SUMMARY
Ovarian and testicular germ cell tumors of young adults are thought to arise from defects in germ cell development, but the molecular mechanisms underlying malignant transformation are poorly understood. In this review, we focus on the biology of germ cell tumor formation in the Drosophila ovary and the mouse testis, for which the evidence supports common underlying mechanisms such as blocking initiation into the differentiation pathway, impaired lineage progression, and sexual identity instability. We then discuss how these concepts inform our understanding of the disease in humans.
Keywords: germline stem cell differentiation, mitotic-meiotic decision, sexual identity, cancer initiation
INTRODUCTION
Germ cell tumors (GCTs) are a relatively rare form of cancer, yet testicular GCTs are the most frequent cause of cancer in men between the ages of 15 and 35 while ovarian GCTs represent the majority of ovarian malignancies in women under the age of 20. GCTs are generally considered derived from germ cells that fail to execute gametogenesis correctly, but the molecular mechanisms underlying malignant transformation are poorly understood.
Not surprisingly, some of the genes that regulate reproduction when mutated lead to GCTs. Drosophila melanogaster and mice are both established and suitable experimental organisms for investigating the genetic, developmental, and molecular mechanisms behind GCTs. In this review, we highlight recent work that illustrates how disruptions in the pathways necessary for gametogenesis lead to GCTs, first in the Drosophila ovary and then in the mouse testis. We then discuss how the concepts emerging from each of these experimental systems informs our current understanding of the disease in humans.
DROSOPHILA OVARIAN GCTS
Normal germ cell development
Gametogenesis begins early in embryogenesis, when primordial germ cells are specified as distinct from somatic cells. Specified primordial germ cells then migrate into the embryonic gonad, where the germ cells exhibit sex-specific division rates and expression programs. Initiation of the differentiation pathway leading to meiosis and egg development, however, only begins in adulthood.
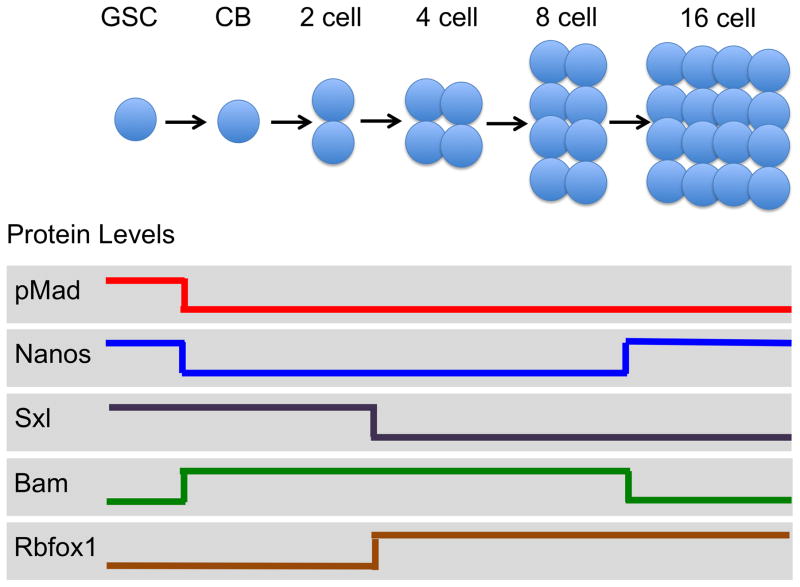
An adult Drosophila female contains a pair of ovaries of simple organization, in which the different cell types can be identified unequivocally by their location, morphology, and expression of molecular markers (Fig. 1). Each ovary is composed of about 16 individual strands of progressively developing egg chambers called ovarioles. Continuous egg production is assured by the presence of a steady population of two to three germ-line stem cells located at the apical tip of the ovariole, in a structure called the germarium. When the stem cell divides, the anterior daughter cell retains contact with the somatic cap cells through adherens and gap junctions, thereby remaining a stem cell. The posterior daughter dissociates from the cap cells, becomes a cystoblast, and divides four more times to produce a cyst of 16 interconnected cells. One of the 16 cyst cells will become the oocyte and initiate meiosis, whereas the remaining 15 cells will become polyploid nurse cells. An egg chamber is formed as the somatic follicle cells surround the 16-cell cyst and bud off from the germarium. (For comprehensive reviews of fly oogenesis see Eliazer and Buszczak 2011; Spradling et al. 2011; Hudson and Cooley 2014; Slaidina and Lehmann 2014; Gilboa 2015; Greenspan et al. 2015).
Figure 1.
Germ cell development in the Drosophila ovary. In the adult ovary, two to three germ-line stem cells (GSCs) give rise to cytoblasts (CBs), then divide four times to form 16-cell cysts. One cell within the 16-cell cyst undergoes meiosis and differentiates into an oocyte (not shown). The level of key regulatory proteins (illustrated as high or low) changes rapidly as the germ cell passes through each stage. Bam, Bag of marbles; pMad, phosphorylated Mothers against Decapentaplegic; Sxl, Sex-lethal.
Ovarian GCTs
The use of Drosophila as a genetic system to study the origin and biology of GCTs was first proposed in 1957 by King and Burnett, in a short publication in Science (King and Burnett 1957). They noted that while flies rarely developed tumors spontaneously, an unusual mutation in the fused locus caused all females to develop tumors in their ovaries. Since that time, directed genetic screens for female-sterile alleles have identified well over 100 genes that, when mutated, produce GCTs (Gans et al. 1975; Mohler 1977; Perrimon et al. 1986; Schüpbach and Wieschaus 1989; Swan et al. 2001; Yan et al. 2014; Teixeira et al. 2015). Although only a small subset of these mutations was studied in detail, their analysis thus far has provided significant insight into the mechanisms underlying tumor formation (Table 1). As summarized below, the three major themes emerging from these studies suggest that GCTs arise when initiation into the differentiation pathway is blocked, when there are defects in the orderly progression of the steps leading to oocyte differentiation, and when germ cells fail to maintain their female identity.
Table 1.
Drosophila GCT genes discussed in this review
| Gene | Function | Select references |
|---|---|---|
| tkv | Control of Dpp diffusion | Luo et al. 2015 |
| fused | Control of BMP signaling | Xia et al. 2010 |
| smurf | Control of BMP signaling | Xia et al. 2010 |
| mir-184 | Control of BMP signaling | Iovino et al. 2009 |
| bam | Differentiation Sexual identity |
McKearin and Spradling 1990 Shapiro-Kulnane et al. 2015 |
| bgcn | Required for bam function | Ohlstein et al. 2000 |
| mei-P26 | Required for bam function | Li et al. 2013 |
| twin | Required for bam function | Fu et al. 2015 |
| Sxl | Required for bam function Sexual identity |
Chau et al. 2009 Shapiro-Kulnane et al., 2015 |
| rbfox1 | lineage progression towards oocyte/nurse cell choice | Carreira-Rosario et al., 2016 |
Differentiation block
In the adult ovary, cell fate switching from a self-renewing stem cells to a differentiation-competent cystoblast cell is initiated by expression of the key differentiation-promoting protein Bag of marbles (Bam). Loss of bam in germ cells leads to a GCT phenotype, whereas ubiquitous overexpression prevents stem cell self-renewal and forces all stem cells to differentiate (Mckearin and Spradling 1990; Ohlstein and McKearin 1997). Accordingly, mutations in any number of genes that ultimately lead to the failure to activate bam transcription, or prevent the Bam protein from functioning appropriately, will display a GCT phenotype.
bam transcription is tightly regulated by bone morphogenetic (BMP) signaling emanating from the neighboring somatic gonadal cells (Xie and Spradling 1998; Chen and McKearin 2003a; Chen and McKearin 2003b; Song et al. 2004). When signaling is high, as in the neighborhood of germ-line stem cells, bam transcription is repressed. The somatic cap cells secrete the BMP ligands Decapentaplegic (Dpp) and Glass-bottom boat (Gbb), which are received in the germ-line stem cells by the receptors Thickveins (Tkv), Saxophone (Sax), and Punt, and thus trigger phosphorylation of Mothers against Dpp (Mad). Phospho-Mad is then transported into the nucleus, where it associates with the bam promoter to repress transcription. Cystoblast cells are refractory to BMP signaling, which allows bam to be transcribed and differentiation to begin; however, GCTs can arise when cystoblast cells inappropriately respond to circulating BMP ligands. The first described GCT mutation in the fused locus falls into this category (King and Burnett 1957; Xia et al. 2010): Fused encodes a serine/threonine kinase that, in wild-type cystoblast cells, partners with the E3 ubiquitin ligase Smurf to antagonize BMP signaling by promoting the degradation of the receptor Tkv. Similarly, mutations in the microRNA mir-184, which in wild-type cystoblast cells inhibits translation of the receptor Sax, also lead to GCTs (Iovino et al. 2009).
Despite these safeguards, GCTs form when the neighboring somatic cells are forced to ectopically transcribe the BMP ligand-encoding dpp gene (Xie and Spradling 1998; Decotto and Spradling 2005; López-Onieva et al. 2008; Wang et al. 2008; Liu et al. 2010; Eliazer et al. 2011; Kirilly et al. 2011; Wang et al. 2011; Xuan et al. 2013; Jin et al. 2013; Eliazer et al. 2014; Mottier-Pavie et al. 2016). GCTs can also form if cap cell-expressed Dpp ligand is able to reach the cystoblast cells. During normal development, somatic gonadal cells use two primary strategies to restrict how far Dpp can travel: In cap cells, the proteoglycan Division abnormally delayed (Dally) functions at the cell surface to limit the distribution of biologically active Dpp to the extracellular space adjacent to the germ-line stem cells (Guo and Wang 2009; Hayashi et al. 2009; Liu et al. 2010). In the adjoining escort cells, the cell-surface Tkv receptor functions to remove Dpp from the extracellular space, thereby preventing Dpp from reaching the cystoblast cells (Luo et al. 2015). Accordingly, expanding Dpp’s range, by either forcing escort cells to ectopically express dally or by knocking down tkv expression in escort cells, leads to an increased number of stem-like cells, which fills the entire germarium as the animal ages.
Disrupting Bam function is another underlying cause of GCTs. Bam orchestrates the stem cell-to-cystoblast cell-fate switch by repressing stem cell-maintenance factors. Bam does not contain any sequence motifs that directly predict its biochemical function, although Bam’s interaction partners – Benign gonial cell neoplasm (Bgcn), Sex-lethal (Sxl), Mei-P26, and Twin – all have documented functions in RNA processing (Ohlstein et al. 2000; Chau et al. 2009; Li et al. 2009; Chau et al. 2012; Li et al. 2013; Fu et al. 2015). Whether these Bam-associated proteins form a single complex or form multiple Bam-containing complexes with distinct target specificities is unclear. Nevertheless, observation that the loss of each partner individually gives rise to GCTs in which the Bam protein is present, but unable to drive differentiation, indicates an essential requirement for Bam function(s).
One of Bam’s roles in cystoblast cells is to repress translation of the stem cell-maintenance factor Nanos (Li et al. 2009; Chau et al. 2012; Li et al. 2013). Nanos represses the translation of differentiation promoting mRNAs in stem cells (Forbes and Lehmann 1998; Gilboa and Lehmann 2004; Wang and Lin 2004; Harris et al. 2011). By preventing the accumulation of Nanos protein, Bam enables the stem cell-to-cystoblast cell switch. Yet, forcing Nanos protein expression in cystoblast cells does not interfere with gametogenesis (Li et al. 2009; Harris et al. 2011; Chau et al. 2012). Thus, nanos dysregulation does not drive GCT formation; instead, other pathways under Bam control are likely necessary to elicit GCTs. In this regard, it is intriguing that a number of studies documented the anomalous expression of testis-specific genes in bam GCTs (Wei et al. 1994; Chau et al. 2009; Shapiro-Kulnane et al. 2015). Whether or not the global depression of testis genes observed in bam mutants is the driving force behind GCT formation is an open question.
Impaired lineage progression
Following commitment to the differentiation pathway, the cystoblast cell divides four times to form a 16-cell cyst. As the cysts divide, they exhibit distinct combinations of molecular markers, suggesting that differentiation requires passing through unique intermediate fates (Chau et al. 2009; Tastan et al. 2010; Carreira-Rosario et al. 2016). For example, the cystoblast and 2-cell cysts express both Sxl and Bam. In the 4- and 8- cell cysts, Sxl protein is not longer detectable whereas Bam protein continues to be present as a new protein marker, Rbfox1, is detectable. In the 16-cell cysts, Bam protein is absent, while the Rbfox1 protein abundance is maintained until the germ cells make their final oocyte/nurse cell-fate choice. GCTs are formed when lineage progression is blocked by inactivating rbfox1 (Tastan et al. 2010; Carreira-Rosario et al. 2016). Interestingly, these mutant cysts break apart into single cells that express both Bam and Sxl, suggesting that the mutant cells have dedifferentiated towards a more immature cell fate. Furthermore, and unlike normal Sxl/Bam-expressing germ cells, these mutant cells remain mitotically active. What distinguishes dedifferentiated Sxl/Bam-expressing cells from normal Sxl/Bam-expressing cells remains unknown.
Sexual cell fate instability
The sexual identity of both germ cells and somatic cells is first established early in embryogenesis. While somatic cells make a cell-autonomous decision based only on their chromosome constitution, the sex of the embryonic germ cells (called primordial germ cells) initially reflects the sex of the surrounding somatic cells. For example, the gene expression program and behavior of XX primordial germ cells is masculinized when placed in a male somatic environment (Horabin et al. 1995; Staab et al. 1996; Wawersik et al. 2005; Casper and van Doren 2009; Hashiyama et al. 2011). Dictation by somatic signals continues through the larval period, after which extrinsic control is lost and XX germ cells control their own sexual development in a cell-autonomous manner (Casper and van Doren 2009).
Recent work established that the failure to maintain sexual identity in the adult female germ line leads to GCTs. Although expression of the female-specific Sxl protein is the central female-determination event in somatic cells, germ cell expression of Sxl is not required for establishing sexual identity in the female germ line (Salz and Erickson 2010; Salz 2011). In the absence of Sxl, female primordial germ cells develop normally through the end of the larval period (Steinmann-Zwicky 1994; Casper and van Doren 2009; Chau et al. 2009). Only in the adult does Sxl function become essential, when its loss leads to a global up-regulation of spermatogenesis genes and a GCT phenotype (Chau et al. 2009; Shapiro-Kulnane et al. 2015).
The degree to which these GCTs are masculinized is illustrated by the sex-inappropriate presence of the testis-specific PHD finger 7 (Phf7) protein (Shapiro-Kulnane et al. 2015). Phf7 is a regulator of male germ cell fate (Yang et al. 2012). Accordingly, forced expression in adult germ cells also leads to GCTs (Shapiro-Kulnane et al. 2015). phf7 is reported to encode an H3K4me2-binding protein (Yang et al. 2012), thus Phf7 likely engages the spermatogenesis gene expression program by interpreting, or reading, the underlying histone code in the male germ line. How ectopic expression of this chromatin reader leads to the reprogramming of female germ cells towards a male fate remains an open question. One possibility is that ectopic Phf7 overrides female identity by recruiting chromatin remodelers to its target genes. Although entirely speculative at this time, a causal relationship between unscheduled chromatin remodeling and GCT formation is consistent with the results of a large-scale RNA-interference-based screen showing that germ cell-specific knockdowns of several different chromatin remodelers yield GCT phenotypes (Yan et al. 2014).
One of the male-like characteristics acquired by GCTs lacking Sxl protein is aberrant activation of the Janus kinase/Signal transducer and activator of transcription (Jak/Stat) signaling pathway (Shapiro-Kulnane et al. 2015). In the testis, secretion of the Unpaired family of cytokines from the somatic gonadal cells activates Jak/Stat signaling in neighboring somatic and germ cells (Kiger et al. 2001; Tulina and Matunis 2001; Leatherman and DiNardo 2008; Leatherman and DiNardo 2010). The situation is different in the ovary, where somatic cytokine production activates Jak/Stat signaling in cap and escort cells, but not in the adjacent germ cells (Decotto and Spradling 2005; López-Onieva et al. 2008; Wang et al. 2008). Yet, depleting just one of the somatically derived ligands in GCTs reverts the tumor phenotype (Shapiro-Kulnane et al. 2015), suggesting that the mutant germ cells respond to the circulating ligands in their environment as if they were male, leading to sex-inappropriate Jak/Stat activation. Some intriguing questions remain, including: How do these mutant cells acquire the male-like ability to receive activating signals from the surrounding somatic cells? Once they receive those signals, why does activation of the Jak/Stat pathway lead to GCT formation? In the testis, Jak/Stat signaling is only needed for adhesion to the somatic hub cells (Leatherman and DiNardo 2010). Perhaps the mutant germ cells express a Stat-regulated pathway that is unrelated to the pathway normally expressed in male germ cells.
The importance of sex-concordant interactions between germ cells and the adjacent somatic cells is further highlighted by a study showing that reprogramming the sexual identity of escort cells in the adult ovary leads to GCTs (Ma et al. 2016). In these studies, female-to-male reprogramming was caused by ectopic expression of the transcription factor Chronologically inappropriate morphogenesis (Chinmo) in escort cells of the adult ovary. Whether the resulting GCTs exhibit a global depression of spermatogenesis genes on the scale observed in GCTs caused by the absence of Sxl or Bam remains to be determined.
MURINE TESTICULAR GCTS
Normal germ cell development
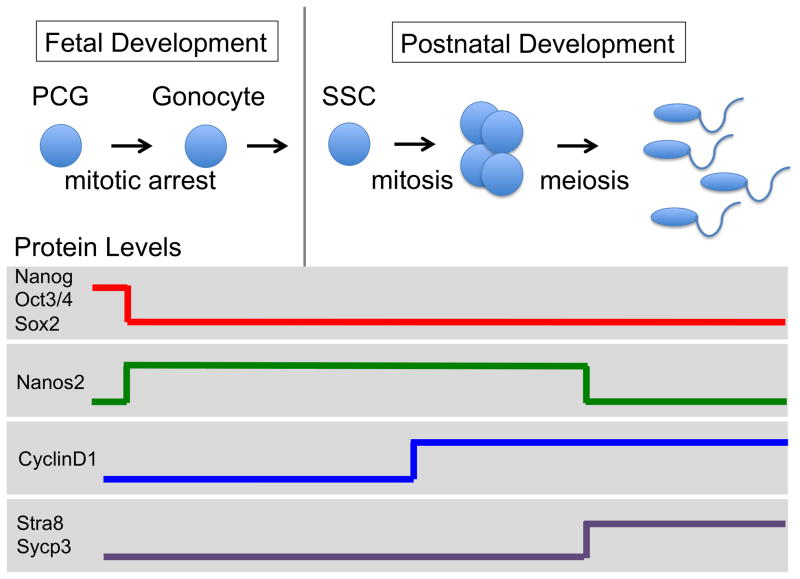
Germ cells are specified in mice from the proximal epiblast during embryogenesis. These nascent germ cells, which express the core pluripotency genes (Nanog, Sox2, and Oct3/4 [also known as Pou5f1]), migrate from the base of the allantois, through the hindgut, into the developing gonad. Once they reach the genital ridge, at around embryonic day (E) 10.5–11.5, the primordial germ cells continue to proliferate to establish a population of approximately 25,000 cells. Sex-specific differentiation, leading to either sperm or egg development, takes place shortly thereafter, at around E12–13.5 (Fig. 2). This cell-fate decision is called the mitotic-meiotic switch because the female germ cells initiate meiosis, whereas the male germ cells (called gonocytes) cease to divide and remain quiescent until after birth. Shortly after birth the gonocytes re-enter mitosis, forming a large pool of undifferentiated germ cells, referred to as spermatogonia stem cells, that provide continuous sperm production throughout the majority of postnatal life by dividing asymmetrically to produce one daughter cell that remains a spermatogonia stem cell and a second daughter cell that enters meiosis to begin the process of spermatogenesis. (For comprehensive reviews of mouse spermatogenesis, see Oatley and Brinster 2012; Saitou and Yamaji 2012; Kanatsu-Shinohara and Shinohara 2013; Yang and Oatley 2014; Boitani et al. 2016).
Figure 2.
Germ cell development in the mouse testis. In the male embryo, the primordial germ cells (PCGs) give rise to quiescent gonocytes. At birth, the gonocytes give rise to spermatogonia stem cells (SSCs) that proliferate, enter meiosis, and differentiate into spermatids. The level of key regulatory proteins (illustrated as high or low) changes as the germ cell passes through each stage. Stra8, Stimulated by retinoic acid 8; Sycp3, Synaptonemal complex protein 3.
Testicular GCTs
In the 1950’s, Leroy Stevens identified an inbred mouse strain, 129/Sv, that spontaneously develops testicular teratomas (Stevens and Little 1954). Teratomas are GCTs that contain patches of somatic tissues, such as hair, bone, teeth, and neurons. The first sign of tumorigenesis, however, is during embryogenesis (starting around E15.5), when germ cells transform into embryonal carcinoma cells, the proliferative and pluripotent stem cells of teratomas (Stevens and Hummel 1957; Stevens 1962). Although the incidence of teratoma formation is only 1–10% in the “wild-type” 129 family of inbred strains, there are a number of mutations that, when crossed into this background, substantially increase teratoma frequency (Table 2) (Heaney and Nadeau 2008; Bustamante-Marin et al. 2013); however, these mutations only influence risk in 129 males, implying that there are as-yet-unidentified strain-specific variants that influence tumorigenesis. Despite this genetic complexity, studies focused on the 129/Sv family of inbred mice suggest that blocking the first differentiation step (mitotic arrest), impaired lineage progression, and sexual identity instability all underlie GCT formation, as they do in the fly ovary.
Table 2.
Genetic variants that affect GCT incidence in the 129/Sv mouse
| Gene | Type of Variant | Increase vs Decrease incidence | Function (in wild-type) | References |
|---|---|---|---|---|
| Ago2Gt(XE344)Byg/Mmucd | Engineered Knockout | Increase | Biogenesis of non-coding RNAs | Carouge et al. 2016 |
| Apobec1tm1Ddsn | Engineered Knockout | Increase | Cytidine deaminase | Nelson et al. 2012 |
| Chr19MOLF/Ei (Sf1Gt(XD130)Byg and unknown genes) | Chromosome substitution (Genetrap) | Increase | Sf1, pre-mRNA splicing |
Matin et al. 1999 Zhu et al. 2010 |
| Dmrt1tm1.1Zark | Engineered Knockout | Increase | Transcription factor | Krentz et al., 2013 |
| Dnd1Ter | Spontaneous Point Mutation | Increase | RNA-binding protein | Noguchi and Stevens 1982 Youngren et al. 2005 |
| KitSl, KitSl-J, KitSl-gb | Spontaneous Deletions | Increase | Ligand for KIT receptor |
Stevens 1967 Heaney et al. 2008 |
| Nanos3tm2.1(cre)Ysa | Cre Knockin | Increase | RNA binding protein | Schemmer et al. 2013 |
| Ptentm1Ppp & Ptentm2Mak floxed | Engineered Knockouts | Increase | Lipid phosphatase |
Di Cristofano et al 1998 Kimura et al. 2003 |
| Tfap2ctm1Hsc | Engineered Knockout | Increase | Transcription factor | Schemmer et al. 2013 |
| Trp53tm1Brd | Engineered Knockout | Increase | Tumor suppressor | Harvey et al. 1993 |
| Ay (Eif2s2Gt(XH413)Byg) | Spontaneous Deletion (Genetrap) | Decrease | Translation initiation |
Stevens 1967 Heaney et al. 2009 |
| A1cftm1Ddsn | Engineered Knockout | Decrease | RNA binding co-factor of APOBEC1 | Carouge et al. 2016 |
| Ccnd1tm1Wbg | Engineered Knockout | Decrease | Cell cycle, oncogene | Lanza et al. 2016 |
Differentiation block
The testicular differentiation program begins during embryogenesis, when primordial germ cells enter into a state of mitotic quiescence. The link between the failure to arrest the cell cycle on schedule and GCT formation was first suggested by the observation that an actively dividing population of germ cells were present in the 129 strain past E15.5 (Stevens 1964; Stevens 1967; Noguchi and Stevens 1982; Matin et al. 1999). Additional studies with two 129/Sv-derivative strains with different frequencies of teratoma incidence firmly established a correlation between prolonged gonocyte proliferation in the embryo with teratoma incidence in the adult (Heaney et al. 2012). Finally, prolonged gonocyte proliferation is always correlated with increased teratoma incidence on the 129 background (Noguchi and Stevens 1982; Kimura et al. 2003; Heaney et al. 2009; Cook et al. 2011; Krentz et al. 2011; Lanza et al. 2016).
The mechanism that underlies the failure to execute the decision to exit the cell cycle has only recently begun to be revealed. Profiling experiments show that gonocytes isolated from the 129 strain aberrantly express a number of genes, including the Cyclin D1-encoding gene Ccnd1 (Cook et al. 2011; Heaney et al. 2012). Cyclin D1 is known to be a general driver of mitotic divisions, and is normally first expressed in male germ cells shortly after birth, when mitosis resumes (Beumer et al. 2000). Cyclin D1 is also observed in the teratoma-susceptible gonocytes. Although not the only mis-expressed cell cycle regulator, Cyclin D1 appears to have an outsized role in misdirecting germ cell development. In fact, eliminating Ccnd1 expression in teratoma-susceptible mice permits gonocytes to arrest on schedule and to significantly reduce the risk of teratoma incidence in the adult (Lanza et al. 2016). Importantly, the ability of a Ccnd1 deficiency to reduce, but not prevent, teratoma occurrence suggests that it is but one essential component of a larger tumorigenic network. Nevertheless, these studies establish that circumventing mitotic arrest is a key driver of GCT initiation.
Impaired lineage progression
GCT-susceptible gonocytes are thought to have retained or regained pluripotent potential, allowing the formation of teratomas. Transplantation and cell culture experiments revealed that both male and female primordial germ cells are capable of forming teratomas prior to E13.5 (Stevens 1964; Matsui et al. 1991; Resnick 1992; Labosky et al. 1994; Chuma et al. 2005). This property is lost, however, once germ cells enter their respective differentiation pathways. Although differentiation is normally accompanied by down-regulation of Nanog, Sox2, and Oct3/4, abnormally proliferating GCT-susceptible gonocytes continue to express these markers into adulthood (Krentz et al. 2009; Cook et al. 2011; Heaney et al. 2012; Schemmer et al. 2013; Lanza et al. 2016). Interestingly, whether GCT-susceptible gonocytes arise from a bona fide germ cell or from a precursor cell that has failed to adopt a complete germ cell fate is still debated. Despite the fact that primordial germ cells continue to express pluripotency markers until sex differentiation begins, the prevailing school of thought is that primordial germ cells are lineage-restricted (Magnúsdóttir et al. 2012). If correct, then primordial germ cells must undergo de-differentiation in order to give rise to GCT-susceptible gonocytes and teratomas. On the other hand, emerging evidence indicates that primordial germ cells are not locked into a germ cell fate until they begin to both express the cell identity licensing factor DAZL and down-regulate the pluripotency markers (Chuma et al. 2005; Gill et al. 2011). Thus, GCT-susceptible gonocytes appears to arise from primordial germ cells that have failed to adopt a complete germ cell fate, and that a prolonged, immature, proliferative and pluripotent cell state in the embryo permits inappropriate execution of the somatic differentiation pathway and teratoma formation. The molecular mechanism that impairs lineage progression and leads to a teratoma remains to be elucidated. One important driver may be the failure to enter mitotic arrest because the regulation of pluripotency is tied to cell cycle control (Filipczyk et al. 2007; Singh and Dalton 2009; Kareta et al. 2015). Indeed, Ccnd1 deficiency not only facilitates proper gonocyte cell cycle arrest in teratoma-susceptible mice, it also suppresses gonocyte retention of pluripotency (Lanza et al. 2016).
Sexual fate instability
Several lines of evidence suggest that the failure to execute male-specific programming on schedule is accompanied by embryonic female-like behaviors. For example, teratoma-susceptible gonocytes inappropriately express meiotic genes, including Stimulated by retinoic acid 8 (Stra8) and Synaptonemal complex protein 3 (Sycp3) (Cook et al. 2011; Heaney et al. 2012). In rare instances, these gonocytes prematurely enter meiosis, progressing through the leptotene to early zygotene prophase stages. Like Cyclin D1, embryonic germ cell expression of these meiotic markers is normally restricted to pre-meiotic oocytes (Beumer et al. 2000; Menke et al. 2003; Heaney et al. 2012). Deletion of Stra8 significantly reduces tumor incidence in 129 mice, further suggesting that unscheduled female-like gene expression plays a central role in GCT formation (Heaney et al. 2012).
The idea that the sexual identity of teratoma-susceptible gonocytes is compromised is further supported by the observation that several key male germ cell fate markers are absent (Cook et al. 2011). One of these markers is the male-specific transcription factor Doublesex and mab-3 related transcription factor 1 (DMRT1). Germ cells that lack Dmrt1 precociously enter meiosis in the embryo and form teratomas in adult 129 mice (Krentz et al. 2009; Krentz et al. 2013). Another example is NANOS2, a male-specific RNA-binding protein that plays an important role in achieving mitotic quiescence by repressing the meiotic gene expression program during embryogenesis (Suzuki and Saga 2008; Saba et al. 2014). In the absence of Nanos2, embryonic gonocytes abnormally enter meiosis – yet, no teratomas are observed in the adult, most likely because the analysis was carried out in the tumor-resistant C57BL/6J background. Whether or not teratomas form when the Nanos2 mutant allele is bred onto the 129/Sv background remains to be determined. In this regard, we find it intriguing that the NANOS2 protein physically interacts with Dead end1 (DND1) (Suzuki et al. 2016) because mutations in Dnd1 significant increase the rate of teratoma incidence in the 129/Sv background (Noguchi and Noguchi 1985; Youngren et al. 2005). Furthermore, expression of the autocrine factor NODAL, a TGF-β superfamily member and key inducer of Nanos2 expression in germ cells, is significantly reduced in 129/Sv gonocytes when compared to C57BL/6J gonocytes (Cook et al. 2011).
WHAT CAN THE FLY AND MOUSE STUDIES TELL US ABOUT HUMAN GCTS?
In humans, GCTs can arise in both the ovary and the testis, but malignant tumors occur much more frequently in males than in females. Indeed, testicular GCTs are among the most frequent malignancies in young men. Testicular GCTs represent 98% of all testicular cancer cases, whereas ovarian GCTs account for only 1–3% of all ovarian malignancies. Gonadoblastoma, another rare type of GCTs, occurs in individuals with disorders of sex development. (For comprehensive reviews of germ cell malignancies in humans, see Dolci et al. 2015; Jørgensen et al. 2015; Gonzalez-Exposito et al. 2016; Litchfield et al. 2016; Rajpert-De Meyts et al. 2016).
Most testicular tumors can be classified either as a seminoma or non-seminoma. Seminoma is a homogeneous tumor composed of mitotically active, undifferentiated cells whereas non-seminoma is a heterogeneous tumor composed of undifferentiated and differentiated cells. Non-seminomas can be further subdivided into teratomas (with differentiated somatic tissues) or yolk-sac tumors (with differentiated extra-embryonic tissues). Despite their differences, testicular seminomas and non-seminomas likely share a common etiology because they evolve from the same precursor cell, called germ cell neoplasia in situ (GCNIS); carcinoma in situ (CIS); intratubular germ cell neoplasia, unclassified (IGCNU); or testicular intraepithelial neoplasia (TIN) (Berney et al. 2016; Moch et al. 2016). GCNIS cells retain a number of characteristics of embryonic germ cells, including expression of the stem cell markers Nanog and Oct3/4 (Looijenga et al. 2003; Almstrup et al. 2004; Sonne et al. 2009; Alagaratnam et al. 2011), further suggesting that, as in mice and flies, dysregulation of the earliest steps of pre-meiotic germ cell development underlie tumorigenesis. Genome-wide association studies have also consistently noted that testicular GCT risk tracks with pathways required for germ cell development (Rapley et al. 2009; Kanetsky et al. 2009; Turnbull et al. 2010; Kanetsky et al. 2011; Kratz et al. 2011; Poynter et al. 2012; Chung et al. 2013; Ruark et al. 2013; Koster et al. 2014; Litchfield et al. 2015).
Several gene polymorphisms associated with an increased risk of testicular GCTs fall within the DMRT1 locus, in which defects were previously shown to cause GCTs in male mice. Sex-specific Dmrt1 expression in mice is necessary for controlling the timing of the mitotic-meiotic switch, and is likely to play a similar role in humans (Jørgensen et al. 2012). An examination of DMRT1 and other sex-specific markers in human testicular GCNIS cells provides evidence that the sexual identity of these cells is compromised, and suggests that the failure to execute male-specific programming on schedule drives tumor initiation (Jørgensen et al. 2013). Thus, aberrant sexually dimorphic gene expression programming may be a critical feature of GCT initiation shared by flies, mice, and humans.
The risk for GCTs is greatest for individuals with gonadal dysgenesis (Jørgensen et al. 2015), a term used for a unique set of disorders characterized by incomplete or defective formation of the testis or ovary. The most severe cases occur in individuals with disorders of sex development (DSD), who display genital ambiguity or are sex-reversed in relation to their chromosomal sex. At the other end of the spectrum are chromosomal and phenotypic males with testicular dysgenesis syndrome (TDS), a group of urogenital abnormalities including undescended testis and testicular atrophy. The strong association between gonadal dysgenesis and GCT risk suggests that disturbances in the surrounding somatic environment are responsible. This is certainly the case in Drosophila, where genetic studies have established that GCT formation can be caused by defective somatic-germ line communication. One of the key functions of the somatic gonadal cells in the mouse is to provide the sex-specific instructions to germ cells poised to either enter meiosis or mitotic arrest. Thus, disturbances of the somatic environment during fetal development could disrupt the mitotic-meiotic switch, leading to GCT formation in humans, as it does in the mouse.
The risk for GCTs is lowest in women. Like their male counterparts, ovarian GCTs originate from GCNIS cells that retain characteristics of undifferentiated primordial germ cells, including continued expression of embryonic Nanog and Oct3/4 (Kraggerud et al. 2013). The presumed common cell-of-origin in males and females suggests that parallel mechanisms are responsible for oncogenic transformation. Why then are testicular GCTs significantly more frequent than ovarian GCTs? Once germ cells successfully enter meiosis in Drosophila, they are no longer able to dedifferentiate (Brawley and Matunis 2004; Kai and Spradling 2004), suggesting that this decision branch point imposes a developmental barrier to tumor formation. If the same is true for human germ cells, then GCTs might occur less frequently in females than in males because female germ cells enter meiosis during embryogenesis. Male germ cells, on the other hand, are not exposed to meiotic-inducing signals in utero; instead, the process of entering into mitotic arrest is thought to be gradual and asynchronous, as it is in the mouse (Western et al. 2008). This may leave male germ cells more vulnerable to oncogenic transformation for an extended period of time.
CONCLUDING REMARKS
Given the large evolutionary distances that separate humans, mice, and flies, it is not surprising that the tumors in the Drosophila ovary and the mouse testis do not fully recapitulate human GCTs; nevertheless, research in the fly and mouse experimental systems have revealed shared regulatory principles that have deepened our understanding of how GCTs form in humans. Yet, many important questions remain: Why are pre-meiotic germ cells uniquely vulnerable to oncogenic transformation? What are the steps in the germ cell-to-tumor cell conversion pathway? What signals emanate from the somatic gonad to contribute to tumor initiation and/or progression? The answers to these, and other questions, will continue to provide the foundation needed for understanding GCT initiation in humans, and may, in the future, aid in their prevention, early detection, and treatment.
Acknowledgments
We thank the Salz and Heaney lab members for discussions, and we apologize to our colleagues whose work we did not cover due to space constraints.
Supporting grants: National Institutes of Health: R01GM102141 to HKS; Cancer Prevention and Research Institute of Texas (CPRIT): RP140102 to EPD and RP150081 to JDH.
Abbreviations
- Bam
Bag of marbles
- Dmrt1
Doublesex and mab-3 related transcription factor 1
- E#
embryonic day
- GCNIS
germ cell neoplasia in situ
- GCT
germ cell tumor
- Sxl
Sex-lethal
Footnotes
Quote: [R]esearch in the fly and mouse experimental systems have revealed shared regulatory principles that have deepened our understanding of how GCTs form in humans.
References
- Alagaratnam S, Lind GE, Kraggerud SM, Lothe RA, Skotheim RI. The testicular germ cell tumour transcriptome. Int J Androl. 2011;34:133–501. doi: 10.1111/j.1365-2605.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- Almstrup K, Hoei-Hansen CE, Wirkner U, Blake J, Schwager C, Ansorge W, Nielsen JE, Skakkebaek NE, Rajpert De-Meyts E, Leffers H. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 2004;64:4736–4743. doi: 10.1158/0008-5472.CAN-04-0679. [DOI] [PubMed] [Google Scholar]
- Berney DM, Looijenga LHJ, Idrees M, Oosterhuis JW, Rajpert-De Meyts E, Ulbright TM, Skakkebaek NE. Germ cell neoplasia in situ (GCNIS): evolution of the current nomenclature for testicular pre-invasive germ cell malignancy. Histopathology. 2016;69:7–10. doi: 10.1111/his.12958. [DOI] [PubMed] [Google Scholar]
- Beumer TL, Roepers-Gajadien HL, Gademan IS, Kal HB, de Rooij DG. Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biology of Reproduction. 2000;63:1893–1898. doi: 10.1095/biolreprod63.6.1893. [DOI] [PubMed] [Google Scholar]
- Boitani C, Di Persio S, Esposito V, Vicini E. Spermatogonial cells: mouse, monkey and man comparison. Semin Cell Dev Biol. 2016;59:79–88. doi: 10.1016/j.semcdb.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Bustamante-Marin X, Garness JA, Capel B. Testicular teratomas: an intersection of pluripotency, differentiation and cancer biology. Int J Dev Biol. 2013;57:201–210. doi: 10.1387/ijdb.130136bc. [DOI] [PubMed] [Google Scholar]
- Carouge D, Blanc V, Knoblaugh SE, Hunter RJ, Davidson NO, Nadeau JH. Parent-of-origin effects of A1CF and AGO2 on testicular germ-cell tumors, testicular abnormalities, and fertilization bias. Proc Natl Acad Sci USA. 2016;113:5425–5433. doi: 10.1073/pnas.1604773113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira-Rosario A, Bhargava V, Hillebrand J, Kollipara RK, Ramaswami M, Buszczak M. Repression of Pumilio protein expression by Rbfox1 promotes germ cell differentiation. Dev Cell. 2016;36:562–571. doi: 10.1016/j.devcel.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper AL, van Doren M. The establishment of sexual identity in the Drosophila germline. Development. 2009;136:3821–3830. doi: 10.1242/dev.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau J, Kulnane LS, Salz HK. Sex-lethal facilitates the transition from germline stem cell to committed daughter cell in the Drosophila ovary. Genetics. 2009;182:121–132. doi: 10.1534/genetics.109.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau J, Kulnane LS, Salz HK. Sex-lethal enables germline stem cell differentiation by down-regulating Nanos protein levels during Drosophila oogenesis. Proc Natl Acad Sci USA. 2012;109:9465–9470. doi: 10.1073/pnas.1120473109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Current Biology. 2003a;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003b;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- Chuma S, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Hosokawa M, Nakatsuji N, Ogura A, Shinohara T. Spermatogenesis from epiblast and primordial germ cells following transplantation into postnatal mouse testis. Development. 2005;132:117–122. doi: 10.1242/dev.01555. [DOI] [PubMed] [Google Scholar]
- Chung CC, Kanetsky PA, Wang Z, Hildebrandt MA, Koster R, Skotheim RI, Kratz CP, Turnbull C, Cortessis VK, Bakken AC, et al. Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nat Genet. 2013;45:680–685. doi: 10.1038/ng.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MS, Munger SC, Nadeau JH, Capel B. Regulation of male germ cell cycle arrest and differentiation by DND1 is modulated by genetic background. Development. 2011;138:23–32. doi: 10.1242/dev.057000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Dolci S, Campolo F, De Felici M. Gonadal development and germ cell tumors in mouse and humans. Semin Cell Dev Biol. 2015;45:114–123. doi: 10.1016/j.semcdb.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Eliazer S, Buszczak M. Finding a niche: studies from the Drosophila ovary. Stem Cell Res Ther. 2011;2:45. doi: 10.1186/scrt86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliazer S, Palacios V, Wang Z, Kollipara RK, Kittler R, Buszczak M. Lsd1 restricts the number of germline stem cells by regulating multiple targets in escort cells. PLoS Genet. 2014;10:e1004200. doi: 10.1371/journal.pgen.1004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliazer S, Shalaby NA, Buszczak M. Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc Natl Acad Sci USA. 2011;108:7064–7069. doi: 10.1073/pnas.1015874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipczyk AA, Laslett AL, Mummery C, Pera MF. Differentiation is coupled to changes in the cell cycle regulatory apparatus of human embryonic stem cells. Stem Cell Res. 2007;1:45–60. doi: 10.1016/j.scr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Forbes AA, Lehmann RR. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development. 1998;125:679–690. doi: 10.1242/dev.125.4.679. [DOI] [PubMed] [Google Scholar]
- Fu Z, Geng C, Wang H, Yang Z, Weng C, Li H, Deng L, Liu L, Liu N, Ni J, et al. Twin promotes the maintenance and differentiation of germline stem cell lineage through modulation of multiple pathways. Cell Reports. 2015;13:1366–1379. doi: 10.1016/j.celrep.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Gans M, Audit C, Masson M. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics. 1975;81:683–704. doi: 10.1093/genetics/81.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L. Organizing stem cell units in the Drosophila ovary. Current Opinion in Genetics & Development. 2015;32:31–36. doi: 10.1016/j.gde.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. Repression of primordial germ cell differentiation parallels germ line stem cell maintenance. Curr Biol. 2004;14:981–986. doi: 10.1016/j.cub.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Gill ME, Hu Y-C, Lin Y, Page DC. Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc Natl Acad Sci USA. 2011;108:7443–7448. doi: 10.1073/pnas.1104501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Exposito R, Merino M, Aguayo C. Molecular biology of testicular germ cell tumors. Clin Transl Oncol. 2016;18:550–556. doi: 10.1007/s12094-015-1423-7. [DOI] [PubMed] [Google Scholar]
- Greenspan LJ, de Cuevas M, Matunis E. Genetics of gonadal stem cell renewal. Annu Rev Cell Dev Biol. 2015;31:291–315. doi: 10.1146/annurev-cellbio-100913-013344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Wang Z. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 2009;136:3627–3635. doi: 10.1242/dev.036939. [DOI] [PubMed] [Google Scholar]
- Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell. 2011;20:72–83. doi: 10.1016/j.devcel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiyama K, Hayashi Y, Kobayashi S. Drosophila Sex-lethal gene initiates female development in germline progenitors. Science. 2011;333:885–888. doi: 10.1126/science.1208146. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kobayashi S, Nakato H. Drosophila glypicans regulate the germline stem cell niche. J Cell Biol. 2009;187:473–480. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JD, Anderson EL, Michelson MV, Zechel JL, Conrad PA, Page DC, Nadeau JH. Germ cell pluripotency, premature differentiation and susceptibility to testicular teratomas in mice. Development. 2012;139:1577–1586. doi: 10.1242/dev.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JD, Lam M-YJ, Michelson MV, Nadeau JH. Loss of the transmembrane but not the soluble kit ligand isoform increases testicular germ cell tumor susceptibility in mice. Cancer Res. 2008;68:5193–5197. doi: 10.1158/0008-5472.CAN-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JD, Michelson MV, Youngren KK, Lam MY, Nadeau JH. Deletion of eIF2beta suppresses testicular cancer incidence and causes recessive lethality in agouti-yellow mice. Hum Mol Genet. 2009;18:1395–1404. doi: 10.1093/hmg/ddp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JD, Nadeau JH. Testicular germ cell tumors in mice: new ways to study a genetically complex trait. Methods Mol Biol. 2008;450:211–231. doi: 10.1007/978-1-60327-214-8_15. [DOI] [PubMed] [Google Scholar]
- Horabin JI, Bopp D, Waterbury J, Schedl P. Selection and maintenance of sexual identity in the Drosophila germline. Genetics. 1995;141:1521–1535. doi: 10.1093/genetics/141.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AM, Cooley L. Methods for studying oogenesis. Methods. 2014;68:207–217. doi: 10.1016/j.ymeth.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino N, Pane A, Gaul U. miR-184 has multiple roles in Drosophila female germline development. Dev Cell. 2009;17:123–133. doi: 10.1016/j.devcel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Jin Z, Flynt AS, Lai EC. Drosophila piwi mutants exhibit germline stem cell tumors that are sustained by elevated Dpp signaling. Curr Biol. 2013;23:1442–1448. doi: 10.1016/j.cub.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A, Lindhardt Johansen M, Juul A, Skakkebaek NE, Main KM, Rajpert-De Meyts E. Pathogenesis of germ cell neoplasia in testicular dysgenesis and disorders of sex development. Semin Cell Dev Biol. 2015;45:124–137. doi: 10.1016/j.semcdb.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Nielsen JE, Almstrup K, Toft BG, Petersen BL, Rajpert-De Meyts E. Dysregulation of the mitosis-meiosis switch in testicular carcinoma in situ. J Pathol. 2013;229:588–598. doi: 10.1002/path.4154. [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Nielsen JE, Blomberg Jensen M, Graem N, Rajpert-De Meyts E. Analysis of meiosis regulators in human gonads: a sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol Hum Reprod. 2012;18:523–534. doi: 10.1093/molehr/gas030. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–187. doi: 10.1146/annurev-cellbio-101512-122353. [DOI] [PubMed] [Google Scholar]
- Kanetsky PA, Mitra N, Vardhanabhuti S, Li M, Vaughn DJ, Letrero R, Ciosek SL, Doody DR, Smith LM, Weaver J, et al. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat Genet. 2009;41:811–815. doi: 10.1038/ng.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsky PA, Mitra N, Vardhanabhuti S, Vaughn DJ, Li M, Ciosek SL, Letrero R, D’Andrea K, Vaddi M, Doody DR, et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet. 2011;20:3109–3117. doi: 10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareta MS, Gorges LL, Hafeez S, Benayoun BA, Marro S, Zmoos AFCMJ, Spacek D, Batista L, O’Brien M, Ng Y, et al. Inhibition of pluripotency networks by the Rb tumor suppressor restricts reprogramming and tumorigenesis. Cell Stem Cell. 2015;16:39–50. doi: 10.1016/j.stem.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–1700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- King RC, Burnett RG. Hereditary ovarian tumors in Drosophila melanogaster. Science. 1957;126:562. doi: 10.1126/science.126.3273.562. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Wang S, Xie T. Self-maintained escort cells form a germline stem cell differentiation niche. Development. 2011;138:5087–5097. doi: 10.1242/dev.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster R, Mitra N, D’Andrea K, Vardhanabhuti S, Chung CC, Wang Z, Erickson RL, Vaughn DJ, Litchfield K, Rahman N, et al. Pathway-based analysis of GWAS data identifies association of sex determination genes with susceptibility to testicular germ cell tumors. Hum Mol Genet. 2014;23:6061–6068. doi: 10.1093/hmg/ddu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, Skotheim RI, Abeler VM, Rajpert-De Meyts E, Lothe RA. Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr Rev. 2013;34:339–376. doi: 10.1210/er.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Han SS, Rosenberg PS, Berndt SI, Burdett L, Yeager M, Korde LA, Mai PL, Pfeiffer R, Greene MH. Variants in or near KITLG, BAK1, DMRT1, and TERT-CLPTM1L predispose to familial testicular germ cell tumour. J Med Genet. 2011;48:473–476. doi: 10.1136/jmedgenet-2011-100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Kim S, Cook MS, Capel B, Zhu R, Matin A, Sarver AL, Parker KL, Griswold MD, et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci USA. 2009;106:22323–22328. doi: 10.1073/pnas.0905431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol. 2011;356:63–70. doi: 10.1016/j.ydbio.2011.05.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Zhang T, Sarver AL, Jain S, Griswold MD, Bardwell VJ, Zarkower D. Interaction between DMRT1 function and genetic background modulates signaling and pluripotency to control tumor susceptibility in the fetal germ line. Dev Biol. 2013;377:67–78. doi: 10.1016/j.ydbio.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120:3197–3204. doi: 10.1242/dev.120.11.3197. [DOI] [PubMed] [Google Scholar]
- Lanza DG, Dawson EP, Rao P, Heaney JD. Misexpression of cyclin D1 in embryonic germ cells promotes testicular teratoma initiation. Cell Cycle. 2016:1–12. doi: 10.1080/15384101.2016.1149272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, DiNardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, DiNardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nature Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Minor NT, Park JK, Mckearin DM, Maines JZ. Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proceedings of the National Academy of Sciences. 2009;106:9304–9309. doi: 10.1073/pnas.0901452106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Q, Carreira-Rosario A, Maines JZ, McKearin DM, Buszczak M. mei-p26 cooperates with bam, bgcn and Sxl to promote early germline development in the Drosophila ovary. PLoS ONE. 2013;8:e58301. doi: 10.1371/journal.pone.0058301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield K, Holroyd A, Lloyd A, Broderick P, Nsengimana J, Eeles R, Easton DF, Dudakia D, Bishop DT, Reid A, et al. Identification of four new susceptibility loci for testicular germ cell tumour. Nature Commun. 2015;6:8690. doi: 10.1038/ncomms9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield K, Levy M, Huddart RA, Shipley J, Turnbull C. The genomic landscape of testicular germ cell tumours: from susceptibility to treatment. Nat Rev Urol. 2016;13:409–419. doi: 10.1038/nrurol.2016.107. [DOI] [PubMed] [Google Scholar]
- Liu M, Lim TM, Cai Y. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci Signal. 2010;3:57. doi: 10.1126/scisignal.2000740. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van DH, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- López-Onieva L, Fernández-Miñán A, González-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–540. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- Luo L, Wang H, Fan C, Sen Liu, Cai Y. Wnt ligands regulate Tkv expression to constrain Dpp activity in the Drosophila ovarian stem cell niche. J Cell Biol. 2015;209:595–608. doi: 10.1083/jcb.201409142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, de Cuevas M, Matunis EL. Chinmo is sufficient to induce male fate in somatic cells of the adult Drosophila ovary. Development. 2016;143:754–763. doi: 10.1242/dev.129627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnúsdóttir E, Gillich A, Grabole N, Surani MA. Combinatorial control of cell fate and reprogramming in the mammalian germline. Current Opinion in Genetics & Development. 2012;22:466–474. doi: 10.1016/j.gde.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Matin A, Collin GB, Asada Y, Varnum D, Nadeau JH. Susceptibility to testicular germ-cell tumours in a 129.MOLF-Chr 19 chromosome substitution strain. Nat Genet. 1999;23:237–240. doi: 10.1038/13874. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Toksoz D, Nishikawa S, Nishikawa S, Williams D, Zsebo K, Hogan BL. Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature. 1991;353:750–752. doi: 10.1038/353750a0. [DOI] [PubMed] [Google Scholar]
- Mckearin DM, Spradling AC. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- Mohler JD. Developmental genetics of the Drosophila egg. I. Identification of 59 sex-linked cistrons with maternal effects on embryonic development. Genetics. 1977;85:259–272. doi: 10.1093/genetics/85.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottier-Pavie VI, Palacios V, Eliazer S, Scoggin S, Buszczak M. The Wnt pathway limits BMP signaling outside of the germline stem cell niche in Drosophila ovaries. Dev Biol. 2016;417:50–62. doi: 10.1016/j.ydbio.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson VR, Heaney JD, Tesar PJ, Davidson NO, Nadeau JH. Transgenerational epigenetic effects of the Apobec1 cytidine deaminase deficiency on testicular germ cell tumor susceptibility and embryonic viability. Proc Natl Acad Sci USA. 2012;109:2766–2773. doi: 10.1073/pnas.1207169109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Noguchi M. A recessive mutation (ter) causing germ cell deficiency and a high incidence of congenital testicular teratomas in 129/Sv-ter mice. J Natl Cancer Inst. 1985;75:385–392. [PubMed] [Google Scholar]
- Noguchi T, Stevens LC. Primordial germ cell proliferation in fetal testes in mouse strains with high and low incidences of congenital testicular teratomas. J Natl Cancer Inst. 1982;69:907–913. [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92:577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Lavoie CA, Vef O, Gateff E, Mckearin DM. The Drosophila cystoblast differentiation factor, benign gonial cell neoplasm, is related to DExH-box proteins and interacts genetically with bag-of-marbles. Genetics. 2000;155:1809–1819. doi: 10.1093/genetics/155.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Mohler D, Engstrom L, Mahowald AP. X-linked female-sterile loci in Drosophila melanogaster. Genetics. 1986;113:695–712. doi: 10.1093/genetics/113.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter JN, Hooten AJ, Frazier AL, Ross JA. Associations between variants in KITLG, SPRY4, BAK1, and DMRT1 and pediatric germ cell tumors. Genes, Chromosomes & Cancer. 2012;51:266–271. doi: 10.1002/gcc.20951. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, McGlynn KA, Okamoto K, Jewett MAS, Bokemeyer C. Testicular germ cell tumours. Lancet. 2016;387:1762–1774. doi: 10.1016/S0140-6736(15)00991-5. [DOI] [PubMed] [Google Scholar]
- Rapley EA, Turnbull C, Olama Al AA, Dermitzakis ET, Linger R, Huddart RA, Renwick A, Hughes D, Hines S, Seal S, et al. A genome-wide association study of testicular germ cell tumor. Nat Genet. 2009;41:807–810. doi: 10.1038/ng.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- Ruark E, Seal S, McDonald H, Zhang F, Elliot A, Lau K, Perdeaux E, Rapley E, Eeles R, Peto J, et al. Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat Genet. 2013;45:686–689. doi: 10.1038/ng.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R, Kato Y, Saga Y. NANOS2 promotes male germ cell development independent of meiosis suppression. Dev Biol. 2014;385:32–40. doi: 10.1016/j.ydbio.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol. 2012;4:a008375. doi: 10.1101/cshperspect.a008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz HK. Sex determination in insects: a binary decision based on alternative splicing. Current Opinion in Genetics & Development. 2011;21:395–400. doi: 10.1016/j.gde.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz HK, Erickson JW. Sex determination in Drosophila: The view from the top. Fly. 2010;4:60–70. doi: 10.4161/fly.4.1.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemmer J, Araúzo-Bravo MJ, Haas N, Schäfer S, Weber SN, Becker A, Eckert D, Zimmer A, Nettersheim D, Schorle H. Transcription factor TFAP2C regulates major programs required for murine fetal germ cell maintenance and haploinsufficiency predisposes to teratomas in male mice. PLoS ONE. 2013;8:e71113–e71113. doi: 10.1371/journal.pone.0071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Kulnane L, Smolko AE, Salz HK. Maintenance of Drosophila germline stem cell sexual identity in oogenesis and tumorigenesis. Development. 2015;142:1073–1082. doi: 10.1242/dev.116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AM, Dalton S. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell. 2009;5:141–149. doi: 10.1016/j.stem.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina M, Lehmann R. Translational control in germline stem cell development. J Cell Biol. 2014;207:13–21. doi: 10.1083/jcb.201407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Sonne SB, Almstrup K, Dalgaard M, Juncker AS, Edsgard D, Ruban L, Harrison NJ, Schwager C, Abdollahi A, Huber PE, et al. Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res. 2009;69:5241–5250. doi: 10.1158/0008-5472.CAN-08-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harbor Perspectives in Biology. 2011;3:a002642. doi: 10.1101/cshperspect.a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab S, Heller A, Steinmann-Zwicky M. Somatic sex-determining signals act on XX germ cells in Drosophila embryos. Development. 1996;122:4065–4071. doi: 10.1242/dev.122.12.4065. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky M. Sxl in the germline of Drosophila: a target for somatic late induction. Dev Genet. 1994;15:265–274. doi: 10.1002/dvg.1020150308. [DOI] [PubMed] [Google Scholar]
- Stevens LC. Testicular teratomas in fetal mice. J Natl Cancer Inst. 1962;28:247–267. [PubMed] [Google Scholar]
- Stevens LC. Experimental produciton of testicular teratomas in mice. Proc Natl Acad Sci USA. 1964;52:654–661. doi: 10.1073/pnas.52.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LC. Origin of testicular teratomas from primordial germ cells in mice. J Natl Cancer Inst. 1967;38:549–552. [PubMed] [Google Scholar]
- Stevens LC, Hummel KP. A description of spontaneous congenital testicular teratomas in strain 129 mice. J Natl Cancer Inst. 1957;18:719–747. [PubMed] [Google Scholar]
- Stevens LC, Little CC. Spontaneous testicular teratomas in an inbred strain of mice. Proc Natl Acad Sci USA. 1954;40:1080–1087. doi: 10.1073/pnas.40.11.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Niimi Y, Shinmyozu K, Zhou Z, Kiso M, Saga Y. Dead end1 is an essential partner of NANOS2 for selective binding of target RNAs in male germ cell development. EMBO Rep. 2016;17:37–46. doi: 10.15252/embr.201540828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22:430–435. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A, Hijal S, Hilfiker A, Suter B. Identification of new X-chromosomal genes required for Drosophila oogenesis and novel roles for fs(1)Yb, brainiac and dunce. Genome Res. 2001;11:67–77. doi: 10.1101/gr.156001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastan OY, Maines JZ, Li Y, McKearin DM, Buszczak M. Drosophila ataxin 2-binding protein 1 marks an intermediate step in the molecular differentiation of female germline cysts. Development. 2010;137:3167–3176. doi: 10.1242/dev.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira FK, Sanchez CG, Hurd TR, Seifert JRK, Czech B, Preall JB, Hannon GJ, Lehmann R. ATP synthase promotes germ cell differentiation independent of oxidative phosphorylation. Nature Cell Biol. 2015;17:689–696. doi: 10.1038/ncb3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Turnbull C, Rapley EA, Seal S, Pernet D, Renwick A, Hughes D, Ricketts M, Linger R, Nsengimana J, Deloukas P, et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet. 2010;42:604–607. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Z, Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol. 2008;180:721–728. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pan L, Wang S, Zhou J, McDowell W, Park J, Haug J, Staehling K, Tang H, Xie T. Histone H3K9 trimethylase Eggless controls germline stem cell maintenance and differentiation. PLoS Genet. 2011;7:e1002426. doi: 10.1371/journal.pgen.1002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lin H. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science. 2004;303:2016–2019. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, van Doren M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature. 2005;436:563–567. doi: 10.1038/nature03849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Oliver B, Pauli D, Mahowald AP. Evidence for sex transformation of germline cells in ovarian tumor mutants of Drosophila. Dev Biol. 1994;161:318–320. doi: 10.1006/dbio.1994.1032. [DOI] [PubMed] [Google Scholar]
- Xia L, Jia S, Huang S, Wang H, Zhu Y, Mu Y, Kan L, Zheng W, Wu Di, Li X, et al. The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell. 2010;143:978–990. doi: 10.1016/j.cell.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xuan T, Xin T, He J, Tan J, Gao Y, Feng S, He L, Zhao G, Li M. dBre1/dSet1-dependent pathway for histone H3K4 trimethylation has essential roles in controlling the germline stem cell maintenance and germ cell differentiation in the Drosophila ovary. Dev Biol. 2013;379:167–181. doi: 10.1016/j.ydbio.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Yan D, Neumüller RA, Buckner M, Ayers K, Li H, Hu Y, Yang-Zhou D, Pan L, Wang X, Kelley C, et al. A regulatory network of Drosophila germline stem cell self-renewal. Dev Cell. 2014;28:459–473. doi: 10.1016/j.devcel.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q-E, Oatley JM. Spermatogonial stem cell functions in physiological and pathological conditions. Curr Top Dev Biol. 2014;107:235–267. doi: 10.1016/B978-0-12-416022-4.00009-3. [DOI] [PubMed] [Google Scholar]
- Yang SY, Baxter EM, van Doren M. Phf7 controls male sex determination in the Drosophila germline. Dev Cell. 2012;22:1041–1051. doi: 10.1016/j.devcel.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren KK, Coveney D, Peng X, Bhattacharya C, Schmidt LS, Nickerson ML, Lamb BT, Deng JM, Behringer RR, Capel B, et al. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435:360–364. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Heaney J, Nadeau JH, Ali S, Matin A. Deficiency of splicing factor 1 suppresses the occurrence of testicular germ cell tumors. Cancer Res. 2010;70:7264–7272. doi: 10.1158/0008-5472.CAN-10-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]