Abstract
OBJECTIVE
We previously reported that in the absence of hormone therapy (HT) or calcium/vitamin D (Ca/D) supplementation, earlier menopause age was associated with decreased bone mineral density (BMD) and increased fracture risk in healthy post-menopausal women. Treatment with HT and Ca/D are protective against fractures after menopause. In this analysis, we asked if age of menopause onset alters fracture risk in healthy post-menopausal women receiving HT, Ca/Vit D, or the combination.
METHODS
Hazard ratios (HR) for any fracture among 21,711 healthy post-menopausal women enrolled in the Women’s Health Initiative Clinical Trial (WHI-CT), who were treated with HT, Ca/Vit D, or HT + Ca/D, and who reported age of non-surgical menopause of <40, 40-49, and ≥50 years, were compared.
RESULTS
Women with menopause <40 y had significantly higher HR for fracture compared to women with menopause 40-49 or ≥50, regardless of treatment intervention [HR (95% CI): menopause < 40 y vs. ≥50 y, 1.36 (1.11, 1.67); menopause < 40 y vs. 40-49 y, 1.30 (1.06, 1.60).
CONCLUSIONS
In the overall WHI-CT cohort and within each treatment group, women with younger menopause age (<40) had a higher risk of any fracture compared to women reporting older menopause ages. The effect of menopause age on fracture risk was not altered by any of the treatment interventions (HT, Ca/D, HT+Ca/D), suggesting that early age of menopause is an independent contributor to postmenopausal fracture risk.
Keywords: menopause, fracture, bone mineral density, estrogen plus progestogen therapy, calcium, vitamin D, Women’s Health Initiative Clinical Trial
INTRODUCTION
Osteoporotic fractures are a significant cause of morbidity and mortality in menopausal women [1-7]. The Women’s Health Initiative Clinical Trial (WHI CT) was comprised of three prospective, randomized, controlled trials investigating the effects of (1) hormone therapy (HT), (2) calcium and vitamin D supplementation (Ca/D), and (3) a low fat, high fruit/ vegetable/grain diet on morbidity and mortality in postmenopausal women. Eligible women were able to participate in one, two, or all three trials. Among the WHI CT’s primary outcome measures were the occurrence of osteoporotic fractures and modification of fracture rate by intervention with HT, Ca/D supplementation, or both (HT + Ca/D). Results from the WHI CT showed that HT reduced the risk for osteoporotic fractures compared to placebo [8-10]. Ca/D treatment alone significantly decreased risk for hip fracture in women aged 60 or older, suggesting a longer duration of menopause may be associated with greater efficacy of treatment [8]. On the other hand, all participants treated with HT experienced a decrease in fracture rate compared to women taking placebo, and this effect was independent of participants’ age or baseline risk for fracture [9,10].
We recently reported that age of non-surgical menopause onset is a risk factor for fracture among healthy post-menopausal women in the WHI Observational Study (WHI OS), in which there was no treatment intervention [11]. In the absence of HT or Ca/D supplementation, and after adjusting for known risk factors for fracture, women in the WHI OS who experienced early menopause (<40 years) had higher fracture rates compared to women who experienced menopause between ages 40-49 or ≥50 years [11]. Building on these findings, here we sought to examine the effect of age of non-surgical menopause on fracture risk in women randomized within the WHI CT to HT, Ca/D, or HT + Ca/D, understanding that these exposures might attenuate fracture risk related to age of menopause and thus offer a therapeutic intervention to reduce fracture risk in women who experience younger age of natural menopause.
METHODS
The WHI CT recruited 68,132 postmenopausal women between October 1, 1993, and December 31, 1998, at 40 different study sites across the United States. Women aged 50-79 years without medical co-morbidities that precluded survival for at least three years were enrolled. Women were initially enrolled into the HT and/or dietary modification arms of the trial, and if eligible, were invited to also participate in the Ca/D arm at their first or second annual follow up visit. Each participant provided written informed consent through the approving Institutional Review Board (IRB) at her respective study site. Local IRBs at each study site, the NIH, and the Coordinating Center IRB approved the WHI CT protocols. Detailed descriptions of the WHI CT eligibility criteria, study design and methods have been previously reported [12].
At study enrollment, the following information was collected from all participants using standardized questionnaires: demographic information; complete personal medical history including medication history and current medication use, smoking status, alcohol consumption, and physical activity level (quantified in METS/week based on the number of weekly sessions of recreational physical activity); and family history. Patients were instructed to bring all of their medications, including over-the-counter medications and dietary supplements, to the clinic for data recording. Current or prior use of HT was carefully documented. At baseline, information was collected regarding risk for fractures (such as personal history of fractures and personal history of an osteoporosis diagnosis) using standardized questionnaires. This included an assessment of total daily calcium and vitamin D intake from dietary sources and supplements. Physical and clinical measurements, performed by a certified WHI staff member, were collected at study entry using standardized protocols to assess height, weight, and blood pressure.
Fracture occurrences were collected from all responding WHI participants annually using standardized questionnaires. Fractures on which our analyses were based were only those that occurred after study enrollment. Fractures were categorized by site: hip/pelvis/upper leg, lower leg/ankle/knee, foot, upper arm/shoulder/elbow, lower arm/wrist/hand, and spine/tailbone. Hip fractures were adjudicated using medical record data by centrally trained WHI physician adjudicators. Fracture events were adjudicated during the main WHI study (1993-2005) and through the end of the first WHI Extension study (2005 – 2010).
All women in the Ca/D Trial (n=36,282) were randomized using a permuted-block algorithm (stratified by study site and age) to receive either two tablets containing 500 mg of elemental calcium plus 200 IU of vitamin D daily (total daily dose 1,000 mg calcium and 400 IU vitamin D), or placebo pills daily. Women were allowed to enroll in the study if they took personal supplements of calcium and/or vitamin D at doses of not more than 1,000 mg calcium and 600 IU vit D daily. Among women in the Ca/D trial, 40% (n=14,507) self-reported use of both calcium and vitamin D, 11% (n=3,809) reported use of calcium only, 4% (n=1,272) reported use of vitamin D only, and 46% (n=16,693) reported no supplement use. The HT trial consisted of two possible interventions: i) estrogen alone (E2) and ii) estrogen + progestin (E+P). Women who had undergone hysterectomy were eligible for the E2 trial (n=10,739), while women with an intact uterus were eligible for the E+P trial (n=16,608). Among the women in the E+P trial, participants were randomly assigned to receive 0.625 mg/day of conjugated equine estrogen (CEE) in combination with 2.5 mg/day of medroxyprogesterone acetate (MPA) in a single pill (Prempro; Wyeth Philadelphia, PA) (n= 8,506) or matching placebo (n=8,102). All HT trial participants who were using HT at the time of enrollment were required to undergo a 3-month washout period prior to study initiation.
For this analysis, we included only women with an intact uterus to avoid confounding data with women who had undergone surgical menopause and for whom natural menopause age was consequently not able to be determined. Thus, we included only women in the E+P arms of the HT and HT + Ca/D trials. Self-reported age of menopause was grouped as follows: menopause <40, 40-49, and ≥50 years. These age categories were pre-specified by the WHI CT. Clinically, these age categories also divide women between those with menopause onset at the population mean age (≥50 years) and those with younger ages of menopause onset. For this analysis, all women who were randomized to the placebo arms for the HT Trial, the Ca/D Trial, and the HT + Ca/D Trial were combined into one ‘placebo control’ group, creating four treatment levels: control, HT intervention, Ca/D intervention, and HT + Ca/D intervention. Our primary aim was to determine whether age of menopause altered fracture risk among women treated with HT, Ca/D, or both.
Statistical Analyses
Overall baseline demographic characteristics were first summarized by age of menopause group (<40, 40-49, and ≥50). We then determined the number of fractures (hip only and any fracture) in the overall cohort and among the treatment groups (HT, Ca/D, HT + Ca/D, and control) across the 3 menopausal ages (<40, 40-49, and ≥50 yrs). Cumulative hazard plots using age at fracture as the primary endpoint were calculated. Cox proportional hazard models using age as the time scale were used to assess the impact of age of menopause and treatment on any fracture. First, an unadjusted model with age at menopause and treatment received (control, HT, Ca/D, HT + Ca/D) as the only covariates was applied to the data. Using this model, the significance of the interaction between age at menopause and treatment received was determined. Next, we checked for the presence of significant confounders or effect modifiers to the relationship between age at menopause and fracture using a risk factor modeling approach [13]. Each of the variables listed in Table 1 with <20% missing data were added one at a time to the unadjusted model. To be a confounder, the addition of the variable in the model would have to change the hazard ratios for fracture between the different menopause age groups by at least 10%. To be an effect modifier, the two-way interaction between the variable and age at menopause would have to be significant at a P<0.01 level. All analyses were performed using SAS/STAT software, Version 9.4 of the SAS System for Windows (SAS Institute Inc., Cary, NC).
Table 1.
Baseline Characteristics of Uterus-Intact Women in the WHI Intervention Trial (HT and Ca/VitD Alone) Based on Age of Menopause
| Characteristic | Age of Menopause
|
Total | ||
|---|---|---|---|---|
| <40 | 40-49 | ≥50 | ||
|
| ||||
| Number Randomized | 537 | 8,449 | 16,513 | 25,499 |
|
| ||||
| Region: | ||||
| Northeast | 119 (22%) | 1,984 (23%) | 4,058 (25%) | 6,161 |
| South | 141 (26%) | 1,936 (23%) | 3,524 (21%) | 5,601 |
| Midwest | 103 (19%) | 2,048 (24%) | 4,136 (25%) | 6,287 |
| West | 174 (32%) | 2,481 (29%) | 4,795 (29%) | 7,450 |
|
| ||||
| Trial Membership: | ||||
| E+P Trial Alone | 137 (26%) | 2,119 (25%) | 3,744 (23%) | 6,000 |
| Ca/D Alone | 182 (34%) | 3,266 (39%) | 7,020 (43%) | 10,468 |
| E+P and Ca/D | 218 (41%) | 3,064 (36%) | 5,749 (35%) | 9,031 |
|
| ||||
| Dietary Modification Trial | 288 (54%) | 4,710 (56%) | 9,571 (58%) | 14,569 |
|
| ||||
| Age Group at Screening, Years: | ||||
|
| ||||
| <50 – 59 | 180 (34%) | 3,446 (41%) | 5,553 (34%) | 9,179 |
| 60 – 69 | 243 (45%) | 3,447 (41%) | 7,855 (48%) | 11,545 |
| 70 – 79+ | 114 (21%) | 1,556 (18%) | 3,105 (19%) | 4,775 |
| Mean (SD) | 63.1 (7.3) | 62.0 (6.7) | 63.0 (6.7) | |
| (range) | (50 – 79) | (50 – 79) | (50 – 79) | |
|
| ||||
| Time from Menopause to Screening, Years: | ||||
| <10 | 0 | 2,133 (25%) | 8,261 (50%) | 10,394 |
| 10 – 19 | 95 (18%) | 3,330 (39%) | 6,484 (39%) | 9,909 |
| > 19 | 442 (82%) | 2,986 (35%) | 1,768 (11%) | 5,196 |
| Mean (SD) | 27.4 (8.2) | 16.0 (8.3) | 10.2 (6.7) | |
| (range) | (11 – 55) | (1 – 39) | (0 – 29) | |
|
| ||||
| Race/Ethnicity: | ||||
| Missing | 1 (<1%) | 22 (<1%) | 30 (<1%) | 53 |
| White | 414 (77%) | 7,104 (84%) | 14,204 (86%) | 21,722 |
| Black | 62 (12%) | 610 (7%) | 1,063 (6%) | 1,735 |
| Other* | 60 (11%) | 713 (8%) | 1,216 (7%) | 1,989 |
|
| ||||
| Education: | ||||
| Missing | 4 (1%) | 44 (1%) | 100 (1%) | 148 |
| None – some HS | 49 (9%) | 443 (5%) | 745 (5%) | 1,237 |
| HS diploma/GED | 97 (18%) | 1,488 (18%) | 2,869 (17%) | 4,454 |
| Vocational, training school, some college or associate degree | 234 (44%) | 3,273 (39%) | 6,169 (37%) | 9,676 |
| College graduate or more | 153 (28%) | 3,201 (38%) | 6,630 (40%) | 9,984 |
|
| ||||
| Body Mass Index (BMI), kg/m2: | ||||
| Missing | 1 (<1%) | 49 (1%) | 82 (<1%) | 132 |
| <25 | 163 (30%) | 2,656 (31%) | 4,909 (30%) | 7,728 |
| 25 - <30 | 182 (34%) | 2,964 (35%) | 5,875 (36%) | 9,021 |
| 30+ | 191 (36%) | 2,780 (33%) | 5,647 (34%) | 8,618 |
| Mean (SD) | 28.8 (5.9) | 28.3 (5.8) | 28.6 (5.9) | |
| (range) | (15.0 – 49.8) | (13.8 – 69.6) | (14.6 – 69.6) | |
|
| ||||
| Physical Activity (MET-hours/week): | ||||
| Missing | 41 (8%) | 306 (4%) | 624 (4%) | 971 |
| None | 106 (20%) | 1,569 (19%) | 2,747 (17%) | 4,422 |
| >0 – <3.75 | 90 (17%) | 1,351 (16%) | 2,480 (15%) | 3,921 |
| 3.75 – <8.75 | 109 (20%) | 1,688 (20%) | 3,376 (20%) | 5,173 |
| 8.75 – <17.5 | 90 (17%) | 1,696 (20%) | 3,506 (21%) | 5,292 |
| 17.5+ | 101 (19%) | 1,839 (22%) | 3,780 (23%) | 5,720 |
| Median (range) | 5.3 (0 – 75.8) | 6.9 (0 – 119.0) | 7.5 (0 – 134.2) | |
|
| ||||
| Any Fracture Ever: | ||||
| Missing | 44 (8%) | 334 (4%) | 695 (4%) | 1,073 |
| No | 294 (55%) | 4,953 (59%) | 9,820 (59%) | 15,067 |
| Yes | 199 (37%) | 3,162 (37%) | 5,998 (36%) | 9,359 |
|
| ||||
| Hip Fracture Ever: | ||||
| Missing | 374 (70%) | 5,993 (71%) | 11,894 (72%) | 18,261 |
| No | 162 (30%) | 2,391 (28%) | 4,475 (27%) | 7,028 |
| Yes | 1 (<1%) | 65 (1%) | 144 (1%) | 210 |
|
| ||||
| Main Occupation: | ||||
| Missing | 62 (12%) | 585 (7%) | 1,161 (7%) | 1,808 |
| Managerial/Professional | 173 (32%) | 3,189 (38%) | 6,366 (39%) | 9,728 |
| Technical/Sales/Admin | 159 (30%) | 2,442 (29%) | 4,702 (28%) | 7,303 |
| Service/Labor | 88 (16%) | 1,498 (18%) | 2,721 (16%) | 4,307 |
| Homemaker only | 55 (10%) | 735 (9%) | 1,563 (9%) | 2,353 |
|
| ||||
| First Fracture Age 55+**: | ||||
| Missing | 65 (14%) | 752 (11%) | 1,675 (11%) | 2,492 |
| No | 323 (69%) | 5,051 (74%) | 11,224 (75%) | 16,598 |
| Yes | 77 (17%) | 1,003 (15%) | 2,007 (13%) | 3,087 |
|
| ||||
| Hip Fracture Age 55+**: | ||||
| Missing | 72 (15%) | 822 (12%) | 1,870 (13%) | 2,764 |
| No | 392 (84%) | 5,941 (87%) | 12,957 (87%) | 19,290 |
| Yes | 1 (<1%) | 43 (1%) | 79 (1%) | 123 |
|
| ||||
| History of Osteoporosis: | ||||
| Missing | 49 (9%) | 627 (7%) | 1,160 (7%) | 1,836 |
| No | 489 (91%) | 7,903 (94%) | 15,573 (94%) | 23,965 |
| Yes | 36 (7%) | 438 (5%) | 735 (4%) | 1,209 |
|
| ||||
| Parity: | ||||
| Missing | 0 | 25 (<1%) | 57 (<1%) | 82 |
| Never pregnant | 67 (12%) | 865 (10%) | 1,220 (7%) | 2,152 |
| Never had a term pregnancy | 24 (4%) | 284 (3%) | 364 (2%) | 672 |
| 1 – 2 | 192 (36%) | 2,832 (34%) | 5,125 (31%) | 8,149 |
| 3+ | 254 (47%) | 4,443 (53%) | 9,747 (59%) | 14,444 |
|
| ||||
| Number of Falls in the Previous 12 Months: | ||||
| Missing | 35 (7%) | 266 (3%) | 547 (3%) | 848 |
| None | 322 (60%) | 5,588 (66%) | 10,856 (66%) | 16,766 |
| 1 | 108 (20%) | 1,644 (19%) | 3,203 (19%) | 4,955 |
| 2 | 48 (9%) | 624 (7%) | 1,300 (8%) | 1,972 |
| 3+ | 24 (4%) | 327 (4%) | 607 (4%) | 958 |
|
| ||||
| Smoking Status: | ||||
| Missing | 5 (1%) | 107 (1%) | 157 (1%) | 269 |
| Never or Former | 464 (86%) | 7,362 (87%) | 15,226 (92%) | 23,052 |
| Current | 68 (13%) | 980 (12%) | 1,130 (7%) | 2,178 |
|
| ||||
| ETOH Use: | ||||
| Missing | 4 (1%) | 47 (1%) | 116 (1%) | 167 |
| Non-drinker or past drinker | 162 (30%) | 2,257 (27%) | 4,155 (25%) | 6,574 |
| <1 drink/month to <7 drinks/week | 313 (58%) | 5,097 (60%) | 10,239 (62%) | 15,649 |
| 7+ drinks/week | 58 (11%) | 1,048 (12%) | 2,003 (12%) | 3,109 |
|
| ||||
| Hormone Use: | ||||
| Missing | 0 | 6 (<1%) | 6 (<1%) | 12 |
| Never | 235 (44%) | 4,774 (57%) | 10,243 (62%) | 15,252 |
| Past User | 211 (39%) | 1,812 (21%) | 2,426 (15%) | 4,449 |
| Current User | 91 (17%) | 1,857 (22%) | 3,838 (23%) | 5,786 |
|
| ||||
| History of Diabetes: | ||||
| Missing | 0 | 1 (<1%) | 5 (<1%) | 6 |
| No | 498 (93%) | 8,043 (95%) | 15,627 (95%) | 24,168 |
| Yes | 39 (7%) | 405 (5%) | 881 (5%) | 1,325 |
|
| ||||
| Baseline Glucocorticosteroid Taken Orally and Daily: | ||||
| Missing | 104 (19%) | 1,782 (21%) | 3,340 (20%) | 5,226 |
| No | 433 (81%) | 6,656 (79%) | 13,156 (80%) | 20,245 |
| Yes | 0 | 11 (<1%) | 17 (<1%) | 28 |
|
| ||||
| Baseline Bisphosphonate Use: | ||||
| Missing | 104 (19%) | 1,782 (21%) | 3,340 (20%) | 5,226 |
| No | 433 (81%) | 6,646 (79%) | 13,143 (80%) | 20,222 |
| Yes | 0 | 21 (<1%) | 30 (<1%) | 51 |
|
| ||||
| Baseline SERM Use: | ||||
| Missing | 104 (19%) | 1,782 (21%) | 3,340 (20%) | 5,226 |
| No | 433 (81%) | 6,658 (79%) | 13,158 (80%) | 20,249 |
| Yes | 0 | 9 (<1%) | 15 (<1%) | 24 |
|
| ||||
| Baseline Thiazide Use: | ||||
| Missing | 104 (19%) | 1,782 (21%) | 3,340 (20%) | 5,226 |
| No | 409 (76%) | 6,278 (74%) | 12,314 (75%) | 19,001 |
| Yes | 24 (4%) | 389 (5%) | 859 (5%) | 1,272 |
|
| ||||
| Baseline Levothyroxine Use: | ||||
| Missing | 104 (19%) | 1,782 (21%) | 3,340 (20%) | 5,226 |
| No | 379 (71%) | 5,666 (67%) | 11,130 (67%) | 17,175 |
| Yes | 54 (10%) | 1,001 (12%) | 2,043 (12%) | 3,098 |
|
| ||||
| Baseline Vitamin D Intake (mcg/day): | ||||
| Missing | 21 (4%) | 199 (2%) | 326 (2%) | 546 |
| Median | 5.7 | 6.5 | 7.3 | |
| (range) | (0.2 – 54.9) | (0.04 – 111.6) | (0.1 – 67.4) | |
|
| ||||
| Baseline Calcium Intake (mg/day): | ||||
| Missing | 21 (4%) | 199 (2%) | 326 (2%) | 546 |
| Median | 866 | 976 | 1031 | |
| (range) | (114 – 5339) | (96 – 11556) | (107 – 14642) | |
American Indian or Alaskan Native, Asian or Pacific Islander, Hispanic/Latino, Other
Among participants who were at least 55 at the time of screening
RESULTS
A total of 47,540 women participated in the WHI HT and/or Ca/D trials. We first excluded 19,912 women with self-reported total hysterectomy (TAH) and/or bilateral oophorectomy, or who were enrolled in the E2 alone trial (indicating prior TAH) because of differential estrogen exposure that is known to influence fracture risk. Next, we removed 2,087 women who were missing menopause information and 42 women who were missing data from at least 1 study follow-up visit, resulting in 25,499 women to be included in the analytical sample. The average follow-up time for women in our study cohort was 11.3 years (SD=4.1 years, range: 0.003 – 16.8 years). Within this sample, approximately 2% (n=537 women) reported age of non-surgical menopause < 40 years, compared to 33% (n=8,449) reporting menopause between age 40 and 49 years and 65% (n=16,513) reporting menopause at age 50 or later (Figure 1, Table 1).
Figure 1.
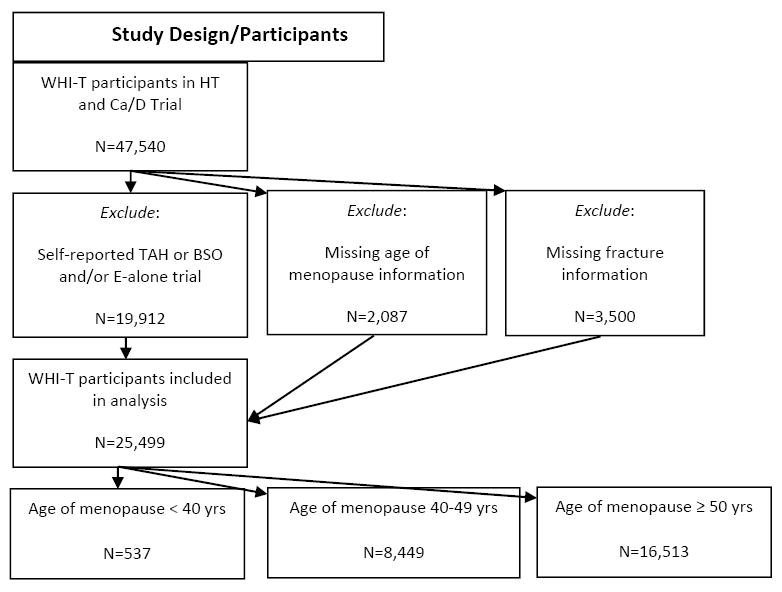
Flowchart of study design and participant inclusion/exclusion.
Demographic characteristics, risk factors for fracture, and treatment group by menopause age group are detailed in Table 1. We observed several differences between the menopause groups. More women with menopause ≥50 y reported having 3+ children compared to women with menopause < 40 y (59% vs. 47%, respectively). A larger proportion of women with menopause < 40 y were current smokers at baseline compared to women with menopause ≥50 y (13% vs. 7%, respectively). Women with menopause ≥50 y reported the highest rate of physical activity compared to women with menopause < 40 y (17.5+ MET-hours/week, 23% vs. 19%, respectively). Women with menopause < 40 y had higher rates of past HT use but lower rates of current HT use compared to women with menopause ≥50 y (past HT use, 39% vs. 15%; current HT use, 17% vs. 23%, respectively). In addition, baseline calcium and vitamin D intake (combined dietary and supplemental) was higher in the menopause ≥50 y group compared to the < 40 y group (median calcium/day = 1031 vs. 868 mg/day, respectively; median vitamin D/day = 7.3 vs. 5.7 mcg/day, respectively).
Confounding and effect modification were evaluated among the variables listed in Table 1 with <20% missing data [i.e., dietary modification trial participation, race/ethnicity, region, education, occupation, BMI, physical activity, parity, number of falls in the previous 12 months, smoking status, ETOH use, HT use, history of diabetes, personal history of fracture (overall and at age 55 or later), history of osteoporosis, total baseline vitamin D intake, total baseline calcium intake], as described in the Statistical Analyses section. Individually, none of the variables listed met the criterion for effect modification, as the minimum p-value for any two-way interaction was >0.05 (see Table S1, Supplemental Digital Content 1, which shows p-values for effect modification of all variables). Similarly, none of the variables was deemed a confounding variable, as none of the variables elicited more than a 6% change from the unadjusted HR for menopause age (see Table S2, Supplemental Digital Content 2, which shows changes in hazard ratios with and without adjustment for each variable in the univariate model). Therefore, we report hazard ratios for fracture calculated using models unadjusted for these variables.
The total number of fractures of any type and the total number of adjudicated hip fractures, overall and by treatment received, and by age of menopause group, are presented in Table 2. Of note, only 21 women reporting age of menopause < 40 years had a hip fracture, thus there was not sufficient statistical power to compare rates of hip fracture across menopause age groups. We therefore focused our analysis on rates of any fracture among study participants. Overall and within each active treatment group, a greater percentage of women with menopause < 40 had a fracture compared to women with older menopause ages. Thus, regardless of the intervention received (i.e., HT, Ca/D, HT + Ca/D), a larger proportion of women with menopause < 40 y had a fracture compared to women with older menopause ages (~20%, menopause <40 y vs. ~14%, menopause 40-49 y and ≥50 y) (Table 2).
Table 2.
Number of Fractures and Percentage of Women with Fracture, Overall and by Treatment Received
| Group | N | Any Fracture: No. (% with fracture) | Hip Fracture: No. (% with fracture) |
|---|---|---|---|
|
| |||
| Overall: | 25,499 | 3,670 (14%) | 656 (3%) |
|
| |||
| <40 | 537 | 97 (18%) | 21 (4%) |
| 40-49 | 8,449 | 1,231 (15%) | 230 (3%) |
| ≥50 | 16,513 | 2,342 (14%) | 405 (2%) |
|
| |||
| Control: | 10,386 | 1,573 (15%) | 277 (3%) |
|
| |||
| <40 | 210 | 30 (14%) | 4 (2%) |
| 40-49 | 3,433 | 529 (15%) | 107 (3%) |
| ≥50 | 6,743 | 1,014 (15%) | 166 (2%) |
|
| |||
| E+P Intervention: | 5,349 | 744 (14%) | 170 (3%) |
|
| |||
| <40 | 127 | 26 (20%) | 8 (6%) |
| 40-49 | 1,863 | 268 (14%) | 60 (3%) |
| ≥50 | 3,359 | 450 (13%) | 102 (3%) |
|
| |||
| Ca/D Intervention: | 7,519 | 1,071 (14%) | 174 (2%) |
|
| |||
| <40 | 150 | 31 (21%) | 9 (6%) |
| 40-49 | 2,425 | 344 (14%) | 50 (2%) |
| ≥50 | 4,944 | 696 (14%) | 115 (2%) |
|
| |||
| E+P and Ca/D Interventions: | 2,245 | 282 (13%) | 35 (2%) |
|
| |||
| <40 | 50 | 10 (20%) | 0 |
| 40-49 | 728 | 90 (12%) | 13 (2%) |
| ≥50 | 1,467 | 182 (12%) | 22 (1%) |
Results from the unadjusted Cox model for any fracture are shown in Table 3; the cumulative hazard function by menopause group is shown in Figure 2. The interaction between age at menopause (<40 y, 40-49 y, ≥50 y) and treatment received (control, HT, Ca/D, HT + Ca/D) was not significant (p=0.452), implying that the effect of menopause age on fracture risk was not meaningfully different between treatment groups. Regardless of treatment group, women with menopause <40 y had a significantly higher risk for any fracture compared to women with menopause at later ages: the hazard ratio for fracture for menopause < 40 y vs. ≥50 y was 1.36 (95% CI: 1.11, 1.67) and the HR for fracture for menopause < 40 y vs. 40-49 y was 1.30 (95% CI: 1.06, 1.60). Of note, hazard ratios calculated after adjusting for all variables in Table 1 estimated HR’s for fracture across menopause age groups that were not different from HR’s obtained from the unadjusted model [<40 vs. 40-49: HR = 1.33 (1.08, 1.64); <40 vs. 50+: HR = 1.41 (1.15, 1.73); 40-49 vs. 50+: HR = 1.06 (0.99, 1.14)]. Among the treatment groups, regardless of menopause age, the HR for fracture was higher in women randomized to a placebo as compared to women randomized to Ca/D and women randomized to the combination of HT + Ca/D (Table 3).
Table 3.
Hazard Ratios from the Unadjusted Cox Proportional Hazards Model for Any Fracture
| Comparison: | Estimated HR for Any Fracture | 95% CI | p-value |
|---|---|---|---|
|
| |||
| Age at menopause: | 0.007 | ||
| <40 vs. 40-49 | 1.30 | (1.06, 1.60) | |
| <40 vs. 50+ | 1.36 | (1.11, 1.67) | |
| 40-49 vs. 50+ | 1.05 | (0.98, 1.12) | |
|
| |||
| Treatment received: | 0.004 | ||
| Control vs. E+P intervention | 1.08 | (0.99, 1.17) | |
| Control vs. CaD intervention | 1.1 | (1.02, 1.19) | |
| Control vs. E+P and CaD interventions | 1.23 | (1.08, 1.4) | |
| E+P intervention vs. CaD intervention | 1.03 | (0.93, 1.13) | |
| E+P intervention vs. E+P and CaD interventions | 1.14 | (1, 1.31) | |
| CaD intervention vs. E+P and CaD interventions | 1.11 | (0.98, 1.27) | |
Figure 2.
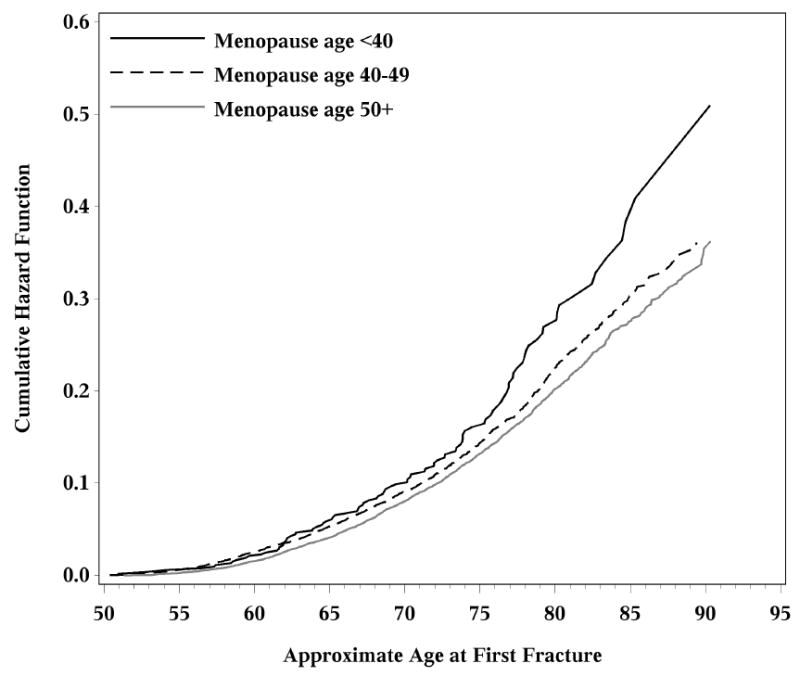
Cumulative hazard functions for participants by age of menopause.
DISCUSSION
In this secondary analysis of the WHI CT, we asked if age of non-surgical menopause altered the risk for fracture among healthy post-menopausal women who were treated with HT, calcium and vitamin D, or both interventions. We found that women with younger ages of menopause (< 40 y) had significantly increased risk for any fracture compared to women reporting menopause at older ages (40-49 y or ≥50 y). This increased fracture risk among women with menopause < 40 remained regardless of study intervention with hormone therapy, calcium and vitamin D supplementation, or both therapies, all of which have the potential to lessen fracture risk in postmenopausal women [8-10]. Our findings are in agreement with some, but not all, of previously published investigations of effects of menopause age on fracture risk. For example, both Svejme et al [14] and van Der Voort et al [15] showed that women with early natural menopause had increased risk of fractures in the later post-menopausal years, and several investigators have shown that early age of menopause is an independent risk factor for fractures [16,17]. On the other hand, Luisetta et al [18] found that chronological age and BMI were stronger predictors of bone mineral density than age of menopause, and further, that after age 60, detrimental effects of early menopause age on BMD were no longer evident. Francucci et al [19] similarly demonstrated that an association between early menopause age and decreased BMD persisted only until age 55 but not beyond, and Ahlborg et al [20] found no influence of menopause age on BMD after age 64.
A potential primary etiology by which earlier menopause age amplifies fracture risk is a longer duration of estrogen deficiency. However, we showed increased fracture risk even among women reporting menopause < 40 who were treated with E+P HT. Together, these data indicate that age of menopause is a significant independent contributor to fracture risk in post-menopausal women. These findings are congruent with our recent observations of lower BMD and higher fracture risk among uterus-intact postmenopausal women enrolled in the WHI Observational Study (OS) who reported menopause < 40 y compared to women in the same study reporting menopause between ages 40-49 y or ≥50 y. In the WHI OS, however, there was no treatment intervention, thus any potential interaction between menopause age and bone-protective treatments could not be ascertained.
In our present analysis, we found that overall, control women had higher risk of fracture compared to women in the E+P and/or Ca/D treatment groups, that is, the effect of treatment as a predictor of fracture was highly significant (P=0.004) (Table 3). However, in considering the effect of treatment relative to the relationship between menopause age and fracture, we found no evidence that treatment with E+P and/or Ca/D significantly altered the relationship between age at menopause and fracture risk, i.e., treatment did not change the effect of menopause age on fracture risk. This suggests that early age of menopause is a potent risk factor for fracture in postmenopausal women and should be taken into account when assessing overall fracture risk in this population. It is possible that earlier initiation of treatment with Ca/D and/or HT, longer duration of treatment, or longer duration of follow-up would show an interaction between menopause age and these bone-protecting treatments. It is also possible that among women treated with E+P, anti-estrogenic effects of the progestin component counteracted any benefits that may have been attributable to the estrogen component of HT on bone. However, our data indicate that early menopause age may be a predominant risk factor predicting fracture risk after menopause despite commonly used therapeutic interventions.
We did not find differences in the percentages of women with fractures (Table 2) or the risk for fracture (Table 3, Figure 2) between women with menopause age 40-49 vs ≥50 y. This was perhaps due to small numbers of fractures within each subgroup. With a larger fracture incidence in the overall cohort, smaller differences in fracture risk that occur on the continuum of menopause age may have become evident. Another contributing factor may be that this time difference in menopause onset—the 5th rather than the 6th decade of life—does not allow sufficient time to result in observable changes in fracture risk. Finally, a longer time of follow up may have demonstrated differences in fracture risk that become more pronounced with advancing chronological age.
In our comparison of demographic characteristics among women in the three menopause age groups, we found several differences, including parity, smoking status, current and past HT use, activity level, and total daily calcium and vitamin D intake, all of which could have altered fracture risk between the groups. However, none of these variables proved to be statistical confounders or effect modifiers in our model analyzing HR for fracture, suggesting the differences we found were due to differences in menopause age rather than differences in other risk factors or demographic factors. Indeed, HRs for fracture were similar in our model with and without adjustment for these variables, indicating age of menopause was the primary predictor of fracture risk.
The number of women reporting menopause < 40 y who reported a hip fracture was very small, precluding an analysis of the HR for adjudicated hip fracture among the menopause age groups in the WHI CT. That said, in the HT and Ca/D intervention groups, women with menopause < 40 y exhibited a higher prevalence of hip fracture compared to women in the older menopause age groups (6% vs. ~3%, Table 2). Further research with larger numbers of postmenopausal women reporting younger menopause age and hip fracture is needed to confirm whether the differences we observed in hip fracture rates across menopause age groups are statistically or clinically significant.
We conducted our primary analysis using data on rates of any fracture among the WHI CT cohort, which limits the specificity of our conclusions. ‘Any fracture’ is used to describe the composite of fractures at the hip/pelvis/upper leg, lower leg/ankle/knee, foot, upper arm/shoulder/elbow, lower arm/wrist/hand, and spine/tailbone. When looking at specific fracture types among our cohort, we found that the absolute numbers for each fracture type were too small within each menopause age group to determine if menopause age altered risk for a particular fracture type, such as wrist or hip fracture. Nevertheless, the distribution of fracture types across the menopause age groups was similar, thus we performed our analysis on the composite of ‘any fracture.’ We were also unable to assess causality of fractures (i.e., traumatic or atraumatic/osteoporotic), therefore we can only speculate that the increase in fracture risk we observed was due to an increase in the prevalence of post-menopausal loss of bone mineral density (BMD). In that regard, we had limited data on bone mineral density as assessed by dual-energy x-ray absorptiometry (DXA) among women in this cohort for meaningful comparisons across groups; however, in our previous report examining the effect of menopause age on fracture risk in naturally postmenopausal women in the WHI Observational Study [11], we found that earlier menopause age not only increased fracture risk, but was also associated with significant decreases in BMD.
Strengths of this study include a large cohort of well-characterized, naturally postmenopausal women with information on many osteoporotic risk factors (including past and current use of calcium, vitamin D, and HT and major co-morbidities), menopausal age, and detailed fracture data. Limitations include its retrospective design; overall small numbers of fractures, particularly among women with age of menopause < 40 years; lack of information on serum 25-hydroxy-vitamin D levels; lack of physician confirmation of menopause onset, and our inability to control for all osteoporotic risk factors in all participants (for example, data on family history of fracture was available for only a small subset of women). Given our study’s strengths and despite its limitations, our data present a clear signal of an increase in overall fracture risk seen in women who experience younger ages of natural menopause.
CONCLUSIONS
This secondary analysis of over 25,000 healthy post-menopausal women suggests that younger menopause age is a strong predictor of increased fracture occurrence in the post-menopausal years and that treatment with HT, Ca/Vit D, or the combination does not influence this association.
Supplementary Material
Table S1. Checking for Effect Modification
Table S2. Change in Hazard Ratios and Parameter Estimates from Univariable Model
Acknowledgments
Funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, and by the United States Department of Health and Human Services.
Financial Support: No additional financial support was obtained for preparation of this manuscript.
Footnotes
Disclosures: No author has a conflict of interest or financial disclosure that is relevant to the subject matter or materials included in this work.
References
- 1.Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38–42. doi: 10.1007/s00198-003-1490-4. [DOI] [PubMed] [Google Scholar]
- 2.Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ., 3rd Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12(12):1042–1049. doi: 10.1007/s001980170015. [DOI] [PubMed] [Google Scholar]
- 3.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 4.Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–1650. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- 5.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 6.Bliuc D, Alarkawi D, Nguyen TV, Eisman JA, Center JR. Risk of Subsequent Fractures and Mortality in Elderly Women and Men With Fragility Fractures With and Without Osteoporotic Bone Density: The Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 2014;30(4):637–46. doi: 10.1002/jbmr.2393. [DOI] [PubMed] [Google Scholar]
- 7.Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. Pharmacoeconomics. 2002;20(5):289–303. doi: 10.2165/00019053-200220050-00001. [DOI] [PubMed] [Google Scholar]
- 8.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 9.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Women’s Health Initiative Investigators: Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290(13):1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Women’s Health Initiative Investigators: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan SD, Lehman A, Thomas F, Johnson KC, Jackson R, Wactawski-Wende J, Ko M, Chen Z, Curb JD, Howard BV. Effects of self-reported age at nonsurgical menopause on time to first fracture and bone mineral density in the Women’s Health Initiative Observational Study. Menopause. 2015;22(10):1035–44. doi: 10.1097/GME.0000000000000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer DW, Lemeshow S. Applied logistic regression. 2. New York: John Wiley & Sons; 2002. [Google Scholar]
- 14.Svejme O, Ahlborg HG, Nilsson JA, Karlsson MK. Early menopause and risk of osteoporosis, fracture and mortality: a 34-year prospective observational study in 390 women. BJOG. 2012;119(7):810–816. doi: 10.1111/j.1471-0528.2012.03324.x. [DOI] [PubMed] [Google Scholar]
- 15.van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int. 2003;14(6):525–530. doi: 10.1007/s00198-003-1408-1. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro MM, Ciconelli RM, Martini LA, Ferraz MB. Clinical risk factors for osteoporotic fractures in Brazilian women and men: the Brazilian Osteoporosis Study (BRAZOS) Osteoporos Int. 2009;20(3):399–408. doi: 10.1007/s00198-008-0680-5. [DOI] [PubMed] [Google Scholar]
- 17.van der Klift M, de Laet CE, McCloskey EV, Johnell O, Kanis JA, Hofman A, Pols HA. Risk factors for incident vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res. 2004;19(7):1172–1180. doi: 10.1359/JBMR.040215. [DOI] [PubMed] [Google Scholar]
- 18.Luisetto G, Zangari M, Bottega F, Peccolo F, Galuppo P, Nardi A, Ziliotto D. Different rates of forearm bone loss in healthy women with early or late menopause. Osteoporos Int. 1995;5(1):54–62. doi: 10.1007/BF01623659. [DOI] [PubMed] [Google Scholar]
- 19.Francucci CM, Romagni P, Camilletti A, Fiscaletti P, Amoroso L, Cenci G, Boscaro M, et al. Effect of natural early menopause on bone mineral density. Maturitas. 2008;59(4):323–328. doi: 10.1016/j.maturitas.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Ahlborg HG, Johnell O, Nilsson BE, Jeppsson S, Rannevik G, Karlsson MK. Bone loss in relation to menopause: a prospective study during 16 years. Bone. 2001;28(3):327–331. doi: 10.1016/s8756-3282(00)00451-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Checking for Effect Modification
Table S2. Change in Hazard Ratios and Parameter Estimates from Univariable Model


