ABSTRACT
Few data on breakthrough candidemia (BC), defined as candidemia that develops on administration of antifungal agents (AFAs), in allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients are available. The medical and microbiological records of recipients of an allo-HSCT obtained between December 2008 and December 2014 were reviewed. Of 768 allo-HSCT cases, 26 developed BC. Among the 26 causative strains, 22 strains were stored and identified by sequencing. The following species were isolated: Candida parapsilosis (9 strains), C. glabrata (4 strains), C. guilliermondii (3 strains), and other Candida species (6 strains). The AFAs being used when BC developed were micafungin (17 cases), liposomal amphotericin B (5 cases), itraconazole (2 cases), and voriconazole (2 cases). All 17 cases who developed BC during micafungin administration were administered 150 mg/day of micafungin. The susceptibilities of the causative Candida species to the administered AFAs when breakthrough occurred ranged from susceptible to resistant. Especially, 85% of the Candida species that caused BC during micafungin administration were susceptible to micafungin. Additionally, 75% of the strains were wild type for susceptibility to the administered AFAs when breakthrough occurred. Systemic steroid administration and a longer severe neutropenic phase (≥5 days) were independent risk factors for BC (P = 0.016 and P = 0.015, respectively). BC developed in allo-HSCT recipients even when they received a sufficient dose of AFA, including micafungin, to which the causative Candida species were susceptible and/or had wild-type susceptibility in vitro. Systemic steroid administration and a longer severe neutropenic phase were host-based factors associated with BC.
KEYWORDS: allogeneic hematopoietic stem cell transplantation, candidemia, breakthrough candidemia, micafungin, steroids
INTRODUCTION
Candidemia is one of the most common nosocomial bloodstream infections and is associated with high rates of morbidity and mortality (1, 2). When such an infection develops during systemic antifungal therapy, it is known as breakthrough candidemia (BC). The rate of BC among all patients with hematological malignancies and candidemia was previously found to range from 19% to 72% (3–5). According to the findings of some previous studies focused on BC, risk factors for BC are severe neutropenia, the use of corticosteroids, the presence of a central venous catheter (CVC), and resistance to antifungal agents (6–10). Furthermore, a recent multicenter study found that the causes of BC, particularly in patients with BC receiving azole or liposomal amphotericin B, might be associated with microbiological factors, such as resistance to antifungal agents, and/or host factors, such as neutropenia or the presence of a CVC (7). However, in the previous study, only two cases of BC were found among patients receiving an echinocandin (7). Thus, further investigations are needed to clarify the causes of BC in the era of new antifungal agents, such as echinocandins. Additionally, no study had been conducted with a cohort of patients receiving antifungal prophylaxis in which the patients who developed BC were directly compared to those who did not develop BC. Current treatment guidelines recommend antifungal prophylaxis for allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients (11–13). Therefore, we decided to conduct a study that focused on BC in a cohort of allo-HSCT recipients in order to clarify the characteristics and causes of BC.
RESULTS
Clinical presentations and characteristics of patients with breakthrough candidemia.
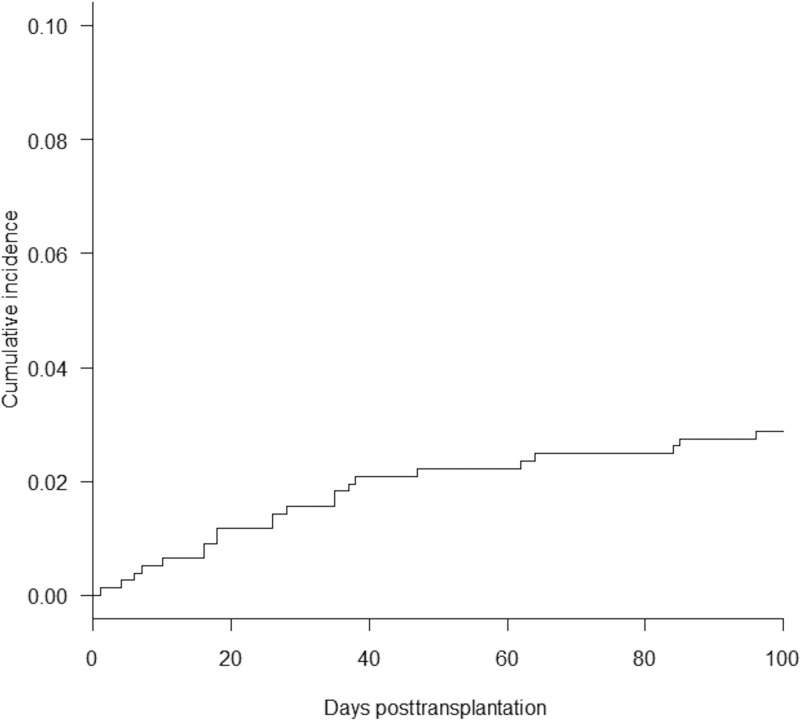
Of 768 patients receiving allo-HSCT, 26 experienced BC. There were no significant differences in the baseline characteristics between the recipients with BC and those without BC, except for the numbers of patients in each group with a longer severe neutropenic phase (LSNP) (P = 0.023) (Table 1). Micafungin was the major antifungal agent that was administered at day 0 (the first day of allo-HSCT) to the cohort (444/768 patients). The clinical presentation and characteristics of the 26 recipients with BC are shown in Table 2. The antifungal agents used when BC developed were micafungin (17 cases), liposomal amphotericin B (5 cases), itraconazole (2 cases), and voriconazole (2 cases). The median duration of prior antifungal exposure before the development of BC was 33 days (range, 4 to 137 days). The median duration of drug exposure before the development of BC in patients receiving micafungin (n = 17) was longer than that in patients receiving the other antifungal agents (n = 9) (34 days versus 22 days), but the difference was not statistically significant (P = 0.13). The median time of onset of BC after allo-HSCT was day 33 (33 days following allo-HSCT). In addition, 22 (85%) of all 26 allo-HCST recipients with BC developed the disease within 100 days following allo-HSCT. The cumulative incidence of BC up to day 100 following allo-HSCT was 2.9% (Fig. 1). Three of 15 recipients with BC whose fundus oculi were examined by ophthalmologists had endophthalmitis. The crude 30-day mortality rate was 38%. The serum (1,3)-beta-d-glucan (BDG) levels in 11 cases (11/26) were below the cutoff.
TABLE 1.
Clinical characteristics of each of the two groups in the allo-HSCT cohorta
| Characteristic | Result for the following cases: |
P value | ||
|---|---|---|---|---|
| Total (n = 768) | Cases with BC (n = 26) | Cases without BC (n = 742) | ||
| Median (range) age (yr) | 55 (16–74) | 53 (22–69) | 55 (16–74) | 0.41 |
| No. (%) male patients/no. (%) female patients | 488/280 | 18 (69)/8 (31) | 470 (63)/272 (37) | 0.68 |
| No. (%) of patients with the following diagnosis: | ||||
| AML | 406 | 15 (58) | 391 (53) | 0.69 |
| MDS | 64 | 1 (3.8) | 63 (8.5) | 0.72 |
| CML | 24 | 1 (3.8) | 23 (31) | 0.57 |
| ALL | 101 | 4 (15) | 97 (13) | 0.77 |
| HL | 14 | 0 | 14 (19) | 1 |
| NHL | 84 | 3 (12) | 81 (11) | 0.76 |
| ATLL | 18 | 0 | 18 (2.4) | 1 |
| SAA | 15 | 1 (3.8) | 14 (1.9) | 0.41 |
| MM | 5 | 0 | 5 (0.7) | 1 |
| Others | 37 | 1 (3.8) | 36 (4.9) | 1 |
| No. (%) of patients with underlying diabetes mellitus | 68 | 2 (7.7) | 66 (8.9) | 1 |
| No. (%) of patients with the following disease risk: | ||||
| Standard risk | 101 | 2 (7.7) | 99 (13) | 0.56 |
| High risk | 667 | 24 (92) | 643 (87) | |
| No. (%) of patients with the following conditioning: | ||||
| RIC | 258 | 9 (35) | 249 (34) | 1 |
| MAC | 510 | 17 (65) | 493 (66) | |
| No. (%) of patients treated with the following agent(s) for GVHD prophylaxis: | ||||
| TAC | 187 | 9 (35) | 178 (24) | 0.24 |
| TAC + MMF | 385 | 14 (54) | 371 (50) | 0.84 |
| TAC + sMTX | 125 | 1 (3.8) | 124 (17) | 0.1 |
| CsA + sMTX | 56 | 2 (7.7) | 54 (7.3) | 0.71 |
| Others | 15 | 0 | 15 (2) | 1 |
| No. (%) of patients with the following transplant type: | ||||
| CBT | 549 | 21 (81) | 528 (71) | 0.38 |
| rPBSCT | 76 | 1 (3.8) | 75 (10) | 0.5 |
| uBMT | 143 | 4 (15) | 139 (19) | 0.8 |
| No. (%) of patients with the following HLA disparity: | ||||
| Matched | 178 | 4 (15) | 174 (23) | 0.48 |
| Mismatched | 590 | 22 (85) | 568 (77) | |
| No. (%) of patients with pretransplant status of LSNP until day 0 of ≥5 days | 276 | 15 (58) | 261 (35) | 0.023 |
| No. (%) of patients treated with the following antifungal agent at day 0: | ||||
| MCFG | 444 | 16 (62) | 428 (52) | 0.84 |
| VRC | 164 | 4 (15) | 160 (22) | 0.63 |
| ITC | 82 | 3 (12) | 79 (11) | 0.75 |
| LAMB | 73 | 3 (12) | 70 (9.4) | 0.73 |
| Others | 5 | 0 | 5 (0.7) | 1 |
allo-HSCT, allogeneic hematopoietic stem cell transplantation; BC, breakthrough candidemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; ALL, acute lymphoblastic leukemia; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; ATLL, adult T cell leukemia/lymphoma; SAA, severe aplastic anemia; MM, multiple myeloma; RIC, reduced-intensity conditioning; MAC, myeloablative conditioning; GVHD, graft-versus-host disease; TAC, tacrolimus; MMF, mycophenolate mofetil; sMTX, short-term methotrexate; CsA, cyclosporine; CBT, cord blood transplantation; rPBSCT, related peripheral blood stem cell transplantation; uBMT, unrelated bone marrow transplantation; LSNP, longer severe neutropenic phase until day 0, defined as severe neutropenic duration until day 0 (the first day of transplantation) of ≥5 days; MCFG, micafungin; VRC, voriconazole; ITC, itraconazole; LAMB, liposomal amphotericin B.
TABLE 2.
Clinical presentation and characteristics of the 26 recipients with BCa
| Characteristic | Result |
|---|---|
| Median (range) body temp (°C) | 38.2 (36.7–40.2) |
| Median (range) time to BC onset following allo-HSCT (days) | 33 (2–242) |
| Median (range) duration of prior antifungal exposureb (days) | 33 (4–137) |
| No. (%) of patients with the following characteristicsc: | |
| Antifungal agents used when BC developedd | |
| Micafungin | 17 |
| Liposomal amphotericin B | 5 |
| Itraconazole | 2 |
| Voriconazole | 2 |
| BC onset within 100 days following allo-HSCT | 22 (85) |
| BC onset before engraftment (neutrophil count, <500/μl) | 8 (31) |
| Septic shock at BC onset | 5 (19) |
| Systemic steroid (PSL at ≥20 mg/day) administration | 17 (65) |
| aGVHD of grade 2 or greater at BC onset | 11 (42) |
| Catheter-related candidemia | 9/22 (41) |
| Endophthalmitis | 3/15 (20) |
| BDG concn of <11 pg/ml (cutoff) | 11 (42) |
| Death within 30 days after BC onset | 10 (38) |
BC, breakthrough candidemia; allo-HSCT, allogeneic hematopoietic stem cell transplantation; PSL, prednisone; aGVHD; acute graft-versus-host disease.
The duration of prior antifungal exposure is the duration of antifungal agent administration when breakthrough candidemia developed.
Data are for the 26 patients with BC.
The distribution of the antifungal agents used when BC developed was different from that of the antifungal agents used at day 0 (Table 1) because for some patients the clinicians changed the antifungal agents that were used during transplantation.
FIG 1.
Cumulative incidence of breakthrough candidemia.
Microbiological characteristics of breakthrough candidemia. (i) Breakthrough candidemia during micafungin therapy.
All 17 recipients who developed BC during micafungin therapy received micafungin at 150 mg/day. Fourteen of the 17 Candida strains that caused BC during micafungin therapy were stored and analyzed (Table 3). The causative Candida strains identified by sequencing were Candida parapsilosis (7 strains), C. glabrata (2 strains), C. guilliermondii (2 strains), C. albicans (1 strain), C. krusei (1 strain), and C. fermentati (1 strain). The sites of infection of these strains were CVCs (7 cases) and unknown (7 cases). The MICs of micafungin for 14 strains ranged from 0.015 to 4 μg/ml. C. fermentati has no established breakpoint, and no epidemiological cutoff value (ECV) for C. fermentati has been established for micafungin. Among the 13 strains for which an ECV was established, 10 strains were wild type for susceptibility to micafungin (Table 3). Two strains of C. glabrata were not susceptible to micafungin. The rest of the causative Candida strains for which a breakpoint was established (85%, 11/13) were susceptible to micafungin in vitro.
TABLE 3.
Microbiological characteristics of BCa
| Treatment when BC occurred | Patient no. | Pathogen | Pathogen identified by Vitek system | Dose | Presence of neutropenia | Steroid administration | Presence of a CVC | CVC culture result | Focus | MIC (μg/ml) of treatment drug when BC occurred | Evaluation | eECV | MIC (μg/ml) |
Therapeutic agent | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCFG | AMB | FLC | ITC | VRC | PSC | |||||||||||||||
| Micafungin | 1 | C. glabrata | C. glabrata | 150 mg/day | + | − | + | Negative | Unknown | 0.12 | I | nWT | 1 | 64 | 2 | 1 | 2 | LAMB | Alive | |
| 2 | C. glabrata | C. glabrata | 150 mg/day | − | + | + | Negative | Unknown | 4 | R | nWT | 2 | 16 | 4 | 1 | 2 | LAMB | Deceased | ||
| 3 | C. guilliermondii | C. guilliermondii | 150 mg/day | − | − | + | Positive | CRBSI | 0.25 | S | WT | 0.5 | 4 | 1 | 0.25 | 0.5 | VRC | Alive | ||
| 4 | C. guilliermondii | C. guilliermondii | 150 mg/day | + | + | + | Negative | Unknown | 0.5 | S | WT | 0.5 | 16 | 1 | 0.5 | 0.5 | LAMB | Deceased | ||
| 5 | C. parapsilosis | C. parapsilosis | 150 mg/day | − | + | + | Positive | CRBSI | 0.5 | S | WT | 1 | 0.5 | 0.12 | 0.015 | 0.25 | VRC | Deceased | ||
| 6 | C. parapsilosis | C. parapsilosis | 150 mg/day | − | − | + | Positive | CRBSI | 0.5 | S | WT | 1 | 0.25 | 0.03 | 0.015 | 0.03 | LAMB | Alive | ||
| 7 | C. parapsilosis | C. parapsilosis | 150 mg/day | − | + | + | Positive | CRBSI | 0.25–0.5 | S | WT | 1 | 1 | 0.25 | 0.03 | NA | VRC | Deceased | ||
| 8 | C. parapsilosis | C. parapsilosis | 150 mg/day | − | + | + | Negative | Unknown | 1 | S | WT | 1 | 0.5 | 0.12–0.25 | 0.015–0.03 | NA | LAMB | Alive | ||
| 9 | C. parapsilosis | C. parapsilosis | 150 mg/day | − | + | + | Positive | CRBSI | 0.5 | S | WT | 1 | 0.25–0.5 | 0.06 | 0.015–0.03 | NA | LAMB | Alive | ||
| 10 | C. parapsilosis | C. parapsilosis | 150 mg/day | − | + | + | Positive | CRBSI | 0.5 | S | WT | 1 | 0.25–0.5 | 0.06 | 0.15 | NA | LAMB | Deceased | ||
| 11 | C. parapsilosis | C. parapsilosis | 150 mg/day | + | + | + | Negative | Unknown | 0.5 | S | WT | 0.5 | 0.5–1 | 0.06–0.12 | 0.015–0.03 | NA | LAMB | Alive | ||
| 12 | C. albicans | C. parapsilosis | 150 mg/day | − | + | + | Positive | CRBSI | 0.015 | S | WT | 1 | >64 | >8 | >8 | >8 | VRC | Alive | ||
| 13 | C. krusei | Candida species | 150 mg/day | − | − | + | Negative | Unknown | 0.25 | S | nWT | 2 | 16–32 | 0.5 | 0.25–0.5 | NA | LAMB | Alive | ||
| 14 | C. fermentati | Candida species | 150 mg/day | + | − | + | Negative | Unknown | 1 | No BP | No ECV | 1 | 0.5 | 0.25 | 0.03 | 0.12 | LAMB | Alive | ||
| Liposomal amphotericin B | 15 | C. glabrata | C. glabrata | 2.5 mg/kg/day | − | + | + | NA | Unknown | 2 | No BP | WT | 0.03 | 8 | 2 | 0.25 | 2 | No change | Deceased | |
| 16 | C. glabrata | C. glabrata | 2.5 mg/kg/day | + | − | + | Negative | Unknown | 2 | No BP | WT | 0.015 | 2 | 1 | 0.12 | 1 | MCFG | Alive | ||
| 17 | C. guilliermondii | C. guilliermondii | 3 mg/kg/day | − | + | + | Negative | Unknown | 0.5 | No BP | WT | 0.25 | >64 | >8 | >8 | 2 | VRC | Deceased | ||
| 18 | C. parapsilosis | C. parapsilosis | 2.5 mg/kg/day | − | + | + | Positive | CRBSI | 1 | No BP | WT | 1 | 0.5 | 0.25 | 0.03 | 0.12 | No change | Deceased | ||
| 19 | C. fermentati | Candida species | 2 mg/kg/day | + | − | − | NA | Unknown | 0.5 | No BP | No ECV | 0.12 | 2 | 0.25–0.5 | 0.06–0.12 | NA | VRC | Deceased | ||
| Itraconazole | 20 | C. parapsilosis | Candida species | 200 mg/day (oral) | − | + | + | Positive | CRBSI | 0.25 | No BP | WT | 2 | 1 | 8 | 0.12 | 0.25 | LAMB | Alive | |
| Voriconazole | 21 | C. albicans | C. albicans | 400 mg/day (oral) | − | + | + | Negative | Peritonitis | >8 | R | nWT | 2 | 1 | >64 | >8 | >8 | LAMB | Deceased | |
| 22 | C. tropicalis | C. tropicalis | 200 mg/day (oral) | + | − | + | Negative | Unknown | >8 | R | nWT | 0.03 | 2 | >64 | 8 | 8 | LAMB | Alive | ||
BC, breakthrough candidemia; CVC, central venous catheter; NA, not available; CRBSI, catheter-related bloodstream infection; eECV, evaluation by determining the epidemiological cutoff value; S, susceptible; I, intermediate; R, resistant; No BP, no breakpoint was available; WT, wild type; nWT, non-wild type; MCFG, micafungin; AMB, amphotericin B; LAMB, liposomal amphotericin B; FLC, fluconazole; ITC, itraconazole; VRC, voriconazole; PSC, posaconazole; Pathogen, the Candida species identified by sequencing; Neutropenia, absolute neutrophil count of <500 cells/μl; Steroid, oral or intravenous prednisone at ≥20 mg/day; Focus, the site of the infection caused by the Candida strain; Evaluation, results of drug susceptibility for the agent that was administered when breakthrough candidemia developed in reference to the established breakpoints; Therapeutic agent, the therapeutic agent used after the appearance of BC; Outcome, patient status 30 days after the onset of breakthrough candidemia. The result of peripheral vein catheter tip culture for recipient 19 was negative. Therapeutic drug monitoring for itraconazole was not performed at the Toranomon Hospital during the study period. The trough levels of voriconazole for recipients 21 and 22 were 4.2 mg/liter and 1.2 mg/liter, respectively. The dose of voriconazole for recipient 22 was decreased from 300 mg/day to 200 mg/day 1 month before the patient received allo-HSCT because the trough level was 4.8 mg/liter at that time. Antifungal susceptibility testing for posaconazole was not performed for strains that were isolated between December 2012 and December 2014.
(ii) Breakthrough candidemia during liposomal amphotericin B therapy.
All five recipients who developed BC during liposomal amphotericin B therapy received liposomal amphotericin B at doses ranging from 2 to 3 mg/kg of body weight per day. A catheter tip culture was performed for three of the four recipients. Only one of the three recipients had catheter-related candidemia. All five causative Candida strains were stored and analyzed (Table 3). The causative Candida strains identified by sequencing were C. glabrata (2 strains), C. guilliermondii (1 strain), C. parapsilosis (1 strain), and C. fermentati (1 strain). The sites of infection with these five strains were a CVC (1 strain) and unknown (4 strains). The MICs of amphotericin B for the five Candida strains ranged from 0.5 to 2 μg/ml. No ECV for C. fermentati has been established for amphotericin B. The rest of the 4 strains had wild-type susceptibility to amphotericin B.
(iii) Breakthrough candidemia during azole therapy.
Among the four recipients who developed BC during azole therapy, two received oral itraconazole (200 mg/day) and the other two received oral voriconazole (200 mg/day or 400 mg/day). Three of the four causative Candida strains were stored and analyzed (Table 3). The causative Candida strains identified by sequencing were C. albicans (1 strain), C. parapsilosis (1 strain), and C. tropicalis (1 strain). The sites of infection with these strains were a CVC (1 strain), the intra-abdominal region (1 strain), and unknown (1 strain). The MIC of itraconazole for one strain that caused BC during itraconazole treatment was 0.25 μg/ml. Thus, the strain had wild-type susceptibility to itraconazole. The two strains that caused BC during voriconazole administration were resistant and non-wild type for susceptibility to voriconazole in vitro.
Analysis of risk factors for breakthrough candidemia that developed within 100 days following allo-HSCT.
The results from the Cox model are shown in Table 4. Systemic steroid administration and the LSNP were identified to be risk factors for BC among allo-HSCT recipients in the univariate analysis (P = 0.01 and P = 0.01, respectively) and in the multivariate analysis (P = 0.016 and P = 0.015, respectively). In contrast, micafungin administration at day 0 was not a risk factor when the administration of micafungin was compared to the administration of the other antifungal agents. Additionally, engraftment within the 30 days following allo-HSCT did not decrease the risk significantly.
TABLE 4.
Analysis of risk factors for BCa
| Analysis type and risk factor | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Univariate analysis | |||
| Pretransplant factors | |||
| Male sex | 1.63 | 0.64–4.17 | 0.31 |
| Age ≥ 60 yr | 0.91 | 0.37–2.24 | 0.84 |
| Diagnosis | |||
| Myeloid malignancy | 1.73 | 0.40–7.41 | 0.46 |
| Nonmyeloid malignancy | 1 (reference) | ||
| Underlying diabetes mellitus | 1.08 | 0.25–4.64 | 0.91 |
| Disease risk | |||
| High risk | 1.7 | 0.40–7.41 | 0.46 |
| Standard risk | 1 (reference) | ||
| Transplant type | |||
| CBT | 2.01 | 0.68–5.93 | 0.21 |
| Non-CBT | 1 (reference) | ||
| Pretransplant status (until day 0) | |||
| LSNP (≥5 days) | 2.97 | 1.27–6.96 | 0.01 |
| Longer AMA exposure (≥30 days) | 1.9 | 0.82–4.39 | 0.13 |
| Longer AFA exposure (≥60 days) | 1.4 | 0.57–3.44 | 0.46 |
| PH-allo-HSCT | 1.63 | 0.64–4.16 | 0.31 |
| RIC | 1.27 | 0.53–3.03 | 0.59 |
| Antimicrobial regimen at day 0 | |||
| Quinolone based | 0.49 | 0.20–1.17 | 0.11 |
| APBL based | 1 (reference) | ||
| Antifungal regimen at day 0 | |||
| Micafungin | 1.44 | 0.59–3.5 | 0.42 |
| Other antifungal agents | 1 (reference) | ||
| Time-dependent factors | |||
| Systemic steroid administration | 3.83 | 1.31–11.1 | 0.01 |
| Engraftment within 30 days | 0.54 | 0.16–1.84 | 0.32 |
| aGVHD of grade 2 or greater | 0.99 | 0.35–2.79 | 0.99 |
| aGVHD of less than grade 2 | 1 (reference) | ||
| Diarrhea | 1.87 | 0.68–5.12 | 0.22 |
| Episode of antimicrobial agent change | 1.91 | 0.35–10.5 | 0.46 |
| Episode of antifungal agent change | 0.93 | 0.35–2.51 | 0.89 |
| Multivariate analysis | |||
| Systemic steroid administration | 3.65 | 1.27–10.5 | 0.016 |
| LSNP (≥5 days) | 2.87 | 1.23–6.73 | 0.015 |
| Longer AMA exposure (≥30days) | 1.41 | 0.59–3.37 | 0.44 |
| Quinolone based | 0.73 | 0.29–1.84 | 0.5 |
CBT, cord blood transplantation; AMA, antimicrobial agent; AFA, antifungal agent; PH-allo-HSCT, history of allogeneic hematopoietic stem cell transplantation; RIC, reduced-intensity conditioning; APBL, antipseudomonal beta-lactam; aGVHD, acute graft-versus-host disease. Myeloid malignancy consists of acute myeloid leukemia, myelodysplastic syndrome, myelodysplastic syndrome with overt acute myeloid leukemia, and chronic myelogenous leukemia. The longer severe neutropenic phase until day 0 (LSNP) was defined as a severe neutropenic duration until day 0 (the first day of transplantation) of ≥5 days. Longer antimicrobial agent exposure means that the recipient received any antimicrobial agent continuously for ≥30 days until day 0 (the first day of transplantation). Longer antifungal agent exposure means that the recipient received any antifungal agent continuously for ≥60 days until day 0 (the first day of transplantation). Recipients with a history of allogeneic hematopoietic stem cell transplantation had received allogeneic hematopoietic stem cell transplantation 2 or more times. Diarrhea was defined as an increase in the number of stools to ≥7 stools per day over the baseline number during allo-HSCT. An episode of antimicrobial agent change means that the antimicrobial regimen that was administered at day 0 was changed to another antimicrobial regimen after the start of allo-HSCT; e.g., a quinolone-based regimen at day 0 was changed to an antipseudomonal beta-lactam-based regimen, an antipseudomonal beta-lactam-based regimen (e.g., a cefepime-based regimen) at day 0 was changed to another antipseudomonal beta-lactam-based regimen (e.g., a meropenem-based regimen), an anti-methicillin-resistant Staphylococcus aureus agent was added to the regimen, and/or amikacin was added to the regimen. An episode of an antifungal agent change means that the antifungal regimen that was administered at day 0 was changed to another antifungal regimen after the start of allo-HSCT.
DISCUSSION
Almost all transplant recipients received antifungal agents at more than the minimum dose recommended for prophylaxis according to current treatment guidelines (11, 12). The incidence of BC in our study was predicted to be higher than that among allo-HSCT recipients found in previous studies (14, 15). The reason for this difference was probably a local factor at the Toranomon Hospital. In fact, there was considerable variability in the incidence of BC across the institutions (range for the various sites, 0.9% to 13.2%), although the overall incidence of invasive fungal infections was 3.4% in a nationwide multicenter prospective study in the United States (16).
In our study, the major antifungal agent that was administered when BC developed was micafungin. However, micafungin treatment was not a risk factor for BC (Table 4). In the present study, all 17 recipients who developed BC during micafungin administration received 150 mg/day micafungin. In general, in current treatment guidelines, a lower dose of micafungin (50 mg/day) has been approved for use for prophylactic administration (11, 12). This is probably why there are only a few studies on the development of BC when a sufficient dose of micafungin (≥100 mg/day) is provided (9, 17). Among the 13 strains that caused BC during micafungin treatment and for which a breakpoint was established and antifungal susceptibility testing was performed, 11 strains were susceptible to micafungin in vitro (85%). A previous study evaluated the development of BC in patients treated with sufficient doses of micafungin according to the previous CLSI breakpoint (17). According to the drug susceptibility results of that study, 2 of the 13 strains causing BC during treatment with 100 mg/day of micafungin (15%) were found to be susceptible by use of the current CLSI M27-S4 breakpoint (17, 18). In this context, BC could develop in allo-HSCT recipients who were administered a sufficient dose of micafungin, even though the causative Candida species were susceptible to micafungin in vitro according to the current CLSI guideline (18). In a recent study from Greece that described BC, BC could develop in antifungal agent-treated allo-HSCT recipients, even though the causative Candida species was susceptible to that agent in vitro (7). The study found that the MICs of the antifungal agents, with the exception of the MIC of echinocandin, were not predictive of the development of BC because most cases of BC developed while the patients were receiving liposomal amphotericin B or an azole (7). Similar results were obtained in our study (Table 3). To our knowledge, this is the first study to show that the current breakpoint for micafungin established by CLSI does not predict the development of BC in patients receiving 150 mg/day of micafungin in the allo-HSCT setting. Additionally, 75% of the causative strains (15/20 strains for which an ECV was established) in our study were wild type for susceptibility to the administered antifungal agents when BC occurred (Table 3). This result indicated that the mechanisms of resistance to the antifungal agents were not often associated with the cause of BC, because the wild type was considered a Candida strain that had no mutational or acquired mechanism of resistance (19). Accordingly, the causes of BC are probably host based and/or pathogen based.
In a previous report, host-based factors were often considered more important than susceptibility test results in determining the clinical outcome of fungal infections (20). Even the Candida strains which were susceptible to the therapeutic antifungal agents did not always respond to those agents (20). Also, in the present study, host-based factors might have been a stronger cause of BC than pathogen-based factors, according to the susceptibility results and evaluation of the ECV. In the multivariate analysis performed in the present study, systemic steroid administration and the LSNP were identified to be independent risk factors for BC. The following risk factors for BC have been reported in previous studies: severe neutropenia, use of corticosteroids, the presence of a CVC, and resistance to antifungal agents (6–10). However, in those studies they were identified to be risk factors on the basis of a comparison of cases with BC to cases without BC (de novo candidemia) (6–10). To our knowledge, no studies identified risk factors for BC by comparing cases with BC to cases without BC in a cohort in which all cases had received antifungal agents. Therefore, this is the first study to show that systemic steroid administration and the LSNP are significant independent risk factors for BC in an allo-HSCT cohort in which all the recipients had received antifungal prophylaxis. Thus, the two risk factors should probably be included in the host-based factors that are associated with BC, at least among allo-HSCT recipients. The therapeutic outcome of candidemia worsens according to host-based factors, such as steroid administration and neutropenia (3, 21), because these factors could inhibit the immune function of the host. Also, the efficacy of antifungal prophylaxis was probably reduced by systemic steroid administration and the LSNP due to similar mechanisms. That was probably why the two factors were identified to be the risk factors for BC, even though the Candida species causing BC were susceptible to the antifungal agents in vitro.
Our study had some limitations. First, it was a single-center retrospective study. A prospective multicenter study should be performed to identify the causes of and exact risk factors for BC. Second, testing of susceptibility to antifungal agents and sequencing-based identification could not be performed with all 26 Candida strains because 4 of the 26 strains were lost. Third, it was difficult to determine if the point mutations in FKS1 and/or FKS2 were associated with the cause of BC during micafungin administration, although the reduced susceptibility of Candida to echinocandins has been associated with point mutations in these genes (22). In our study, the causative Candida species were not examined for these point mutations. The ECVs were used instead of performing the examination that could detect the mutations in our study. Fourth, other host-based factors that could have been associated with BC were not identified in our study. Although systemic steroid administration was identified to be a risk factor, multiple factors could have been responsible for the deteriorated status of the patients, such as acute graft-versus-host disease (aGVHD), a preengraftment immune reaction, or idiopathic pneumonia syndrome, and might have been the true risk factors for BC. A systemic steroid is commonly administered to treat patients with these factors in allo-HSCT settings. Fifth, one suitable variable that reflected the intensity and duration of neutropenia between the pretransplant phase and the posttransplant phase could not be chosen. Thus, the LSNP and neutrophil engraftment were chosen as substitute variables that reflect them during the pretransplant phase and the posttransplant phase, respectively (Table 4). Especially, the LSNP was probably associated with BC that occurred during the early phase of allo-HSCT, because the LSNP was identified to be a risk factor for preengraftment BC (n = 8) that occurred before neutrophil engraftment was achieved (hazard ratio, 3.55; 95% confidence interval, 1.67 to 110.1; P = 0.015). Sixth, CVCs could not be included in the risk factor analysis because the exact times of CVC removal were lacking.
In summary, BC could develop in allo-HSCT recipients even when they received a sufficient dose of antifungal agents, including micafungin, to which the causative Candida species were susceptible and/or had wild-type susceptibility in vitro. Host-based factors, such as systemic steroid administration and the LSNP, were associated with the cause of BC among allo-HSCT recipients.
MATERIALS AND METHODS
A retrospective analysis of BC among allo-HSCT recipients (age, ≥16 years) was conducted between December 2008 and December 2014 at Toranomon Hospital (890 beds; Tokyo, Japan). The medical and microbiological records of the recipients evaluated during the study period were reviewed. CVCs were inserted into all recipients before the start of conditioning therapy. All recipients received antifungal prophylaxis from at least day −7 (7 days before allo-HSCT) up to day 100 (100 days after allo-HSCT), and some recipients received antifungal prophylaxis for a longer duration. Data on the antifungal prophylaxis used before allo-HSCT were limited. During transplantation, routine anti-infective prophylaxis was also provided by the use of broad-spectrum antibiotics (oral fluoroquinolone prophylaxis or intravenous antipseudomonal beta-lactam prophylaxis) and acyclovir. Prophylaxis for Pneumocystis jirovecii pneumonia was provided by the administration of trimethoprim-sulfamethoxazole from day −7 to day −1 and after the engraftment. Recipients were screened weekly for cytomegalovirus (CMV), and antiviral therapy (ganciclovir or foscarnet) was started on the detection of CMV antigenemia. This study was approved by the Institutional Review Board (IRB) of Toranomon Hospital.
Definitions.
Candidemia was defined as the isolation of any Candida species from ≥1 sets of cultures of blood from a patient who had signs and symptoms of infections. BC was defined as candidemia in patients receiving systemic antifungal agents for ≥3 days before the first positive blood culture (7). A second episode of candidemia observed in the same patient within 4 weeks of the first episode was considered the same episode (23, 24). Neutropenia and severe neutropenia were defined as absolute neutrophil counts (ANCs) of ≤500 cells/μl and ≤100 cells/μl, respectively. Neutrophil engraftment was defined as the first three consecutive days with an ANC of ≥500 cells/μl. A longer severe neutropenic phase (LSNP) (ANC, ≤100 cells/μl) until day 0 means that the severe neutropenic duration until day 0 (the first day of transplantation) was ≥5 days. Systemic steroid administration was defined as at least a single dose of an oral or intravenous steroid, which included prednisone at ≥20 mg/day during allo-HSCT. Steroid doses were converted from hydrocortisone to prednisone equivalents and from methylprednisolone to prednisone equivalents using the following conversion: 4 mg of hydrocortisone was equal to 1 mg of prednisone, and 0.80 mg of methylprednisolone was equal to 1 mg of prednisone (25). Diarrhea was defined as an increase in the number of stools to ≥7 stools per day over the baseline number during allo-HSCT. Septic shock was defined as the persistence of sepsis-induced hypotension despite adequate fluid resuscitation or a requirement for vasopressor agents (26). Catheter-related candidemia was diagnosed when there was no other apparent source of infection and the Candida strain was isolated from both peripheral blood and a catheter tip culture. The duration of antifungal exposure was defined as the duration over which recipients with BC received the antifungal agents that were administered when BC developed. Conditioning regimens were classified on the basis of a report by the Center for International Blood and Marrow Transplant Research (27). For disease status, all hematological disorders were defined as either standard risk or high risk (28). Acute graft-versus-host diseases (aGVHD) was defined and graded by the use of standard criteria (29).
Identifications.
Blood culture samples were processed using Bactec 9240 (between December 2008 and December 2014) and Bactec FX (between June 2010 and December 2014) systems (Becton, Dickinson and Company, Sparks, MD, USA). All breakthrough Candida isolates were recovered on Sabouraud dextrose agar (Nippon Becton Dickinson Company, Ltd., Japan) at 35°C and were identified to the species level by using a Vitek or Vitek 2 system (bioMérieux, Marcy l'Etoile, France) for all germ tube-negative Candida yeasts in the Toranomon Hospital. The internal transcribed spacer (ITS) region and D1/D2 region of the rRNA gene of the isolates were sequenced to provide further support (30, 31). Sequencing of the stored Candida strains was performed at the National Institute of Infectious Diseases.
Antifungal agent prophylaxis and treatment strategy.
Clinicians could choose any of the antifungal agents, including itraconazole (oral solution), voriconazole, liposomal amphotericin B, or micafungin, for prophylaxis. Although 50 mg/day micafungin is recommended for use for prophylaxis in HSCT settings (11, 12, 32), 150 mg/day was used as an antimold prophylaxis for some patients in our allo-HSCT setting as part of a clinical trial that was approved by the IRB. Additionally, clinicians could change the antifungal agents empirically when they included fungal infections in the differential diagnosis.
Antifungal susceptibility.
The in vitro susceptibilities of the stored Candida strains obtained from the blood samples to six antifungal agents (fluconazole, itraconazole, voriconazole, posaconazole, amphotericin B, and micafungin) were determined by the broth microdilution method with a commercial frozen plate for antifungal susceptibility testing (Eiken Chemical Co., Ltd.) that complied with Clinical Laboratory and Standards Institute (CLSI) guideline M27-A3 (33), following the manufacturer's instructions, and MIC values were interpreted according to the criteria in CLSI guideline M27-S4 (18) and the epidemiological cutoff value (ECV) (19). C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as quality control isolates.
Serum BDG.
The Wako turbidimetric assay (Wako Pure Chemical Industries, Tokyo, Japan) was used to measure the serum (1,3)-beta-d-glucan (BDG) concentration (34) using the cutoff values for positivity recommended in the manufacturer's instructions, i.e., the conventional cutoff value (11 pg/ml) (35). The sensitivity for detecting candidemia by the Wako turbidimetric assay with the cutoff value was comparable to the sensitivity reported in previous meta-analyses (23, 36). Serum BDG concentrations were routinely measured for all the recipients once a week, on every Wednesday.
Statistical analysis.
Categorical variables were compared by Fisher's exact test. The Mann-Whitney U test was performed to test the equality of continuous variables. The 30-day mortality rates were estimated by using Kaplan-Meier analysis. The incidence of BC was estimated on the basis of cumulative incidence curves by using Gray's test. Competing events were relapse of hematological diseases and death from causes not related to the BC. Univariate Cox regression models were used for pretransplant variables and time-dependent variables (systemic steroid administration, engraftment within 30 days, aGVHD, diarrhea, an episode of a change of an antimicrobial agent, and an episode of a change of an antifungal agent) in order to identify significant risk factors for BC that led to its development within 100 days following allo-HSCT; we planned to provide antifungal prophylaxis from the day before allo-HSCT up to day 100 or more to all the recipients. Variables with P values of <0.20 were included in the multivariate analysis. The same methods were used in a previous study (37). Significance was set at an α value of 0.05. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing) (38).
ACKNOWLEDGMENTS
This research is supported by the Research Program on Emerging and Re-Emerging Infectious Diseases from the Japan Agency for Medical Research and Development, AMED.
We report no conflicts of interest.
We alone are responsible for the content and the writing of the paper.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Verfaillie C, Weisdorf D, Haake R, Hostetter M, Ramsay NK, McGlave P. 1991. Candida infections in bone marrow transplant recipients. Bone Marrow Transplant 8:177–184. [PubMed] [Google Scholar]
- 3.Sipsas NV, Lewis RE, Tarrand J, Hachem R, Rolston KV, Raad II, Kontoyiannis DP. 2009. Candidemia in patients with hematological malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 115:4745–4752. doi: 10.1002/cncr.24507. [DOI] [PubMed] [Google Scholar]
- 4.Uzun O, Ascioglu S, Anaissie EJ, Rex JH. 2001. Risk factors and predictors of outcome in patients with cancer and breakthrough candidemia. Clin Infect Dis 32:1713–1717. doi: 10.1086/320757. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis DP, Raddy BT, Hanna H, Bodey GP, Tarrand J, Raad II. 2002. Breakthrough candidemia in patients with cancer differs from de novo candidemia in host factors and Candida species but not intensity. Infect Control Hosp Epidemiol 23:542–545. doi: 10.1086/502104. [DOI] [PubMed] [Google Scholar]
- 6.Nucci M, Colombo AL. 2002. Risk factors for breakthrough candidemia. Eur J Clin Microbiol Infect Dis 21:209–211. doi: 10.1007/s10096-002-0697-1. [DOI] [PubMed] [Google Scholar]
- 7.Gamaletsou MN, Daikos GL, Walsh TJ, Perlin DS, Ortigosa CJ, Psaroulaki A, Pagoni M, Argyropoulou A, Nepka M, Perivolioti E, Kotsopoulou M, Perloretzou S, Marangos M, Kofteridis D, Grammatikou M, Goukos D, Petrikkos G, Sipsas NV. 2014. Breakthrough candidaemia caused by phenotypically susceptible Candida spp. in patients with haematological malignancies does not correlate with established interpretive breakpoints. Int J Antimicrob Agents 44:248–255. doi: 10.1016/j.ijantimicag.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Bizerra FC, Jimenez-Ortiqosa C, Souza AC, Breda GL, Queiroz-Telles F, Perlin DS, Colombo AL. 2014. Breakthrough candidemia due to multidrug-resistant Candida glabrata during prophylaxis with low dose of micafungin. Antimicrob Agent Chemother 58:2438–2440. doi: 10.1128/AAC.02189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saraya T, Tanabe K, Araki K, Yonetani S, Makino H, Watanabe T, Tsujimoto N, Takata S, Kurai D, Ishii H, Miyazaki Y, Takizawa H, Goto H. 2014. Breakthrough invasive Candida glabrata in patients on micafungin: a novel FKS gene conversion correlated with sequential elevation of MIC. J Clin Microbiol 52:2709–2712. doi: 10.1128/JCM.03593-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Shimono N, Kamimura T, Akashi K. 2012. Fatal candidemia caused by azole-resistant Candida tropicalis in patients with hematological malignancies. J Infect Chemother 18:741–746. doi: 10.1007/s10156-012-0412-9. [DOI] [PubMed] [Google Scholar]
- 11.Tacke D, Buchheidt D, Karthaus M, Krause SW, Maschmeyer G, Neumann S, Ostermann H, Penack O, Rieger C, Ruhnke M, Sandherr M, Schweer KE, Ullmann AJ, Cornely OA. 2014. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann Hematol 93:1449–1456. doi: 10.1007/s00277-014-2108-y. [DOI] [PubMed] [Google Scholar]
- 12.Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Donnelly JP, Garbino J, Groll AH, Hope WW, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Cuenca-Estrella M, ESCMID Fungal Infection Study Group. 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 18:53–67. doi: 10.1111/1469-0691.12041. [DOI] [PubMed] [Google Scholar]
- 13.Freifeld AG, Bow EJ, Sepkowiz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 14.Pagano L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, Aloisi T, Irrera G, Bonini A, Picardi M, Caramatti C, Invernizzi R, Mattei D, Melillo L, de Waure C, Reddiconto G, Fianchi L, Valentini CG, Girmenia C, Leone G, Aversa F. 2007. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study–Sorveglianza epidemiologica infezioni fungine nelle emopatie maligne. Clin Infect Dis 45:1161–1170. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 15.Cornely OA, Gachot B, Akan H, Bassetti M, Uzun O, Kibbler C, Marchetti O, de Burghgraeve P, Ramadan S, Pylkkanen L, Ameye L, Paesmans M, Donnelly JP, EORTC Infectious Diseases Group. 2015. Epidemiology and outcome of fungemia in a cancer cohort of the infectious diseases group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin Infect Dis 61:324–331. doi: 10.1093/cid/civ293. [DOI] [PubMed] [Google Scholar]
- 16.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD. 2010. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol 48:2373–2380. doi: 10.1128/JCM.02390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing yeasts; fourth informational supplement. Document M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rex JH, Pfaller MA. 2002. Has antifungal susceptibility testing come of age? Clin Infect Dis 35:982–989. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 21.Bassetti M, Merelli M, Ansaldi F, de Florentiis D, Sartor A, Scarparo C, Callegari A, Righi E. 2015. Clinical and therapeutic aspects of candidemia: a five year single centre study. PLoS One 10:e0127534. doi: 10.1371/journal.pone.0127534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Abe M, Kimura M, Araoka H, Taniguchi S, Yoneyama A. 2014. Serum (1,3)-beta-d-glucan is an inefficient marker of breakthrough candidemia. Med Mycol 52:835–840. doi: 10.1093/mmy/myu066. [DOI] [PubMed] [Google Scholar]
- 24.Malani A, Hmoud J, Chiu L, Carver PL, Bielaczyc A, Kauffman CA. 2005. Candida glabrata fungemia: experience in a tertiary care center. Clin Infect Dis 41:975–981. doi: 10.1086/432939. [DOI] [PubMed] [Google Scholar]
- 25.Schimmer BP, Parker KL. 2006. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones, p 1587 In Brunton LL, Lazo JS, Parker KL (ed), The pharmacological basis of therapeutics, 11th ed McGraw-Hill, New York, NY. [Google Scholar]
- 26.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 27.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, Sandmaier B. 2009. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto H, Uchida N, Matsuno N, Ota H, Kageyama K, Wada S, Kaji D, Nishida A, Ishiwata K, Takagi S, Tsuji M, Asano-Mori Y, Yamamoto G, Izutsu K, Masuoka K, Wake A, Yoneyama A, Makino S, Taniguchi S. 2014. Anti-HLA antibodies other than against HLA-A, -B, -DRB1 adversely affect engraftment and nonrelapse mortality in HLA-mismatched single cord blood transplantation: possible implications of unrecognized donor-specific antibodies. Biol Blood Marrow Transplant 20:1634–1640. doi: 10.1016/j.bbmt.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan KM. 1994. Graft-versus-host disease, 4th ed Blackwell Science, Cambridge, MA. [Google Scholar]
- 30.White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, NY. [Google Scholar]
- 31.O'Donnell K. 1993. Fusarium and its near relatives, p 225–233. In Reynolds DR, Taylor JW (ed), The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, United Kingdom. [Google Scholar]
- 32.van Burik JA, Ratanatharathom V, Stepan DE, Miller CB, Lipton JH, Vesole DH, Bunin N, Wall DA, Hiemenz JW, Satoi Y, Lee JM, Walsh TJ, National Institute of Allergy and Infectious Diseases Mycoses Study Group. 2004. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 33.Peron IH, Reichert-Lima F, Busso-Lopes AF, Nagasako CK, Lyra L, Moretti ML, Schreiber AZ. 2016. Resistance surveillance in Candida albicans: a five-year antifungal susceptibility evaluation in a Brazilian University Hospital. PLoS One 11:e0158126. doi: 10.1371/journal.pone.0158126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moro H, Tsukada H, Ohara T, Susa R, Tanabe Y, Suzuki E, Gejyo F. 2003. Clinical evaluation of performance of a new diagnostic method for deep mycosis by measuring β-glucan concentration in the blood. Kansenshogaku Zasshi 77:219–226. (In Japanese.) doi: 10.11150/kansenshogakuzasshi1970.77.219. [DOI] [PubMed] [Google Scholar]
- 35.Kawazu M, Kanda Y, Nannya Y, Aoki K, Kurokawa M, Chiba S, Motokura T, Hirai H, Ogawa S. 2004. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-beta-d-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J Clin Microbiol 42:2733–2741. doi: 10.1128/JCM.42.6.2733-2741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. 2011. β-d-Glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 52:750–770. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 37.Omer AK, Ziakas PD, Anaqnostou T, Coughlin E, Kourkoumpetis T, McAfee SL, Dey BR, Attar E, Chen YB, Spitzer TR, Mylonakis E, Ballen KK. 2013. Risk factors for invasive fungal diseases after allogeneic hematopoietic stem cell transplantation: a single center experience. Biol Blood Marrow Transplant 19:1190–1196. doi: 10.1016/j.bbmt.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Kanda Y. 2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]