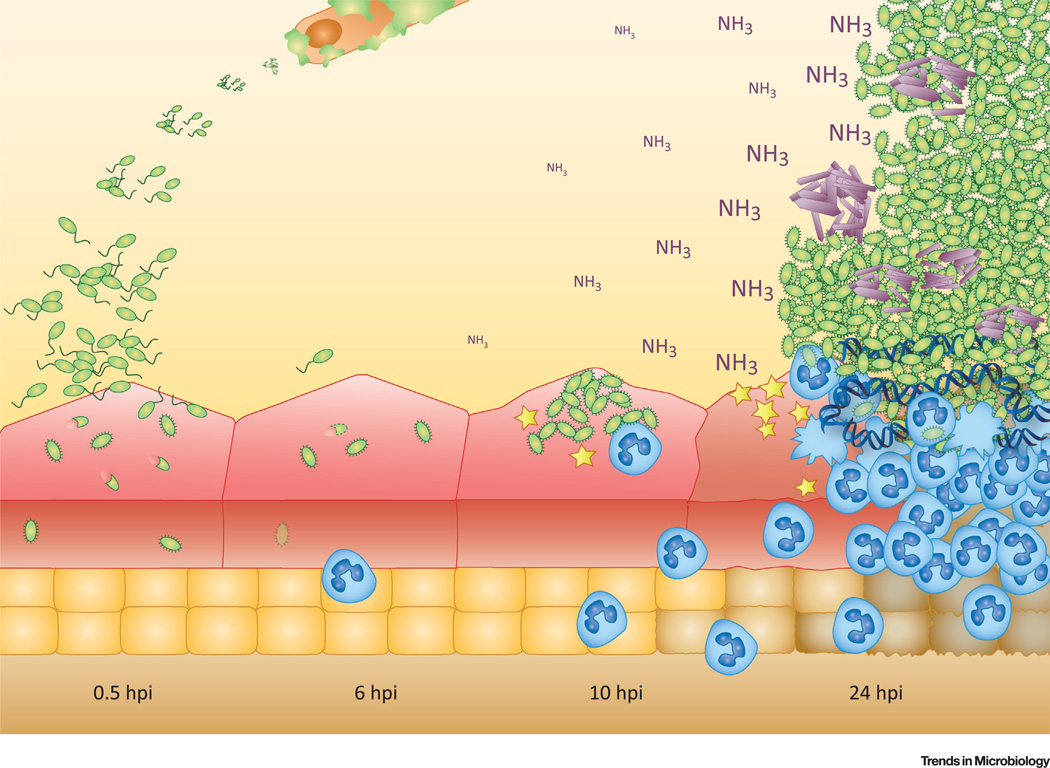
Figure 1. Lifestyle of Proteus mirabilis.
P. mirabilis cells form crystalline biofilms on the surface of catheters. Once inside the bladder (0.5–6 hours post-infection[hpi]), this organism can invade into urothelial cells of the bladder. As early as 10–24 hpi, P. mirabilis forms intraluminal clusters that can extend the length of the bladder and are associated with urothelial cell destruction. Host innate immune cells such as neutrophils (blue) are recruited to the site of infection and can form NETs (neutrophil extracellular traps). Figure used with permission from Schaffer et al. [1].
P. mirabilis CAUTIs are exacerbated by the formation of catheter-associated biofilms, or multicellular communities that are surrounded by a matrix of macromolecules such as polysaccharide, protein, and extracellular DNA [3,24]. Although biofilm formation on catheters by bacterial pathogens is a common feature of CAUTI, the potent urease activity of P. mirabilis in particular leads to crystalline biofilms, which can block catheter flow and are hypothesized to protect cells within crystalline armor, making them particularly difficult to eradicate [25–27].
Once P. mirabilis gains access to the bladder in a mouse model of UTI, it may invade the bladder urothelium as early as a half hour after infection (Figure 1) [1]. Some research has begun to characterize this process through the use of various cell lines [28–34]. However, invasion of the bladder epithelium, or urothelium, does not seem to be the dominant lifestyle for P. mirabilis in the bladder [1,35]. Instead, P. mirabilis forms large intraluminal clusters that can extend the entire length of the bladder. Importantly, these clusters serve as the site of stone formation (Figure 1) [1]. After entering the bladder, P. mirabilis can ascend to the kidney, cause kidney stones, and cross into the bloodstream [36–39].
The lifecycle of P. mirabilis in the bladder is a complicated, multistep process (Figure 1). In the next section, we focus on two virulence factors, urease and mannose-resistant Proteus-like (MR/P) fimbriae, as they exert multiple, key functions during infection, including having a role in the aforementioned bladder clusters. Other known virulence factors required in experimental UTI in mice are listed in Figure 2 and described in detail elsewhere [21,22], but they will not be discussed further in this review.
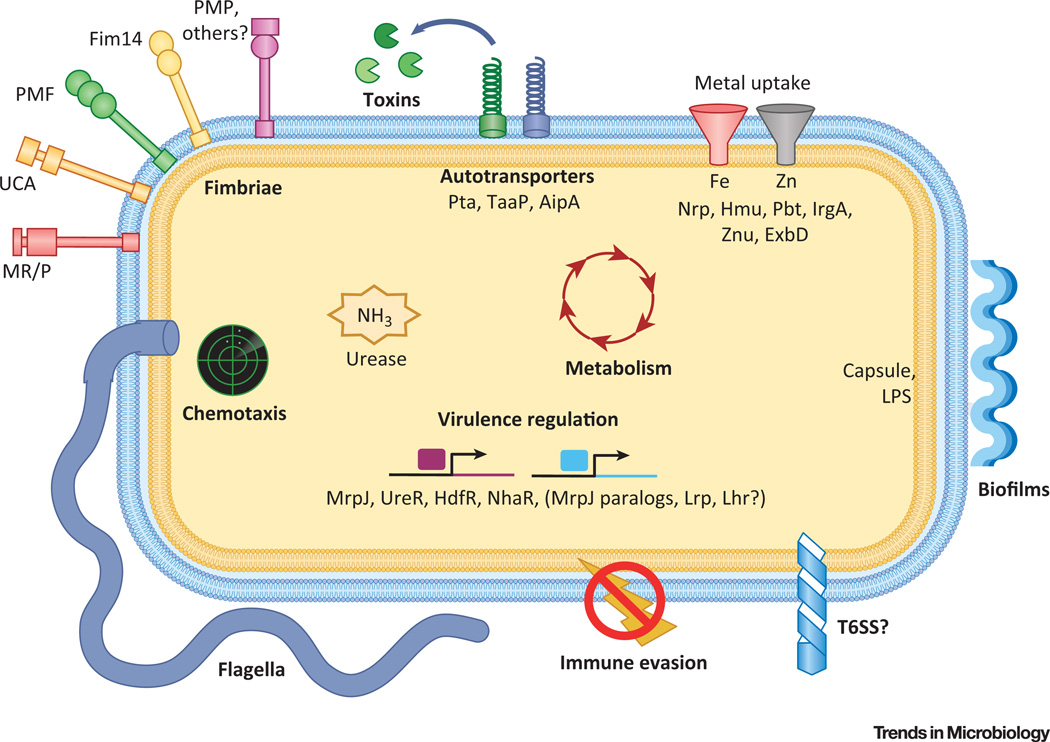
Figure 2. Concepts of Proteus mirabilis Pathogenesis during Urinary Tract Infection (UTI).
Bolded classes of virulence factors have genes that are regulated by MrpJ. For more extensive information on these virulence factors, see references [21,22,99,100]. Adherence: binding catheters, host tissues, and neighboring bacteria may all contribute to disease. Adherence is mediated by chaperone-usher fimbriae and autotransporter adhesins. Urease: involved in stones, crystalline biofilms, and possibly nutrition or host sensing. Motility: P. mirabilis swarms across catheters and may ascend to the kidneys using swimming motility. Both forms of motion are mediated by flagella. Chemotaxis proteins allow the bacteria to follow chemical gradients. Metabolism: likely permits establishment of a nutritional niche, competition with other species, and response to host cues. Metal scavenging: iron and zinc uptake are essential for growth, but are sequestered by the host; therefore, specialized proteins are required for bacteria to scavenge these metals. Toxins: proteins such as HpmA and Pta may aid in nutrient accessibility, immune evasion, or provision of surfaces to colonize. Biofilm formation: Crystalline biofilms readily form on catheters, and bacterial clusters in the bladder may be a biofilm-mediated process. Immune evasion: this can include antibody and antimicrobial peptide degradation, polymyxin resistance, lipopolysaccharide (LPS) variation, and physical obstruction of phagocytosis. Virulence regulation: required to coordinate all steps of infection. MrpJ-controlled systems in this figure are bolded. Type 6 secretion system (T6SS): involved in self-recognition; unknown role during UTI.
Urease
Urease is a nickel metalloenzyme that catalyzes the hydrolysis of urea, a highly water-soluble molecule concentrated into the urine of mammals as a mechanism to eliminate excess nitrogen [40]. Specifically in P. mirabilis, and less potently in Providencia spp., urease is induced by the regulator UreR in the presence of urea (approx. 400 mM in human urine) [41]. Urea hydrolysis via urease results in the production of carbonic acid and two molecules of ammonia, and among uropathogens, the hydrolysis rate of P. mirabilis urease is especially rapid. In aqueous solutions at equilibrium, the two ammonia molecules can dramatically increase the net pH (Figure 1). During CAUTIs, the ammonia and carbon dioxide bind with Mg2+ and Ca2+ found in the urine, respectively, resulting in struvite and carbonate apatite [41]. These minerals precipitate from the urine and form crystalline deposits on catheters and/or aggregate into stones in an organ within the urinary system [6,42]. This biochemical process is important for P. mirabilis to form both crystalline biofilms and urinary stones (Figure 2).
Bacterial catheter colonization can occur in vitro as early as 15 min post-inoculation. As P. mirabilis propagates, and adherent bacteria grow and divide into microcolonies, the pH rises, leading to the formation of crystals, which can further facilitate bacterial attachment to the catheter [43–45]. In fact, the higher the urease activity, the faster a catheter encrusts in vitro [46]. As the layer of crystalline biofilm continues to grow, bacteria and crystals become tightly associated [44]. In a flow system, bacteria can subsequently disseminate from microcolonies across the surface of the catheter, where the crystalline biofilm process can begin again [45]. P. mirabilis strains with a mutation within the ure operon (ureDABCEFG) are unable to form crystalline biofilms, thus solidifying the role of this enzyme in this process (Figure 2) [25,44,45].
In addition to catheter encrustation, P. mirabilis uses urease to establish both bladder and kidney infection in a mouse model of ascending UTI [37]. Indeed, ure gene expression can be seen in both the bladders and kidneys at 7 days post-infection (dpi), and ure genes are consistently expressed in voided urine from 1 to 7 days post-infection [47,48]. A ureC mutant exhibits bladder colonization defects as early as 24 h post-infection (hpi) and fails to form clusters [1]. Urease mutants have difficulty overcoming this early defect, as they exhibit a lower CFU in both the bladder and kidney, have a less severe pathology in these organs, and fail to form urinary stones anywhere from 2 to 14 dpi [36,37,49].
The requirement for urease at early time points (24 hpi) of bladder colonization suggests that urease may be doing more than just supplying mineral deposits within a cluster. This is in accordance with an in vitro analysis of catheter encrustation, which found urease to be important for initiating biofilm formation on catheters seemingly independently of crystal formation [43]. One explanation is that the methods used for these studies were not able to observe microscopic mineral deposits. In contrast, since urease activity produces ammonia, it is possible that P. mirabilis uses urea as a nitrogen source for growth; however, various in vitro conditions do not expose a growth defect for a urease mutant [1,36,49], urease-encoding genes are not induced during nitrogen starvation [50], and in vivo microarray analysis suggests that urease activity is not important for nitrogen uptake and assimilation [48]. Ammonia liberated by urease may also contribute to urothelial damage, potentially exposing niches for adherence or making cellular nutrients available to the bacteria [37]. It is possible that the contribution of urease to CAUTI is multifactorial, and additional functions remain unknown.
Fimbriae
Fimbriae, or pili, are hair-like appendages that coat the exterior of both Gram-negative and Gram-positive bacteria [51]. They can be involved in a number of processes such as biofilm formation, surface attachment, and evasion of the host immune response [52]. One of the five major classes of fimbriae, chaperone-usher, represents the most abundant group of surface appendages on Gram-negative bacteria [53]. These fimbriae are typically composed of many copies of one major protein subunit that polymerizes into the characteristic linear structure. This assembly is coordinated by a periplasmic chaperone and a membrane-bound usher [54]. Many fimbriae possess an adhesin protein at the tip, which mediates direct contact with a specific surface [54].
The P. mirabilis HI4320 type strain genome contains at least 17 different operons that encode chaperone-usher fimbriae, the most within a single strain of any bacterium known to date [55]. In contrast with other fimbria-producing species, most of these 17 operons are conserved among P. mirabilis strains isolated from varied human and animal anatomical sites, as well as distinct parts of the world over a 30-year time frame [56]. Presumably, maintaining a large repertoire of attachment mechanisms is advantageous for P. mirabilis, perhaps by permitting the bacteria to colonize a variety of environmental niches.
Out of these 17 fimbrial operons, five have been characterized in vivo. At least four fimbrial operons are implicated in P. mirabilis virulence in mouse models of UTI: MR/P [57–59], uroepithelial cell adhesin (UCA, also known as nonagglutinating fimbria, or NAF) [60], P. mirabilis fimbriae (PMF) [61,62], and Fimbria 14 [63]. Analysis of a fifth fimbria, the ambient-temperature fimbria (ATF), suggested that it was not required for infection [64]. Although ATF production increases in an MR/P mutant strain in vivo, the significance of this is unknown [35]. Here, we focus on MR/P fimbriae, the best-characterized fimbriae that have a newly elucidated importance for bladder infection.
MR/P Fimbriae
MR/P fimbriae are critical for bladder and kidney infection in mice. Bacteria in the bladder, ureters, and kidneys produce MR/P fimbriae at 24–48 hpi, with the highest percentage found in the bladder [35]. Additionally, mrp genes within the mrp operon (mrpABCDEFGHJ) are the most highly-induced in vivo [48], are required at early steps of infection, including cluster formation [1], and are critical for wild-type levels of bladder colonization at later time points [57–59,65]. Together, these data solidify MR/P fimbriae as a key virulence factor for P. mirabilis to establish and maintain its hold on a host's urinary system.
The bladder and/or kidney receptors for MR/P are unknown. Some evidence suggests that MR/P may be important for urothelial cell adherence and/or proper localization of P. mirabilis in the bladder [35,65,66]. Interestingly, cells constitutively producing MR/P (MR/P ‘on’) and cells that do not express the mrp operon (MR/P ‘off’) associate with distinct locations in the bladder, although ‘on’ and ‘off’ bacteria are recovered in similar numbers [35]. The P. mirabilis MR/P ‘on’ strain and an E. coli strain engineered to express mrp exhibit enhanced hemagglutination, aggregation during growth in culture, and/or biofilm formation [35,59]. These in vitro phenotypes are reminiscent of the in vivo cluster, and suggest that MR/P may be important for initiating or maintaining cluster formation within the bladder. Therefore, MR/P could be responsible for both direct host binding as well as cluster formation.
Without clusters, mineral deposition does not occur, and therefore MR/P is likely important for stone formation. Indeed, scanning electron microscopy (SEM) studies of MR/P ‘on’ and ‘off’ strains in the bladder suggested that the MR/P ‘on’ strain formed more biofilm-like clusters [35]. Conversely, mixed populations of MR/P-OFF and MR/P-ON are more likely to occur in mice with bladder stones whereas the majority of the population is MR/P-ON in mice without bladder stones [67]. This may suggest that MR/P is not important for maintaining the cluster or stone, or that only subpopulations of bacteria expressing mrp are needed during this step in infection. The microenvironment in stones may drive this variation, as pH, leukocyte attack, and nutrient accessibility vary greatly. In particular, MR/P fimbriae are induced during oxygen restriction and they are also immunogenic [68]. Additionally, if clusters represent a form of biofilm, then perhaps MR/P ‘off’ cells are disseminating from the cluster/stone using flagella-mediated motility, which is repressed by an MR/P regulatory protein, MrpJ (described below).
MR/P is critical for biofilms in microtiter plate models [35,69]. However, it remains untested whether MR/P is important for mediating crystalline biofilm formation on catheters. Identifying the role of MR/P and other fimbriae for catheter encrustation is important, as it will create a better overall picture of the UTI lifestyle of P. mirabilis from catheter ascension to urinary dissemination.
To successfully colonize a host, P. mirabilis must appropriately regulate MR/P production during the infection process. Indeed, at the end of the mrp operon is mrpJ, a gene that autoregulates mrp [70]. Additionally, MrpJ controls non-mrp genes, many of which are involved in virulence [70,71]. This suggests that MrpJ may be a more global regulator of virulence in P. mirabilis.
MrpJ Regulation
MrpJ is a predicted helix-turn-helix (HTH) protein that is required for a mouse model of ascending UTI [71,72]. It positively autoregulates the mrp operon by binding to a region within the promoter, which presumably leads to recruitment of the σ70-dependent RNA polymerase holoenzyme [71]. Interestingly, chromatin immunoprecipitation (ChIP) evidence suggests that MrpJ interacts with an additional element approximately 800 bp upstream of the mrp operon [71]. This element is located within the divergently transcribed mrpI gene, which encodes a recombinase that alters the orientation of an invertible element found within the mrpA promoter; the orientation of this invertible element determines whether mrp is expressed [66]. This additional site of DNA-MrpJ interaction suggests that MrpJ may exert further, uncharacterized control of the transcription of mrp, mrpI, or both [71,72]. The possibility that MrpJ controls transcription through looping of DNA between these two binding sites seems particularly compelling, although the mechanism of regulation remains unknown.
In addition to the mrpA promoter, MrpJ directly interacts with the promoter of flhDC, an operon that encodes the master flagellar regulator [70–72]. As opposed to mrp regulation, MrpJ represses the expression of flhDC, thus inhibiting motility [70–72]. This is an intuitive solution that permits P. mirabilis to simultaneously control two opposing actions: adherence and motility. Further ChIP analysis revealed that MrpJ interacts with at least two regions in flhDC [71]. These two regions are located in the promoter and the coding region [71]. Similarly to mrp, MrpJ may regulate flhDC by binding to two different sites and/or through DNA looping.
MrpJ also controls the expression of a variety of other genes, many of which are known or putative virulence factors (Figure 2, bold) [71]. These include cell-surface molecules such as other fimbriae, lipoproteins, and lipopolysaccharide (LPS), the Pta toxin, and the ZapA metalloprotease; the latter two (and PMF fimbriae) have been demonstrated to be important for infection in mice (Figure 2) [28,71,73–75]. P. mirabilis can provoke extensive urinary tissue injury, particularly when urolithiasis occurs [1]. Proteases such as ZapA and Pta may contribute to this damage and, as a result, (i) reveal alternative binding targets for MrpJ-regulated fimbriae, and (ii) release cellular nutrients. Notably, P. mirabilis, unlike uropathogenic Escherichia coli (UPEC), utilizes glycolysis during UTI, implying that this pathogen has access to and relies on different carbon sources in the host [48,76]. ZapA may also aid bacterial survival by cleaving antimicrobial peptides found in urine [73]. LPS modifications, lipoproteins, and other fimbriae can affect bacterial adherence, biofilm formation, or immune evasion in bacterial pathogens, although all of these topics have not been examined for P. mirabilis [77–79].
Interestingly, one of the most highly-upregulated classes of genes encodes type VI secretion system (T6SS) proteins. In P. mirabilis, this system permits bacteria to identify other cells of the same strain in vitro, as strains with different sets of T6SS genes leads to killing of the non-identical strain [80,81]. However, these studies identified T6SS activity during swarming, when the mrp genes are repressed; this suggests that the T6SS is also active under other, MrpJ-activated conditions, such as during UTI, and that T6SS effectors may also target the host. Indeed, T6SS contribute to infection caused by other pathogens [82]. The importance of the T6SS for UTI, or whether MrpJ directly binds to targets identified in the microarray, remain an active area of research.
Overall, the MrpJ regulon is more extensive and complex than originally anticipated. There exist many unknowns, such as whether there is a different mechanism of control for targets of activation versus repression, and the identification of a consensus binding site. These outstanding questions merit further studies on MrpJ to elucidate differential control over its regulon, a process that is key for infection.
Fimbrial Cross-Regulation
Ten of the 17 fimbrial operons encoded by P. mirabilis contain an mrpJ paralog [55]. Similarly to MrpJ, at least two of these factors (AtfJ and Fim8J) autoregulate their own operon, whereas one does not (UcaJ); the remainder are uncharacterized [83]. Most of these paralogs repress motility to varying degrees [70]. Interestingly, mechanistic studies of one paralog, AtfJ, found that this factor requires a different combination of residues for autoregulation than for motility repression [83]. Some of this specificity is mediated by the unique C-terminal domain in AtfJ [83]. Together, these data suggest that MrpJ paralogs may use different protein/DNA interactions to differentially regulate target genes.
Given the degree of similarity among these regulators, it is reasonable to hypothesize that P. mirabilis has mechanisms in place to prevent ineffective regulatory cross-talk in environments that require one or more fimbriae. MrpJ paralogs may control which fimbriae are produced in a hierarchical, temporal fashion. Indeed, fimbrial cross-regulation exists at the transcriptional level [71,83], although the exact mechanisms remain uncharacterized. Another intriguing concept is whether MrpJ paralogs form heterodimers to either prevent or promote transcription at particular promoters. These questions and others may not only lead to a better understanding of CAUTIs and stone formation, but also have a broader relevance for regulatory mechanisms used in other bacterial species.
Polymicrobial Infections
Patients with polymicrobial UTIs (pUTIs), or mixed UTIs, generally have long-term, indwelling catheters; in fact, anywhere from 75 to 85% of cultures isolated from indwelling catheters are polymicrobial (Table 1) [11,84]. Until recently, pUTIs have been overlooked clinically [85], perhaps because it was not clear whether multiple strains represented a true etiology of the UTI, or whether the additional strains were simply contamination [86]. Likewise, because modeling polymicrobial infections is considerably more difficult than single-species infections, there have been relatively few studies in pUTI. There is conflict as to how to address pUTIs associated with CAUTI in the clinic: whereas the IDSA (Infectious Diseases Society of America) emphasizes the total number of bacteria in a urine sample regardless of the number of isolated strains [87], the NHSN (National Healthcare Safety Network) does not analyze samples with more than two bacterial isolates.ii So far, there are no established clinical guidelines specifically discussing polymicrobial urinary stones.
Table 1.
Studies Assessing the Prevalence of Polymicrobial Infections of Indwelling Catheters
| Refs | # of samples |
Population | Catheter duration |
Method | % No detectable bacteria |
% Single species | % Polymicrobial |
|---|---|---|---|---|---|---|---|
| Armbruster, 2016 [88] | 182a | Nursing home patients | 3 to 360 days | Urine culture | 0b | 69 | 31 |
| Breitenbucher, 1984 [11] | 15a | Nursing home patients | ≥1 year | Urine culture | 0c | 14 | 86 |
| Dedeic-Ljubovic and Hukic, 2009 [94] | 1809a | Hospitalized patients with spinal cord injuries |
unknown | Urine culture | 6 | 48 | 46 |
| Frank et al., 2009 [95] | 8 | Men catheterized after total prostatectomy | 2 weeks | rRNA sequencing of catheter surface |
12 | 0 | 88 |
| Ganderton et al., 1992 [84] | 50 | Nursing home patients | 3 to 83 days (median 35 days) |
Culture of catheter surface |
0c | 25 | 75 |
| Holá et al., 2010 [96] | 535 | Hospital patients | NSd | Sonication and culture of catheters |
0b | 13 | 87 |
| Macleod and Stickler, 2007 [12] | 106 | Patients in hospitals or community care | NSd | Sonication and culture of catheters |
0b | 28 | 72 |
| Matsukawa et al., 2005 [97] | 86 | Elderly men | 1–35 days (mean of 3) |
Culture of catheter swabs |
46.5 | 39.5 | 14 |
| Matsukawa et al., 2005 [97] | 86 | Elderly men | 1–35 days (mean of 3) |
Urine culture | 70 | 21 | 9 |
| Tenney and Warren, 1987 [98] | 7 | Afebrile patients | >30 days | Urine culture | 14 | 0 | 86 |
| Warren et al., 1982 [15] | 619a | Patients in a chronic care facility | ≥1 month | Urine culture | 2 | 21 | 77 |
Multiple samples from the same patients were included in the analysis.
Only culture-positive samples were assessed.
Study does not state whether there were samples with no detectable bacteria.
NS, not specified.
P. mirabilis pUTI has been observed with other species such as P. aeruginosa, Providencia stuartii, Klebsiella pneumoniae, and Enterococcus faecalis [12,84,88]. Multispecies interactions may result in synergistic benefits for urinary pathogens. For example, the presence of two urease+ organisms, P. mirabilis and P. stuartii, enhances urease activity in vitro, and coinfection in vivo increased the number of mice with observable urinary stones [85]. Likewise, cocolonization of UPEC and P. mirabilis in mice increased the CFU of E. coli in the bladder and of both of these organisms in the kidneys, particularly at 7 dpi [76]. The authors of this study proposed that these bacteria do not directly compete within the bladder because they utilize different carbon metabolism pathways in the host; this is supported by the observation that the same metabolic gene mutations in each of these organisms resulted in differential fitness defects in vivo, but not in vitro [76]. These results pose a compelling hypothesis, as E. coli often lives within intracellular bacterial communities (IBCs) in host urothelial cells, whereas the majority of P. mirabilis cells exist in luminal clusters [1,89]. It is possible that E. coli can integrate into urinary stones, which could explain its enhanced CFU during mixed UTIs, particularly if the cluster is protecting E. coli from attack by the host's innate immune cells. Antagonistic interactions are also possible during pUTI. In one study, P. mirabilis was isolated less often from catheters colonized by Morganella morganii or Enterobacter cloacae; however, investigation of laboratory-grown biofilms showed that P. mirabilis readily blocked catheters when mixed with these species [12]. Further clinical CAUTI studies, particularly surveys that do not discard samples containing three or more species, will help focus laboratory studies on the most important interactions.
Concluding Remarks
Although research on P. mirabilis has elucidated how this organism may cause CAUTI and urinary stones, there remain many more questions that would provide critical insights into how this organism causes disease (see Outstanding Questions). To paint a better picture of P. mirabilis's lifestyle, further research needs to connect the dots between catheters, bladder, and kidney infections, perhaps by using an indwelling catheter mouse model. This includes better understanding of MR/P fimbriae and urease as their exact functions during all steps of infection remains enigmatic. Additionally, multispecies interactions during pUTIs remain an exciting field with many areas of clinical importance that are ripe for investigating. This is particularly relevant given the recent observations that the healthy bladder is not necessarily sterile [90].
Outstanding Questions.
What is the current incidence of urease+ bacteria in CAUTI and urinary stones? What are the healthcare and economic burdens of these organisms?
How does P. mirabilis form/disseminate from clusters?
What is the lifestyle of P. mirabilis in the kidney?
Does urease have a function outside of crystal/stone formation?
What is the function of MR/P fimbriae during UTI?
How do MrpJ paralogs achieve specificity, and is there crosstalk between these regulators?
What are the lifestyles of other urease+ bacteria in the urine? How might polymicrobial infections alter these lifestyles?
How might the urinary microbiome affect uropathogens’ ability to cause disease?
P. mirabilis uses an amazing assembly of mechanisms to cause urinary tract disease, and further research will certainly lead to a continued appreciation of this bacterium's remarkable approach to causing infection.
Supplementary Material
Trends.
P. mirabilis is a model organism for urease+ organisms that are associated with CAUTIs and infectious stones.
P. mirabilis forms extensive crystalline biofilms on catheters that are infamously difficult to treat.
Once in the bladder, P. mirabilis can form luminal clusters that are the site of bladder stone formation.
At least two virulence factors, urease and MR/P fimbriae, are required for both cluster formation and crystalline biofilm formation on catheters.
The MR/P regulator, MrpJ, controls a variety of other known and putative virulence factors.
Polymicrobial infections can lead to synergistic enhancement of UTI and may have clinically relevant consequences.
Glossary
- Apatite
a common urinary stone mineral with the chemical formula: [Ca10(PO4)6CO3] [6].
- CAUTI
catheter-associated urinary tract infection. A UTI that occurs when a urinary catheter is indwelling for >2 days and symptoms appear during catheterization or a day before removalii.
- Flow system
a method for growing bacterial biofilms utilizing a flow chamber with continuously cycling fresh or spent media [91].
- Hemagglutination
an in vitro assay that assesses whether factors such as bacteria, viruses, or antibodies can agglutinate red blood cells.
- Struvite
a common infection stone mineral with the composition: [(NH4) (MgPO4·6H2O)] [6].
- Urosepsis
a possibly life-threatening systemic inflammatory response (sepsis) to a urogenital infection [92].
- Urothelium
epithelial cells that line the inner surface of the renal pelvis, ureters, bladder, and urethra [93].
Footnotes
Appendix A Resources
www.cdc.gov/HAI/pdfs/progress-report/hai-progress-report.pdf.
Appendix B Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tim.2016.11.015.
References
- 1.Schaffer JN, et al. Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation. Proc. Natl. Acad. Sci. U.S.A. 2016;113:4494–4499. doi: 10.1073/pnas.1601720113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stickler DJ, Feneley RC. The encrustation and blockage of long-term indwelling bladder catheters: a way forward in prevention and control. Spinal Cord. 2010;48:784–790. doi: 10.1038/sc.2010.32. [DOI] [PubMed] [Google Scholar]
- 3.Stickler DJ. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J. Intern. Med. 2014;276:120–129. doi: 10.1111/joim.12220. [DOI] [PubMed] [Google Scholar]
- 4.Marien T, Miller NL. Treatment of the infected stone. Urol. Clin. North Am. 2015;42:459–472. doi: 10.1016/j.ucl.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Sabbuba NA, et al. Genotyping demonstrates that the strains of Proteus mirabilis from bladder stones and catheter encrustations of patients undergoing long-term bladder catheterization are identical. J. Urol. 2004;171:1925–1928. doi: 10.1097/01.ju.0000123062.26461.f9. [DOI] [PubMed] [Google Scholar]
- 6.Bichler KH, et al. Urinary infection stones. Int. J. Antimicrob. Agents. 2002;19:488–498. doi: 10.1016/s0924-8579(02)00088-2. [DOI] [PubMed] [Google Scholar]
- 7.O’Keeffe NK, et al. Severe sepsis following percutaneous or endoscopic procedures for urinary tract stones. Br. J. Urol. 1993;72:277–283. doi: 10.1111/j.1464-410x.1993.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal MW, et al. Contemporary management of struvite stones using combined endourologic and medical treatment: predictors of unfavorable clinical outcome. J. Endourol. 2013;30:771–777. doi: 10.1089/end.2013.0257. [DOI] [PubMed] [Google Scholar]
- 9.Flannigan R, et al. Renal struvite stones – pathogenesis, microbiology, and management strategies. Nat. Rev. Urol. 2014;11:333–341. doi: 10.1038/nrurol.2014.99. [DOI] [PubMed] [Google Scholar]
- 10.Hedelin H. Uropathogens and urinary tract concretion formation and catheter encrustations. Int. J. Antimicrob. Agents. 2002;19:484–487. doi: 10.1016/s0924-8579(02)00095-x. [DOI] [PubMed] [Google Scholar]
- 11.Breitenbucher RB. Bacterial changes in the urine samples of patients with long-term indwelling catheters. Arch. Intern. Med. 1984;144:1585–1588. [PubMed] [Google Scholar]
- 12.Macleod SM, Stickler DJ. Species interactions in mixed-community crystalline biofilms on urinary catheters. J. Med. Microbiol. 2007;56:1549–1557. doi: 10.1099/jmm.0.47395-0. [DOI] [PubMed] [Google Scholar]
- 13.Mufarrij PW, et al. Multibacterial growth from a surgical renal stone culture: a case report and literature review. Rev. Urol. 2012;14:108–114. [PMC free article] [PubMed] [Google Scholar]
- 14.Paonessa JE, et al. Preoperative bladder urine culture as a predictor of intraoperative stone culture results: clinical implications and relationship to stone composition. J. Urol. 2016;196:769–774. doi: 10.1016/j.juro.2016.03.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren JW, et al. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 1982;146:719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- 16.Chow AW, et al. A nosocomial outbreak of infections due to multiply resistant Proteus mirabilis: role of intestinal colonization as a major reservoir. J. Infect. Dis. 1979;139:621–627. doi: 10.1093/infdis/139.6.621. [DOI] [PubMed] [Google Scholar]
- 17.Holá V, et al. Virulence factors in Proteus bacteria from biofilm communities of catheter-associated urinary tract infections. FEMS Immunol. Med. Microbiol. 2012;65:343–349. doi: 10.1111/j.1574-695X.2012.00976.x. [DOI] [PubMed] [Google Scholar]
- 18.Jones BV, et al. Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infect. Immun. 2004;72:3941–3950. doi: 10.1128/IAI.72.7.3941-3950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbuba N, et al. The migration of Proteus mirabilis and other urinary tract pathogens over Foley catheters. BJU Int. 2002;89:55–60. doi: 10.1046/j.1464-4096.2001.01721.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones BV, et al. Role of swarming in the formation of crystalline Proteus mirabilis biofilms on urinary catheters. J. Med. Microbiol. 2005;54:807–813. doi: 10.1099/jmm.0.46123-0. [DOI] [PubMed] [Google Scholar]
- 21.Armbruster CE, Mobley HLT. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat. Rev. Microbiol. 2012;10:743–754. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaffer JN, Pearson MM. Proteus mirabilis and urinary tract infections. Microbiol Spectr. 2015 doi: 10.1128/microbiolspec.UTI-0017-2013. http://dx.doi.org/10.1128/microbiolspec.UTI-0017-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen AM, et al. Visualization of Proteus mirabilis morphotypes in the urinary tract: the elongated swarmer cell is rarely observed in ascending urinary tract infection. Infect. Immun. 2003;71:3607–3613. doi: 10.1128/IAI.71.6.3607-3613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Toole G, et al. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 25.Li X, et al. Ureolytic biomineralization reduces Proteus mirabilis biofilm susceptibility to ciprofloxacin. Antimicrob. Agents Chemother. 2016;60:2993–3000. doi: 10.1128/AAC.00203-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stickler D, et al. Studies on the formation of crystalline bacterial biofilms on urethral catheters. Eur. J. Clin. Microbiol. Infect. Dis. 1998;17:649–652. doi: 10.1007/BF01708349. [DOI] [PubMed] [Google Scholar]
- 27.Morris NS, Stickler DJ. Encrustation of indwelling urethral catheters by Proteus mirabilis biofilms growing in human urine. J. Hosp. Infect. 1998;39:227–234. doi: 10.1016/s0195-6701(98)90262-6. [DOI] [PubMed] [Google Scholar]
- 28.Alamuri P, Mobley HLT. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol. Microbiol. 2008;68:997–1017. doi: 10.1111/j.1365-2958.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- 29.Allison C, et al. Ability of Proteus mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect. Immun. 1992;60:4740–4746. doi: 10.1128/iai.60.11.4740-4746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allison C, et al. The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J. Infect. Dis. 1994;169:1155–1158. doi: 10.1093/infdis/169.5.1155. [DOI] [PubMed] [Google Scholar]
- 31.Mobley HLT, et al. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 1996;64:5332–5340. doi: 10.1128/iai.64.12.5332-5340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oelschlaeger TA, Tall BD. Uptake pathways of clinical isolates of Proteus mirabilis into human epithelial cell lines. Microb. Pathog. 1996;21:1–16. doi: 10.1006/mpat.1996.0037. [DOI] [PubMed] [Google Scholar]
- 33.Peerbooms PG, et al. Vero cell invasiveness of Proteus mirabilis. Infect. Immun. 1984;43:1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scavone P, et al. Role of Proteus mirabilis MR/P fimbriae and flagella in adhesion, cytotoxicity and genotoxicity induction in T24 and Vero cells. Pathog. Dis. 2015 doi: 10.1093/femspd/ftv017. http://dx.doi.org/10.1093/femspd/ftv017. [DOI] [PubMed] [Google Scholar]
- 35.Jansen AM, et al. Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect. Immun. 2004;72:7294–7305. doi: 10.1128/IAI.72.12.7294-7305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones BD, et al. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 1990;58:1120–1123. doi: 10.1128/iai.58.4.1120-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson DE, et al. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 1993;61:2748–2754. doi: 10.1128/iai.61.7.2748-2754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeuchi H, et al. Detection of Proteus mirabilis urease gene in urinary calculi by polymerase chain reaction. Int. J. Urol. 1996;3:202–206. doi: 10.1111/j.1442-2042.1996.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen CY, et al. Proteus mirabilis urinary tract infection and bacteremia: risk factors, clinical presentation, and outcomes. J. Microbiol. Immunol. Infect. 2012;45:228–236. doi: 10.1016/j.jmii.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Weiner ID, et al. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol. 2015;10:1444–1458. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mobley HLT, et al. Molecular biology of microbial ureases. Microbiol. Rev. 1995;59:451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro MR, et al. Development of monoclonal antibodies against the human sodium iodide symporter: immunohistochemical characterization of this protein in thyroid cells. J. Clin. Endocrinol. Metab. 1999;84:2957–2962. doi: 10.1210/jcem.84.8.5871. [DOI] [PubMed] [Google Scholar]
- 43.Stickler DJ, et al. Observations on the adherence of Proteus mirabilis onto polymer surfaces. J. Appl. Microbiol. 2006;100:1028–1033. doi: 10.1111/j.1365-2672.2006.02840.x. [DOI] [PubMed] [Google Scholar]
- 44.Wilks SA, et al. Novel insights into the Proteus mirabilis crystalline biofilm using real-time imaging. PLoS One. 2015 doi: 10.1371/journal.pone.0141711. http://dx.doi.org/10.1371/journal.pone.0141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stickler D, et al. Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol. Res. 2003;31:306–311. doi: 10.1007/s00240-003-0340-3. [DOI] [PubMed] [Google Scholar]
- 46.Broomfield RJ, et al. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J. Med. Microbiol. 2009;58:1367–1375. doi: 10.1099/jmm.0.012419-0. [DOI] [PubMed] [Google Scholar]
- 47.Zhao H, et al. Use of green fluorescent protein to assess urease gene expression by uropathogenic Proteus mirabilis during experimental ascending urinary tract infection. Infect. Immun. 1998;66:330–335. doi: 10.1128/iai.66.1.330-335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearson MM, et al. Transcriptome of Proteus mirabilis in the murine urinary tract: virulence and nitrogen assimilation gene expression. Infect. Immun. 2011;79:2619–2631. doi: 10.1128/IAI.05152-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dattelbaum JD, et al. UreR, the transcriptional activator of the Proteus mirabilis urease gene cluster, is required for urease activity and virulence in experimental urinary tract infections. Infect. Immun. 2003;71:1026–1030. doi: 10.1128/IAI.71.2.1026-1030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholson EB, et al. Proteus mirabilis urease: transcriptional regulation by UreR. J. Bacteriol. 1993;175:465–473. doi: 10.1128/jb.175.2.465-473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria - structure, assembly and their role in disease. Cell. Mol. Life Sci. 2009;66:613–635. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pizarro-Cerda J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Fronzes R, et al. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 2008;27:2271–2280. doi: 10.1038/emboj.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waksman G, Hultgren SJ. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat. Rev. Microbiol. 2009;7:765–774. doi: 10.1038/nrmicro2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearson MM, et al. Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J. Bacteriol. 2008;190:4027–4037. doi: 10.1128/JB.01981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuan L, et al. Characterization of 17 chaperone-usher fimbriae encoded by Proteus mirabilis reveals strong conservation. J. Med. Microbiol. 2014;63:911–922. doi: 10.1099/jmm.0.069971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bahrani FK, et al. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 1994;62:3363–3371. doi: 10.1128/iai.62.8.3363-3371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, et al. Proteus mirabilis mannose-resistant, Proteus-like fimbriae: MrpG is located at the fimbrial tip and is required for fimbrial assembly. Infect. Immun. 1997;65:1327–1334. doi: 10.1128/iai.65.4.1327-1334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, et al. Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis. Infect. Immun. 1999;67:2822–2833. doi: 10.1128/iai.67.6.2822-2833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pellegrino R, et al. Proteus mirabilis uroepithelial cell adhesin (UCA) fimbria plays a role in the colonization of the urinary tract. Pathog. Dis. 2013;67:104–107. doi: 10.1111/2049-632X.12027. [DOI] [PubMed] [Google Scholar]
- 61.Massad G, et al. Proteus mirabilis fimbriae: identification, isolation, and characterization of a new ambient-temperature fimbria. Infect. Immun. 1994;62:1989–1994. doi: 10.1128/iai.62.5.1989-1994.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zunino P, et al. Proteus mirabilis fimbriae (PMF) are important for both bladder and kidney colonization in mice. Microbiology. 2003;149:3231–3237. doi: 10.1099/mic.0.26534-0. [DOI] [PubMed] [Google Scholar]
- 63.Himpsl SD, et al. Identification of virulence determinants in uropathogenic Proteus mirabilis using signature-tagged mutagenesis. J. Med. Microbiol. 2008;57:1068–1078. doi: 10.1099/jmm.0.2008/002071-0. [DOI] [PubMed] [Google Scholar]
- 64.Zunino P, et al. Virulence of a Proteus mirabilis ATF isogenic mutant is not impaired in a mouse model of ascending urinary tract infection. FEMS Immunol. Med. Microbiol. 2000;29:137–143. doi: 10.1111/j.1574-695X.2000.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 65.Zunino P, et al. New aspects of the role of MR/P fimbriae in Proteus mirabilis urinary tract infection. FEMS Immunol. Med. Microbiol. 2001;31:113–120. doi: 10.1111/j.1574-695X.2001.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 66.Li X, et al. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol. Microbiol. 2002;45:865–874. doi: 10.1046/j.1365-2958.2002.03067.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhao H, et al. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol. Microbiol. 1997;23:1009–1019. doi: 10.1046/j.1365-2958.1997.2791645.x. [DOI] [PubMed] [Google Scholar]
- 68.Lane MC, et al. Oxygen-limiting conditions enrich for fimbriate cells of uropathogenic Proteus mirabilis and Escherichia coli. J. Bacteriol. 2009;191:1382–1392. doi: 10.1128/JB.01550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scavone P, et al. Fimbriae have distinguishable roles in Proteus mirabilis biofilm formation. Pathog. Dis. 2016 doi: 10.1093/femspd/ftw033. http://dx.doi.org/10.1093/femspd/ftw033. [DOI] [PubMed] [Google Scholar]
- 70.Pearson MM, Mobley HLT. Repression of motility during fimbrial expression: identification of 14 mrpJ gene paralogues in Proteus mirabilis. Mol. Microbiol. 2008;69:548–558. doi: 10.1111/j.1365-2958.2008.06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bode NJ, et al. Transcriptional analysis of the MrpJ network: modulation of diverse virulence-associated genes and direct regulation of mrp fimbrial and flhDC flagellar operons in Proteus mirabilis. Infect. Immun. 2015;83:2542–2556. doi: 10.1128/IAI.02978-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, et al. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 2001;20:4854–4862. doi: 10.1093/emboj/20.17.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belas R, et al. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infect. Immun. 2004;72:5159–5167. doi: 10.1128/IAI.72.9.5159-5167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phan V, et al. ZapA, a virulence factor in a rat model of Proteus mirabilis-induced acute and chronic prostatitis. Infect. Immun. 2008;76:4859–4864. doi: 10.1128/IAI.00122-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Massad G, et al. Proteus mirabilis fimbriae: construction of an isogenic pmfA mutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect. Immun. 1994;62:536–542. doi: 10.1128/iai.62.2.536-542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alteri CJ, et al. Preferential use of central metabolism in vivo reveals a nutritional basis for polymicrobial infection. PLoS Pathog. 2015 doi: 10.1371/journal.ppat.1004601. http://dx.doi.org/10.1371/journal.ppat.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bishop RE. The lipid A palmitoyltransferase PagP: molecular mechanisms and role in bacterial pathogenesis. Mol. Microbiol. 2005;57:900–912. doi: 10.1111/j.1365-2958.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 78.Kovacs-Simon A, et al. Lipoproteins of bacterial pathogens. Infect. Immun. 2011;79:548–561. doi: 10.1128/IAI.00682-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tan Y, Kagan JC. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol. Cell. 2014;54:212–223. doi: 10.1016/j.molcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alteri CJ, et al. Multicellular bacteria deploy the type VI secretion system to preemptively strike neighboring cells. PLoS Pathog. 2013 doi: 10.1371/journal.ppat.1003608. http://dx.doi.org/10.1371/journal.ppat.1003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wenren LM, et al. Two independent pathways for self-recognition in Proteus mirabilis are linked by type VI-dependent export. MBio. 2013 doi: 10.1128/mBio.00374-13. http://dx.doi.org/10.1128/mBio.00374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hachani A, et al. Type VI secretion and anti-host effectors. Curr. Opin. Microbiol. 2016;29:81–93. doi: 10.1016/j.mib.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Bode NJ, et al. Distinct residues contribute to motility repression and autoregulation in the Proteus mirabilis fimbria-associated transcriptional regulator AtfJ. J. Bacteriol. 2016;198:2100–2112. doi: 10.1128/JB.00193-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ganderton L, et al. Scanning electron microscopy of bacterial biofilms on indwelling bladder catheters. Eur. J. Clin. Microbiol. Infect. Dis. 1992;11:789–796. doi: 10.1007/BF01960877. [DOI] [PubMed] [Google Scholar]
- 85.Armbruster CE, et al. Increased incidence of urolithiasis and bacteremia during Proteus mirabilis and Providencia stuartii coinfection due to synergistic induction of urease activity. J. Infect. Dis. 2014;209:1524–1532. doi: 10.1093/infdis/jit663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bartlett RC, Treiber N. Clinical significance of mixed bacterial cultures of urine. Am. J. Clin. Pathol. 1984;82:319–322. doi: 10.1093/ajcp/82.3.319. [DOI] [PubMed] [Google Scholar]
- 87.Hooton TM, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 88.Armbruster CE, et al. How often do clinically diagnosed catheter-associated urinary tract infections in nursing homes meet standardized criteria? J. Am. Geriatr. Soc. 2016 doi: 10.1111/jgs.14533. http://dx.doi.org/10.1111/jgs.14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mulvey MA, et al. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas-White K, et al. The bladder is not sterile: History and current discoveries on the urinary microbiome. Curr. Bladder Dysfunct. Rep. 2016;11:18–24. doi: 10.1007/s11884-016-0345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tolker-Nielsen T, Sternberg C. Growing and analyzing biofilms in flow chambers. Curr. Protoc. Microbiol. 2011;Chapter 1(Unit 1B):2. doi: 10.1002/9780471729259.mc01b02s21. [DOI] [PubMed] [Google Scholar]
- 92.Wagenlehner FM, et al. Diagnosis and management for urosepsis. Int. J. Urol. 2013;20:963–970. doi: 10.1111/iju.12200. [DOI] [PubMed] [Google Scholar]
- 93.Wu XR, et al. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009;75:1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dedeić-Ljubović A, Hukić M. Catheter-related urinary tract infection in patients suffering from spinal cord injuries. Bosn J. Basic Med. Sci. 2009;9:2–9. doi: 10.17305/bjbms.2009.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frank DN, et al. Culture-independent microbiological analysis of Foley urinary catheter biofilms. PLoS One. 2009 doi: 10.1371/journal.pone.0007811. http://dx.doi.org/10.1371/journal.pone.0007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holá V, et al. Microbial diversity in biofilm infections of the urinary tract with the use of sonication techniques. FEMS Immunol. Med. Microbiol. 2010;59:525–528. doi: 10.1111/j.1574-695X.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- 97.Matsukawa M, et al. Bacterial colonization on intraluminal surface of urethral catheter. Urology. 2005;65:440–444. doi: 10.1016/j.urology.2004.10.065. [DOI] [PubMed] [Google Scholar]
- 98.Tenney JH, Warren JW. Long-term catheter-associated bacteriuria: species at low concentration. Urology. 1987;30:444–446. doi: 10.1016/0090-4295(87)90376-1. [DOI] [PubMed] [Google Scholar]
- 99.Hatt JK, Rather PN. Role of bacterial biofilms in urinary tract infections. Curr. Top. Microbiol. Immunol. 2008;322:163–192. doi: 10.1007/978-3-540-75418-3_8. [DOI] [PubMed] [Google Scholar]
- 100.Subashchandrabose S, Mobley HLT. Back to the metal age: battle for metals at the host-pathogen interface during urinary tract infection. Metallomics. 2015;7:935–942. doi: 10.1039/c4mt00329b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.