ABSTRACT
Despite its toxicity and low efficacy in the chronic phase, benznidazole is the drug of choice in Chagas disease. Scarce information about pharmacokinetics and pharmacodynamics of benznidazole has been published. We performed a phase I, open-label, nonrandomized pharmacokinetic study of benznidazole (Abarax) conducted with 8 healthy adult volunteers at the Infectious Diseases Department of the Vall d'Hebron University Hospital (Barcelona, Spain). The separation and detection of benznidazole were performed on a Waters Acquity ultraperformance liquid chromatography system (UPLC) coupled with a Waters Xevo TQ MS triple quadrupole mass spectrometer. The pharmacokinetic parameters were calculated based on a noncompartmental body model using Phoenix WinNonlin version 6.3 software. Furthermore, computational simulations were calculated for the multiple-dose administration at two dose regimens: 100 mg of benznidazole administered every 8 h and 150 mg of benznidazole administered every 12 h. After benznidazole administration, the median area under the concentration-time curve from time zero to time t (AUC0–t) and extrapolated to infinity (AUC0–∞) were about 46.4 μg · h/ml and 48.4 μg · h/ml, respectively. Plasma benznidazole concentrations peaked at 3.5 h, with maximal concentrations of 2.2 μg/ml, and benznidazole exhibited a terminal half-life of 12.1 h. The median maximum concentration (Cmax) of benznidazole was lower in men than in women (1.6 versus 2.9 μg/ml), and median volume of distribution (V) as a function of bioavailability (F) was higher in men than in women (125.9 versus 88.6 liters). In conclusion, dose regimens (150 mg/12 h or 100 mg/8 h) reached a steady-state range concentration above of the minimum experimental therapeutic dose. Sex differences in the benznidazole pharmacokinetics were observed; mainly, men had lower Cmax and higher V/F than women.
KEYWORDS: benznidazole, pharmacokinetics, Trypanosoma cruzi, Chagas disease
INTRODUCTION
Benznidazole (N-benzyl-2-nitroimidazole acetamide) is a nitroimidazole derivative with trypanocidal activity used in Chagas disease. Grunberg et al. showed in 1967 for the first time that benznidazole was active against Trypanosoma cruzi (1). Despite its toxicity and low efficacy in the chronic phase, benznidazole is the drug of choice in Chagas disease (2).
Scarce information about pharmacokinetics and pharmacodynamics of benznidazole has been published. In 1979, Raaflaub and Zeigler published a single-dose pharmacokinetic study of benznidazole in 6 healthy volunteers (3), and in 1980, Raaflaub published a multiple-dose study with 8 Chagas disease patients receiving treatment with benznidazole (4). These studies were performed with tablets containing 100 mg of benznidazole (Radanil; Laboratorio Roche). In November 2012, Laboratorio Elea (Argentina) started the manufacture and distribution of a new formulation of benznidazole (Abarax).
Two recent studies performed in Argentina and Spain including pediatric and adult populations, respectively, with Chagas disease used a population pharmacokinetic approach (5, 6). One of the main differences regarding population pharmacokinetics is that data are obtained for patients treated with benznidazole, while nonpopulation pharmacokinetic studies are performed with healthy volunteers. Thus, one of the advantages is that interindividual and intraindividual variability can be distinguished. On the other hand, those studies are more complex and time-consuming, and a large number of patients (>40) are required (2).
Due to the scarce information available for benznidazole, and taking into account that most of it comes from studies with Radanil (a drug no longer manufactured), we performed a pharmacokinetic study of benznidazole (Abarax) in 8 healthy volunteers. Clinical implications for the treatment regimen and for future clinical trial design are also discussed.
RESULTS
Ten subjects were screened, and 8 participants met the inclusion criteria and were therefore included. One of them was excluded because of the use of other drugs. There were 4 men and 4 women, the average age was 27.2 years, and mean time of residence in our country was 32.6 months. Table 1 shows the main clinical and epidemiological data for participants. All participants completed the study protocol. Blood cell and biochemical parameters as well as serological tests were in the normality range or tested negative. Regarding safety and tolerability, two participants reported mild side effects. One participant reported headache and nausea around 6 h after drug administration, probably related to the study medication, which disappeared without treatment in less than 6 h. The other participant reported muscle pain around 48 h after drug administration, not related to the study medication. After 72 h post-benznidazole intake, the general biochemistry and cell blood count of all participants were within the normal range.
TABLE 1.
Demographic and epidemiological data for healthy volunteers
| Participant | Country | Age (yrs) | Sex | Ht (m) | Wt (kg) | Body mass index (kg/m2) |
|---|---|---|---|---|---|---|
| 1 | Argentina | 26 | Female | 1.60 | 54.8 | 21.41 |
| 2 | Colombia | 27 | Male | 1.79 | 80.5 | 25.12 |
| 3 | Peru | 28 | Female | 1.59 | 58.8 | 23.26 |
| 4 | Bolivia | 29 | Female | 1.63 | 61.0 | 22.96 |
| 5 | Paraguay | 32 | Male | 1.76 | 89.2 | 28.80 |
| 6 | Ecuador | 19 | Male | 1.75 | 63.9 | 20.87 |
| 7 | Colombia | 27 | Female | 1.64 | 54.4 | 20.23 |
| 8 | Bolivia | 26 | Male | 1.83 | 91.0 | 27.17 |
Figure 1 shows the plasma concentration-versus-time profiles in 8 healthy volunteers after the single oral administration of 100 mg of benznidazole; the main pharmacokinetic parameters are displayed in Table 2. Results are compared with those from previous pharmacokinetic studies performed with adults. The pharmacokinetic parameters of benznidazole by sex are summarized in Table 3.
FIG 1.
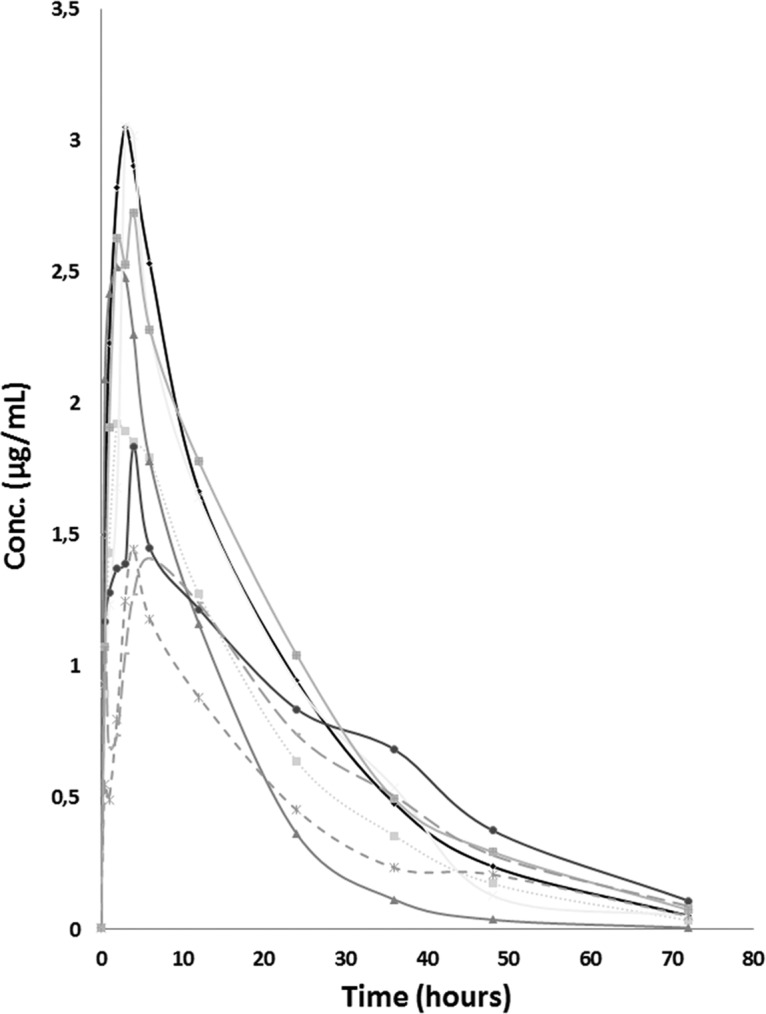
Pharmacokinetic curves of benznidazole after oral administration at fixed dose of 100 mg.
TABLE 2.
Pharmacokinetic parameters of benznidazole in healthy volunteers in comparison with previously published pharmacokinetics in adultsa
| Parameter | Value in indicated study |
|||
|---|---|---|---|---|
| This study | Raaflaub and Zeigler (3) | Raaflaub (4) | Soy et al. (6) | |
| No. of subjects (age range, yrs) | 8 (19–32) | 6 (22–24) | 8 | 49 (19–55) |
| Dose | 100 mg, single dose | 100 mg, single dose | 3.5 mg/kg/12 h, multiple doses | 2.5 mg/kg/12 h, multiple doses |
| t1/2 (h) | 12.1 (13.7–11.1) | 11.7 (13–11.1) | 13.75 (15–12) | 36 |
| Tmax, h (range) | 3.5 (4–2.7) | 3.5 (4–3) | ||
| Cmax (μg · ml−1) | 2.2 (2.8–1.7) | 2.61 (2.6–2.3) | SS max: 12.54 (14–11) SS min: 8.02 (9.6–7.1) | |
| AUC0–t (μg · h/ml−1) | 46.4 (56.3–40.5) | |||
| AUC0–∞ (μg · h/ml−1) | 48.4 (57.2–40.9) | |||
| V/F (liters) | 34.5 (39.7–29.5) | 89.6 | ||
| CL/F (liters/h) | 2.07 (2.4–1.7) | 0.56 (0.6–0.5) | 1.73 | |
Data are reported as medians and interquartile ranges. Tmax data are shown as medians and ranges. t1/2, elimination half-life; Tmax, observed time to reach the maximum concentration in plasma; Cmax, observed maximum concentration in plasma; AUC0–t, area under the plasma drug concentration-time curve from 0 to the last measurable concentration; AUC0–∞, area under plasma drug concentration-time curve from 0 to infinity; V/F, apparent volume of distribution; CL/F, apparent oral clearance; SS max, steady-state maxima; SS min, steady-state minima.
TABLE 3.
Pharmacokinetic parameters of benznidazole by sex after a single oral dose of 100 mg of benznidazole in the healthy volunteers (n = 8)
| Parameter | Value ina: |
P value | |
|---|---|---|---|
| Men (n = 4) | Women (n = 4) | ||
| BMIb (kg/m2) | 25.4 | 22 | 0.34 |
| Cmax (μg/ml)* | 1.6 (1.4–1.9) | 2.9 (2.6–3.1) | 0.02 |
| tmax (h) (range) | 4.0 (2.5–5.5) | 3.0 (2.3–3.8) | 0.42 |
| AUC0–t (μg · h/ml) | 42.5 (33.2–48.3) | 57.5 (40.3–60.7) | 0.2 |
| AUC0–∞ (μg · h/ml) | 43.7 (34.2–50.4) | 58.4 (40.5–61.9) | 0.2 |
| t1/2 (h) | 13.9 (11.4–15.1) | 11.3 (8.9–12.5) | 0.2 |
| V/F (liters)* | 125.9 (109.1–193.9) | 88.6 (82.2–96.6) | 0.02 |
| CL/F (liters/h) | 6.9 (5.9–8.9) | 5.1 (4.8–7.7) | 0.2 |
Data are reported as medians and interquartile ranges unless otherwise noted. *, P < 0.05 between men and women.
BMI, body mass index.
The mean area under the concentration-time curve from time zero to time t (AUC0–t) and extrapolated to infinity (AUC0–∞) obtained for benznidazole were about 46.4 and 48.4 μg · h/ml, respectively. Plasma benznidazole concentrations peaked at 3.5 h, with maximum concentrations of 2.2 μg/ml, and benznidazole exhibited a terminal half-life of 12.1 h. These results are similar to those from another previously reported single-dose pharmacokinetic study (3). After benznidazole administration, the median peak plasma benznidazole concentration was lower in men than in women (1.6 versus 2.9 μg/ml), and the median volume of distribution (V) as a function of bioavailability (F) was smaller in women than those in men (88.6 versus 125.9 liters).
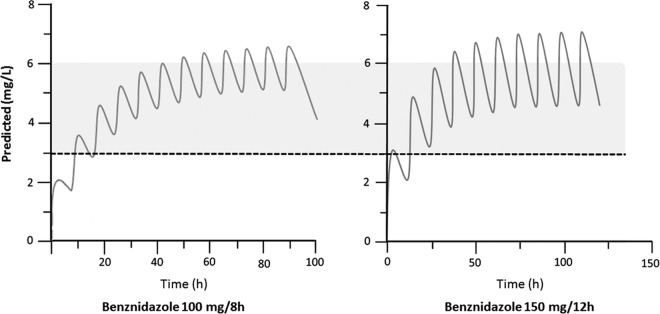
According to simulation, both regimens will attain the classically accepted trypanocidal concentration. (Steady-state concentrations for 150-mg/12-h regimen were as follows: maximum concentration (Cmax), 7 μg/ml, and minimum concentration (Cmin), 4.5 μg/ml. Steady-state concentrations for the 100-mg/8-h regimen were as follows: Cmax, 6.5 μg/ml, and Cmin, 5 μg/ml) (Fig. 2).
FIG 2.
Computational simulation of median benznidazole versus time at steady state for oral administration of benznidazole at 100 mg every 8 h and benznidazole at 150 mg every 12 h. Shaded areas between dashed lines represent the classically accepted the optimal therapeutic range between 3 and 6 mg/liter.
DISCUSSION
Despite the recent clinical trials and efforts toward discovery of drugs against Chagas disease (7), the current standard of care is benznidazole, and according to the pipeline and preliminary results for experimental drugs, there is not going to be any new drug commercially available in the forthcoming years.
Therefore, to gather data for such an old drug must be a priority in order to fully understand both mechanisms of action and toxicity.
In this study, we have conducted a pharmacokinetic evaluation of benznidazole (Abarax) with healthy volunteers. Results obtained do not differ substantially from those previously published for benznidazole from Roche. Although the studies of Raaflaub (4) and Soy et al. (6) were performed with patients with chronic Chagas disease and the influence of T. cruzi infection on pharmacokinetic parameters has not been assessed, it seems that the drug used now has the same pharmacokinetics properties as the one used more than 40 years ago.
After analysis of the effect of sex differences in the benznidazole pharmacokinetics, our study indicated that men had lower a Cmax and higher V/F than women. These results suggest that sex-related differences in pharmacokinetics of benznidazole might be due to variations in the gastric pH, rate of gastric emptying, gut transit time, intestinal expression of transport proteins (i.e., P-glycoprotein), and body composition (8, 9). However, further studies using a larger sample size will be required to understand implications regarding the dose adjustment and toxic profile of benznidazole and to discriminate between the mechanisms underlying these sex differences.
From these data and using computational simulations and considering a monocompartmental model, the therapeutic regimen which reaches a higher concentration range is the one in which benznidazole is administered at 150 mg every 12 h.
At present, there are no data regarding the relationship between the dose of benznidazole and its efficacy, as the great majority of studies have used the standard dose.
It is classically accepted that the optimal therapeutic range is between 3 and 6 mg/liter. The first time this was stated was in the paper authored by Richle and Raaflaub in 1980 (10). Unfortunately, there is no further information about the experimental design, and all subsequent studies have used this result as a reference.
A 6-mg/liter concentration of benznidazole is equivalent to 23 μM benznidazole. Most of the laboratory strains used in experimental assays have a much lower 50% inhibitory concentration (IC50), but it is not strange to find in vitro susceptibility above this therapeutic experimental range (11). Moreover, if the strains are isolated from clinical samples, higher IC50s can be observed, in both the amastigote and epimastigote stages of the parasite (12, 13). Therefore, it could be thought that in some cases, the standard dose used could not be enough to reach the therapeutic concentration range.
From an opposite point of view, two recent studies based on population pharmacokinetics came to the fore because of a novel hypothesis (5, 6). Both studies reach to the conclusion that the benznidazole treatment regimen against Chagas disease in adults might be overdosed.
One of the reasons argued by Altcheh et al. (5) is that children have markedly lower plasma benznidazole concentrations than those reported for adults. Therefore, taking into account the drug exposure, the high effectiveness, and the excellent tolerance in children, they suggest the possibility to pose a dose reduction in the adult population in order to reduce the incidence of an adverse drug reaction while maintaining its efficacy. Other factors, such as time from infection, should be considered when efficacy is borne in mind. In contrast, two more studies performed with adult patients not only failed to link the occurrence of adverse reaction with the plasma benznidazole levels but also open the theory of a genetic background besides the hypersensitivity reactions to benznidazole (14, 15).
On the other hand, Soy et al. (6), using an interesting dosage regimen simulation under steady-state conditions, found that using the standard treatment protocol (2.5 mg/kg/12 h), approximately 70% of treated patients would be over the maximum experimental therapeutic concentration (6 mg/liter). Unfortunately, according to Soy et al., when the dose regimen was reduced by 50% of the total dose regimen (2.5 mg/kg/day), the simulation showed that one-third of treated patients would be under the minimum experimental therapeutic concentration (3 mg/liter).
Finally, the novel time-to-kill assays used to evaluate drug candidates have shown that benznidazole, as well as nifurtimox and fexinidazole sulfone, is a concentration-dependent trypanocidal drug and therefore more efficacious at high doses. Hence, while the dose of benznidazole seems not to be related to adverse drug reactions but seems to be correlated with the efficacy, theoretically it is difficult to suggest the idea that a benznidazole regimen with lower doses would be as effective as the standard one.
Conclusion.
In light of the computational simulation from our study and the current evidence, either dose regimen (150 mg/12 h or 100 mg/8 h) will reach a steady-state range concentration above the minimum experimental therapeutic concentration.
Sex differences were observed for benznidazole pharmacokinetics; mainly, men had a lower Cmax and higher V/F than women.
Under this uncertain and even contradictory scenario regarding the optimal dose regimen, new clinical trials are needed in order to establish the adequate treatment protocol.
MATERIALS AND METHODS
Study design.
This investigation was a phase I, open-label, nonrandomized pharmacokinetic study conducted with 8 healthy adult volunteers (n = 4 men and 4 women) at the Infectious Diseases Department of the Vall d'Hebron University Hospital (Barcelona, Spain) during January 2014 (EudraCT 2013-003381-14). The participants were healthy men and women, 18 to 35 years of age, coming from Latin America. The following screening protocol was performed in all candidates before they were included: medical history, physical examination, blood cell count, general biochemistry (including liver function, creatinine, electrolytes, and glucose), urine pregnancy test for women, and Trypanosoma cruzi, HIV, and hepatitis B and C virus serological tests.
The participants received a single oral dose of 100 mg of benznidazole (Abarax, Laboratorio Elea, Argentina) on an empty stomach. Drug administration was directly observed by study personnel. Six milliliters of blood was taken for the pharmacokinetic analysis in tubes containing clot activator and gel for serum separation at time zero (predose) and 0.5, 1, 2, 3, 4, 6, 12, 24, 36, 48, and 72 h after drug administration. Blood samples were centrifuged for 10 min at 3,500 rpm, and the serum retrieved was stored at −20°C until analyzed. For safety and tolerability control, vital signs and adverse events were recorded through a specific questionnaire. Blood cell count and general biochemistry were repeated for all participants at the end of the study, matching with the last blood sample extraction (72 h after drug administration). The study protocol was approved by the institutional review board of the hospital (in accordance with the ethical standards of the Helsinki Declaration), and written informed consent was obtained from all participants.
Preparation of standard solutions.
The stock standard solutions of benznidazole were prepared by dissolving the accurately weighed benznidazole standard in methanol. The stock standard solutions were then diluted with methanol to achieve standard working solution at concentration of 1 mg/ml.
The standard working solution was used to spike blank serum samples either for the calibration standards at concentrations of 0.1, 0.3, 0.5, 1, 2, and 3 μg/ml or for the quality control samples at 0.3, 1, and 3 μg/ml.
Determination of benznidazole in serum.
The separation and detection of benznidazole were performed on a Waters Acquity ultraperformance liquid chromatography system (UPLC) coupled with a Waters Xevo TQ MS triple quadrupole mass spectrometer.
Separation was achieved on an Acquity BEH C18 column (2.1 mm by 50 mm; 1.7-μm particle size). A gradient elution program was conducted for chromatographic separation with mobile phase A (acetonitrile) and mobile phase B (0.1% formic acid in water) as follows: 0 to 0.5 min (60–60% mobile phase A), 0.5 to 1.0 min (60–90% mobile phase A), 1.0 to 2.0 min (90–90% mobile phase A), 2.0 to 2.5 min (90–60% mobile phase A), and 2.5 to 3.0 min (60–60% mobile phase A). The flow rate was 0.350 ml/min. The overall run time was 3.0 min. The autosampler was conditioned at 14°C. The injection volume of the sample was 1 μl.
The mass spectrometer was operated using an electrospray source in positive mode. The benznidazole MRM transitions were m/z 261.0→91 and m/z 261.0→148. The system control and data analysis were carried out using MassLynx software (version 4.1) and processed using the TargetLynxTM program.
Before analysis, the serum samples and controls were thawed to room temperature. In a 1.5-ml centrifuge tube, 250 μl of ethyl acetate was added to 50 μl of serum sample, and the tube was vortexed for 1.0 min and centrifuged at 9,000 rpm for 10 min. Two hundred microliters of the supernatant was transferred to a new tube and dried under a high-speed vacuum. The residue was reconstituted in 200 μl of 1% formic acid in acetonitrile, vortexed, and centrifuged at 9,000 rpm for 3 min. Then 190 μl was transferred to a vial ready for UPLC-tandem mass spectrometry (MS/MS) analysis.
Pharmacokinetic analysis.
The pharmacokinetic parameters were calculated based on a noncompartmental body model using Phoenix WinNonlin version 6.3 software.
The maximum plasma peak concentration (Cmax) and time to peak concentration (Tmax) were obtained directly from the individual plasma concentration-time profiles. The area under the concentration-time curve from time zero to time t (AUC0–t) was obtained using the trapezoidal method, and the area under the total plasma concentration-time curve from time zero to infinity (AUC0–∞) was calculated using the equation AUC0–t + (Clast/kel), where Clast is the concentration observed at the last time and kel is the apparent elimination rate constant obtained from the terminal slope of the individual plasma concentration-time curves after logarithmic transformation of the plasma concentrations and application of linear regression. Plasma elimination half-life (t1/2) was calculated as ln 2/kel. Apparent oral clearance (CL) and apparent volume of distribution (V) parameters were obtained from the plasma concentrations and expressed normalized to bioavailability (F) because only oral data are available.
Furthermore, computational simulations were calculated for the multiple-dose administration at two dose regimens: 100 mg of benznidazole administered every 8 h and 150 mg of benznidazole administered every 12 h.
Quantitative results were obtained by integrating the peak height of the specific MRM (multiple reaction monitoring) chromatogram. Calibration curves were assessed in a range of 0.1 to 3.0 μg/ml by linear regression. Origin was excluded, and a weighing factor of 1/x was used. Quality controls were prepared in plasma at three concentrations (0.3, 1.0, and 3.0 μg/ml). The lower limit of quantification (LLOQ) was 0.1 μg/ml according to the criteria of the coefficient of variation (CV) being <20% and an acceptable accuracy of 80 to 120% for the lowest concentration levels in the calibration curves.
ACKNOWLEDGMENTS
This work was supported by the European Union's Seventh Framework Programme for Research, Technological Development and Demonstration under grant agreement no. 30593 (Berenice Project) and Programa Ciências Sem Fronteiras (project no. A082/2013), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Brasil (Luísa Perin and Israel Molina).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have no conflicts of interest to declare.
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/AAC.02410-16.
REFERENCES
- 1.Grunberg E, Beskid G, Cleeland R, DeLorenzo WF, Titsworth E, Scholer HJ, Richle R, Brener Z. 1967. Antiprotozoan and antibacterial activity of 2-nitroimidazole derivatives. Antimicrob Agents Chemother 7:513–519. [PubMed] [Google Scholar]
- 2.WHO Expert Committee. 2002. Control of Chagas disease. World Health Organ Tech Rep Ser 905:i–vi, 1–109, back cover. [PubMed] [Google Scholar]
- 3.Raaflaub J, Ziegler WH. 1979. Single-dose pharmacokinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung 29:1611–1614. [PubMed] [Google Scholar]
- 4.Raaflaub J. 1980. Multiple-dose kinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung 30:2192–2194. [PubMed] [Google Scholar]
- 5.Altcheh J, Moscatelli G, Mastrantonio G, Moroni S, Giglio N, Marson ME, Ballering G, Bisio M, Koren G, García-Bournissen F. 2014. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PLoS Negl Trop Dis 8:e2907. doi: 10.1371/journal.pntd.0002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soy D, Aldasoro E, Guerrero L, Posada E, Serret N, Mejía T, Urbina JA, Gascón J. 2015. Population pharmacokinetics of benznidazole in adult patients with Chagas disease. Antimicrob Agents Chemother 59:3342–3349. doi: 10.1128/AAC.05018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina I, Gómez i Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sánchez-Montalvá A, Vidal X, Pahissa A. 2014. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N Engl J Med 370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 8.Soldin OP, Mattison DR. 2009. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soldin OP, Chung SH, Mattison DR. 2011. Sex differences in drug disposition. J Biomed Biotechnol 2011:187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richle RW, Raaflaub J. 1980. Difference of effective antitrypanosomal dosages of benznidazole in mice and man. Chemotherapeutic and pharmacokinetic results. Acta Trop 37:257–261. [PubMed] [Google Scholar]
- 11.Moraes CB, Giardini MA, Kim H, Franco CH, Araujo-Junior AM, Schenkman S, Chatelain E, Freitas-Junior LH. 2014. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci Rep 4:4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luna KP, Hernández IP, Rueda CM, Zorro MM, Croft SL, Escobar P. 2009. In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole. Biomedica 29:448–455. [PubMed] [Google Scholar]
- 13.Moreno M, D'ávila DA, Silva MN, Galvão LM, Macedo AM, Chiari E, Gontijo ED, Zingales B. 2010. Trypanosoma cruzi benznidazole susceptibility in vitro does not predict the therapeutic outcome of human Chagas disease. Mem Inst Oswaldo Cruz 105:918–924. doi: 10.1590/S0074-02762010000700014. [DOI] [PubMed] [Google Scholar]
- 14.Pinazo M-J, Guerrero L, Posada E, Rodríguez E, Soy D, Gascon J. 2013. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic Chagas disease. Antimicrob Agents Chemother 57:390–395. doi: 10.1128/AAC.01401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvador F, Sánchez-Montalvá A, Martínez-Gallo M, Sala-Cunill A, Viñas L, García-Prat M, Aparicio G, Sao Avilés A, Artaza MÁ, Ferrer B, Molina I. 2015. Evaluation of cytokine profile and HLA association in benznidazole related cutaneous reactions in patients with Chagas disease. Clin Infect Dis 61:1688–1694. [DOI] [PubMed] [Google Scholar]