Of the ten most highly cited papers published in Schizophrenia Research during 2015–2016, four addressed the role of GABA neurons in schizophrenia (Berretta et al., 2015; Cohen et al., 2015; Dong et al., 2015; Schubert et al., 2015). These reports illustrate the breadth of GABA-related abnormalities in schizophrenia, with alterations identified in multiple brain regions using a variety of techniques. Thus, it appears timely to comment on certain aspects of what we currently know, and do not know, about GABA neurotransmission in the disease process of schizophrenia.
In individuals with schizophrenia, alterations in multiple GABA-related markers have been widely reported in the prefrontal cortex (PFC), one of the most studied brain regions in the illness given its key role in cognitive control (Lewis et al., 2012). Recent postmortem human studies demonstrate GABA-related alterations in epigenetic (Dong et al., 2015), transcript (Chung et al., 2016b), protein (Berretta et al., 2015; Chung et al., 2016a; Enwright et al., 2016; Schubert et al., 2015), and synaptic (Chung et al., 2016a) markers, especially for the subpopulation of GABA neurons containing the calcium-binding protein parvalbumin (PV) (Figure 1). In layer 3 of the PFC, the microcircuit between glutamatergic pyramidal cells and GABAergic PV cells plays an essential role in generating the gamma oscillations thought to be essential for cognitive processing (Lewis et al., 2012). In many subjects with schizophrenia, PFC PV neurons have 1) lower transcript and protein levels of PV and of a key GABA-synthesizing enzyme, GAD67 (Lewis et al., 2012; Mitchell et al., 2015), 2) compromised integrity of perineuronal nets (PNNs) which regulate structural and synaptic functions (Berretta et al., 2015; Enwright et al., 2016), and 3) lower density of excitatory inputs onto their somata (Chung et al., 2016a). These molecular and structural alterations are thought to impair GABA neurotransmission and to contribute to the lower power of PFC gamma oscillations seen in individuals with schizophrenia (McNally and McCarley, 2016). Indeed, in vivo studies indicate that measures of extracellular GABA, which more closely approximate synaptic GABA than do measures of total GABA levels, are lower in subjects with schizophrenia (Frankle et al., 2015). In aggregate, these and other findings strongly implicate altered function of GABAergic PV neurons in schizophrenia, but they also raise important questions regarding the nature of these alterations in the underlying disease process.
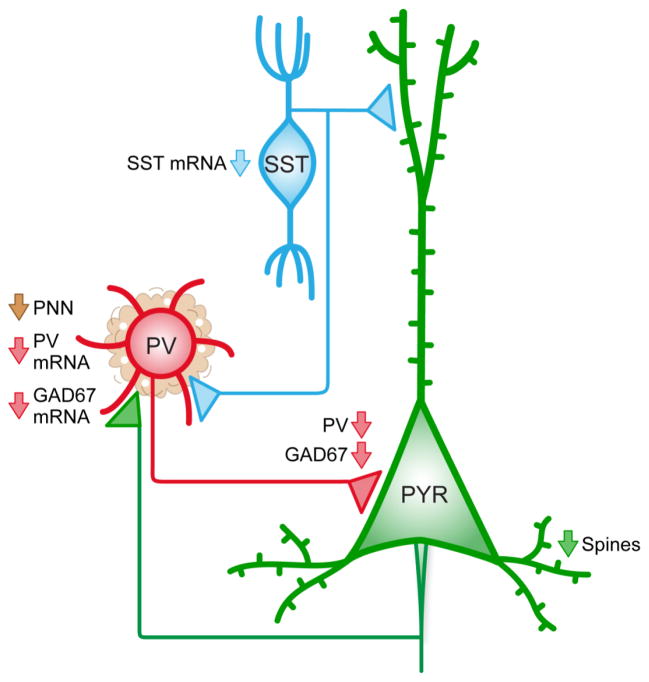
Figure 1. Schematic depiction of a subset of GABA-related abnormalities identified in schizophrenia.
In the prefrontal cortex (PFC), GABAergic neurons containing parvalbumin (PV) innervate the perisomatic region of glutamatergic pyramidal cells (PYR), which in turn, innervate PV neurons. This reciprocal microcircuit is key for the generation of gamma oscillations. GABAergic neurons containing somatostatin (SST) can innervate both PV and PYR cells. In schizophrenia, all three neuronal populations show alterations, including 1) lower density of dendritic spines on PYR cells, 2) less PV and GAD67 mRNA and protein in PV neurons, and 3) lower expression of SST mRNA.
The observed changes in GABA-related markers could represent a cause, consequence, compensation, comorbidity, or confound in schizophrenia. Multiple control experiments provide convergent evidence that markers of lower GABA neurotransmission from PV neurons are likely not due to confounds or comorbidities (Berretta et al., 2015; Chung et al., 2016a; Enwright et al., 2016; Lewis et al., 2012). Measures consistent with lower inhibitory output from PV neurons in schizophrenia could represent a compensation to restore excitatory-inhibitory balance in response to an upstream deficit in PFC excitatory pyramidal cells (Lewis et al., 2012). This interpretation is supported by findings in schizophrenia that PFC layer 3 pyramidal cells have a lower complement of dendritic spines, and thus likely receive fewer excitatory synapses (Lewis et al., 2012). The resulting lower activity of pyramidal cells would preferentially reduce the excitation of PV cells, resulting in the deficits observed in GAD67, PV and PNNs. Alternatively, the changes in pyramidal and PV cells might both be consequences of another disturbance. For example, somatostatin (SST)-containing GABAergic neurons, which are thought to innervate both pyramidal and PV cells, have significant reductions in SST mRNA in schizophrenia (Figure 1) (Fung et al., 2014). As this finding may reflect lower inhibition from SST neurons, a predicted result would be disinhibition and greater activity of both pyramidal and PV cells. However, increased activity of PV cells is not congruent with the observed deficits in GAD67, PV and PNNs. Finally, impairments intrinsic to PV neurons might cause the deficits in measures of GABA neurotransmission. For example, schizophrenia is associated with a shift in the alternative splicing of ErbB4 that is selective to PV neurons, and this ErbB4 variant may impair the formation of excitatory inputs onto PV neurons (Chung et al., 2016b). The reduction in excitation of PV neurons would lead to the observed deficits in GAD67, PV and PNNs. Consistent with this interpretation, PV neurons in the PFC of schizophrenia subjects receive fewer excitatory inputs (Chung et al., 2016a).
Discriminating among these possible interpretations can be aided by the conduct of experimental studies in animal models that are explicitly informed by the disease state and that are ultimately required to identify the specific neural circuits that underlie, and can be engaged to treat, schizophrenia (Gordon, 2016). Specifically, the experimental production in a relevant animal model of one alteration identified in postmortem human studies can then be used to determine if other disease-related findings occur. For example, studies using genetic manipulations to lower GAD67 expression do not recapitulate the other GABA-related findings in schizophrenia, suggesting that less GABA synthesis is not a cause in schizophrenia (Georgiev et al., 2016). However, manipulations in mice that mimic the schizophrenia-associated reduction of the actin cytoskeleton regulatory protein Arp2/3 in PFC pyramidal cells result in a progressive loss of dendritic spines that is followed by other molecular and behavioral alterations seen in schizophrenia, including elevated subcortical dopamine levels and antipsychotic-sensitive increased locomotion (Yan et al., 2016). Future tests of GABA-related markers in this, and other disease-informed animal models, can be effectively used to test whether the alterations in GABA-related markers in schizophrenia represent cause, consequence or compensation in the disease process.
Acknowledgments
Role of the funding sources
Cited work by the authors was supported by NIMH grants MH107735 to JRG and MH043784 and MH103204 to DAL.
Footnotes
Contributors
Glausier JR and Lewis DA
Jill R. Glausier, PhD, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh, Biomedical Science Tower W1654, 3811 O’Hara Street, Pittsburgh, PA 15213, Tel: 412-624-7869; Fax: 412-624-9910, glausierjr@upmc.edu
David A. Lewis, MD, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh, Biomedical Science Tower W1654, 3811 O’Hara Street, Pittsburgh, PA 15213, Tel: 412-246-6010; Fax: 412-624-9910, lewisda@upmc.edu
Conflicts of Interest
David A. Lewis currently receives investigator-initiated research support from Pfizer. In 2013-2015, he served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals and Sunovion. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015;167(1–3):18–27. doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DW, Fish KN, Lewis DA. Pathological Basis for Deficient Excitatory Drive to Cortical Parvalbumin Interneurons in Schizophrenia. Am J Psychiatry. 2016a;173(11):1131–1139. doi: 10.1176/appi.ajp.2016.16010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DW, Volk DW, Arion D, Zhang Y, Sampson AR, Lewis DA. Dysregulated ErbB4 Splicing in Schizophrenia: Selective Effects on Parvalbumin Expression. Am J Psychiatry. 2016b;173(1):60–68. doi: 10.1176/appi.ajp.2015.15020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 2015;167(1–3):98–107. doi: 10.1016/j.schres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Ruzicka WB, Grayson DR, Guidotti A. DNA-methyltransferase1 (DNMT1) binding to CpG rich GABAergic and BDNF promoters is increased in the brain of schizophrenia and bipolar disorder patients. Schizophr Res. 2015;167(1–3):35–41. doi: 10.1016/j.schres.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwright JF, Sanapala S, Foglio A, Berry R, Fish KN, Lewis DA. Reduced Labeling of Parvalbumin Neurons and Perineuronal Nets in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia. Neuropsychopharmacology. 2016;41(9):2206–2214. doi: 10.1038/npp.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Prasad KM, Mason NS, Paris J, Himes ML, Walker C, Lewis DA, Narendran R. In Vivo Measurement of GABA Transmission in Healthy Subjects and Schizophrenia Patients. Am J Psychiatry. 2015;172(11):1148–1159. doi: 10.1176/appi.ajp.2015.14081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Fillman SG, Webster MJ, Shannon Weickert C. Schizophrenia and bipolar disorder show both common and distinct changes in cortical interneuron markers. Schizophr Res. 2014;155(1–3):26–30. doi: 10.1016/j.schres.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Georgiev D, Yoshihara T, Kawabata R, Matsubara T, Tsubomoto M, Minabe Y, Lewis DA, Hashimoto T. Cortical Gene Expression After a Conditional Knockout of 67 kDa Glutamic Acid Decarboxylase in Parvalbumin Neurons. Schizophr Bull. 2016;42(4):992–1002. doi: 10.1093/schbul/sbw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA. On being a circuit psychiatrist. Nat Neurosci. 2016;19(11):1385–1386. doi: 10.1038/nn.4419. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JM, McCarley RW. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry. 2016;29(3):202–210. doi: 10.1097/YCO.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AC, Jiang Y, Peter C, Akbarian S. Transcriptional regulation of GAD1 GABA synthesis gene in the prefrontal cortex of subjects with schizophrenia. Schizophr Res. 2015;167(1–3):28–34. doi: 10.1016/j.schres.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert KO, Focking M, Cotter DR. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: potential roles in GABAergic interneuron pathology. Schizophr Res. 2015;167(1–3):64–72. doi: 10.1016/j.schres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Yan Z, Kim E, Datta D, Lewis DA, Soderling SH. Synaptic Actin Dysregulation, a Convergent Mechanism of Mental Disorders? J Neurosci. 2016;36(45):11411–11417. doi: 10.1523/JNEUROSCI.2360-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]