ABSTRACT
Ceftolozane-tazobactam was tested against 58 multidrug-resistant nonfermenting Gram-negative bacilli (35 Pseudomonas aeruginosa, 11 Achromobacter xylosoxydans, and 12 Stenotrophomonas maltophilia isolates) isolated from cystic fibrosis patients and was compared to ceftolozane alone, ceftazidime, meropenem, and piperacillin-tazobactam. Ceftolozane-tazobactam was the most active agent against P. aeruginosa but was inactive against A. xylosoxydans and S. maltophilia. In time-kill experiments, ceftolozane-tazobactam had complete bactericidal activity against 2/6 clinical isolates (33%).
KEYWORDS: ceftolozane, Pseudomonas, cystic fibrosis, bactericidal activity, Achromobacter, Stenotrophomonas
TEXT
Nonfermenting Gram-negative bacilli (NF-GNB) are well-known pulmonary pathogens that colonize the airways of patients with cystic fibrosis (CF) (1, 2). Acute pulmonary exacerbations and chronic infections due to Pseudomonas aeruginosa, Achromobacter xylosoxidans, and Stenotrophomonas maltophilia may result in decreases in pulmonary function and high morbidity and mortality rates among CF patients (3–5). It was shown that multidrug-resistant (MDR) P. aeruginosa strains were associated with accelerated progression of CF and unfavorable outcomes (6).
Although the ceftolozane-tazobactam combination was tested previously in vitro against a large panel of MDR Enterobacteriaceae and P. aeruginosa isolates (7–10), there is little information concerning its activity against MDR strains isolated from CF patients. The activity of ceftolozane alone against P. aeruginosa isolates with high rates of β-lactam susceptibility from chronically infected CF patients was reported in two studies with encouraging results (11, 12). Only one study reported the activity of ceftolozane in combination with tazobactam, which is the currently marketed combination (Zerbaxa; Merck & Co., Inc.) (13). To our knowledge, ceftolozane-tazobactam has never been tested against MDR Achromobacter and S. maltophilia isolates from CF patients.
In this study, we measured the MIC and bactericidal activity of ceftolozane, alone and in combination with tazobactam, against various NF-GNB isolated from chronically infected CF patients that were highly resistant to most antipseudomonal β-lactams. Although tazobactam was not expected to enhance the activity of ceftolozane against MDR NF-GNB from CF patients, ceftolozane and ceftolozane-tazobactam were tested in parallel, in order to compare our results with those of other studies that evaluated ceftolozane alone and/or ceftolozane-tazobactam.
Bacterial strains and susceptibility results.
Fifty-eight nonduplicate clinical isolates of NF-GNB (35 P. aeruginosa, 11 A. xylosoxydans, and 12 S. maltophilia isolates) from 55 adult patients with CF were collected in two French teaching hospitals. All isolates were from respiratory tract samples and were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker Daltonics, Bremen, Germany). All strains showed a high level of resistance to β-lactams, defined by resistance to ceftazidime determined with the disk diffusion method (inhibition zone diameter of <16 mm), according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints (http://www.eucast.org/clinical_breakpoints). MICs were determined using the agar dilution method, according to the 2015 recommendations of the Clinical and Laboratory Standards Institute (CLSI) (14). The MICs of the following antibiotics were determined: ceftolozane (MSD, Courbevoie, France), alone and in combination with tazobactam (Sigma-Aldrich, Lyon, France), piperacillin-tazobactam (Panpharma, Luitré, France), ceftazidime (Panpharma), and meropenem (Fresenius Kabi, Sèvres, France). Tazobactam was used at the fixed concentration of 4 μg/ml. The MICs of all antibiotics were determined for all strains in triplicate, after 24 h of incubation at 35 ± 2°C, as recommended (14). When bacterial growth was insufficient, incubation was extended to 48 h. P. aeruginosa ATCC 27853 and Klebsiella pneumoniae ATCC 700603 (an SHV-18-producing strain for the control of tazobactam combinations) were used as reference strains throughout this study.
Ceftolozane was the most active compound against P. aeruginosa isolates, with MIC50 and MIC90 values of 4 μg/ml and 128 μg/ml, respectively (Table 1). The combination with 4 μg/ml tazobactam did not improve the activity of ceftolozane against P. aeruginosa; this is not surprising, since CF isolates rarely produce acquired class A β-lactamases, which are the main targets for tazobactam (15, 16). The MIC50 of ceftolozane-tazobactam was 5 doubling dilutions lower than those of ceftazidime and piperacillin-tazobactam and 2 doubling dilutions lower than that of meropenem. Considering the EUCAST clinical breakpoints, the highest rate of susceptibility (54%) was among the P. aeruginosa isolates with the ceftolozane-tazobactam combination (Table 1). Among 24 P. aeruginosa isolates that were nonsusceptible (i.e., intermediate or resistant) to all comparator antibiotics, ceftolozane-tazobactam remained active against 10/24 isolates (42%). Among the 16 P. aeruginosa isolates that were resistant to ceftolozane-tazobactam (MIC range, 8 to >128 μg/ml; median MIC, 8 μg/ml), piperacillin-tazobactam and meropenem were active against only one isolate each, while all 16 isolates were resistant to ceftazidime (MICs of ≥16 μg/ml) (data not shown). These results are consistent with previous studies of P. aeruginosa isolates from CF patients, which showed (i) greater activity of ceftolozane against strains for which ceftazidime MIC50 values ranged from 1 to 8 μg/ml (11, 13) and (ii) a ceftolozane MIC50 value 3 doubling dilutions lower than that of ceftazidime for strains with decreased susceptibility to ceftazidime (MIC50 of 32 μg/ml) (12).
TABLE 1.
Susceptibility data for ceftolozane-tazobactam and β-lactam comparators in a collection of 58 nonfermenting Gram-negative bacilli isolated from cystic fibrosis patients
| Species and antimicrobial agent(s) | MIC (μg/ml) |
EUCAST clinical breakpoint (μg/ml) | No. of strains |
Proportion of susceptible strains (%) | ||||
|---|---|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | Resistant | Intermediate | Susceptible | |||
| All isolates (n = 58) | ||||||||
| Ceftolozane | 0.5 to >128 | 16 | >128 | |||||
| Ceftolozane + tazobactam | 0.5 to >128 | 16 | >128 | |||||
| Ceftazidime | 4 to >128 | 128 | >128 | |||||
| Piperacillin + tazobactam | 1 to >128 | 64 | >128 | |||||
| Meropenem | 0.5 to >128 | 32 | >128 | |||||
| Pseudomonas aeruginosa (n = 35) | ||||||||
| Ceftolozane | 0.5 to >128 | 4 | 128 | |||||
| Ceftolozane + tazobactama | 0.5 to >128 | 4 | 128 | ≤4 to >4 | 16 | 19 | 54 | |
| Ceftazidime | 4 to >128 | 128 | >128 | ≤8 to >8 | 31 | 4 | 11 | |
| Piperacillin + tazobactam | 2 to >128 | 128 | >128 | ≤16 to >16 | 26 | 9 | 26 | |
| Meropenem | 1 to >128 | 16 | 128 | ≤2 to >8 | 26 | 6 | 3 | 9 |
| Stenotrophomonas maltophilia (n = 12) | ||||||||
| Ceftolozane | 16 to >128 | 64 | >128 | |||||
| Ceftolozane + tazobactam | 16 to >128 | 64 | >128 | ≤4 to >4 | 12 | 0 | 0 | |
| Ceftazidime | 16 to >128 | 128 | >128 | ≤8 to >16 | 11 | 1 | 0 | 0 |
| Piperacillin + tazobactam | 8 to >128 | 128 | >128 | ≤4 to >16 | 11 | 0 | 1 | 8 |
| Meropenem | 4 to >128 | >128 | >128 | ≤2 to >8 | 10 | 2 | 0 | 0 |
| Achromobacter xylosoxydans (n = 11) | ||||||||
| Ceftolozane | >128 | >128 | >128 | |||||
| Ceftolozane + tazobactam | >128 | >128 | >128 | ≤4 to >4 | 11 | 0 | 0 | |
| Ceftazidime | 32 to >128 | >128 | >128 | ≤4 to >8 | 11 | 0 | 0 | 0 |
| Piperacillin + tazobactam | 1 to 64 | 16 | 32 | ≤4 to >16 | 3 | 6 | 2 | 18 |
| Meropenem | 0.5 to >128 | 32 | 128 | ≤2 to >8 | 9 | 1 | 1 | 9 |
When CLSI clinical breakpoints were applied, the numbers of P. aeruginosa isolates resistant, intermediate, and susceptible to ceftolozane-tazobactam were 8, 8, and 19, respectively. The proportion of susceptible strains remained unchanged (54%).
Piperacillin-tazobactam was the most active antibiotic against A. xylosoxydans, followed by meropenem, while ceftolozane-tazobactam and ceftazidime showed no activity (Table 1). None of the β-lactams tested was active against MDR S. maltophilia isolates (Table 1), which is consistent with previous results obtained for stains isolated from non-CF patients (9).
Bactericidal activity of ceftolozane-tazobactam.
Considering the lower MICs of ceftolozane-tazobactam, in comparison with other antipseudomonal β-lactams, for P. aeruginosa, time-kill assays were performed to evaluate the bactericidal activity of ceftolozane-tazobactam against various P. aeruginosa isolates. The bactericidal activity of ceftolozane in combination with 4 μg/ml tazobactam was determined as described previously (17). Briefly, a bacterial suspension incubated overnight at 35°C in brain heart infusion broth (BHI-B) was diluted 1/1,000 in fresh BHI-B and incubated for 2 h at 35°C, with agitation, to reach a bacterial density of 105 to 106 CFU/ml. Antibiotics were added at concentrations corresponding to 4 or 8 times their MIC values, and cultures were incubated for 24 h at 35°C, with agitation. CFU counts were performed at 0, 2, 6, 8, and 24 h, using adequate dilutions plated on Mueller-Hinton (MH) agar. Antibiotic-free cultures were included as growth controls in all assays. Bactericidal activities of antipseudomonal β-lactam antibiotics (ceftolozane alone, ceftazidime, piperacillin-tazobactam, and meropenem) were compared with those of ceftolozane-tazobactam against the reference strain P. aeruginosa ATCC 27853. A complete bactericidal effect was defined as a ≥3-log10 decrease from the initial bacterial colony count. For the reference strain P. aeruginosa ATCC 27853 (ceftolozane-tazobactam MIC of 0.5 μg/ml), a complete bactericidal effect (defined as a ≥3-log10 decrease from the initial colony count) was observed 6 h after the addition of ceftolozane-tazobactam at both 4× MIC and 8× MIC (Fig. 1). Ceftolozane alone showed similar results, indicating that tazobactam did not improve the bactericidal activity of ceftolozane. Ceftazidime had comparable bactericidal activity. Meropenem achieved complete bactericidal activity more quickly, with a 3-log10 reduction at 8× MIC after 4 h, compared to approximately 6 to 7 h for the other compounds (Fig. 1B). No regrowth was observed within 24 h in the presence of ceftolozane, ceftolozane-tazobactam, or meropenem, while an increase in colony counts was observed with piperacillin-tazobactam, especially at 4× MIC.
FIG 1.
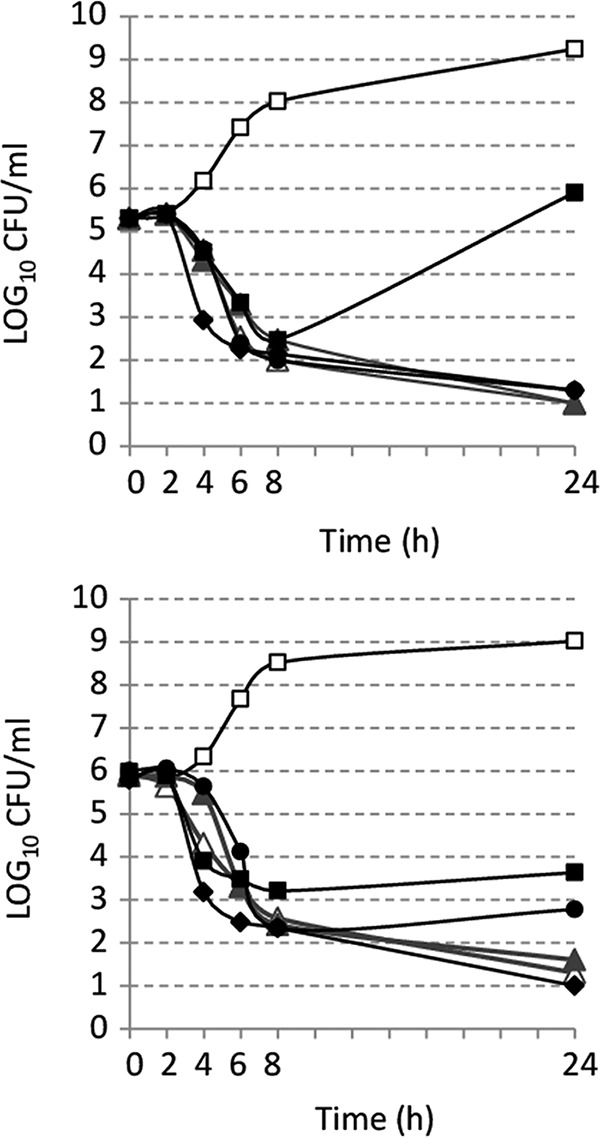
Time-kill curves for P. aeruginosa ATCC 27853 exposed to antimicrobial agents at 4× MIC (A) or 8× MIC (B). Antibiotics were added to the medium after 2 h of incubation. □, growth control; ▲, ceftolozane alone; △, ceftolozane-tazobactam; ●, ceftazidime; ■, piperacillin-tazobactam; ◆, meropenem.
The bactericidal activity was also studied with six P. aeruginosa clinical isolates for which the MICs of ceftolozane-tazobactam were 4 μg/ml (upper limit of susceptibility [three strains]) or 8 μg/ml (resistance [three strains]) (Fig. 2). The highest concentration of ceftolozane-tazobactam tested for these strains (64 μg/ml in the case of the three resistant strains) remained in the ranges achievable in human serum, according to previous pharmacokinetic studies conducted with standard dosing regimens of 1,000 mg/500 mg three times a day (18, 19). For all six strains, a >1.5-log10 decrease in the number of CFU per milliliter was observed after 6 h of incubation in the presence of antibiotic (Fig. 2). However, complete bactericidal activity was achieved for only two strains (Fig. 2A and D) using 8× MIC of ceftolozane-tazobactam; only partial bactericidal activity was achieved for all isolates at 4× MIC. Regrowth was observed for all P. aeruginosa isolates after 24 h of incubation.
FIG 2.

Time-kill curves for clinical P. aeruginosa isolates exposed to ceftolozane-tazobactam. Antibiotics were added to the medium after 2 h of incubation. The MICs, for three isolates each, were 4 μg/ml (A to C) or 8 μg/ml (D to F). □, growth control; ▲, 4× MIC; ●, 8× MIC.
Similar regrowth was observed with other combinations of β-lactams, such as piperacillin-tazobactam and ceftazidime-avibactam, in previous studies (20) and also with piperacillin-tazobactam at 4× MIC against P. aeruginosa ATCC 27853 in the present study. As the wild-type P. aeruginosa ATCC 27853 showed no regrowth when exposed to ceftolozane-tazobactam (Fig. 1), an inoculum effect due to AmpC overexpression cannot be excluded for clinical isolates. The amplification of a ceftolozane-resistant subpopulation was previously suggested in time-kill assays performed with Escherichia coli (21). Therefore, 24-h colonies obtained after regrowth were plated on brain heart infusion (BHI) agar with increasing concentrations of ceftolozane-tazobactam. Growth at concentrations higher than the initial MIC was observed for 3/6 isolates (50%), for which the initial and final MICs were 4 and 32 mg/liter, 4 and >64 mg/liter, and 8 and >64 mg/liter, respectively. This finding reveals the in vitro selection of ceftolozane-tazobactam-resistant P. aeruginosa populations, suggesting that resistant strains could easily emerge in everyday clinical practice, especially among CF patients, who are usually subjected to long courses of antibiotic treatments. Appropriate and thoughtful use of this antibiotic should be considered by prescribers.
In conclusion, based on MIC determinations as well as time-kill assays, the ceftolozane-tazobactam combination appears to be a major antipseudomonal drug, especially for CF patients, who are often chronic carriers of P. aeruginosa strains that are highly resistant to other antipseudomonal β-lactams. The ceftolozane-tazobactam activity is insufficient, however, against other CF NF-GNB, such as S. maltophilia and A. xylosoxidans.
ACKNOWLEDGMENT
Ceftolozane was kindly provided by MSD.
REFERENCES
- 1.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 2.Lyczak JB, Cannon CL, Pier GB. 2002. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flume PA, Mogayzel PJ, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC. 2009. Cystic fibrosis pulmonary guidelines. Am J Respir Crit Care Med 180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 4.Waters V, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. 2013. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros 12:482–486. doi: 10.1016/j.jcf.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 5.De Baets F, Schelstraete P, Van Daele S, Haerynck F, Vaneechoutte M. 2007. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros 6:75–78. doi: 10.1016/j.jcf.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Lechtzin N, John M, Irizarry R, Merlo C, Diette GB, Boyle MP. 2006. Outcomes of adults with cystic fibrosis infected with antibiotic-resistant Pseudomonas aeruginosa. Respiration 73:27–33. doi: 10.1159/000087686. [DOI] [PubMed] [Google Scholar]
- 7.Juan C, Zamorano L, Pérez JL, Ge Y, Oliver A. 2010. Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob Agents Chemother 54:846–851. doi: 10.1128/AAC.00834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livermore DM, Mushtaq S, Ge Y. 2010. Chequerboard titration of cephalosporin CXA-101 (FR264205) and tazobactam versus β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother 65:1972–1974. doi: 10.1093/jac/dkq248. [DOI] [PubMed] [Google Scholar]
- 9.Farrell DJ, Sader HS, Flamm RK, Jones RN. 2014. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 43:533–539. doi: 10.1016/j.ijantimicag.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN. 2014. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-12). J Antimicrob Chemother 69:2713–2722. doi: 10.1093/jac/dku184. [DOI] [PubMed] [Google Scholar]
- 11.Zamorano L, Juan C, Fernández-Olmos A, Ge Y, Cantón R, Oliver A. 2010. Activity of the new cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa isolates from chronically-infected cystic fibrosis patients. Clin Microbiol Infect 16:1482–1487. doi: 10.1111/j.1469-0691.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- 12.Livermore DM, Mushtaq S, Ge Y, Warner M. 2009. Activity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolates. Int J Antimicrob Agents 34:402–406. doi: 10.1016/j.ijantimicag.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Kuti JL, Pettit RS, Neu N, Cies JJ, Lapin C, Muhlebach MS, Novak KJ, Nguyen ST, Saiman L, Nicolau DP. 2015. Microbiological activity of ceftolozane/tazobactam, ceftazidime, meropenem, and piperacillin/tazobactam against Pseudomonas aeruginosa isolated from children with cystic fibrosis. Diagn Microbiol Infect Dis 83:53–55. doi: 10.1016/j.diagmicrobio.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2015. Methods for dilution susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. CLSI document M7-A8 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Llanes C, Pourcel C, Richardot C, Plésiat P, Fichant G, Cavallo J-D, Mérens A, Vu-Thien H, Leclercq R, Romaszko J-P, Poyard C, Marchandin H, Bingen E, Segonds C, Caillon J, Roussel-Delvallez M, Vergnaud G, Hocquet D, Plésiat P, Cavallo J-D. 2013. Diversity of β-lactam resistance mechanisms in cystic fibrosis isolates of Pseudomonas aeruginosa: a French multicentre study. J Antimicrob Chemother 68:1763–1771. doi: 10.1093/jac/dkt115. [DOI] [PubMed] [Google Scholar]
- 16.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grohs P, Houssaye S, Aubert A, Gutmann L, Varon E. 2003. In vitro activities of garenoxacin (BMS-284756) against Streptococcus pneumoniae, viridans group streptococci, and Enterococcus faecalis compared to those of six other quinolones. Antimicrob Agents Chemother 47:3542–3547. doi: 10.1128/AAC.47.11.3542-3547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 56:3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge Y, Whitehouse MJ, Friedland I, Talbot GH. 2010. Pharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusions. Antimicrob Agents Chemother 54:3427–3431. doi: 10.1128/AAC.01753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keepers TR, Gomez M, Celeri C, Nichols WW, Krause KM. 2014. Bactericidal activity, absence of serum effect, and time-kill kinetics of ceftazidime-avibactam against β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:5297–5305. doi: 10.1128/AAC.02894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soon RL, Lenhard JR, Bulman ZP, Holden PN, Kelchlin P, Steenbergen JN, Friedrich LV, Forrest A, Tsuji BT. 2016. Combinatorial pharmacodynamics of ceftolozane-tazobactam against genotypically defined β-lactamase-producing Escherichia coli: insights into the pharmacokinetics/pharmacodynamics of β-lactam–β-lactamase inhibitor combinations. Antimicrob Agents Chemother 60:1967–1973. doi: 10.1128/AAC.02635-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


