ABSTRACT
Cryptococcus neoformans is a pathogen that is common in immunosuppressed patients. It can be treated with amphotericin B and fluconazole, but the mortality rate remains 15 to 30%. Thus, novel and more effective anticryptococcal therapies are needed. The troponoids are based on natural products isolated from western red cedar, and have a broad range of antimicrobial activities. Extracts of western red cedar inhibit the growth of several fungal species, but neither western red cedar extracts nor troponoid derivatives have been tested against C. neoformans. We screened 56 troponoids for their ability to inhibit C. neoformans growth and to assess whether they may be attractive candidates for development into anticryptococcal drugs. We determined MICs at which the compounds inhibited 80% of cryptococcal growth relative to vehicle-treated controls and identified 12 compounds with MICs ranging from 0.2 to 15 μM. We screened compounds with MICs of ≤20 μM for cytotoxicity in liver hepatoma cells. Fifty percent cytotoxicity values (CC50s) ranged from 4 to >100 μM. The therapeutic indexes (TI, CC50/MIC) for most of the troponoids were fairly low, with most being <8. However, two compounds had TI values that were >8, including a tropone with a TI of >300. These tropones are fungicidal and are not antagonistic when used in combination with fluconazole or amphotericin B. Inhibition by these two tropones remains unchanged under conditions favoring cryptococcal capsule formation. These data support the hypothesis that troponoids may be a productive scaffold for the development of novel anticryptococcal therapies.
KEYWORDS: antifungal agents, Cryptococcus neoformans, tropolones
INTRODUCTION
Cryptococcus neoformans is a fungal pathogen found in immunocompromised people that causes up to 1 million infections each year among HIV-positive patients worldwide, resulting in up to 600,000 deaths annually (1). Much of the disease burden occurs in sub-Saharan Africa, where deaths from cryptococcal infections may exceed those from tuberculosis in some areas (1). Cryptococcal infections are also a major problem among solid organ transplant patients, where up to 3% develop an invasive fungal infection within the first year, with an overall mortality rate of 25 to 40% (2). Transplant patients remain susceptible to C. neoformans infections for 5 years due to its presence in the environment (3). Cryptococcal infections can be treated with amphotericin B and fluconazole, but the treatment course is long and has significant toxicity. Importantly, the mortality rate remains 15 to 30% for treated patients, even in the context of antiviral treatments for HIV (4–6).
Currently, the only new therapeutic candidate for treating cryptococcal infections in clinical trials is sertraline, an antidepressive agent, which has been shown to increase the rate of Cryptococcus clearance from the cerebral spinal fluid of patients with cryptococcal meningitis (7). It is being tested as an adjunct antifungal in a phase III clinical trial (NCT01802385; https://clinicaltrials.gov/). Recent preclinical studies have also identified several additional compounds or molecular scaffolds with anticryptococcal activities (8–12). There are several antifungal small molecules in preclinical development, but only two of these show efficacies against C. neoformans (13).
The tropolones (Fig. 1) have a broad range of antimicrobial activities. They are based on natural products isolated from the heartwood of western red cedar (Thuja plicata), whose natural function is to inhibit fungal growth. Extracts of western red cedar inhibit the growth of a number of unrelated fungal species (14), and β-thujaplicin (Fig. 1D, compound [Cmpd] no. 47) inhibits Candida albicans (15). To date, neither western red cedar extracts nor chemical derivatives of β-thujaplicin have been tested for inhibiting C. neoformans. β-Thujaplicinol (Fig. 1E, Cmpd no. 46), another natural product from T. plicata, has an additional hydroxyl group on the tropolone ring compared with β-thujaplicin, and it can inhibit the HIV RNase H (RNaseH) (16). More recently, β-thujaplicinol was shown to inhibit hepatitis B virus (HBV) replication by blocking the activity of the virally encoded RNaseH (17, 18) and to inhibit herpes simplex virus replication (19, 20). The α-hydroxytropolones (αHTs) have a broad range of beneficial effects against other diseases, including malaria, bipolar disorder, and diabetes (21, 22). However, their use as therapeutic agents has been limited by the lack of efficient synthetic approaches to expand their chemical diversity for structure-activity relationship (SAR) and efficiency optimization studies that are essential for drug development. Recently, we pioneered a novel approach to generate poly substituted αHTs from readily available intermediate compounds (23). Here, 56 troponoids were screened for their capacities for inhibiting C. neoformans growth to assess whether they may be attractive candidates for the development of anticryptococcal drugs.
FIG 1.
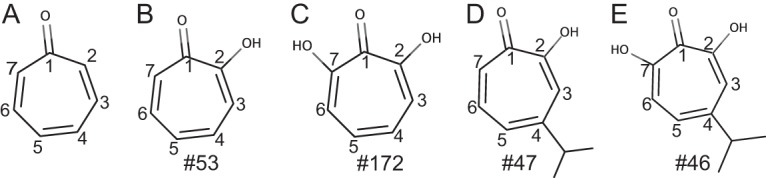
Structures of (A) tropone, (B) tropolone, (C) α-hydroxytropolone, (D) β-thujaplicin, and (E) β-thujaplicinol with the R-group numbering and the natural products. Numbered structures were tested for inhibition and are found in Fig. S2 and S3 in the supplemental material.
RESULTS
Development of a C. neoformans growth inhibition assay.
We set out to develop an assay that enabled us to cheaply and reproducibly measure the inhibition of C. neoformans growth using a 96-well format. We initially tested C. neoformans growth in yeast extract-peptone-dextrose (YPD) versus YPD plus 1% dimethyl sulfoxide (DMSO) at 25°C with shaking for 24 and 48 h. The DMSO-treated cells showed a significant lag in growth for the first 24 h but caught up with untreated cells after 48 h, resulting in more consistent and higher levels of cell growth. We tested inhibition by β-thujaplicin and β-thujaplicinol in YPD plus 1% DMSO at 25°C at 3.75, 15, and 60 μM and demonstrated that they almost completely inhibited C. neoformans growth at 60 μM (see Fig. S1A in the supplemental material). However, these conditions are unlike conditions encountered by C. neoformans in mammalian infections, where nutrients are limited, and the pH and temperature are higher than in YPD media. The cryptococcal stress response to low glucose and high pH and temperature may alter its susceptibility to the inhibitors. It is also possible that the enzymes targeted by the inhibitors may render the cell temperature sensitive when inhibited. To better mimic the growth in mammals, we tested growth without shaking in nutrient-limited media at 35°C. We first tested the cell culture media, RPMI 1640 plus 0.4% glucose and 1% DMSO as defined by National Committee for Clinical Laboratory Standards (NCCLS) for antifungal susceptibility testing. However, the cells grew very poorly under these conditions. We then tested growth in YNB (pH 7.0) with 0.2% glucose plus 1% DMSO without shaking at 35°C, which has been identified as an appropriate substitute for C. neoformans susceptibility testing (24), and saw more consistent cell growth over the 48-h assay. We tested β-thujaplicin and β-thujaplicinol at 3.75, 15, and 60 μM in YNB-02 plus 1% DMSO at 35°C and observed some inhibition at 15 and 60 μM (Fig. S1B), but did not observe the nearly complete lack of growth at 60 μM as was observed in YPD at 25°C (Fig. S1A). However, as the nutrient-limited medium and higher temperature is more similar to mammalian cell growth conditions. We used these conditions for all further assays.
Determination of the MIC for troponoids.
Since we had only 56 troponoids (Table 1; see also Fig. S2 and S3 in the supplemental material) available to test, we determined MICs for all of the compounds to provide an accurate measure of their inhibitory potentials. We measured the MIC employing the limiting dilution assay and defined the MIC as the concentration at which cells were inhibited ≥80% relative to vehicle-treated cells. All values were measured at least two times in independent experiments and the average MIC is reported.
TABLE 1.
Troponoids tested for inhibiting growth of C. neoformans
| Compound No. | Name/catalog no. | MIC80 (μM) | CC50 (μM) | TI |
|---|---|---|---|---|
| Tropones | ||||
| 57 | 2-Chlorotropone | 50 | 57.0 | 1 |
| 60 | Chembridge 5942159 | 50 | >100 | 2 |
| 61 | Chembridge 5940946 | 41 | 30.5 | <1 |
| 63 | Chembridge 5938894 | 17 | 100.0 | 6 |
| 281 | Chembridge 5947055 | 50 | 100.0 | 2 |
| 282 | Chembridge 5942369 | 36 | 96.5 | 3 |
| 283 | Chembridge 5940028 | 50 | 74.0 | 1 |
| 284 | Vitas-M Lab STK526992 | 0.25 | 91.0 | >300 |
| 285 | Sigma 378400 | 49 | >100 | 2 |
| Tropolones | ||||
| 47 | β-Thujaplicin | 21 | 66.5 | 3 |
| 48 | γ-Thujaplicin | 8 | 45.0 | 5 |
| 49 | Nootkatin | 18 | 18.5 | 1 |
| 50 | 5-Nitrosotropolone | 24 | 17.5 | 1 |
| 51 | Tropolone p-nitrobenzoate | 24 | >100 | 4 |
| 52 | NSC 79556 | 50 | >100 | 2 |
| 53 | Tropolone | 24 | 100.0 | 4 |
| 54 | 3-Bromotropolone | 7 | 95.0 | 13 |
| 55 | NSC 282885 | 6 | 4.3 | <1 |
| 195 | Purpurogallin | 50 | 92.0 | 2 |
| α-Hydroxytropolones | ||||
| 56 | Manicol | 50 | 27.0 | <1 |
| 106 | CM1012-6a | 41 | 24.5 | <1 |
| 107 | CM1012-6b | 50 | 85.5 | 2 |
| 108 | CM1012-6c | 50 | 90.5 | 2 |
| 109 | CM1012-6d | 50 | 87.5 | 2 |
| 110 | CM1012-6e | 50 | 19.0 | <1 |
| 111 | CM1012-6f | 36 | 26.3 | 1 |
| 112 | CM1012-6i | 49 | 74.0 | 1 |
| 113 | RM-YM-1-0613 | 24 | 37.5 | 2 |
| 114 | RM-YM-2-0613 | 11 | 18.6 | 1 |
| 118 | RM-MD-2-0813 | 18 | 17.5 | 1 |
| 120 | RM-MD-1-0713 | 12 | 21.5 | 2 |
| 143 | MD-1-138 | 50 | >100 | 2 |
| 144 | DH-1-148 | 50 | NDa | |
| 145 | DH-1-163 | 15 | 11.0 | 1 |
| 146 | DH-2-8 | 20 | 20.0 | 1 |
| 147 | DH-2-4 | 15 | 13.1 | 1 |
| 172 | 7-Hydroxytropolone | 24 | 100.0 | 4 |
| 210 | MolMoll 19617 | 15 | 71.0 | 5 |
| 261 | AG40 | 49 | 100 | 2 |
| 262 | AG51 | 18 | 96.0 | 5 |
| 264 | AG44 | 35 | 28.0 | <1 |
| 267 | AG59 | 24 | 17.0 | <1 |
| 273 | DH-4-116 | 50 | 100.0 | 2 |
| 274 | DH-4-117 | 50 | 92.5 | 2 |
| 280 | AG77 | 50 | 66.0 | 1 |
| 308 | AG-II-18-P | 11 | 24.0 | 2 |
| 309 | AG-I-183-P | 24 | 35.0 | 1 |
| 310 | AG-II-21-P | 11 | 35.0 | 3 |
| 311 | AG-II-3-P | 15 | 20.0 | 1 |
| 312 | AG-II-4-P | 18 | 18.0 | 1 |
| 313 | AG-II-5-P | 24 | 46.0 | 2 |
| 315 | AG-I-186-P | 24 | ND | |
| 317 | AG-II-22-P | 50 | ND | |
| 318 | AG-II-17-P | 50 | ND | |
| 319 | AB-1-111 | 50 | ND |
ND, not determined.
The library consists of tropolone (no. 53), which had moderate activity (MIC, 24 μM), and derivatives thereof. Eight of the tropolone analogs had moderate substitutions on the tropone ring (no. 47 to 50, 52, 54, 55, and 195), and were, for the most part, at least as good as tropolone itself, with three of these seven compounds (no. 48, 54, and 55) having MIC values almost 3-fold lower than no. 53, and only two (no. 52 and 195) showing decreased activity. Another 10 of the troponoids tested were variants of no. 53 with modifications to the tropolone hydroxyl (-OH), and most of these had decreased activities, such as compounds with a change to a chlorine (no. 57), an aniline (no. 60), and a sulfonyl ester (no. 61). The activity of several benzoylated variants (no. 51, 63, 281 to 283, and 285) seemed to be impacted by the electronics of the phenyl ring. Those with electron-donating groups (no. 281, 283, and 285) had higher MIC values, whereas those with electron-withdrawing groups (no. 51, 63, 282) had values more similar to that of tropolone. By far, the most potent inhibition of C. neoformans growth was observed when the tropolone hydroxyl (-OH) was instead a thioester (no. 284), which led to activity 100-fold more potent than that of tropolone.
Another 37 of the molecules are αHTs (see Fig. S3 in the supplemental material), which have a third contiguous oxygen atom on their troponoid rings. The αHT with no substitutions (no. 172) had an MIC of 24 μM, which was consistent with tropolone (no. 54), demonstrating these are likely comparable as a starting pharmacophore. Six of the molecules tested had substitutions at only C-4 (no. 46, 210, 260, 262, 264, and 265), and half of these (no. 46, 210, and 265) had activities at ≤15 μM. Of note, β-thujaplicinol (no. 46) and γ-thujaplicin (no. 48) share similar isopropyl appendages and had comparable activities. Five of the αHTs had substitutions at C-4, C-5, and C-6 (no. 56, 106 to 108 and 280), and all had MICs higher than no. 172. Most of the synthetic αHTs made through the Murelli laboratory's synthetic strategy (23, 25, 26) had a methyl substitution at C-5 and varied at C-4 with the tropolone directly linked to an aromatic (no. 112 to 114 and 144 to 147) or a carbonyl functional (no. 109 to 111, 118, 120, 143, 308 to 313, 315, and 319) group. Most of these molecules had MIC values higher than no. 172, although four of the 14 carbonyl-appended compounds (no. 118, 120, 310, and 311) and three of the seven aromatic-linked compounds (no. 114, 145, and 147) were slightly more potent. Three additional α-methoxytropolones (no. 172, 317, and 318), synthetic precursors to αHTs, were also tested, and these had little activity. We measured the MICs of two of our best hits, no. 54 and no. 284, in two independent clinical strains and confirmed that they showed very similar levels of inhibitor activity, with an MIC of 6 to 12 μM for no. 54 and an MIC of 0.16 to 0.32 μM for no. 284 (see Fig. S4 in the supplemental material).
Hydrolyzed compound no. 284 remains active.
Compound no. 284 (Fig. S2) has a labile thioester linkage that is likely to be hydrolyzed in vivo. This would release a tropothione, possibly in multiple oxidation states, as well as p-benzoic acid, any of which could potentially act as inhibitors. Tropothione was not available commercially for testing its inhibition of C. neoformans, but we tested p-benzoic acid and the hydrolysis products of no. 284 in MIC assays. p-Benzoic acid up to 50 μM did not inhibit fungal growth (data not shown), whereas the hydrolyzed products were able to inhibit growth with an MIC of 0.78 μM (Fig. 2). We examined the hydrolysis products of no. 284 by mass spectrometry and observed the loss of the starting material, but could not resolve the hydrolysis products (data not shown). The fact that the hydrolysis products still inhibited the growth of C. neoformans, whereas p-benzoic acid did not, suggests that tropothione or oxidized derivatives of tropothione are effective inhibitors of C. neoformans growth.
FIG 2.
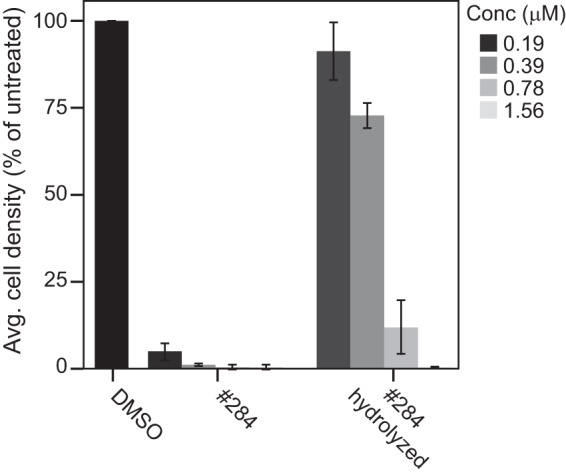
Inhibition of KN99α cells with no. 284 or hydrolyzed products of no. 284 measured by cell densities after 48 h at 35°C in YNB-02 plus 1% DMSO. Values are means ± standard deviations from three replicates.
Inhibition under capsule-inducing conditions.
We conducted our screening in the nutrient-limited media YNB-02 at 35°C to mimic some aspects of the growth conditions in humans. However, it is possible the compounds may demonstrate less potency under conditions that induce the cryptococcal yeast cells to elaborate a polysaccharide capsule (27). We tested two of our best hits (Fig. S2, no. 284 and 54) under conditions that induce capsule elaboration (nutrient limiting, 37°C and 5% CO2). We measured MICs for no. 54 and no. 284 in cells grown for 48 h in either YNB-02 plus 1% DMSO or RPMI plus 1% DMSO at 37°C with 5% CO2. We observed no growth in RPMI under these conditions, but cells grew in YNB-02. The MIC for no. 54 was between 6 and 12 μM (Fig. 3A), while the MIC for no. 284 was between 0.16 and 0.31 μM (Fig. 3B). The untreated cells grown in YNB-02 elaborated the capsule (Fig. 3D) compared with that of cells grown overnight in YPD at 30°C (Fig. 3C). The MICs for no. 54 and no. 284 were only slightly higher when cells were grown under conditions that elaborate the capsule, suggesting the capsule induction does not block the inhibitors from entering the cells or substantially impair their activity.
FIG 3.
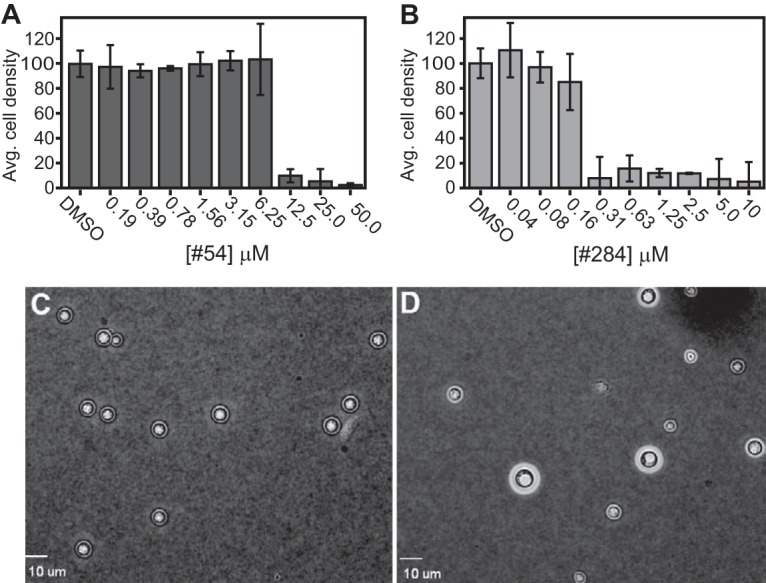
Inhibition of KN99α cells as measured by cell densities after 48 h at 37°C with 5% CO2 in YNB-02 plus 1% DMSO for (A) no. 54 or (B) no. 284. Values are means ± standard deviations from three replicates. India ink exclusion assay of capsule production of the cells cultured in YPD (C) or in YNB-02 plus 1% DMSO (D) at 37°C plus 5% CO2.
Compounds no. 54 and no. 284 are not antagonistic with fluconazole or amphotericin B.
An important consideration of any new antifungal is whether it will be compatible with existing antifungal therapies. Therefore, we measured the MICs of amphotericin B (AMB), fluconazole (FLC), no. 54, and no. 284 alone and in combination. The MIC at which 80% of cells were inhibited relative to vehicle-treated cells was 3 μM (0.9 μg/ml), 1.5 μM (1.4 μg/ml), 12 μM, and 0.31 μM for FLC, AMB, no. 54, and no. 284, respectively. These values remained unchanged for either no. 54 or no. 284 when used in combination with either FLC or AMB (data not shown). This results in fractional inhibitory concentration (FIC) values of 1 for each compound in combination with either FLC or AMB and an FIC index of 2. Thus, there are no differences in the interactions of either no. 54 or no. 284 combined with either FLC or AMB (28). Importantly, neither no. 54 nor no. 284 was antagonistic when used in combination with FLC or AMB.
Tropolones are fungicidal.
We evaluated compounds no. 54 and no. 284 for fungicidal versus fungistatic activities. This was done in a qualitative assay in parallel with FLC, which is known to be fungistatic (29) and AMB, which is known to be fungicidal (30). The MICs were 3 μM (0.9 μg/ml), 1.5 μM (1.4 μg/ml), 12 μM, and 0.31 μM for FLC, AMB, no. 54, and no. 284, respectively. (Fig. 4A and B). Following the drug exposure for 48 h, 30 μl of each of the remaining replicate cultures at 2×, 4×, and 8× the MIC were spotted on YPD plates and incubated at 30°C for 2 days. Significant cell growth was observed for FLC-treated cells up to 8× the MIC (Fig. 4C), whereas no cell growth was observed for AMB-, no. 54-, or no. 284-treated cells at 2 to 8× the MIC (Fig. 4D to F). Based on these data, we conclude that no. 54 and no. 284 are fungicidal against C. neoformans.
FIG 4.
KN99α cells were treated with FLC, AMB or no. 54 at 0.19 to 50 μM (A) or no. 284 at 0.04 to 10 μM (B). (C to F) Following inhibition, 30-μl samples of the each of four replicates at 2×, 4×, and 8× the MIC were spotted on YPD agar and incubated for 48 h at 30°C.
Cytotoxicity in mammalian cells.
Finally, we tested the abilities of key C. neoformans inhibitors to induce cytotoxicity in mammalian cells to begin evaluating the potential use of troponoids as antifungals in humans. HepDES19 cells were chosen because they are a derivative of the HepG2 hepatoblastoma cell line that is commonly used for initial evaluations of potential hepatocytotoxicity (31). Cytotoxicity was measured using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay that measures mitochondrial function, because some troponoids have been reported to reduce mitochondrial function (32) and because the MTS assay has been the most sensitive of the assays we have employed for identifying potential adverse effects on the functions of mammalian cells for this compound class.
HepDES19 cells were treated with medium containing various concentrations of key Cryptococcus inhibitors and select additional compounds for comparison in a final concentration of DMSO of 1%. Three days later, the MTS reagent was added for 90 min prior to terminating the incubation period and the reading of the optical density at 480 nm (OD480). We measured 50 percent cytotoxicity values (CC50s) for 52 of the 56 compounds, and the CC50 values ranged from 4 to >100 μM (Table 1). The tropones were relatively nontoxic, with the lowest CC50 being 30.5 μM for no. 61. Of the 10 tropolones, 3 had CC50 values of ≤20 μM, with no. 55 being the most toxic with a CC50 of 4 μM. The αHTs had a range of toxicity from 11 to >100 µM. Twenty-three of the 32 αHTs tested had CC50 values of >20 μM.
A comparison of the MIC and CC50 data permitted the calculation of a therapeutic index (TI; CC50/MIC) for the Cryptococcus inhibitors. TI values for most of the tropolones were fairly low, with TIs for 30 of the 32 compounds being <8. However, promising TI values of >8 were found for two compounds, no. 54 and no. 284. Most notably, a TI of >300 was observed for no. 284, the most effective inhibitor we identified.
Therefore, substantial cytotoxicity might be induced by most of the troponoids in a liver-derived cell line, but at least one compound, no. 284, had a high TI value of >300 that opens a window for its development into a potentially clinically useful cryptococcal inhibitor.
DISCUSSION
Tropolone bioactivity is often associated with the ability to bind to and inhibit metalloenzymes (33, 34). This binding takes place through bidentate chelation of the metal between the tropolone carbonyl and the tropolone -OH, which is likely deprotonated at physiological pH and enhances coordination to the metal center (35). As such, if the activity against C. neoformans were due to similar metalloenzyme inhibition, modifications to this hydroxyl would be expected to result in a loss of activity. Indeed, most analogs modified at this group led to decreased or a complete loss of activity, such as the change to a chloride (no. 57), aniline (no. 60), or sulfonyl ester (no. 61), as well as several benzoylated analogs (no. 281, 283, and 285). However, benzoylated derivatives with an electron-withdrawing appendage (no. 51, 63, and 282) maintained activities comparable to that of tropolone (no. 53). Since electron-withdrawing groups destabilize the carbonyl ester, it is possible that these molecules are hydrolyzing in the assays, though further tests are needed to evaluate this hypothesis.
The most potent inhibitor was compound no. 284, which had the lowest MIC (0.2 μM) and the best TI of all of the compounds tested. The presence of the high-energy thioester linkage strongly suggests that this molecule is hydrolyzed within cells or in the growth medium. The hydrolysis of no. 284 would likely result in the release of p-benzoic acid and tropone with a free sulfhydryl group (thiotropolone) that may react with free cysteines in enzymes or attenuate the metal-binding abilities of the troponoid. The p-benzoic acid by itself was not effective as an inhibitor, whereas the hydrolyzed products of no. 284 remained effective inhibitors, with an MIC of 0.75 μM. These studies imply that the thiotropolone and/or one of its derivatives are effective inhibitors of C. neoformans growth. Furthermore, the almost 100-fold increase in activity between no. 284 and tropolone (no. 53) suggests an enormous benefit to the sulfur atom, possibly due to the increased affinity of heavy metals, such as iron or zinc, which may prefer coordination to the sulfur of thiotropolone over the oxygen of the tropolone. Further efforts are under way to develop chemistry that would enable a greater SAR of thiotropolones against C. neoformans.
The remaining library that was tested centered around substituted tropolones. The tropolone with no other substitutions (no. 53) was a moderate inhibitor and was also relatively noncytotoxic. Substitutions to the tropone ring led to a variety of effects on these activities. For example, the electron-withdrawing substitution of Br (no. 54) at position C-3 decreased the MIC without increasing cytotoxicity, and an isopropyl group at position C-5 (no. 48 and γ-thujaplicin) also decreased the MIC with a moderate increase in cytotoxicity. A similar isopropyl substitution at C-4 (no. 47) did not decrease the MIC value relative to no. 53, suggesting the importance of the positioning of the appendage. However, a larger and more complex appendage at C-4 containing both an amide and a dichlorophenyl (no. 55) was similarly as potent as β-thujaplicinol, but also substantially more cytotoxic. Another fairly common tropolone natural product, purpurogallin (no. 195), which has a fused polyphenol appendage, was largely ineffective against C. neoformans.
In previous studies of tropolones and αHTs against various fungi, the activities between the two classes were either equivalent or the tropolones were superior growth inhibitors (36). Against C. neoformans, tropolone and αHTs are roughly equivalent in their inhibitory activities, as is evidenced by the comparable activities of no. 53 and 172, as well as the comparable activities between γ-thujaplicin (no. 46) and β-thujaplicinol (no. 48). γ-Thujaplicin (no. 46) and β-thujaplicinol (no. 48) share identical potency enhancements when a single isopropyl group is added, and thus both scaffolds are viable starting points for future optimization. As such, 30 αHTs with diverse substitutions were tested against C. neoformans. αHTs with substitutions only at position C-4 were, for the most part, better or at least equivalent in their ability to inhibit C. neoformans growth. A smaller aliphatic or aromatic substitution (no. 46, 210, 262, and 265) showed activities that were equivalent or moderately improved compared with that of no. 172. An electron-withdrawing (no. 261) or large hydrophobic (no. 264) group, on the other hand, decreased inhibition. All five of the αHTs with a substitution at only C-3 (no. 56, 106 to 108, and 280) had decreased inhibition compared with that of no. 172. The remaining αHTs all had substitutions at position C-4 plus a methyl group at C-5. Seven had a benzene ring at C-4 (no. 112 to 114 and 144 to 146). While the presence of a Br or trifluoromethyl group at the para position on the benzene improved inhibition but also increased cytotoxicity, other electron-withdrawing groups (Cl or nitro) decreased inhibition. The substitution with a naphthalene group (no. 146 and 147) improved inhibition, but also increased cytotoxicity. The remaining 14 αHTs had a carbonyl at C-3, (no. 109 to 111, 118, 120, 143, 274, 308 to 313, 315, and 318). Smaller esters (no. 109 and 274) and the carboxylate (no. 319) showed very little inhibition (MIC, ≥50 μM), as was the case with smaller methyl- (no. 110) and isopropyl-ketones (no. 143). However, the activities were restored (MIC, ≤36 μM) among the remaining 10 ketones, and in some instances (no. 120, 308, and 310), approached that of β-thujaplicinol (MIC, 11 to 12 μM). Three additional α-methoxytropolones (no. 273, 317, and 318) were also tested and showed no activity. Specifically, the lack of activity of no. 318, which is a close analog of one of the more active αHTs, no. 308, suggests that the C-7 hydroxylate may be involved in metalloenzyme binding for the αHTs.
This screen was conducted with the aim of determining if tropones or tropolones could be a potential scaffold for developing new inhibitors of C. neoformans. The α-hydroxytropolones are likely to inhibit at least in part through their ability to coordinate divalent cations in metalloenzyme active sites. They are effective inhibitors of HIV, HBV, and herpes simplex viruses, most likely via coordination of the Mg2+ ions in the active sites of enzymes that are members of the nucleotidyl transferase superfamily (18, 20, 37). There are at least 40 proteins encoded by the C. neoformans genome that likely belong to this class of enzymes (38), and of the various αHTs tested, nine (no. 46, 114, 120, 145, 147, 265, 308, 310, and 311) have MICs of ≤15 μM. Given the large number of potential enzyme targets, it is possible that they inhibit more than one enzyme in C. neoformans.
Tropolone itself (Fig. 1B) is bacteriostatic and bactericidal against a wide range of bacterial species and is known to inhibit metalloproteases, with particularly high activity against carboxypeptidase A, a Zn2+-dependent matrix metalloprotease (35). Tropolone and β-thujaplicin (Fig. 1D) also inhibit the Zn2+-dependent glyoxalase I of Plasmodium falciparum at low μM concentrations (39). There are over 30 potential Zn2+-binding proteins, including members of the carboxypeptidase and glyoxalase protein families, in the C. neoformans genome. Thus, the tropones and tropolones appear to be a promising scaffold to explore for anticryptococcal inhibitors, as 4 of the 19 tested have MICs of <10 μM, including no. 284 which has an MIC of <1 μM with a TI of >300. However, at this time, the limited number of available compounds and the unknown targets of inhibition preclude the development of a thorough SAR.
Our best hit (no. 284) inhibited >80% of cryptococcal growth at 0.2 μM, and was relatively nontoxic in liver cells (CC50, 71 μM), giving it a therapeutic index of >300 under these conditions. It is fungicidal and does not interact antagonistically with either approved antifungal, namely FLC or AMB. Compound no. 284 is a tropone with a thioester linkage. The high-energy nature of thioester bonds makes it likely that this compound is hydrolyzed in the cells or culture medium resulting in a tropothione and p-benzoic acid. The fact that the hydrolyzed products are still potent inhibitors of cryptococcal growth (Fig. 2) and p-benzoic acid is not suggests strongly that the tropothione or its oxidized derivatives are functionally active derivatives of this compound. Therefore, the development of the tropothione scaffold into novel anticryptococcal drugs may be possible.
MATERIALS AND METHODS
Strains and media.
All inhibition assays were performed with C. neoformans var. grubii, KN99 (serotype A, MATα; kindly provided by Jennifer Lodge). Two clinical strains of C. neoformans var. grubii, serotype A, were kindly provided by Tamara Doering and Andre Spec. Cells were passaged on YPD (1% yeast extract, 2% yeast peptone, 2% dextrose) agar plates and grown overnight at 30°C in YPD liquid medium prior to diluting for the limiting dilution assays to determine the MIC of inhibition. YNB-02 (0.67% yeast nitrogen base, 0.2% dextrose, pH 7.0 with 50 mM MOPS [morpholinepropanesulfonic acid]) was used for all limiting dilution inhibition assays unless otherwise noted. cRPMI is RPMI-1650 (Sigma) with glutamine plus 0.625% fetal bovine serum.
Compound acquisition and synthesis.
The compounds employed are listed in Table 1, and their structures are in Fig. S2 and S3 in the supplemental material. Compounds no. 46 to 57 and 195 were acquired from the National Cancer Institute (NCI) Developmental Therapeutics Program. Compounds no. 60 to 63, 281 to 285, and 305 were purchased. α-Hydroxytropolone (compound no. 172) was synthesized in 3 steps from tropolone based on the procedure of Takeshita et al. (40). Compounds no. 106 to 120, 143 to 147, and 273 to 274 were synthesized in 5 to 7 steps from kojic acid as previously described (20, 23, 25, 41, 42). Compounds no. 280, 308 to 313, 315, and 317 to 319 were synthesized using an analogous strategy and are described in the supplemental material. Compounds no. 261 to 264 were made from 1,4-cyclohexadiene using the Banwell αHT synthesis method (43, 44), and specifics can be found in the supplemental material. Compounds were ≥95% pure by 1H NMR analysis. They were dissolved in DMSO (Sigma) and stored at −80°C.
Inhibition of C. neoformans growth.
Compounds were tested in a limiting dilution assay with a starting optical density (at 650 nm) of 0.001 in YNB-02 plus 1% DMSO. Cells were incubated without shaking for 48 h at 35°C and cell densities were measured at 650 nm. The MICs were determined using compound concentrations from 0.19 to 50 μM in YNB-02 plus 1% DMSO. Each assay was done in triplicates and all values are the averages from two or more independent assays. The data are presented as the average cell densities as percentages of DMSO-only treated cells. MICs are reported as the minimal concentration needed to inhibit 80% of C. neoformans growth relative to vehicle-treated controls.
Cytotoxicity in hepatoma cells.
HepDES19 cells (104 cells per well) were seeded in 96-well plates and incubated in DMEM with 10% fetal bovine serum (FBS) plus 1% penicillin and streptomycin, 1% nonessential amino acids, and 1% glutamine. The compounds were diluted in the medium at concentrations ranging from 0.78 to 100 μM plus 1% DMSO and added to the cells 48 h after plating, with each of the concentrations tested in triplicates. Cells were incubated with the compounds for 72 h, and cytotoxicity was measured using a mitochondrial metabolic assay with MTS (Promega). The data were transformed to log[inhibitor] and fit to a 4-variable slope curve using GraphPad Prism (v6; GraphPad Software, Inc.). The concentration at which 50% of cells were inhibited relative to vehicle-treated control is reported as the CC50 value.
Synergy assay.
The MICs of compounds no. 54 and no. 284 were measured in combination with FLC and AMB in a checkerboard assay (45, 46). The MICs of no. 54, FLC, and AMB were measured using compound concentrations from 0.19 to 50 μM, while the MIC of no. 284 was measured using concentrations from 0.04 to 10 μM. Each of the assays was performed in triplicate and all values are the averages from two or more independent assays. The fractional inhibitory concentration index (FICI) model is expressed as ΣFIC = FICA + FICB = MICA′/MICA + MICB′/MICB, where MICA, and MICB are the MIC values of agents A and B used alone and MICA′ and MICB′ are the MICs of agents A and B used in combination. The interactions between FLC or AMB and the test compounds were interpreted as synergistic when FICI was ≤0.5, as indifferent when FICI was between >0.5 and 4, and as antagonistic when FICI was >4 (28, 47).
Fungicidal assay.
To test for fungicidal activity, we removed aliquots of cells after 48 h of growth in the presence of test compounds at 2×, 4×, and 8× the respective MICs and spotted them on YPD plates. The plates were incubated at 30°C for 2 days and checked for growth. The growth of cells treated with test compounds was compared with that of cells treated with FLC, which is known to be fungistatic, and with that of cells treated with AMB, which is known to be fungicidal (29, 30).
Hydrolysis of compound no. 284.
To hydrolyze no. 284, 0.1 M NaOH was added to a solution of 10 mM no. 284 to a final concentration of 20 mM. The solution was mixed by vortexing until a prominent color change (pale yellow to dark orange) was apparent. These reactions were performed in triplicates. One aliquot of the reaction mixture was stored at room temperature, one at 35°C, and one at 40°C. After 24 h, the reaction mixtures were neutralized with HCl. MIC assays were prepared in triplicates for each aliquot in a dilution series from 0.19 to 50 μM. Assays were incubated for 48 h at 35°C, and cell densities were measured at 650 nm.
Growth in capsule-inducing conditions.
KN99α cells were grown overnight in YPD at 30°C with shaking. The cells were diluted to an OD650 of 1 in YNB-02 or cRPMI, then diluted 1:100 in YNB-02 plus 1% DMSO or cRPMI plus 1% DMSO and incubated with the compounds at 37°C with 5% CO2 for 48 h. Capsules were visualized using bright-field microscopy as halos surrounding the yeast cells through India ink exclusion.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Saint Louis University Department of Biochemistry and Molecular Biology, the Saint Louis University School of Medicine, and the Presidents Research Fund for seed grants. We thank Nika Juricic, Elena Lomonosova, Tiffany Edwards, Rajendra Upadhya, and Woei Lam for their technical assistance. We thank Jennifer Lodge, Tamara Doering, and Andre Spec for providing C. neoformans strains.
NMR data of no. 280 was in part collected at the City University of New York Advanced Science Research Center (CUNY ASRC) Biomolecular NMR facility, and we thank James Aramini for his assistance with collecting these data. Mass spectrometry data of no. 284 was collected by the Center for World Health and Medicine at Saint Louis University, and we thank Rich Heier for his assistance with collecting these data.
M.J.D., R.P.M., M.J.M, and J.E.T. are inventors on U.S. provisional patent application USTL.P0071US.P1 that encompasses the inhibitors reported here.
The Saint Louis University School of Medicine summer fellowship program provided support to A.Z. The National Institute of Allergy and Infectious Diseases (NIAID) provided funding to J.E.T. under grant number R01 AI104494. The National Institute of General Medical Sciences (NIGMS) provided funding to R.P.M. under grant number SC1 GM111158.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02574-16.
REFERENCES
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Shoham S, Marr KA. 2012. Invasive fungal infections in solid organ transplant recipients. Future Microbiol 7:639–655. doi: 10.2217/fmb.12.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neofytos D, Fishman JA, Horn D, Anaissie E, Chang CH, Olyaei A, Pfaller M, Steinbach WJ, Webster KM, Marr KA. 2010. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis 12:220–229. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 4.Lortholary O. 2007. Management of cryptococcal meningitis in AIDS: the need for specific studies in developing countries. Clin Infect Dis 45:81–83. doi: 10.1086/518583. [DOI] [PubMed] [Google Scholar]
- 5.Mdodo R, Brown K, Omonge E, Jaoko W, Baddley J, Pappas P, Kempf MC, Aban I, Odera S, Suleh A, Jolly PE. 2010. The prevalence, clinical features, risk factors and outcome associated with cryptococcal meningitis in HIV positive patients in Kenya. East Afr Med J 87:481–487. [PMC free article] [PubMed] [Google Scholar]
- 6.Bratton EW, El Husseini N, Chastain CA, Lee MS, Poole C, Sturmer T, Weber DJ, Juliano JJ, Perfect JR. 2013. Approaches to antifungal therapies and their effectiveness among patients with cryptococcosis. Antimicrob Agents Chemother 57:2485–2495. doi: 10.1128/AAC.01800-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhein J, Morawski BM, Hullsiek KH, Nabeta HW, Kiggundu R, Tugume L, Musubire A, Akampurira A, Smith KD, Alhadab A, Williams DA, Abassi M, Bahr NC, Velamakanni SS, Fisher J, Nielsen K, Meya DB, Boulware DR. 2016. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 16:809–818. doi: 10.1016/S1473-3099(16)00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butts A, Martin JA, DiDone L, Bradley EK, Mutz M, Krysan DJ. 2015. Structure-activity relationships for the antifungal activity of selective estrogen receptor antagonists related to tamoxifen. PLoS One 10:e0125927. doi: 10.1371/journal.pone.0125927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mor V, Rella A, Farnoud AM, Singh A, Munshi M, Bryan A, Naseem S, Konopka JB, Ojima I, Bullesbach E, Ashbaugh A, Linke MJ, Cushion M, Collins M, Ananthula HK, Sallans L, Desai PB, Wiederhold NP, Fothergill AW, Kirkpatrick WR, Patterson T, Wong LH, Sinha S, Giaever G, Nislow C, Flaherty P, Pan X, Cesar GV, de Melo Tavares P, Frases S, Miranda K, Rodrigues ML, Luberto C, Nimrichter L, Del Poeta M. 2015. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio 6:e00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Festa RA, Helsel ME, Franz KJ, Thiele DJ. 2014. Exploiting innate immune cell activation of a copper-dependent antimicrobial agent during infection. Chem Biol 21:977–987. doi: 10.1016/j.chembiol.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samantaray S, Correia JN, Garelnabi M, Voelz K, May RC, Hall RA. 2016. Novel cell-based in vitro screen to identify small-molecule inhibitors against intracellular replication of Cryptococcus neoformans in macrophages. Int J Antimicrob Agents 48:69–77. doi: 10.1016/j.ijantimicag.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartland K, Pu J, Palmer M, Dandapani S, Moquist PN, Munoz B, DiDone L, Schreiber SL, Krysan DJ. 2016. High-throughput screen in Cryptococcus neoformans Identifies a novel molecular scaffold that inhibits cell wall integrity pathway signaling. ACS Infect Dis 2:93–102. doi: 10.1021/acsinfecdis.5b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roemer T, Krysan DJ. 2014. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:pii=a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim YW, Kim JJ, Chedgy R, Morris PI, Breuil C. 2005. Fungal diversity from western redcedar fences and their resistance to beta-thujaplicin. Antonie Van Leeuwenhoek 87:109–117. doi: 10.1007/s10482-004-1729-x. [DOI] [PubMed] [Google Scholar]
- 15.Komaki N, Watanabe T, Ogasawara A, Sato N, Mikami T, Matsumoto T. 2008. Antifungal mechanism of hinokitiol against Candida albicans. Biol Pharm Bull 31:735–737. doi: 10.1248/bpb.31.735. [DOI] [PubMed] [Google Scholar]
- 16.Budihas SR, Gorshkova I, Gaidamakov S, Wamiru A, Bona MK, Parniak MA, Crouch RJ, McMahon JB, Beutler JA, Le Grice SF. 2005. Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones. Nucleic Acids Res 33:1249–1256. doi: 10.1093/nar/gki268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y, Cheng X, Cao F, Huang A, Tavis JE. 2013. Beta-thujaplicinol inhibits hepatitis B virus replication by blocking the viral ribonuclease H activity. Antiviral Res 99:221–229. doi: 10.1016/j.antiviral.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Lu G, Lomonosova E, Cheng X, Moran EA, Meyers MJ, Le Grice SF, Thomas CJ, Jiang JK, Meck C, Hirsch DR, D'Erasmo MP, Suyabatmaz DM, Murelli RP, Tavis JE. 2015. Hydroxylated tropolones inhibit hepatitis B virus replication by blocking viral ribonuclease H activity. Antimicrob Agents Chemother 59:1070–1079. doi: 10.1128/AAC.04617-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavis JE, Wang H, Tollefson AE, Ying B, Korom M, Cheng X, Cao F, Davis KL, Wold WS, Morrison LA. 2014. Inhibitors of nucleotidyltransferase superfamily enzymes suppress herpes simplex virus replication. Antimicrob Agents Chemother 58:7451–7461. doi: 10.1128/AAC.03875-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ireland PJ, Tavis JE, D'Erasmo MP, Hirsch DR, Murelli RP, Cadiz MM, Patel BS, Gupta AK, Edwards TC, Korom M, Moran EA, Morrison LA. 2016. Synthetic alpha-hydroxytropolones inhibit replication of wild-type and acyclovir-resistant herpes simplex viruses. Antimicrob Agents Chemother 60:2140–2149. doi: 10.1128/AAC.02675-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwatsuki M, Takada S, Mori M, Ishiyama A, Namatame M, Nishihara-Tsukashima A, Nonaka K, Masuma R, Otoguro K, Shiomi K, Omura S. 2011. In vitro and in vivo antimalarial activity of puberulic acid and its new analogs, viticolins A-C, produced by Penicillium sp. FKI-4410. J Antibiot (Tokyo) 64:183–188. doi: 10.1038/ja.2010.124. [DOI] [PubMed] [Google Scholar]
- 22.Piettre SR, Andre C, Chanal MC, Ducep JB, Lesur B, Piriou F, Raboisson P, Rondeau JM, Schelcher C, Zimmermann P, Ganzhorn AJ. 1997. Monoaryl- and bisaryldihydroxytropolones as potent inhibitors of inositol monophosphatase. J Med Chem 40:4208–4221. doi: 10.1021/jm9701942. [DOI] [PubMed] [Google Scholar]
- 23.Meck C, Mohd N, Murelli RP. 2012. An oxidopyrylium cyclization/ring-opening route to polysubstituted alpha-hydroxytropolones. Org Lett 14:5988–5991. doi: 10.1021/ol302892g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghannoum MA, Ibrahim AS, Fu Y, Shafiq MC, Edwards JE Jr, Criddle RS. 1992. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol 30:2881–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams YD, Meck C, Mohd N, Murelli RP. 2013. Triflic acid-mediated rearrangements of 3-methoxy-8-oxabicyclo(3.2.1)octa-3,6-dien-2-ones: synthesis of methoxytropolones and furans. J Org Chem 78:11707–11713. doi: 10.1021/jo401617r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Erasmo MP, Smith WB, Munoz A, Mohandas P, Au AS, Marineau JJ, Quadri LE, Bradner JE, Murelli RP. 2014. 7,9-Diaryl-1,6,8-trioxaspiro(4.5)dec-3-en-2-ones: readily accessible and highly potent anticancer compounds. Bioorg Med Chem Lett 24:4035–4038. doi: 10.1016/j.bmcl.2014.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doering TL. 2009. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu Rev Microbiol 63:223–247. doi: 10.1146/annurev.micro.62.081307.162753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 29.Klepser ME, Wolfe EJ, Pfaller MA. 1998. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B against Cryptococcus neoformans. J Antimicrob Chemother 41:397–401. doi: 10.1093/jac/41.3.397. [DOI] [PubMed] [Google Scholar]
- 30.Mesa-Arango AC, Trevijano-Contador N, Roman E, Sanchez-Fresneda R, Casas C, Herrero E, Arguelles JC, Pla J, Cuenca-Estrella M, Zaragoza O. 2014. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob Agents Chemother 58:6627–6638. doi: 10.1128/AAC.03570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo H, Jiang D, Zhou T, Cuconati A, Block TM, Guo JT. 2007. Characterization of the intracellular deproteinized relaxed circular DNA of hepatitis B virus: an intermediate of covalently closed circular DNA formation. J Virol 81:12472–12484. doi: 10.1128/JVI.01123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavis JE, Cheng X, Hu Y, Totten M, Cao F, Michailidis E, Aurora R, Meyers MJ, Jacobsen EJ, Parniak MA, Sarafianos SG. 2013. The hepatitis B virus ribonuclease H is sensitive to inhibitors of the human immunodeficiency virus ribonuclease H and integrase enzymes. PLoS Pathog 9:e1003125. doi: 10.1371/journal.ppat.1003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobsen JA, Major Jourden JL, Miller MT, Cohen SM. 2010. To bind zinc or not to bind zinc: an examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta 1803:72–94. doi: 10.1016/j.bbamcr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Nakano K, Chigira T, Miyafusa T, Nagatoishi S, Caaveiro JM, Tsumoto K. 2015. Discovery and characterization of natural tropolones as inhibitors of the antibacterial target CapF from Staphylococcus aureus. Sci Rep 5:15337. doi: 10.1038/srep15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobsen FE, Lewis JA, Heroux KJ, Cohen SM. 2007. Characterization and evaluation of pyrone and tropolone chelators for use in metalloprotein inhibitors. Inorganica Chim Acta 360:264–272. doi: 10.1016/j.ica.2006.07.044. [DOI] [Google Scholar]
- 36.Roff JW, Whittaker EI. 1959. Toxicity tests of a new tropolone, β-thujaplicinol (7-hydroxy-4-isopropyltropolone) occuring in Western red cedar. Can J Bot 37:1132–1134. doi: 10.1139/b59-089. [DOI] [Google Scholar]
- 37.Meck C, D'Erasmo MP, Hirsch DR, Murelli RP. 2014. The biology and synthesis of alpha-hydroxytropolones. MedChemComm 5:842–852. doi: 10.1039/c4md00055b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janbon G, Ormerod KL, Paulet D, EJByrnes 3rd, Yadav V, Chatterjee G, Mullapudi N, Hon CC, Billmyre RB, Brunel F, Bahn YS, Chen W, Chen Y, Chow EW, Coppee JY, Floyd-Averette A, Gaillardin C, Gerik KJ, Goldberg J, Gonzalez-Hilarion S, Gujja S, Hamlin JL, Hsueh YP, Ianiri G, Jones S, Kodira CD, Kozubowski L, Lam W, Marra M, Mesner LD, Mieczkowski PA, Moyrand F, Nielsen K, Proux C, Rossignol T, Schein JE, Sun S, Wollschlaeger C, Wood IA, Zeng Q, Neuveglise C, Newlon CS, Perfect JR, Lodge JK, Idnurm A, Stajich JE, Kronstad JW, Sanyal K, Heitman J, Fraser JA, Cuomo CA, Dietrich FS. 2014. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet 10:e1004261. doi: 10.1371/journal.pgen.1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishiyama A, Iwatsuki M, Yamamoto T, Miura H, Omura S, Otoguro K. 2014. Antimalarial tropones and their Plasmodium falciparum glyoxalase I (pfGLOI) inhibitory activity. J Antibiot (Tokyo) 67:545–547. doi: 10.1038/ja.2014.28. [DOI] [PubMed] [Google Scholar]
- 40.Takeshita H, Mori A, Kusaba T. 1986. An improved synthesis of 2,7-dihydroxytropone (3-hydroxytropolone). Synthesis 1986:578–579. doi: 10.1055/s-1986-31713. [DOI] [Google Scholar]
- 41.Hirsch DR, Cox G, D'Erasmo MP, Shakya T, Meck C, Mohd N, Wright GD, Murelli RP. 2014. Inhibition of the ANT(2″)-Ia resistance enzyme and rescue of aminoglycoside antibiotic activity by synthetic alpha-hydroxytropolones. Bioorg Med Chem Lett 24:4943–4947. doi: 10.1016/j.bmcl.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Erasmo MP, Masaoka T, Wilson JA, Hunte EM, Beutler JA, Le Grice SFJ, Murelli RP. 2016. Traceless solid-phase alpha-hydroxytropolone synthesis. MedChemComm 7:1789–1792. doi: 10.1039/C6MD00237D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amon CM, Banwell MG, Gravatt GL. 1987. Oxidation of vicinal diols to.alpha.-dicarbonyl compounds by trifluoroacetic anhydride-activated dimethyl sulfoxide. J Org Chem 52:4851–4855. doi: 10.1021/jo00231a005. [DOI] [Google Scholar]
- 44.Banwell M, Cameron J, Collis M, Crisp G, Gable R, Hamel E, Lambert J, Mackay M, Reum M, Scoble J. 1991. The palladium-mediated cross coupling of bromotropolones with organostannanes or arylboronic acids: applications to the synthesis of natural products and natural product analogs. Aust J Chem 44:705–728. doi: 10.1071/CH9910705. [DOI] [Google Scholar]
- 45.White RL, Burgess DS, Manduru M, Bosso JA. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother 40:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banerjee D, Burkard L, Panepinto JC. 2014. Inhibition of nucleotide biosynthesis potentiates the antifungal activity of amphotericin B. PLoS One 9:e87246. doi: 10.1371/journal.pone.0087246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berenbaum MC. 1978. A method for testing for synergy with any number of agents. J Infect Dis 137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.