The American Society for Colposcopy and Cervical Pathology (ASCCP), in collaboration with the National Cancer Institute (NCI), has begun to prepare for the next update to its cervical screening and management consensus guidelines. Here, we describe the current plans for this multi-year process.
Since 2001, the ASCCP has sponsored several rounds of cervical consensus guidelines; each has made extensive use of epidemiologic data from clinical trials and epidemiologic studies from the NCI and other sources (1–6). The recommendations have been developed by expert representatives of many cooperating clinical societies after extensive public comment periods. Initially, the ASCCP-sponsored guidelines concentrated on the management of cytologic and histologic abnormalities found during screening. More recently, the ASCCP has cooperated with the American Cancer Society (ACS) and more than 20 other clinical organizations to consider general population screening issues as well (3). The next round of guidelines might continue to consider both cervical screening and management of abnormal results, pending discussion and in cooperation with other groups.
While it is undesirable to change clinical recommendations unless necessary, revision is in fact needed soon for several reasons. The introduction of human papillomavirus (HPV) testing into U.S. screening programs almost 15 years ago was a major change (7); initially it was not possible to judge how repeated rounds of screening would perform. There are now enough data to judge and guide the realistic clinical performance of multiple rounds of “cotesting” combining HPV testing with cytology. Second, young women vaccinated prophylactically against HPV have reached the age of screening, and HPV vaccination will increasingly and profoundly affect screening performance (8). Given the post-vaccination prevalence of precancer, cytology and pooled HPV tests perform less effectively (e.g., are less predictive of cervical precancer) in vaccinated populations than in unvaccinated populations. Also, new tests and strategies for triage of screen-positive women have been introduced and evaluated sufficiently to consider their role in new recommendations (9). Finally, the current guidelines are already very complex but still incomplete. As we strive for even greater precision to maximize the benefits and minimize the harms of screening, producing more algorithm trees is not a practical answer. Given available evidence, it is possible, as explained below, to push simultaneously toward greater precision and simplicity, supported by a computer-based decision tool. The risk database will be publicly available, permitting access to those wishing to use it to create such tools.
In preparing for the next round of guidelines, we recognize that several groups offer cervical screening and/or management recommendations, most prominently the US Preventive Services Task Force (USPSTF), ACS, and the American College of Obstetrics and Gynecology (ACOG). Consistency in recommendations, preferably unanimity regarding the important issues, is very important. Nonetheless, the ASCCP-sponsored guidelines provide a unique perspective in the following ways. The guidelines aim to be as comprehensive as possible, covering such a large number of specific clinical situations, such that reliance on data from the reference standard of evidence, randomized clinical trials addressing individual questions, are not conceivable for most recommendations. Less emphasis will be on formal review of individual published studies with grading of the published literature provided by a separate group of evaluators. Instead, observational “big data” as well as trial data, pooled from all available sources, will form the basis of the updated recommendations. Epidemiologic research targeted specifically to support the guidelines will be conducted and reviewed by cervical screening and management experts.
The most recent set of ASCCP-sponsored consensus guidelines introduced risk of CIN2, CIN3, AIS, and cancer as the unifying principle of the many recommendations and algorithm “trees” (10). Regardless of the screening test or algorithm (e.g., screening followed by triage), there should be equal management of equal risk. A prime example is the equal management of cytologic low-grade squamous intraepithelial lesion (LSIL) and HPV-positive atypical squamous cells of undetermined significance (ASC-US).
Anticipated improvements planned for the next set of ASCCP-sponsored guidelines will include: a) More detailed risk estimates, for greater numbers of test combinations, and for a greater number of clinical scenarios, such that the guidelines better address the variety of clinical situations commonly encountered; b) Greater simplicity of presentation, through recognition that the first goal of guidelines is to provide clear, scientifically justified recommendations; c) Fuller and more transparent consideration, in meetings and web-based discussions, of what level of reassurance against cancer is reasonably achievable given that no program can provide absolute reassurance; d) Broad stakeholder representation in setting the consensus risk thresholds (e.g., what level of risk merits colposcopic referral, and how soon should screen-negative women be rescreened); e) Greater communication with the clinical community during guideline development and afterward to improve clinical applicability and acceptance; and f) Detailed determination of the portability of risk-based recommendations to diverse clinical settings within the U.S. and internationally.
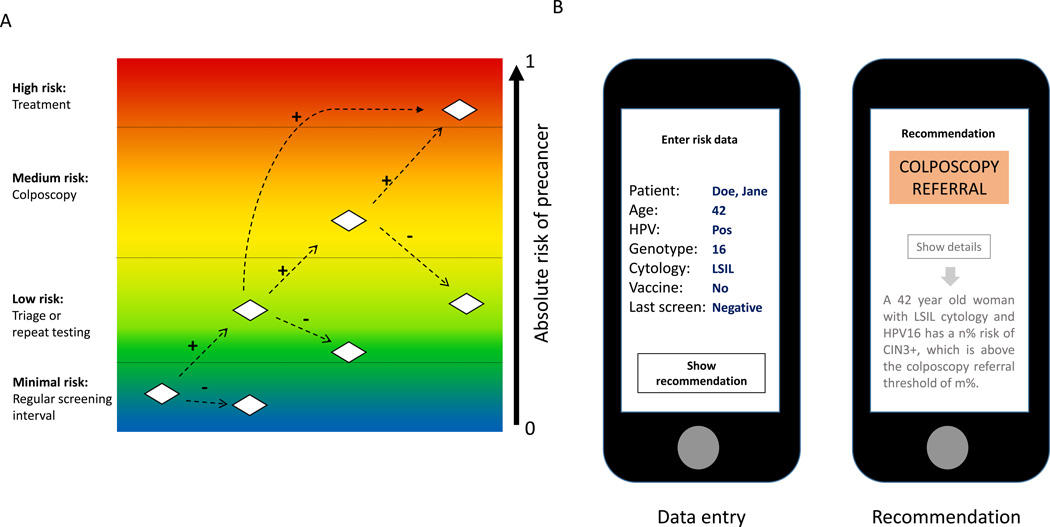
The planned operational format of the next round of guidelines, presented by an app, is illustrated in Figure 1. As currently conceived, the clinician or staff member will be prompted to enter into a smartphone, web-based, or electronic medical record application (“app”) a limited set of risk data for a given woman, including her screening test results and important modifying characteristics to be determined. This electronic format, replacing pages of algorithm trees, represents a multi-dimensional search tool. It maintains decision-making by the clinician and functions as a decision aid. The software will access the estimate of the patient’s risk of cervical cancer or precancer by accessing a database of risk estimates for the patient’s combination of screening test results and individual risk factors. This database will be derived from the analysis of the pooled data sets, and updated on an ongoing basis as new data becomes available.
Figure 1. Principle of risk-based screening and management for cervical cancer prevention.
A: There is a continuous range of absolute risk of cervical precancer that can be precisely estimated based on screening tests, triage tests, clinical diagnostics, vaccination status, prior screening results and possibly other co-factors. However, there are currently only 4 clinically relevant risk ranges that imply different management options. B: Example of a clinical-decision app on a smartphone. The provider enters important risk information and based on the data entered, risks are retrieved from the risk matrix and related to the clinical decision thresholds. A simple recommendation is given to the provider. If desired, the underlying risks and detailed rationale of the recommendation can be shown.
The primary output will be the recommended management for a woman of that risk level. The output will be very simple, unless the clinician wants to see the underlying complexity. It is critical to note that there is a limited set of management options that apply regardless of where the woman is in the screening process, i.e., whether she is undergoing general screening, follow-up or triage of an abnormal result, management in the colposcopy clinic, or follow-up post treatment. Depending on level of risk, the options in decreasing order of risk are to treat, perform colposcopy and biopsies, retest at an intensified shortened interval, re-screen at the standard interval, or discharge from screening.
After the recommended management is shown, the clinician will be able to choose, if desired, to see a display of the risk estimate leading to the recommendation. Part of the guideline development process will be to determine the risk range for which each management or follow-up option is appropriate. If even more detail is preferred, a note explaining the specific support for the recommendation will be presented.
Some recommendations will be based on stronger evidence and more data than others. The clinician will be alerted by the app when the data leading to the recommendation are scarce or judged to be otherwise sub-optimally reliable, and when the risk for the woman places her on the borderline of two possible management options.
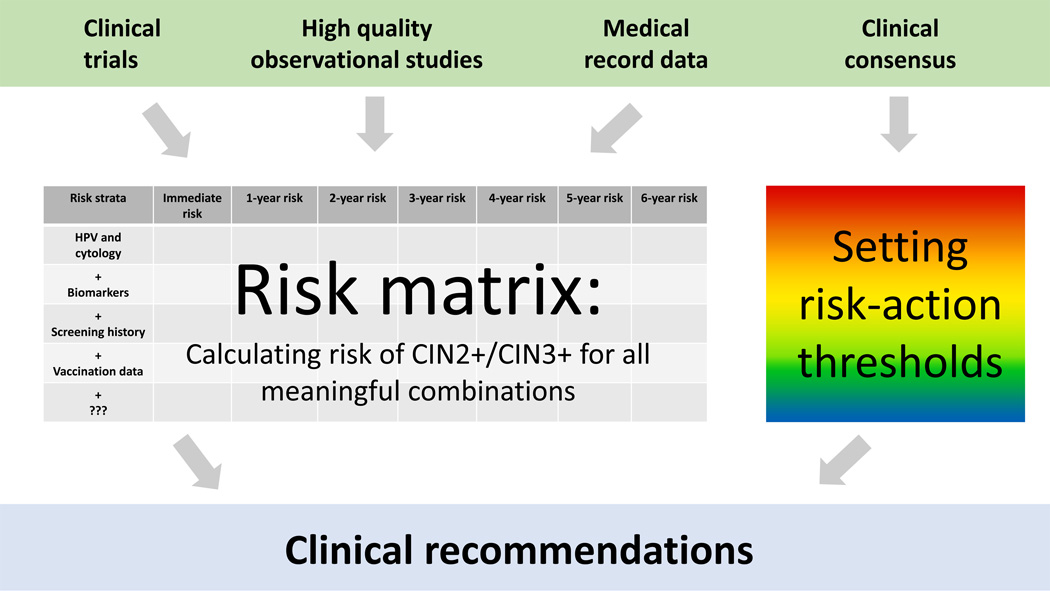
Figure 2 shows how the guidelines will be supported and decided, as planned by the collaborating researchers and clinicians. The steps in the cervical screening process include screening of the general population, triage or follow-up of positive results, colposcopy, post-colposcopy management when treatment is not indicated initially, and post-treatment management. Regardless of where in the process a woman is, a given risk should generally lead to a given clinical action. Nonetheless, for presentation, the guidelines will be divided into the settings of screening/triage, colposcopy clinic, and post-treatment.
Figure 2. Approach to developing new cervical cancer screening guidelines.
Risk information is compiled from various sources into a comprehensive risk matrix for all relevant combinations of screening tests, triage tests, clinical diagnostics, vaccination status, prior screening results and possibly other co-factors. In parallel, risk-action thresholds are set by clinical consensus committees. Together, the risk estimates and action thresholds are used to form the new management guidelines.
Several important decisions and extensive data analyses must be completed to arrive at the next round of guidelines. The steps include:
The choice of test data and other variables to include (i.e., which factors affect a woman’s risk, and how finely those data should be categorized to produce a risk estimate);
How to handle missing or incomplete information, e.g., if HPV testing and/or partial typing are not known, or past history is unknown;
Standardizing use of a proper statistical method to calculate risks from different data sources, which will include clinical databases requiring specialized statistical methods that handle “interval censoring” and loss to follow-up;
The calculation of risk of CIN2 or worse, CIN3 or worse, and cancer, at the time of the visit, and within 1, 2, 3, 4, 5, or 6 years as requested to permit detailed and flexible choice of appropriate clinical management.
The scientific review and publication of the risk calculations in peer-reviewed journals, including the Journal of Lower Genital Tract Disease in an open-access format permitting broad use and credibility;
An extensive, open discussion of risk-action thresholds that warrant each of the management options. This is not a scientific judgment, but rather involves risk tolerance, incorporating client and clinician preference;
Cooperation with other U.S. and international groups to share data, risk estimation approaches, and to assure mutual understanding of terms and classifications;
Adaptation of the guidelines in a relevant and useful manner to lower-resource settings.
In conclusion, the goal of the ASCCP-sponsored guidelines will be to develop recommendations that derive from a common, open-source epidemiologic database available to other guidelines groups internationally, but which take into account the specifics of U.S. cancer risk tolerance and resources. It is not yet known exactly how long the guidelines development process will take; we estimate a few years.
Subsequent changes can be handled by updates to the software of the apps, without disruption of the output seen by the clinicians. New tests can be incorporated as data regarding their performance are available. The pace and responsibility for continued improvement of the risk matrix is a matter for future consideration; what is clear is that it will be relatively simple to add recommendations for new methods and strategies, as adequate data become available. Because we will follow the principle of equal management of equal risks, and risk-action thresholds will be set, the introduction of new technology or protocols can be incorporated in software versions that are made available to app users in a standard manner. In other words, we recognize that guidelines improvement will be an ongoing, dynamic process, in line with ongoing improvement in cervical cancer prevention.
References
- 1.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis. 2015;19(2):91–96. doi: 10.1097/LGT.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 2.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 3.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ Conference AS-SC. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287(16):2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 5.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D, et al. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J Low Genit Tract Dis. 2007;11(4):223–239. doi: 10.1097/LGT.0b013e318159408b. [DOI] [PubMed] [Google Scholar]
- 6.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D, et al. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11(4):201–222. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 7.Schiffman M, Doorbar J, Wentzensen N, de Sanjose S, Fakhry C, Monk BJ, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 8.Wentzensen N, Arbyn M, Berkhof J, Bower M, Canfell K, Einstein M, et al. Eurogin 2016 Roadmap: How HPV knowledge is changing screening practice. Int J Cancer. 2016 doi: 10.1002/ijc.30579. [DOI] [PubMed] [Google Scholar]
- 9.Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol. 2016;76(Suppl 1):S49–S55. doi: 10.1016/j.jcv.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S28–S35. doi: 10.1097/LGT.0b013e318285423c. [DOI] [PMC free article] [PubMed] [Google Scholar]