ABSTRACT
We report the feasibility of enterocin AS-48, a circular cationic peptide produced by Enterococcus faecalis, as a new leishmanicidal agent. AS-48 is lethal to Leishmania promastigotes as well as to axenic and intracellular amastigotes at low micromolar concentrations, with scarce cytotoxicity to macrophages. AS-48 induced a fast bioenergetic collapse of L. donovani promastigotes but only a partial permeation of their plasma membrane with limited entrance of vital dyes, even at concentrations beyond its full lethality. Fluoresceinated AS-48 was visualized inside parasites by confocal microscopy and seen to cause mitochondrial depolarization and reactive oxygen species production. Altogether, AS-48 appeared to have a mixed leishmanicidal mechanism that includes both plasma membrane permeabilization and additional intracellular targets, with mitochondrial dysfunctionality being of special relevance. This complex leishmanicidal mechanism of AS-48 persisted even for the killing of intracellular amastigotes, as evidenced by transmission electron microscopy. We demonstrated the potentiality of AS-48 as a new and safe leishmanicidal agent, expanding the growing repertoire of eukaryotic targets for bacteriocins, and our results provide a proof of mechanism for the search of new leishmanicidal bacteriocins, whose diversity constitutes an almost endless source for new structures at moderate production cost and whose safe use on food preservation is well established.
KEYWORDS: enterocin AS-48, intracellular parasite, antimicrobial peptide, bioenergetics
INTRODUCTION
The current antibiotic crisis has led to a rampant decline of effective drugs against infectious diseases, including those caused by protozoans (1, 2). This shortage of chemotherapeutical resources is of special concern for the so-called neglected tropical diseases (NTDs) (3), whose treatment is almost exclusively limited to already meager chemotherapeutic arsenals, and with a pipeline of new leads barely populated (4). As a result, treatments for NTDs are extremely vulnerable to the loss of effectiveness of just a single drug.
In this regard, leishmaniasis is a case in point. It is a protozoan disease caused by infection with different species of the genus Leishmania. Leishmaniasis encompasses a broad spectrum of clinical symptoms with an incidence of 10 to 12 million people infected worldwide, mostly in tropical or subtropical regions (5). At present, its clinical treatment is based on six drugs, which are threatened by growing resistance, severe side effects, and implementation cost (6). In the short term, combination therapy (7) and drug repurposing (8) may temporally delay this menace until new leads are approved for clinical usage.
In the quest for new antileishmanial agents, several eukaryotic antimicrobial peptides (EAMPs) were successfully assayed at a preclinical stage on this protozoan (9, 10). Many EAMPs kill the target organisms by disruption of the phospholipid matrix of the membrane, mediated through their privileged interaction with the anionic phospholipids exposed at the external medium, with ensuing loss of the membrane's function as a permeability barrier (11, 12). Membrane-active EAMPs constitute an appealing alternative to the shortage of antibiotics, due to their broad spectra of susceptible pathogens and extremely scarce induction of resistance compared to that seen with classical antibiotics (13). Interestingly, membrane disruption of the targeted cell is also the final lethal outcome for many antibiotic bacterial peptides, either encoded by genes as bacteriocins or synthesized by the nonribosomal peptide synthases.
In the last years, there has been an increasing awareness of bacteriocins as a new source of anti-infective agents (14–16), even transcending those infections from a bacterial origin (antifungal or antiviral), substantiated by their staggering structural diversity, feasibility of genetic engineering, safe use as food biopreservatives (17, 18), and safe toxicity profile on higher eukaryotic cells (19). In this trend, the number of reports concerning bacteriocins as antiprotozoal agents (20–23) is surprisingly lower than that of reports aiming at other eukaryotic targets such as fungi (24–26) or even tumoral cells (27–29).
With this background in mind, we sought to investigate the leishmanicidal activity of AS-48, a 70-residue circular bacteriocin (molecular mass, 7.149 kDa), highly cationic (pI = 10.09) and with an amphipatic structure (30, 31). The choice of AS-48 for this goal is due to its low immunogenicity (32) and to its wide bactericidal activity on Gram-positive bacteria (17, 30). AS-48 is a highly compact and stable molecule (31), preserving its activity under harsh environmental conditions, as such suitable for effectiveness either inside the parasitophorous vacuole of the macrophage, where the amastigote dwells, or under tropical climate conditions. In addition, AS-48 does not require a cognate receptor at the target membrane, being active even on planar (33) and liposomal (34) model membranes. Furthermore, AS-48 has an excellent record in preventing human food spoilage (17). More importantly, a patent was issued for the use of AS-48 against acne and other skin bacterial infections (35), paving the way for a clinical application of this bacteriocin on the ulcers of nondisseminated cutaneous leishmaniasis.
Altogether, AS-48 was lethal on both axenic promastigotes and amastigotes of Leishmania at low micromolar concentrations with scarce toxicity on the host cell. Furthermore, it was active on intracellular amastigotes without prior encapsulation, a rare feature in bacteriocins. This work provides a proof of mechanism for the use of membrane-active bacteriocins as a new group of leishmanicidal agents, with little host cytotoxicity and affordable costs of production.
RESULTS
Leishmanicidal and cytotoxic activities of AS-48.
The leishmanicidal activities of AS-48 were assessed by the inhibition of MTT [(3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reduction. When measured immediately after 4 h of incubation with AS-48, the resulting 50% inhibitory concentrations (IC50s) and IC90s were 3.9 ± 1.1 μM and 9.4 ± 1.2 μM, respectively, for Leishmania donovani promastigotes, whereas for Leishmania pifanoi axenic amastigotes, these values were 10.2 ± 1.2 μM and 19.5 ± 2.1 μM, respectively. To assess inhibition of proliferation, the surviving parasites after the 4 h of incubation with AS-48 were allowed to grow in the absence of AS-48; IC50s and IC90s for promastigotes decreased to 1.3 ± 0.2 μM and 2.7 ± 0.4 μM and for axenic amastigotes to 7.5 ± 0.7 μM and 15.5 ± 2.1 μM, respectively. Thus, axenic amastigotes were almost 6-fold more resistant than promastigotes to AS-48. In addition, the deleterious effect of AS-48 on Leishmania was not fully completed after 4 h of incubation.
Variation of intracellular ATP levels in L. donovani promastigotes caused by AS-48.
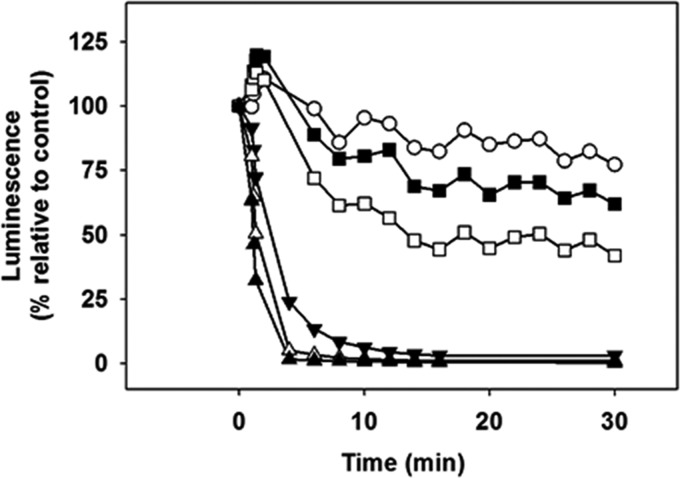
The content of intracellular ATP is an excellent parameter to assess the viability of the parasites. Real-time variation of cytoplasmic free ATP was monitored on living L. donovani promastigotes of the 3-Luc strain that express a cytoplasmic form of firefly luciferase. In the presence of DMNPE-luciferin [D-luciferin, 1-(4,5-dimethoxy-2-nitrophenyl)ethyl ester], a free-membrane caged substrate of luciferase, ATP was the limiting substrate for the luminescence output. The luminescence of 3-Luc promastigotes underwent a concentration-dependent decrease of luminescence after AS-48 addition. At concentrations close to the IC50 for AS-48 (3.1 μM), the luminescence decreased by nearly 50% with respect to untreated promastigotes (Fig. 1). To rule out inhibition of firefly luciferase, a commercial luciferase was assayed in vitro in the presence of AS-48. At 50 μM, the highest AS-48 concentration tested, reduction of luciferase activity was 4% (data not shown).
FIG 1.
In vivo monitoring of intracellular ATP levels of L. donovani 3-Luc promastigotes after AS-48 addition. Promastigotes were resuspended at 2 × 107 cells/ml. DMNPE-luciferin was added at 25 μM. Once the readout became stable, AS-48 was added (t = 0), and variation in luminescence was expressed as the percentage of luminescence with respect to the luminescence of control, untreated parasites. AS-48 micromolar concentration is indicated by symbols: ○, 0.8; ■, 1.6; □, 3.1; ▼, 6.2; △, 12.5; ▲, 25.0. These results are from an experiment representative of three other experiments performed independently.
Permeabilization of the plasma membrane of L. donovani promastigotes by AS-48.
The two more-feasible alternatives to account for the decrease of intracellular ATP were either plasma membrane permeabilization or inhibition of ATP synthesis. Membrane permeabilization was assessed by the entrance of vital dyes impermeable to organisms with an intact plasma membrane as well as by membrane depolarization, accounting for the dissipation of ionic gradients across the membrane (Fig. 2).
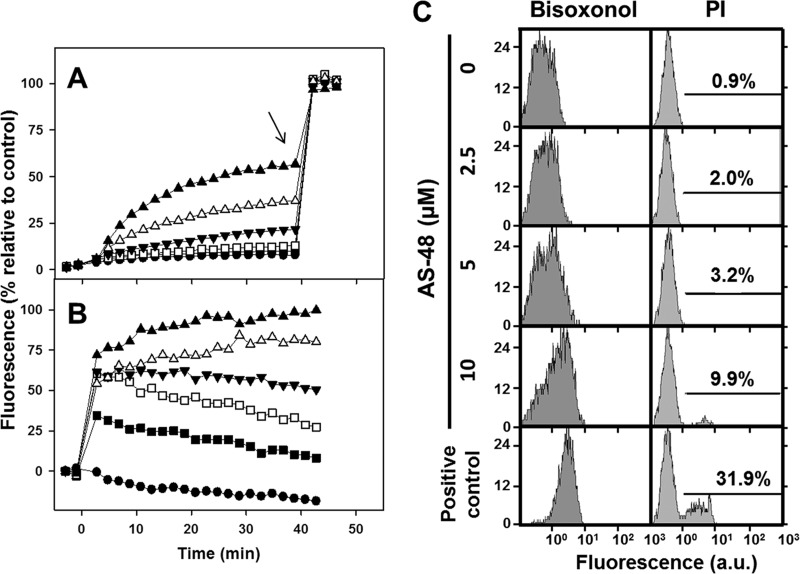
FIG 2.
Permeabilization of the plasma membrane of L. donovani promastigotes by AS-48. Parasites were resuspended at 2 × 107 cells/ml in HBSS-Glc with the corresponding probe. AS-48 was added (t = 0), and fluorescence is noted as a percentage relative to that of fully permeabilized parasites. (A) Kinetics of intracellular entrance of the vital dye Sytox green. Promastigotes were resuspended in HBSS-Glc–1 μM Sytox green. Fluorescence settings: λEXC = 485 nm, λEM = 520 nm. The arrow shows the time of the addition of TX-100 (final concentration, 0.1%), taken as fully permeabilized parasites. (B) Kinetics of plasma membrane depolarization. Parasites were resuspended in HBSS-Glc–0.1 μM bisoxonol. Fluorescence settings: λEXC = 544 nm, λEM = 584 nm. Fully depolarized parasites (100% fluorescence) were considered to be those treated with 10 μM CA(1-8)M(1-18). AS-48 micromolar concentration is indicated by symbols: ●, 0.78; ■, 1.6; □, 3.1; ▼, 6.2; △, 12.5; ▲, 25.0. (C) Endpoint cytofluorometric determination for both parameters after 4 h of incubation. Propidium iodide (PI; 1 μg/ml) and bisoxonol (0.1 μM) were added to the parasites 5 min before the cytofluorometric analysis. Fluorescence settings: for bisoxonol, λEXC = 488 nm, λEM = 525 nm; for PI, λEXC = 488 nm, λEM = 620 nm. Positive controls for permeabilization were 0.1% TX-100 (PI) and 10 μM CA(1-8)M(1-18) (bisoxonol). The percentage of the fully permeabilized population is shown inside the corresponding histogram. a.u., arbitrary units.
After addition of AS-48 to the promastigotes suspension, Sytox green fluorescence increased in a concentration-dependent manner (Fig. 2A). Nevertheless, even at 25 μM AS-48, the highest concentration tested, fluorescence never reached the value obtained for fully permeabilized parasites, obtained by treatment with 0.1% TX-100.
To assess subtler membrane damage by AS-48, plasma membrane depolarization was next assayed. After AS-48 addition, the fluorescence of the potential-sensitive dye bisoxonol increased rapidly, due to its insertion into the hydrophobic matrix of the membrane, precluded in polarized parasites (Fig. 2B). This effect occurred even at 1.5 μM AS-48, the lowest concentration tested. Only at 25 μM AS-48 did the fluorescence match that obtained with 10 μM CA(1-8)M(1-18), a membrane-active leishmanicidal peptide causing full depolarization of promastigotes (36). Nevertheless, for AS-48 concentrations of <3.1 μM, the promastigotes experimented a slow repolarization, suggesting a partially reversible membrane injury by the bacteriocin at this concentration range.
Additionally, both parameters were simultaneously assayed for endpoint values by two-channel cytofluorometry. Sytox green was replaced by propidium iodide (PI) as a vital dye to avoid fluorescence overlapping with bisoxonol. Both dyes were added to the parasite suspension after the end of the incubation (Fig. 2C). Parasites treated with increasing AS-48 concentrations showed a progressive shift toward higher fluorescence values of bisoxonol. Only a single parasite population was conspicuous throughout the range of concentrations assayed. In contrast, two clearly differentiated parasite populations were discerned for PI fluorescence at the highest AS-48 concentration tested. Both populations were characterized by a characteristic mean fluorescence value. The increase in AS-48 concentration led to higher percentages of parasites into the upper fluorescence population, without a shift in the fluorescence position, assimilated to an all-or-none process for PI entry.
Internalization of AS-48 into L. donovani promastigotes.
The previous results suggested the involvement of additional intracellular targets to the membrane permeabilization damage in the final leishmanicidal outcome of AS-48. For this, access of AS-48 into the intracellular milieu would be required.
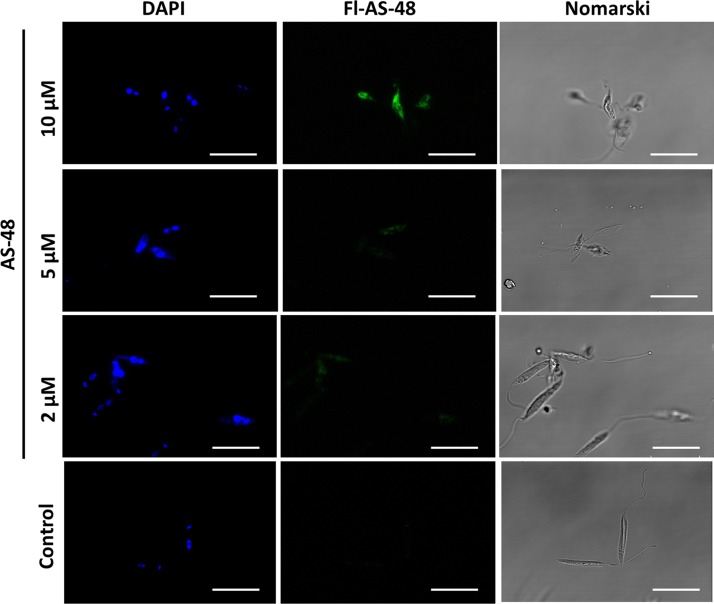
Thus, the Fl-AS-48 accumulated inside the promastigotes after 4 h of incubation, as assessed by confocal microscopy (Fig. 3). Bacteriocin uptake was perceptible even at 2 μM, a concentration lower than its IC50 under these conditions. The intracellular accumulation of Fl-AS-48 increased with higher concentrations (5 μM and 10 μM). Nevertheless, severely distorted morphology was observed only at 10 μM AS-48 and not at lower concentrations.
FIG 3.
Confocal microscopy of L. donovani promastigotes treated with fluoresceinated AS-48. Promastigotes were incubated with fluoresceinated AS-48 (Fl-AS-48) at different concentrations. Incubation was carried out with 2 × 107 cells/ml for 4 h in HBSS-Glc. Promastigotes were stained with DAPI (10 μg/ml, 5 min) prior to their observation as unfixed parasites in confocal microscopy. Nomarski, differential interference contrast microscopy. Fluorescence settings: Fl-AS-48, λEXC = 488 nm, λEM = 519 nm; DAPI, λEXC = 358 nm, λEM = 461 nm. Bar, 10 μm.
The intracellular accumulation of AS-48 into L. donovani promastigotes was severely inhibited at 4°C (see Fig. S1 in the supplemental material). This supports an AS-48 uptake mediated by endocytosis rather than through its direct translocation across the membrane.
Damage to the mitochondria of L. donovani promastigotes caused by AS-48.
The study of a dysfunctional mitochondrion in Leishmania induced by AS-48 was prompted by two results: (i) the fast and severe bioenergetic collapse in AS-48-treated Leishmania parasites in the absence of a substantial plasma membrane permeabilization and (ii) the capacity of AS-48 to access the intracellular space of Leishmania.
AS-48 induced the depolarization of the single mitochondrion of Leishmania, as assessed by rhodamine 123 (Rho123) accumulation. AS-48 at 2.5 μM (lower than its IC50 under the standard assay conditions) decreased the intracellular accumulation of Rho123 by 40% (Fig. 4A) in the absence of Sytox green entrance (Fig. 2A).
FIG 4.
Assessment of mitochondrial damage to L. donovani promastigotes by AS-48. (A) Inhibition of ΔΨm and respiration (inset). Parasites were incubated with AS-48 for 4 h (HBSS-Glc, 2 × 107 cells/ml), loaded with rhodamine 123, and analyzed by cytofluorometry. Parasites incubated with 10 mM KCN were used for positive control of mitochondrial depolarization. (A) (Inset) Oxygen consumption rate of promastigotes treated with AS-48. Respiration was measured at 108 cells/ml in a Clark oxygen electrode, and values were expressed as percentages with respect to respiration in untreated cells. Lanes 1, 2, and 3, parasites treated with AS-48 at 12.5, 25, and 50 μM, respectively; lane 4, parasites treated with 10 μM CA(1-8)M(1-18) as a positive internal control. (B) Production of mitochondrial ROS induced by AS-48. Parasites were loaded with 0.5 μM Mitosox Red. Control parasites (black [left] bars). Parasites treated either with 3 μg/ml antimycin A (light-gray [middle] bars) as a positive control or with 5 μM AS-48 (dark-gray [right] bars). a.u., arbitrary units. Samples were taken at different times and analyzed by cytofluorometry (λEXC = 488 nm, λEM = 520 nm); *, P < 0.05; **, P < 0.01.
The mitochondrial accumulation of Rho123 is driven by the mitochondrial electrochemical potential (ΔΨm), a parameter governing the respiration rate. AS-48 decreased the oxygen consumption rate of promastigotes in a concentration-dependent manner (Fig. 4A, inset). At 12.5 and 50 μM AS-48, promastigote respiration was inhibited by 30% and 75%, respectively. The higher cellular density required by this technique (5-fold higher than the standard value) accounted for the higher AS-48 concentrations required.
AS-48 increased local production of ROS, assessed by the increase in fluorescence of Mitosox Red. After AS-48 (5 μM) addition to the parasites, the accumulated reactive oxygen species (ROS) production at 30 min was approximately 40% of that elicited by antimycin A, used as a positive control (Fig. 4B).
ROS overproduction in Leishmania is frequently associated with programmed cell death processes through mitochondrial dysfunction (37). After 4 h of incubation, AS-48 induced sub-G1 appearance at 5 μM. The percentage of sub-G1 cells at 25 μM AS-48 closely matched that induced by hexadecylphosphocholine (HePC) (Miltefosine), a typical apoptotic inducer in Leishmania spp. (38–40) (see Fig. S2 in the supplemental material).
Leishmanicidal activity of AS-48 on intracellular L. pifanoi amastigotes.
The cidal activity of AS-48 was first assayed on cells of the Raw 264.7 murine monocytic cell line, used as host cells for Leishmania. The inhibition of MTT reduction by this bacteriocin was 22.0% ± 2.6% at 50 μM, the highest concentration tested (data not shown).
At 7.0 μM, AS-48 decreased the parasitization index of the macrophage from 3.42 ± 0.14 in untreated macrophages to 0.41 ± 0.04, a reduction close to 90%. Also, the percentage of infected macrophages decreased from 55.3 ± 7.0 in untreated macrophages to 18.3 ± 3.2 after AS-48 treatment.
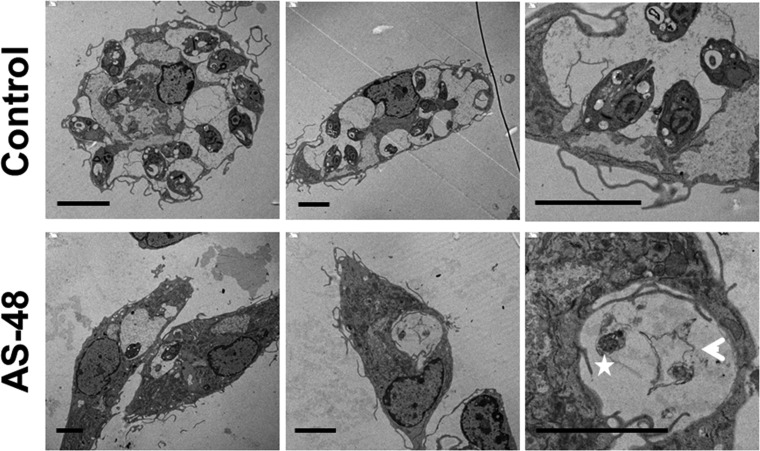
The decrease in intracellular L. pifanoi parasites was also confirmed by electron microscopy. Cellular debris from killed intracellular amastigotes were spotted inside AS-48-treated macrophages (Fig. 5, lower row) but not in control parasites (Fig. 5, upper row). Furthermore, Fl-AS-48 accumulated preferentially in intracellular amastigotes rather than in the host cell (see Fig. S3 in the supplemental material). Thus, a direct action of AS-48 on the intracellular amastigotes is feasible, even when AS-48 may trigger additional leishmanicidal effects mediated by the macrophage.
FIG 5.
Electron microscopy of RAW 264.7 murine macrophage cells infected with L. pifanoi and treated with AS-48. Raw 264.7 cells were infected with L. pifanoi axenic amastigotes as described in Materials and Methods. Once the infection was established, infected macrophages were left untreated or were treated with 7 μM AS-48 for 24 h and processed for electron microscopy. The white arrow and star indicate the cellular debris from killed amastigotes inside the parasitophorous vacuole of AS-48-treated macrophages. Upper row, control parasites; lower row, AS-48-treated parasites. Bar, 10 μm.
DISCUSSION
In this work, we have explored the leishmanicidal activity of AS-48, a bacteriocin produced by Enterococcus faecalis strains, as a feasible candidate for further pharmacological development. The IC50 of AS-48 on promastigote proliferation is 1.3 μM, a value similar to those reported for some Gram-positive bacteria (17, 34, 41, 42) but significantly lower than many membrane-active eukaryotic peptides (9, 13). AS-48 shares with many EAMPs the permeation of the cell membrane as an essential step of its bactericidal mechanism. Furthermore, the entrance of vital dyes, induction of a bioenergetic collapse, and release of cytoplasmic material induced by AS-48 in Leishmania also support this mode of action.
AS-48 does not require a cognate macromolecular receptor at the targeted cell for its bactericidal activity, which is mandatory for other bacteriocins at low concentration (43). The specificity of AS-48 and of most EAMPs to bacterial membranes is achieved by the recognition of acid phospholipids of the target membrane exposed to the external medium (33, 34), a feature present also in Leishmania. Thus, the higher efficacy of AS-48 than that of other EAMPs should rely on its mode of interaction with the membrane. In fact, a very low number of bacteriocin molecules is required to kill bacteria (17). How AS-48 induces membrane permeabilization is not yet fully understood. Both the formation of canonical pores (34, 44, 45) and the induction of phospholipid discontinuities by insertion of AS-48 molecules (44, 46) have been proposed to account for this process. Regardless of how AS-48 achieved membrane permeabilization, both modes were highly dependent on the local density of AS-48 in the membrane. As AS-48 is oligomeric in aqueous solution (47), mostly as dimers (45), once they are inserted into the membrane the number of local AS-48 molecules will be already disruptive, or they will become so once a few additional AS-48 molecules are recruited into this incipient permeating structure, as envisaged by coarse graining modeling (44). This mode of insertion and membrane permeation by AS-48 is highly advantageous over that of other EAMPs, whose monomers insert independently into the membrane, for which a further assembly into a functional membrane-permeating structure is mandatory (48).
AS-48 has a lower activity on axenic amastigotes than on promastigotes. This trend was also described for some EAMPs (10, 49), but its underlying molecular rationale has not yet been uncovered. Differences between the two stages on phospholipid composition or its asymmetrical distribution at the two layers of the plasma membrane may be considered, but a detailed report on this issue is available only for promastigotes (50). Membrane potential influences the bactericidal activity of AS-48 (41) but not the permeation of artificial vesicles (34). Furthermore, the values reported for membrane potential in amastigotes and in promastigotes were quite similar (51), despite the significant difference between their respective proliferation rates. In contrast, lipophosphoglycan (LPG), the main oligosaccharide constituent of the glycocalyx of Leishmania promastigotes, is almost absent in amastigotes (52). The phosphate groups of LPG will create an anionic environment that would facilitate a high density of AS-48 close to the plasma membrane, promoting after AS-48 insertion the formation of the “putative” active permeating structures (47).
Nevertheless, the leishmanicidal activity of AS-48 cannot be solely ascribed to a membrane permeation mechanism; intracellular targets were envisaged as well. The entrance of Sytox green was only partial, even at AS-48 concentrations close to full lethality in promastigotes. The leishmanicidal mechanism of AS-48 was not finished after 4 h, while for a sheer typical membrane permeation, including AS-48 on bacteria, completion is often reached in a few minutes. Finally, fluoresceinated AS-48 was spotted inside Leishmania below its IC50. The inhibition of AS-48 uptake at 4°C ruled out its entrance through a direct translocation across the plasma membrane of the parasite. The membrane pores formed by AS-48 were unlikely to be used as a gate for intracellular entrance of new additional bacteriocin molecules in Leishmania. The reported dimensions for AS-48 pores, 2.1 nm (44) and 0.7 nm (34), will allow entrance of only monomeric forms of AS-48 (20 Å) (31). In addition, even a small molecule such as Sytox green (molecular mass, 600) showed only partial entrance in AS-48-treated parasites. The formation of a mixed phospholipid-peptide toroidal pore, predicted by molecular dynamics (44), will easily afford the translocation of AS-48 across the membrane, similar to some other cell-penetrating peptides (53).
The single mitochondrion of Leishmania is a likely target for AS-48. Aside from its evolutionary resemblance with bacteria, cardiolipin, a typical mitochondrial phospholipid, interacts strongly with AS-48 (42). AS-48 induced a fast depletion of ATP, whose synthesis in Leishmania is carried out by oxidative phosphorylation. Damage to the functionality of the respiratory chain by AS-48 is also supported by the decrease in respiration, with a concomitant rise in mitochondrial ROS production measured by MitoSox Red fluorescence (54). The use of mitochondria as targets for bacteriocins was also described for microcin J25 (55), both inside tumoral cells and as an isolated organelle.
To note, AS-48 also reduced the parasite burden of infected macrophages, even at concentrations lower than those required for axenic amastigotes. We may surmise a privileged accumulation of the cationic AS-48 at the parasitophorous vacuole, achieved by its binding to the anionic oligosaccharides of the extracellular matrix of the macrophage. Furthermore, oligomerization of AS-48 is dependent on the concentration of the bacteriocin and on the pH (47).
By electron microscopy, intracellular amastigotes in AS-48-treated macrophages showed a mixed pattern of morphological alterations suitable for a membrane damage scenario but also consistent with involvement of other intracellular targets. Subtle changes in the local concentration of AS-48 may be responsible for this difference. To note, bacteriocins or bacteriocin-like peptides were active on other intracellular pathogens, such as Legionella (56) and Mycobacterium (57), but only on axenic cultures or after pharmacological vehiculation (58). In this case, AS-48 is a rare exception, being able to reach and kill intracellular amastigotes without further manipulation. Even though AS-48 is considered safe for the preservation of human food (59), we cannot discard an indirect leishmanicidal mechanism of the macrophage, triggered by the bacteriocin. Induction of nitric oxide synthase (NOS2), key for the leishmanicidal activity of the macrophage, was effected by other bacteriocins, such as BacSp222 from Staphylococcus pseudintermedius (60), as well as by the cecropin A-melittin hybrid peptide CA(1-8)M(1-18), a surrogate of natural EAMPs (61), through subtle and reversible plasma membrane permeation.
The leishmanicidal activity of AS-48 paves the way for the use of bacteriocins not only against this parasite but also to treat other trypanosomatid infections. A caveat regarding the wider spectra of protozoan targets came from AS-48 inactivity against Acanthamoeba and Naegleria, two free-living parasitic amoebae (41). Several characteristics of AS-48 are quite advantageous for its further pharmacological development as a new leishmanicidal agent. AS-48 can be produced in large amounts from industrial fermentation broths at a reasonable cost (62, 63) and possesses a very highly stable and compact structure, resistant to exopeptidase degradation, as well as low immunogenicity (32). Furthermore, AS-48 was recently patented as a topical antibacterial agent for skin infections (35), suggesting a relatively easy extrapolation into the ulcers in nondisseminated cutaneous leishmaniasis, inasmuch as AS-48 maintains its activity on high ionic strength, suitable to the hypertonic microenvironment of the skin immune cells (64). Nowadays, the native form of AS-48 is mandatory for its use. All the cutting-edge strategies aimed to improve the microbicidal activity of AS-48 by mutational analysis or to get minimalist AS-48 analogues were unsuccessful (65), accounting for the dubbing of AS-48 as “close to perfection.” Nevertheless, two new aspects for AS-48 optimization have been recently tackled: first, the molecular basis for dimer stability may allow the design of new AS-48 mutants using bioinformatics tools, with improved antimicrobial activity that would facilitate its insertion into the membrane (46); second, the report of the full chemical synthesis of AS-48, including a final enzymatic circularization (66), opens the possibility of modification of residues otherwise essential for the correct biosynthesis and processing of AS-48, as well as the incorporation of nonproteinogenic amino acids into its structure.
MATERIALS AND METHODS
Reagents.
All reagents were of the highest quality available and purchased from Sigma-Aldrich (Madrid, Spain), unless otherwise stated. Fetal calf serum was obtained from Gibco-BRL (Paisley, UK). Bisoxonol [bis-(1,3-diethylthiobarbituric) trimethineoxonol], DAPI (4′,6-diamidino-2-phenylindole), propidium iodide (PI), and rhodamine 123 (Rho123) [2-(6-amino-3-imino-3H-xanthen-9-yl) benzoic acid methyl ester chloride] were purchased from Invitrogen (Carlsbad, CA). DMNPE-luciferin [D-luciferin, 1-(4,5-dimethoxy-2-nitrophenyl)ethyl ester] was obtained from GoldBio (St. Louis, MO).
Cells.
Leishmania donovani promastigotes (strain MHOM/SD/00/1S-2D) were grown at 25°C in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (RPMI-HIFCS). Growth of 3-Luc promastigotes, with episomal expression of a cytoplasmic form of Photinus pyralis luciferase, was carried out as described above except for the addition of 30 μg/ml Geneticin (G-418; Gibco-BRL) into the growth medium. L. pifanoi axenic amastigotes (strain MHOM/VE/60/Ltrod) were grown at 32°C in 199 medium (Gibco-BRL) supplemented with 20% HIFCS, as described previously (36). This cell line was selected due to its well-established record of similarity between axenic and lesion amastigotes, as evidenced by a set of morphological, antigenic, biochemical, and metabolic markers, which were maintained through long-term culture (reviewed in reference 67). This ensures the homogeneity of the target organisms throughout the whole set of experiments. Quantitation of intracellular parasites is easily achieved inside the large and distended parasitophorous vacuole in the macrophage, a feature shared by all the Leishmania species of the mexicana complex, which also encompasses L. amazonensis and L. venezuelensis, with close pathological (68) and genetic (69) proximity to L. pifanoi.
Cells of the tumoral murine macrophage line RAW 264.7 (Cell Culture Facility, CIB; obtained in March 2015) were grown in the same medium as the L. donovani promastigotes, at 37°C in a 5% CO2 atmosphere.
AS-48 purification.
AS-48 was purified from cultures of the Enterococcus faecalis strain UGRA10 (70) grown on Esprion-300 (E-300; DMV Int., Veghel, Netherlands) medium supplemented with 1% glucose (E-300-G) under controlled pH 6.5 conditions (71). The resulting supernatant was concentrated, and AS-48 was purified to homogeneity by cationic exchange chromatography followed by reversed-phase high-performance liquid chromatography (RP-HPLC), as described previously (72). The protein concentration of the purified AS-48 was determined by UV absorption at 280 nm.
Assessment of leishmanicidal activities.
Parasites were harvested at late exponential phase of growth, washed twice in Hanks' balanced salt solution supplemented with 10 mM d-glucose (pH 7.2) (HBSS-Glc) at 4°C, and resuspended in the same medium. Assays were carried out with 2 × 107 cells/ml in HBSS-Glc medium. Parasites were incubated for 4 h with the corresponding AS-48 concentration at 26°C or 32°C for promastigotes or amastigotes, respectively. These conditions were defined as the standard assay, unless otherwise stated.
Parasites resuspended in HBSS-Glc were aliquoted into a 96-microwell plate (120 μl/well) and incubated with AS-48 under the standard conditions described above. After the incubation with AS-48, 20 μl from each well was transferred into a replica microplate containing 180 μl/well of the corresponding growth medium. The surviving parasites were allowed to proliferate (promastigotes, 72 h, 25°C; axenic amastigotes, 120 h, 32°C) and evaluated by MTT reduction. To this end, MTT was added to each well (final concentration, 0.5 mg/ml), and its reduction by the cells was allowed to proceed for 2 h. The resulting formazan was solubilized by addition of SDS (final concentration, 0.5%) and read at 595 nm in a 680 microplate reader (Bio-Rad). The short-term effects of the bacteriocin on the parasites were evaluated by adding MTT into each well of the plate containing the remaining parasites (100 μl) immediately after the 4 h of incubation with AS-48. The cytotoxicity of AS-48 on RAW 264 cells was assayed by MTT reduction. Cells were seeded in a 96-microwell plate (5 × 104 cells/well); once they had adhered, the medium was replaced by HBSS-Glc and AS-48 was added. After incubation with the bacteriocin for 4 h, the cells were washed and inhibition of MTT reduction was measured as described above.
Leishmanicidal activity of AS-48 on RAW 264.7 macrophages infected with L. pifanoi amastigotes.
Cells of the tumoral murine cell line RAW 264.7 were resuspended in RPMI-HIFCS and seeded into sterile coverglasses at the bottom of a 24-well microplate (2.5 × 105 cells in 500 μl/well). Cells were allowed to attach overnight (37°C, 5% CO2). Afterwards, L. pifanoi axenic amastigote infection was carried out at a 2:1 amastigote-to-macrophage ratio. Once phagocytosed (4 h, 32°C, 5% CO2) the noninternalized amastigotes were removed by washing. Finally, 1 ml of RPMI–2% HIFCS was added to each well, and intracellular amastigotes were allowed to proliferate for 72 h at 32°C. Then, AS-48 was added into the plate, which was incubated for 48 h. Finally, coverglasses were removed and stained with Giemsa stain. The percentages of infected macrophages and average numbers of amastigotes per macrophage were calculated by counting at least 500 macrophages per sample in four different fields of the preparation, using an optical microscope.
Real-time variation of intracellular ATP in living L. donovani promastigotes by AS-48.
For quantitation of intracellular ATP, a procedure described previously (36) was followed. Briefly, promastigotes from the 3-Luc strain, expressing a cytoplasmic firefly luciferase, were incubated (2 × 107 cells/ml) with AS-48 under the standard conditions at 25°C in the presence of the 25 μM DMNPE-luciferin, a membrane-permeable caged substrate of luciferase. Under these conditions, the limiting substrate for luminescence output of living parasites is their free cytoplasmic ATP. Once the luminescence reached a steady value, AS-48 was added and the variation of luminescence was monitored in a Polarstar Galaxy Microwell plate reader (Offenburg, DE) using the luminescence setting for reading.
Permeabilization of the plasma membrane of L. donovani promastigotes by AS-48.
Two complementary parameters were measured (36): plasma membrane depolarization and entrance of vital dyes.
Variations in the plasma membrane potential were monitored using the anionic dye bisoxonol. The fluorescence of this dye increases when inserted into the hydrophobic matrix of the membrane, precluded in parasites with a polarized membrane. Assays were performed under standard conditions, except for the presence of 0.1 μM bisoxonol in the incubation medium. Once the fluorescence readout became stable, AS-48 was added and fluorescence changes were registered in a Polarstar Galaxy microplate spectrofluorometer (BGM Labotechnologies, Offenburg, Germany) (excitation wavelength [λEXC], 544 nm; emission wavelength [λEM], 584 nm). The maximal depolarization was considered to be that obtained with 10 μM CA(1-8)M(1-18), a membrane-active leishmanicidal peptide (13).
The entrance of the cell-impermeable dye Sytox green (molecular mass, 600) into L. donovani promastigotes was assayed under the standard assay conditions. Briefly, parasites were resuspended at 2 × 107 cells/ml in HBSS-Glc containing 1 μM Sytox green. Once a stable readout of the fluorescence was reached, AS-48 was added, and the increase in fluorescence due to binding of the dye into intracellular nucleic acids was measured in a Polarstar Galaxy microplate spectrofluorometer (λEXC = 485 nm; λEM = 520 nm). The maximal permeabilization (100%) was considered to be that obtained in the presence of 0.1% Triton X-100.
In addition, simultaneous assessments of the endpoint variations for both parameters in Leishmania were carried out by cytofluorometry. Promastigotes were incubated with AS-48 under the standard conditions for 4 h. Afterwards, parasites were washed in HBSS-Glc, diluted in the same medium at 106 cells/ml, and incubated with 1 μg/ml PI plus 0.1 μM bisoxonol for 5 min. Then, samples were immediately analyzed in an FC500 Coulter cytofluorometer (fluorescence settings: for bisoxonol, λEXC of 488 nm and λEM of 525 nm; for PI, λEXC of 488 nm and λEM of 620 nm).
Mitochondrial dysfunction in L. donovani promastigotes caused by AS-48.
The variation of ΔΨm caused by AS-48 was monitored by the intracellular accumulation of Rho123. After parasite incubation with AS-48 under the standard conditions, cells were diluted to 106 cells/ml in HBSS-Glc, loaded with Rho123 (0.3 μg/ml, 10 min, 26°C), and analyzed by cytofluorometry as described previously (73). Parasites with a fully depolarized mitochondrion were obtained by incubation with KCN (10 mM, 30 min) prior to Rho123 loading.
Oxygen consumption rates were measured using a Clark oxygen electrode (Hansatech, King's Lynn, UK) as described previously (73). Briefly, promastigotes were resuspended at 108 cells/ml in respiration buffer containing 2 mM succinate and 10 mM glucose. Experiments were carried out at 26°C. Once the respiration of the promastigotes reached a steady value, AS-48 was added at the corresponding concentration and variations in the respiration rate were recorded.
The production of reactive oxygen species (ROS) in the mitochondrion was evaluated with Mitosox Red (54). Parasites were loaded with Mitosox Red (0.5 μM, 30 min, 26°C). AS-48 was added at 5 μM, and samples were removed at different times (3 min to 90 min) for fluorescence measurement by flow cytometry in an FC500 Coulter cytofluorometer (Becton-Dickinson, San Jose, CA) (λEXC = 488 nm; λEM = 520 nm). Antimycin A (0.3 μg/ml) was used as a positive control.
Confocal microscopy of L. donovani promastigotes treated with fluoresceinated AS-48.
Fluorescein-labeled AS-48 (Fl-AS-48) was obtained by reaction of AS-48 with fluorescein isothiocyanate (FITC) in 50 mM carbonate buffer (pH 9.2). The final concentration of AS-48 in the reaction mixture was adjusted to 35 μM; FITC was added at an equimolar concentration, and conjugation was allowed to proceed at 4°C overnight. Afterwards, nonconjugated FITC was removed by successive cycles of HBSS addition and centrifugation on a Biomax-5K 0.5 ml (Millipore). The absorbance ratio of the resulting Fl-AS-48 at 495 nm and 280 nm was used to calculate the degree of fluorescein dye conjugation. Only a single Fl-AS-48 batch was used for the whole set of experiments, with an average conjugation of 0.39 fluorescein molecules per AS-48 monomer.
Promastigotes were incubated under the standard conditions with 5 μM Fl-AS-48 for 4 h, at 26°C and 4°C. Confocal microscopy of RAW 264.7 macrophage-infected L. pifanoi amastigotes was carried out according to the protocol for leishmanicidal activity (see above). Fl-AS-48 in full growth medium was added to the infected cells at 5 μM, and the mixture was incubated at 32°C for 14 h.
Regardless of the Leishmania stage analyzed, all samples were stained with 10 μg/ml DAPI (5 min, 26°C for promastigotes; 30 min, 32°C for infected macrophages) prior to their microscopic observation. Nonincorporated dye was removed by washing, and cells were observed unfixed in a laser confocal microscope Leica TCS SP2 (λEXC = 488 nm/λEM = 519 nm and λEXC = 358 nm/λEM = 461 nm for Fl-AS-48 and DAPI, respectively).
Transmission electron microscopy of L. pifanoi-infected macrophages treated with AS-48.
Macrophages were infected as described for the measurement of intracellular leishmanicidal activity, except that AS-48 was added at 5 μM and the incubation time was shortened to 14 h. Afterwards, the samples were processed as described previously (36). Briefly, parasites were fixed with OsO4 (2.5%, wt/vol), gradually dehydrated in increasing concentrations of ethanol, included in propylene oxide, and embedded in Epon 812 resin. Samples were observed and photographed under a Philips 2200 electron microscope.
DNA content analysis in L. donovani promastigotes.
Aliquots of 100 μl of a suspension of L. donovani promastigotes were incubated with AS-48 according to the standard assay conditions. Afterwards, parasites were collected by centrifugation and resuspended in full growth medium at 4 × 106 cells/ml. Promastigotes were transferred into 1 ml of growth medium and incubated for 18 h. Parasites were fixed in ice-cold ethanol overnight at 4°C, washed twice with 1 ml of HBSS-Glc, resuspended in 500 μl of the same medium containing 20 μg/ml propidium iodide (PI) and 3 mg/ml bovine RNase A, and incubated for 30 min at 26°C. DNA content was analyzed by flow cytometry in an FC500 Coulter cytofluorometer (λEXC = 488 nm/λEM = 620 nm) (36).
Statistical analysis.
Data were represented as the means ± standard deviations (SD) from triplicate samples. Experiments were repeated at least twice. The inhibitory concentrations (ICx, where x stands for the percentage of inhibition) were calculated by the four-parameter logistic curve fitting using Sigma Plot versus 11.0. Statistical comparisons were assessed by the Student t test. Differences were considered significant at P values of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank Fernando Escolar and Begoña Pou for their technical assistance in electron microcopy and Teresa Seisdedos and Gema Elvira for their help in confocal microscopy.
L.R. was supported by grants from the Fondo de Investigaciones Sanitarias-ISCIII-FEDER (PI12-02706), Plan Estatal de Investigación Científica y Técnica y de Innovación 2013-2016 (SAF2015-65740-R and SAF2013-48971-C2-1-R), Subdirección General de Redes y Centros de Investigación Cooperativa-FEDER (RICET RD12/0018/0007, RD12/0018/0011, RD16CIII/003/004, and RD16/0027/0010) and CSIC (PIE 201620E038), the European Regional Development Funds (ERDF), and the Research Group General (BIO160, UGR).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02288-16.
REFERENCES
- 1.Caljon G, De Muylder G, Durnez L, Jennes W, Vanaerschot M, Dujardin JC. 2016. Alice in microbes' land: adaptations and counter-adaptations of vector-borne parasitic protozoa and their hosts. FEMS Microbiol Rev 40:664–685. doi: 10.1093/femsre/fuw018. [DOI] [PubMed] [Google Scholar]
- 2.Tilley L, Straimer J, Gnadig NF, Ralph SA, Fidock DA. 2016. Artemisinin action and resistance in Plasmodium falciparum. Trends Parasitol 32:682–696. doi: 10.1016/j.pt.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackey TK, Liang BA, Cuomo R, Hafen R, Brouwer KC, Lee DE. 2014. Emerging and reemerging neglected tropical diseases: a review of key characteristics, risk factors, and the policy and innovation environment. Clin Microbiol Rev 27:949–979. doi: 10.1128/CMR.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goupil LS, McKerrow JH. 2014. Introduction: drug discovery and development for neglected diseases. Chem Rev 114:11131–11137. doi: 10.1021/cr500546h. [DOI] [PubMed] [Google Scholar]
- 5.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagle AS, Khare S, Kumar AB, Supek F, Buchynskyy A, Mathison CJ, Chennamaneni NK, Pendem N, Buckner FS, Gelb MH, Molteni V. 2014. Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem Rev 114:11305–11347. doi: 10.1021/cr500365f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M. 2010. Combination therapy for visceral leishmaniasis. Lancet Infect Dis 10:184–194. doi: 10.1016/S1473-3099(10)70011-6. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira LG, Andricopulo AD. 2016. Drug repositioning approaches to parasitic diseases: a medicinal chemistry perspective. Drug Discov Today 21:1699–1710. doi: 10.1016/j.drudis.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Rivas L, Luque-Ortega JR, Andreu D. 2009. Amphibian antimicrobial peptides and Protozoa: lessons from parasites. Biochim Biophys Acta 1788:1570–1581. doi: 10.1016/j.bbamem.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Mangoni ML, Papo N, Saugar JM, Barra D, Shai Y, Simmaco M, Rivas L. 2006. Effect of natural L- to D-amino acid conversion on the organization, membrane binding, and biological function of the antimicrobial peptides bombinins H. Biochemistry 45:4266–4276. doi: 10.1021/bi052150y. [DOI] [PubMed] [Google Scholar]
- 11.Epand RM, Walker C, Epand RF, Magarvey NA. 2016. Molecular mechanisms of membrane targeting antibiotics. Biochim Biophys Acta 1858:980–987. doi: 10.1016/j.bbamem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Sahoo N, Bhunia A. 2016. Antimicrobial peptides and their pore/ion channel properties in neutralization of pathogenic microbes. Curr Top Med Chem 16:46–53. [DOI] [PubMed] [Google Scholar]
- 13.Torrent M, Pulido D, Rivas L, Andreu D. 2012. Antimicrobial peptide action on parasites. Curr Drug Targets 13:1138–1147. doi: 10.2174/138945012802002393. [DOI] [PubMed] [Google Scholar]
- 14.Cavera VL, Arthur TD, Kashtanov D, Chikindas ML. 2015. Bacteriocins and their position in the next wave of conventional antibiotics. Int J Antimicrob Agents 46:494–501. doi: 10.1016/j.ijantimicag.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Drider D, Bendali F, Naghmouchi K, Chikindas ML. 2016. Bacteriocins: not only antibacterial agents. Probiotics Antimicrob Proteins 8:177–182. doi: 10.1007/s12602-016-9223-0. [DOI] [PubMed] [Google Scholar]
- 16.Montalbán-López M, Sánchez-Hidalgo M, Valdivia E, Martínez-Bueno M, Maqueda M. 2011. Are bacteriocins underexploited? Novel applications for old antimicrobials. Curr Pharm Biotechnol 12:1205–1220. [DOI] [PubMed] [Google Scholar]
- 17.Grande Burgos MJ, Pulido RP, López Aguayo MDC, Gálvez A, Lucas R. 2014. The cyclic antibacterial peptide enterocin AS-48: isolation, mode of action, and possible food applications. Int J Mol Sci 15:22706–22727. doi: 10.3390/ijms151222706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woraprayote W, Malila Y, Sorapukdee S, Swetwiwathana A, Benjakul S, Visessanguan W. 2016. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci 120:118–132. doi: 10.1016/j.meatsci.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Belguesmia Y, Madi A, Sperandio D, Merieau A, Feuilloley M, Prevost H, Drider D, Connil N. 2011. Growing insights into the safety of bacteriocins: the case of enterocin S37. Res Microbiol 162:159–163. doi: 10.1016/j.resmic.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhari A, Fialho AM, Ratner D, Gupta P, Hong CS, Kahali S, Yamada T, Haldar K, Cho W, Chauhan VS, Gupta TKD, Chakrabarty AM. 2006. Azurin, Plasmodium falciparum malaria and HIV/AIDS: inhibition of parasitic and viral growth by azurin. Cell Cycle 5:1642–1648. doi: 10.4161/cc.5.15.2992. [DOI] [PubMed] [Google Scholar]
- 21.Amer EI, Mossallam SF, Mahrous H. 2014. Therapeutic enhancement of newly derived bacteriocins against Giardia lamblia. Exp Parasitol 146:52–63. doi: 10.1016/j.exppara.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Mossallam SF, Amer EI, Diab RG. 2014. Potentiated anti-microsporidial activity of Lactobacillus acidophilus CH1 bacteriocin using gold nanoparticles. Exp Parasitol 144:14–21. doi: 10.1016/j.exppara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Porrini MP, Audisio MC, Sabate DC, Ibarguren C, Medici SK, Sarlo EG, Garrido PM, Eguaras MJ. 2010. Effect of bacterial metabolites on microsporidian Nosema ceranae and on its host Apis mellifera. Parasitol Res 107:381–388. doi: 10.1007/s00436-010-1875-1. [DOI] [PubMed] [Google Scholar]
- 24.Belguesmia Y, Choiset Y, Rabesona H, Baudy-Floc'h M, Le Blay G, Haertle T, Chobert JM. 2013. Antifungal properties of durancins isolated from Enterococcus durans A5-11 and of its synthetic fragments. Lett Appl Microbiol 56:237–244. doi: 10.1111/lam.12037. [DOI] [PubMed] [Google Scholar]
- 25.Mohr KI, Volz C, Jansen R, Wray V, Hoffmann J, Bernecker S, Wink J, Gerth K, Stadler M, Müller R. 2015. Pinensins: the first antifungal lantibiotics. Angew Chem Int Ed Engl 54:11254–11258. doi: 10.1002/anie.201500927. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Srivastava S. 2014. Anti-Candida activity of two-peptide bacteriocins, plantaricins (Pln E/F and J/K) and their mode of action. Fungal Biol 118:264–275. doi: 10.1016/j.funbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Cornut G, Fortin C, Soulieres D. 2008. Antineoplastic properties of bacteriocins: revisiting potential active agents. Am J Clin Oncol 31:399–404. doi: 10.1097/COC.0b013e31815e456d. [DOI] [PubMed] [Google Scholar]
- 28.Kaur S, Kaur S. 2015. Bacteriocins as potential anticancer agents. Front Pharmacol 6:272. doi: 10.3389/fphar.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagos R, Tello M, Mercado G, Garcia V, Monasterio O. 2009. Antibacterial and antitumorigenic properties of microcin E492, a pore-forming bacteriocin. Curr Pharm Biotechnol 10:74–85. doi: 10.2174/138920109787048643. [DOI] [PubMed] [Google Scholar]
- 30.Maqueda M, Gálvez A, Bueno MM, Sánchez-Barrena MJ, González C, Albert A, Rico M, Valdivia E. 2004. Peptide AS-48: prototype of a new class of cyclic bacteriocins. Curr Protein Pept Sci 5:399–416. doi: 10.2174/1389203043379567. [DOI] [PubMed] [Google Scholar]
- 31.González C, Langdon GM, Bruix M, Gálvez A, Valdivia E, Maqueda M, Rico M. 2000. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc Natl Acad Sci U S A 97:11221–11226. doi: 10.1073/pnas.210301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maqueda M, Gálvez A, Martínez-Bueno M, Guerra I, Valdivia E. 1993. Neutralizing antibodies against the peptide antibiotic AS-48: immunocytological studies. Antimicrob Agents Chemother 37:148–151. doi: 10.1128/AAC.37.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abriouel H, Sánchez-González J, Maqueda M, Gálvez A, Valdivia E, Gálvez-Ruíz MJ. 2001. Monolayer characteristics of bacteriocin AS-48, pH effect and interactions with dipalmitoyl phosphatidic acid at the air-water interface. J Colloid Interface Sci 233:306–312. doi: 10.1006/jcis.2000.7239. [DOI] [PubMed] [Google Scholar]
- 34.Gálvez A, Maqueda M, Martínez-Bueno M, Valdivia E. 1991. Permeation of bacterial cells, permeation of cytoplasmic and artificial membrane vesicles, and channel formation on lipid bilayers by peptide antibiotic AS-48. J Bacteriol 173:886–892. doi: 10.1128/jb.173.2.886-892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maqueda AM, Martínez BM, Valdivia ME, Ananou JS, Cebrián CR. July 2014. Composition for treating bacterial infections of the skin and mucous membranes. Patent WO2014006253 A1.
- 36.Luque-Ortega JR, Rivas L. 2010. Characterization of the leishmanicidal activity of antimicrobial peptides. Methods Mol Biol 618:393–420. doi: 10.1007/978-1-60761-594-1_25. [DOI] [PubMed] [Google Scholar]
- 37.Roy A, Ganguly A, BoseDasgupta S, Das BB, Pal C, Jaisankar P, Majumder HK. 2008. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharmacol 74:1292–1307. doi: 10.1124/mol.108.050161. [DOI] [PubMed] [Google Scholar]
- 38.Paris C, Loiseau PM, Bories C, Breard J. 2004. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother 48:852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khademvatan S, Gharavi MJ, Rahim F, Saki J. 2011. Miltefosine-induced apoptotic cell death on Leishmania major and L. tropica strains. Korean J Parasitol 49:17–23. doi: 10.3347/kjp.2011.49.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marinho Fde A, Goncalves KC, Oliveira SS, Oliveira AC, Bellio M, d'Avila-Levy CM, Santos AL, Branquinha MH. 2011. Miltefosine induces programmed cell death in Leishmania amazonensis promastigotes. Mem Inst Oswaldo Cruz 106:507–509. doi: 10.1590/S0074-02762011000400021. [DOI] [PubMed] [Google Scholar]
- 41.Gálvez A, Maqueda M, Martínez-Bueno M, Valdivia E. 1989. Bactericidal and bacteriolytic action of peptide antibiotic AS-48 against gram-positive and gram-negative bacteria and other organisms. Res Microbiol 140:57–68. doi: 10.1016/0923-2508(89)90060-0. [DOI] [PubMed] [Google Scholar]
- 42.Gálvez A, Valdivia E, Martínez M, Maqueda M. 1989. Effect of peptide AS-48 on Enterococcus faecalis subsp. liquefaciens S-47. Antimicrob Agents Chemother 33:641–645. doi: 10.1128/AAC.33.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cotter PD. 2014. An ‘Upp’-turn in bacteriocin receptor identification. Mol Microbiol 92:1159–1163. doi: 10.1111/mmi.12645. [DOI] [PubMed] [Google Scholar]
- 44.Cruz VL, Ramos J, Melo MN, Martinez-Salazar J. 2013. Bacteriocin AS-48 binding to model membranes and pore formation as revealed by coarse-grained simulations. Biochim Biophys Acta 1828:2524–2531. doi: 10.1016/j.bbamem.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-Barrena MJ, Martínez-Ripoll M, Gálvez A, Valdivia E, Maqueda M, Cruz V, Albert A. 2003. Structure of bacteriocin AS-48: from soluble state to membrane bound state. J Mol Biol 334:541–549. doi: 10.1016/j.jmb.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 46.Cebrián R, Martínez-Bueno M, Valdivia E, Albert A, Maqueda M, Sánchez-Barrena MJ. 2015. The bacteriocin AS-48 requires dimer dissociation followed by hydrophobic interactions with the membrane for antibacterial activity. J Struct Biol 190:162–172. doi: 10.1016/j.jsb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Abriouel H, Valdivia E, Gálvez A, Maqueda M. 2001. Influence of physico-chemical factors on the oligomerization and biological activity of bacteriocin AS-48. Curr Microbiol 42:89–95. [DOI] [PubMed] [Google Scholar]
- 48.Sal-Man N, Oren Z, Shai Y. 2002. Preassembly of membrane-active peptides is an important factor in their selectivity toward target cells. Biochemistry 41:11921–11930. doi: 10.1021/bi0260482. [DOI] [PubMed] [Google Scholar]
- 49.Silva T, Abengózar MA, Fernández-Reyes M, Andreu D, Nazmi K, Bolscher JG, Bastos M, Rivas L. 2012. Enhanced leishmanicidal activity of cryptopeptide chimeras from the active N1 domain of bovine lactoferrin. Amino Acids 43:2265–2277. doi: 10.1007/s00726-012-1304-0. [DOI] [PubMed] [Google Scholar]
- 50.Wassef MK, Fioretti TB, Dwyer DM. 1985. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids 20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- 51.Glaser TA, Utz GL, Mukkada AJ. 1992. The plasma membrane electrical gradient (membrane potential) in Leishmania donovani promastigotes and amastigotes. Mol Biochem Parasitol 51:9–15. doi: 10.1016/0166-6851(92)90195-P. [DOI] [PubMed] [Google Scholar]
- 52.Turco SJ, Descoteaux A. 1992. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol 46:65–94. [DOI] [PubMed] [Google Scholar]
- 53.Kauffman WB, Fuselier T, He J, Wimley WC. 2015. Mechanism matters: a taxonomy of cell penetrating peptides. Trends Biochem Sci 40:749–764. doi: 10.1016/j.tibs.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piacenza L, Irigoin F, Alvarez MN, Peluffo G, Taylor MC, Kelly JM, Wilkinson SR, Radi R. 2007. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem J 403:323–334. doi: 10.1042/BJ20061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niklison-Chirou MV, Dupuy F, Saavedra L, Hebert E, Banchio C, Minahk C, Morero RD. 2011. Microcin J25-Ga induces apoptosis in mammalian cells by inhibiting mitochondrial RNA-polymerase. Peptides 32:832–834. doi: 10.1016/j.peptides.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Verdon J, Labanowski J, Sahr T, Ferreira T, Lacombe C, Buchrieser C, Berjeaud J-M, Héchard Y. 2011. Fatty acid composition modulates sensitivity of Legionella pneumophila to warnericin RK, an antimicrobial peptide. Biochim Biophys Acta 1808:1146–1153. doi: 10.1016/j.bbamem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Carroll J, Draper LA, O'Connor PM, Coffey A, Hill C, Ross RP, Cotter PD, O'Mahony J. 2010. Comparison of the activities of the lantibiotics nisin and lacticin 3147 against clinically significant mycobacteria. Int J Antimicrob Agents 36:132–136. doi: 10.1016/j.ijantimicag.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 58.Sosunov V, Mischenko V, Eruslanov B, Svetoch E, Shakina Y, Stern N, Majorov K, Sorokoumova G, Selishcheva A, Apt A. 2007. Antimycobacterial activity of bacteriocins and their complexes with liposomes. J Antimicrob Chemother 59:919–925. doi: 10.1093/jac/dkm053. [DOI] [PubMed] [Google Scholar]
- 59.Abriouel H, Lucas R, Omar NB, Valdivia E, Gálvez A. 2010. Potential applications of the cyclic peptide enterocin AS-48 in the preservation of vegetable foods and beverages. Probiotics Antimicrob Proteins 2:77–89. doi: 10.1007/s12602-009-9030-y. [DOI] [PubMed] [Google Scholar]
- 60.Wladyka B, Piejko M, Bzowska M, Pieta P, Krzysik M, Mazurek L, Guevara-Lora I, Bukowski M, Sabat AJ, Friedrich AW, Bonar E, Miedzobrodzki J, Dubin A, Mak P. 2015. A peptide factor secreted by Staphylococcus pseudintermedius exhibits properties of both bacteriocins and virulence factors. Sci Rep 5:14569. doi: 10.1038/srep14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arias C, Guizy M, Luque-Ortega JR, Guerrero E, de la Torre BG, Andreu D, Rivas L, Valenzuela C. 2006. The induction of NOS2 expression by the hybrid cecropin A-melittin antibiotic peptide CA(1-8)M(1-18) in the monocytic line RAW 264.7 is triggered by a temporary and reversible plasma membrane permeation. Biochim Biophys Acta 1763:110–119. doi: 10.1016/j.bbamcr.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Gálvez A, Valdivia E, González-Segura A, Lebbadi M, Martínez-Bueno M, Maqueda M. 1993. Purification, characterization, and lytic activity against Naegleria fowleri of two amoebicins produced by Bacillus licheniformis A12. Appl Environ Microbiol 59:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bali V, Panesar PS, Bera MB. 2016. Trends in utilization of agro-industrial byproducts for production of bacteriocins and their biopreservative applications. Crit Rev Biotechnol 36:204–214. doi: 10.3109/07388551.2014.947916. [DOI] [PubMed] [Google Scholar]
- 64.Jantsch J, Schatz V, Friedrich D, Schroder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger KJ, Gebhardt M, Heinig M, Neubert P, Fischer F, Teufel S, David JP, Neufert C, Cavallaro A, Rakova N, Kuper C, Beck FX, Neuhofer W, Muller DN, Schuler G, Uder M, Bogdan C, Luft FC, Titze J. 2015. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 21:493–501. doi: 10.1016/j.cmet.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sánchez-Hidalgo M, Montalbán-López M, Cebrián R, Valdivia E, Martínez-Bueno M, Maqueda M. 2011. AS-48 bacteriocin: close to perfection. Cell Mol Life Sci 68:2845–2857. doi: 10.1007/s00018-011-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hemu X, Qiu Y, Nguyen GK, Tam JP. 2016. Total synthesis of circular bacteriocins by butelase 1. J Am Chem Soc 138:6968–6971. doi: 10.1021/jacs.6b04310. [DOI] [PubMed] [Google Scholar]
- 67.Pan AA, Duboise SM, Eperon S, Rivas L, Hodgkinson V, Traub-Cseko Y, McMahon-Pratt D. 1993. Developmental life cycle of Leishmania—cultivation and characterization of cultured extracellular amastigotes. J Eukaryot Microbiol 40:213–223. doi: 10.1111/j.1550-7408.1993.tb04906.x. [DOI] [PubMed] [Google Scholar]
- 68.Colmenares M, Constant SL, Kima PE, McMahon-Pratt D. 2002. Leishmania pifanoi pathogenesis: selective lack of a local cutaneous response in the absence of circulating antibody. Infect Immun 70:6597–6605. doi: 10.1128/IAI.70.12.6597-6605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevenson LG, Fedorko DP, Zelazny AM. 2010. An enhanced method for the identification of Leishmania spp. using real-time polymerase chain reaction and sequence analysis of the 7SL RNA gene region. Diagn Microbiol Infect Dis 66:432–435. doi: 10.1016/j.diagmicrobio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cebrián R, Banos A, Valdivia E, Pérez-Pulido R, Martínez-Bueno M, Maqueda M. 2012. Characterization of functional, safety, and probiotic properties of Enterococcus faecalis UGRA10, a new AS-48-producer strain. Food Microbiol 30:59–67. doi: 10.1016/j.fm.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Ananou S, Muñoz A, Gálvez A, Martínez-Bueno M, Maqueda M, Valdivia E. 2008. Optimization of enterocin AS-48 production on a whey-based substrate. Int Dairy J 18:923–927. doi: 10.1016/j.idairyj.2008.02.001. [DOI] [Google Scholar]
- 72.Abriouel H, Valdivia E, Martinez-Bueno M, Maqueda M, Galvez A. 2003. A simple method for semi-preparative-scale production and recovery of enterocin AS-48 derived from Enterococcus faecalis subsp. liquefaciens A-48-32. J Microbiol Methods 55:599–605. doi: 10.1016/S0167-7012(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 73.Luque-Ortega JR, Reuther P, Rivas L, Dardonville C. 2010. New benzophenone-derived bisphosphonium salts as leishmanicidal leads targeting mitochondria through inhibition of respiratory complex II. J Med Chem 53:1788–1798. doi: 10.1021/jm901677h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.