ABSTRACT
The objective of this study was to evaluate the impact of pharmacist-ordered methicillin-resistant Staphylococcus aureus (MRSA) PCR testing on the duration of empirical MRSA-targeted antibiotic therapy in patients with suspected pneumonia. This is a retrospective analysis of patients who received vancomycin or linezolid for suspected pneumonia before and after the implementation of a pharmacist-driven protocol for nasal MRSA PCR testing. Patients were included if they were adults of >18 years of age and initiated on vancomycin or linezolid for suspected MRSA pneumonia. The primary endpoint was the duration of vancomycin or linezolid therapy. After screening 368 patients, 57 patients met inclusion criteria (27 pre-PCR and 30 post-PCR). Baseline characteristics were similar between the two groups, with the majority of patients classified as having health care-associated pneumonia (68.4%). The use of the nasal MRSA PCR test reduced the mean duration of MRSA-targeted therapy by 46.6 h (74.0 ± 48.9 h versus 27.4 ± 18.7 h; 95% confidence interval [CI], 27.3 to 65.8 h; P < 0.0001). Fewer patients in the post-PCR group required vancomycin serum levels and dose adjustment (48.1% versus 16.7%; P = 0.02). There were no significant differences between the pre- and post-PCR groups regarding days to clinical improvement (1.78 ± 2.52 versus 2.27 ± 3.34; P = 0.54), length of hospital stay (11.04 ± 9.5 versus 8.2 ± 7.8; P = 0.22), or hospital mortality (14.8% versus 6.7%; P = 0.41). The use of nasal MRSA PCR testing in patients with suspected MRSA pneumonia reduced the duration of empirical MRSA-targeted therapy by approximately 2 days without increasing adverse clinical outcomes.
KEYWORDS: methicillin-resistant Staphylococcus aureus, vancomycin, pneumonia, antimicrobial stewardship, Staphylococcus aureus
INTRODUCTION
Despite a relatively low prevalence, methicillin-resistant Staphylococcus aureus (MRSA) remains an important pathogen in considering empirical antibiotic therapy for patients hospitalized with pneumonia (1–3). The most recent iteration of the Infectious Diseases Society of America (IDSA) guidelines for hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) recommend empirical coverage of MRSA in patients with intravenous antibiotic exposure in the past 90 days, hospitalization at an institution with >20% of S. aureus isolates identified as MRSA, or in patients at high risk of mortality (e.g., need for mechanical ventilation or presence of shock) (4). The prior version of these guidelines introduced the category of health care-associated pneumonia (HCAP) (5). This term was intended to identify community-dwelling patients at risk for drug-resistant pathogens, such as MRSA, and included risk factors, such as hospitalization within the past 90 days, outpatient dialysis therapy, wound care, or immunosuppression. Additionally, MRSA-targeted therapy should be considered for patients with severe community-acquired pneumonia (CAP) (6). Thus, based on guideline recommendations, a substantial percentage of patients hospitalized for pneumonia may have cause for empirical vancomycin or linezolid.
In order to appropriately tailor antibiotic therapy, microbiological evaluation is recommended with a high-quality lower respiratory tract culture, such as high-quality expectorated sputum, endotracheal aspirate, or bronchoalveolar lavage fluid (4, 5). However, such samples may be invasive and difficult to obtain in the clinical setting and are thus performed in less than half of cases (7). As a result of these challenges, the most common culture obtained is a sputum culture, which often lacks reliability as a proper sputum sample requires deep expectoration (8, 9). Furthermore, even if a respiratory culture is obtained, a causative pathogen may not be recovered (1, 7). Thus, despite the uncommon incidence of MRSA pneumonia, MRSA-targeted antibiotics are often started and potentially continued for prolonged periods due to a lack of objective microbiology data allowing for de-escalation (1).
In recent years, there has been a growing interest in utilizing nasal MRSA screening as a surrogate marker for MRSA lower respiratory tract infections. These studies have consistently shown that MRSA nasal colonization has a high negative predictive value (>94%) for MRSA recovered from lower respiratory sources, suggesting that the absence of MRSA in the nares can be used to predict the absence of MRSA in lower respiratory tract cultures (10–13). A prior retrospective study from our group demonstrated that MRSA nasal culture screening had a 98.5% negative predictive value for MRSA lower respiratory tract cultures. This study was conducted in 165 medical intensive care unit (ICU) patients who met objective clinical criteria for pneumonia. Of the 165 patients, only 2 patients (1.2%) had a negative MRSA nasal culture but had MRSA isolated from a lower respiratory tract culture (10). In a similarly designed study, Dangerfield and colleagues evaluated MRSA nasal PCR and lower respiratory tract cultures in 435 patients with evidence of pneumonia (12). This group concluded that the MRSA PCR test had a 99.2% negative predictive value for MRSA cultured from a lower respiratory site. Three of the 435 patients (0.7%) had a negative PCR result but grew MRSA from culture (12).
Despite the literature surrounding negative nasal MRSA results correlating with a negative MRSA respiratory culture in patients with pneumonia, there have yet to be any data evaluating its impact on duration of therapy and clinical outcomes. Therefore, our study was designed to evaluate the clinical outcomes of the implementation of a nasal MRSA PCR testing protocol.
RESULTS
Patients and baseline data.
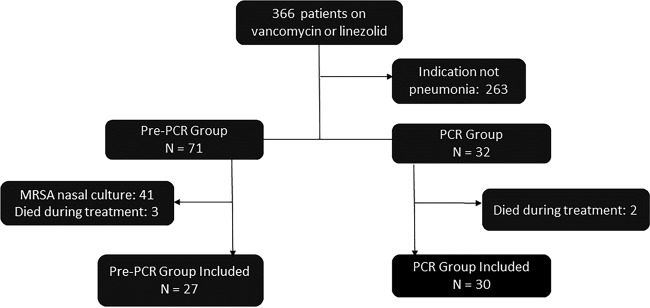
A total of 366 patients were identified during the prespecified time periods. As shown in Fig. 1, 263 (71.8%) patients were excluded due to extrapulmonary indication for anti-MRSA therapy. After excluding patients who either died during initial therapy or had a MRSA nasal culture performed, 27 patients remained in the pre-PCR group and 30 patients remained in the PCR group.
FIG 1.
Study flow.
There were no statistically significant differences in baseline demographics between the two groups (Table 1). The most common variant of pneumonia diagnosed was HCAP (70.4% versus 66.7%), and the majority of patients had non-hospital-acquired infections. ICU admission was required in 37% and 36.7% of the pre-PCR group and PCR group, respectively, with 26% of the pre-PCR group and 26.7% of the PCR group needing invasive mechanical ventilation. The mean simplified acute physiology score (SAPS II) was 35.2 ± 14.7 in the pre-PCR group and 36.3 ± 15.4 in the PCR group (P = 0.79).
TABLE 1.
Baseline characteristics
| Characteristica | Pre-PCR (n = 27) | PCR (n = 30) | P value |
|---|---|---|---|
| Age (yr) | 70.9 ± 17.2 | 63.3 ± 18.8 | 0.12 |
| No. male (%) | 13 (48.2) | 14 (46.7) | 1.0 |
| No. CHF (%) | 9 (33.3) | 10 (33.3) | 1.0 |
| No. DM (%) | 6 (22.2) | 12 (40) | 0.17 |
| No. COPD (%) | 4 (14.8) | 4 (13.3) | 1.0 |
| No. immunosuppression (%) | 10 (37) | 8 (26.7) | 0.57 |
| No. LTAC (%) | 9 (33.3) | 6 (20) | 0.37 |
| No. antibiotics (%) | 12 (44.4) | 14 (46.7) | 1.0 |
| No. wound care (%) | 0 (0) | 0 (0) | 1.0 |
| No. dialysis (%) | 1 (3.7) | 6 (20) | 0.11 |
| No. home infusion (%) | 0 (0) | 2 (6.7) | 0.49 |
| No. recent hospitalization (%) | 19 (70.4) | 16 (53.3) | 0.28 |
| No. CAP (%) | 3 (11.1) | 4 (13.3) | 1.0 |
| No. HCAP (%) | 19 (70.4) | 20 (66.7) | 0.78 |
| No. HAP (%) | 5 (18.5) | 6 (20) | 1.0 |
| No. VAP (%) | 0 (0) | 0 (0) | 1.0 |
| No. ICU admission (%) | 10 (37) | 11 (36.7) | 1.0 |
| No. pulse oximetry of ≤90% (%) | 14 (51.9) | 13 (43.3) | 0.60 |
| No. intubation (%) | 7 (26) | 8 (26.7) | 1.0 |
| No. vasopressor (%) | 5 (18.5) | 2 (6.7) | 0.28 |
| SAPS II score | 35.2 ± 14.7 | 36.3 ± 15.4 | 0.79 |
CHF, congestive heart failure; DM, diabetes mellitus; COPD, chronic obstructive lung disease; LTAC, long-term acute care; CAP, community-acquired pneumonia; HCAP, health care-associated pneumonia; HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia; ICU, intensive care unit; SAPS II, simplified acute physiology score II.
Outcomes.
For the primary endpoint, the use of nasal MRSA PCR reduced the mean duration of empirical MRSA-targeted therapy by 46.6 h (74 ± 48.9 h for the pre-PCR group versus 27.4 ± 18.7 h for the PCR group; 95% confidence interval [CI], 27.3 to 65.8 h; P < 0.0001). As such, patients in the PCR group were exposed to significantly less total intravenous vancomycin (Table 2). Subsequently, the PCR group also required fewer vancomycin serum levels. At least one serum vancomycin was obtained in 48.1% of the pre-PCR patients compared to 16.7% of patients managed with the nasal PCR. No patient in the PCR group required more than one serum level, whereas 5/27 (18.5%) of pre-PCR patients remained on vancomycin long enough to require two levels.
TABLE 2.
MRSA-targeted antibiotic therapy outcomes
| Parameter | Pre-PCR (n = 27) | PCR (n = 30) | Difference | P value | 95% CI |
|---|---|---|---|---|---|
| Duration of therapy | |||||
| Hours | 74 ± 48.9 | 27.4 ± 18.7 | 46.6 | <0.0001 | 27.3–65.8 |
| Days | 4.0 ± 2.0 | 2.13 ± 0.86 | 1.9 | <0.0001 | 1.06–2.67 |
| Total i.v.a vancomycin doses | 4.2 ± 3.1 | 1.7 ± 1.5 | 2.44 | 0.005 | 1.12–3.77 |
| Total i.v. vancomycin (mg) | 5,394.4 ± 3,483.5 | 2,865 ± 2,579.8 | 2,529.4 | 0.003 | 912.9–4,145.9 |
| Vancomycin level obtained (%) | 13 (48.1) | 5 (16.7) | 31.4 | 0.02 | |
| No. of vancomycin levels (%) | |||||
| 0 | 14 (51.9) | 25 (83.3) | |||
| 1 | 8 (29.6) | 5 (16.7) | |||
| 2 | 5 (18.5) | 0 (0) | |||
| Per patient avg no. of levels | 0.67 | 0.17 | 0.50 |
i.v., intravenous.
As displayed in Table 3, there was no significant difference in days to clinical improvement (1.78 ± 2.52 days for pre-PCR versus 2.27 ± 3.34 days for PCR; 95% CI, −2.07 to 1.10; P = 0.54). Similarly, there were no significant differences in length of hospital stay (11.04 ± 9.5 days versus 8.2 ± 7.8 days; P = 0.22) or mortality (14.8% versus 6.7%; P = 0.41). Patients in the PCR group had a significantly lower incidence of acute kidney injury (AKI) during treatment (26% versus 3.3%; P = 0.02).
TABLE 3.
Clinical outcomes
| Outcome | Pre-PCR | PCR | Difference | P value | 95% CI |
|---|---|---|---|---|---|
| Days to clinical improvement | 1.78 ± 2.52 | 2.27 ± 3.34 | −0.49 | 0.54 | −2.07–1.10 |
| No. (%) with acute kidney injury | 7 (26) | 1 (3.3) | 22.7 | 0.02 | |
| Length of hospital stay (days) | 11.04 ± 9.5 | 8.2 ± 7.8 | 2.84 | 0.22 | −1.75–7.43 |
| Mortality (no. [%]) | 4 (14.8) | 2 (6.7) | 8.1 | 0.41 |
Microbiological data are displayed in Table 4. All patients in the nasal MRSA PCR group were negative for MRSA. Despite being ordered in at least 90% of patients, quality respiratory cultures were only obtained in approximately 50% of the total patient population (44.4% pre-PCR versus 56.7% PCR; P = 0.43). No patient in either group grew MRSA from a respiratory or blood culture. In the pre-PCR group, culture results were available in 12 patients. Eight of 12 patients grew either normal flora or yeast. In the PCR group, cultures were obtained in 17 patients. Fourteen of the 17 patients grew either normal flora or yeast.
TABLE 4.
Microbiological outcomes
| Parameter | Pre-PCR (n = 27) | PCR (n = 30) | P value |
|---|---|---|---|
| Respiratory culture ordered (no. [%]) | 25 (92.6) | 27 (90) | 1.0 |
| Respiratory cultures obtained (no. [%]) | 12 (44.4) | 17 (56.7) | 0.43 |
| Type of respiratory culture (no. [%]) | |||
| Sputum (expectorated) | 8 (66.7) | 6 (35.3) | |
| Sputum (induced) | 2 (16.7) | 6 (35.3) | |
| Tracheal aspirate | 1 (8.3) | 5 (29.4) | |
| Bronchoalveolar lavage | 1 (8.3) | 0 (0) | |
| Culture results (no. [%]) | |||
| Normal flora | 6 (50) | 13 (76.5) | |
| Yeast | 2 (16.7) | 1 (5.9) | |
| Klebsiella pneumoniae | 1 (8.3) | 0 (0) | |
| Haemophilus influenzae | 1 (8.3) | 0 (0) | |
| Pseudomonas aeruginosa | 1 (8.3) | 2 (11.8) | |
| Methicillin-susceptible Staphylococcus aureus | 1 (8.3) | 1 (5.9) |
DISCUSSION
Based on our results, the use of nasal MRSA PCR testing reduced the duration of vancomycin and linezolid by approximately 2 days without any deleterious effects to patients' clinical courses. The reduction in vancomycin duration was accompanied by a reduction in the number of vancomycin levels drawn for pharmacokinetic monitoring. Thus, there are significant benefits for antimicrobial stewardship efforts and allocation of pharmacist resources.
While MRSA remains a significant concern in patients who develop pneumonia while hospitalized and even more so while mechanically ventilated, MRSA as a causative pathogen in patients admitted for pneumonia is uncommon (1, 2, 14, 15). Jones and colleagues demonstrated a rate of MRSA-associated pneumonia of 2.0% to 2.5% over a 5-year period (2). In a multicentered observational study, S. aureus was isolated in 37 of 2,259 (1.6%) patients and was more common in winter months (coinciding with influenza season), critically ill patients, and older patients (1). Despite the relative rarity, many of these patients receive empirical MRSA-targeted therapy. Since high-quality respiratory cultures are not always collected in a timely fashion, objective data to allow the de-escalation of empirical therapy may not exist. Thus, there has been interest in MRSA nasal colonization as a less-invasive and easier-to-obtain surrogate for MRSA pneumonia.
In a prior study conducted at our institution, nasal MRSA cultures obtained from 165 medical ICU patients with pneumonia were found to have a negative predictive value of 98.5% (95% CI, 94.3% to 99.7%) compared to respiratory cultures (10). Of the 137 patients with negative MRSA nasal cultures, two patients grew MRSA in a respiratory culture. By comparison, 28 patients were colonized with MRSA but only eight had MRSA isolated in a respiratory culture (positive predictive value, 28.6%). Although the nasal MRSA culture has a high negative predictive value, its clinical application may be hindered by the 24- to 48-h time period required for culture results and may be impacted by decolonization efforts (16).
With the advent of PCR technology, nasal MRSA PCR testing offers the benefits of increased sensitivity as well as a rapid turnaround time of less than 4 h (9). In a trial by Dangerfield et al. that evaluated patients with pneumonia and a positive MRSA respiratory culture, nasal MRSA PCR testing was found to have a negative predictive value of 99.2% (12). Similar to nasal MRSA culture, the positive predictive value of the nasal MRSA PCR was low at 35.4%. These findings were further supported by later studies by Langsjoen et al. and Johnson et al., both of which found a negative predictive value above 94% in patients with pneumonia (11, 13). Based on these studies, there is strong evidence to support the negative predictive value of the nasal MRSA PCR test in pneumonia. However, our study is the first to demonstrate the ability of nasal MRSA PCR testing to reduce empirical MRSA-targeted therapy without causing deleterious effects to patient care.
We believe our study was the first to assess a pragmatic approach to incorporating nasal MRSA PCR screening into de-escalation efforts and evaluated patient outcomes as well as duration of therapy. By utilizing staff pharmacists to order the test as well as evaluate and follow up on the results by communicating with the ordering provider, the protocol provided the consistency that is necessary for a successful reduction in unnecessary antibiotic use. In addition, the vast majority of patients in the two groups were classified as having HCAP, which is consistent with clinical practice guidelines (5).
However, our study had several limitations that may limit its applicability to outside institutions. All diagnoses of pneumonia were based on individual physicians' clinical judgements and were not independently adjudicated for the purposes of this study. It is possible that some patients without true pneumonia were included in this study. We had a relatively small sample size in the two groups with some disparities in baseline characteristics, although none of these differences were statistically significant. As more patients in the pre-PCR group were long-term care facility residents, required vasopressor therapy, or had recent hospital admission, it may be argued that physicians were more aggressive with antibiotic therapy in these patients. However, this is offset by the similarity in the SAPS II score in the two groups, which is a marker of clinical severity of illness. In addition, the proportions of patients requiring ICU admission or intubation were approximately equal between the two groups. Furthermore, the PCR group included patients who were admitted in November 2015 through early December 2015, which is shortly after the implementation of the PCR protocol. Anecdotally, pharmacist compliance and comfort with the protocol as well as physician acceptance of recommendations for discontinuation has improved dramatically since this time frame. Therefore, selecting a later time period for the PCR group may have resulted in finding an even shorter duration of therapy. Additionally, the PCR test was not performed during the laboratory's overnight shift. Thus, if the PCR was run 24 h per day, there may have been additional reductions in duration. However, as the MRSA PCR test has a relatively higher cost than the MRSA culture, a complete cost-benefit analysis would be needed prior to implementing around-the-clock testing capabilities. This analysis was not performed as part of this study. Lastly, we observed that approximately 50% of patients did not have respiratory cultures obtained. While this number seems high, it is consistent with the observations from Jones and colleagues, who documented only 34% of patients in their epidemiology study (2). Respiratory virus PCR testing was ordered inconsistently between the two groups (data not presented), but a predominance of viral pneumonia may explain the low yield of bacterial pathogens.
In addition to these limitations, it should be noted that the PCR group did not have any patients who had a positive MRSA PCR result. Although having a patient in the sample with a positive result may have increased the duration of therapy, such an uncommon occurrence of a positive result is consistent with what is observed in clinical practice at our institution and, more importantly, correlates with the relatively small incidence of true MRSA pneumonia.
In conclusion, this retrospective chart review found that nasal MRSA PCR testing resulted in a significant reduction in the duration of vancomycin and linezolid without any adverse impact on clinical outcomes. In addition, the study found a reduction in the incidence of AKI during therapy, which correlates with the shorter duration of vancomycin. These findings, although limited by a small study population, serve to support the growing role of nasal MRSA PCR testing to promote the early de-escalation of therapy for patients on empirical MRSA-targeted therapies for pneumonia.
MATERIALS AND METHODS
Patient selection.
This retrospective study was conducted among all patients receiving vancomycin or linezolid for pneumonia between 1 March 2015 and 31 March 2015 (pre-PCR group) and 1 November 2015 and 4 December 2015 (PCR group). The study was conducted at the Texas Health Presbyterian Hospital of Dallas, which is a large, community teaching hospital with an established comprehensive antimicrobial stewardship team (17). The study protocol was approved by the Institutional Review Board at the Texas Health Research and Education Institute.
Patients were included if they were adults above 18 years of age and had an order for vancomycin or linezolid for the indication of pneumonia. The diagnosis of pneumonia was determined from physician documentation in the medical record. For the PCR group, patients were only included if they had a MRSA PCR test ordered. Patients were excluded if they had any extrapulmonary indications for MRSA-targeted therapy, such as bacteremia or cellulitis. In addition, all patients who died during treatment with vancomycin or linezolid were excluded. For the pre-PCR group, patients were excluded if they had a MRSA nasal culture performed, as this may have impacted the duration of therapy.
Intervention.
In early 2014, all intravenous vancomycin dosing outside surgical prophylaxis became automatic pharmacist dosing consults. As an extension of this hospital-approved protocol, the pharmacy department implemented a Pharmacy and Therapeutics committee-approved pharmacist-driven nasal MRSA PCR protocol in October 2015. This protocol allowed pharmacists to order a nasal MRSA PCR test without a direct physician order for any patient initiated on intravenous vancomycin or intravenous/oral linezolid for the indication of pneumonia. Per this protocol, if a pharmacist receives an order for vancomycin or linezolid with an indication of pneumonia or potential pneumonia, the pharmacist then orders the nasal MRSA PCR test. Pharmacists were only authorized to order PCR testing on patients with potential pneumonia. Pharmacists would then flag patients with a PCR order in our electronic health record via a pharmacy intervention system to review the patients' microbiological data periodically until the MRSA PCR results were posted. This allowed continued communication from shift to shift. There was no automated process to call or forward these results from microbiology to the pharmacy. Once the test results returned, the pharmacists were instructed to follow up with the appropriate provider to recommend discontinuation of the MRSA-targeted antibiotic if the test was negative. The provider could make the decision to stop antibiotics solely based on the PCR test result at that time or defer until additional data were obtained. The pre-PCR and PCR groups were managed with the same vancomycin dosing protocol (goal trough, 15 to 20 μg/ml), and there were no other significant changes in antimicrobial management made between the time periods, with the exception of the pharmacist-driven nasal MRSA PCR order protocol.
The nasal MRSA PCR was collected by nursing staff with a swab set (Copan ESwab [Copan Diagnostics, Inc.] with Remel Stuart transport medium [Thermo Fisher Scientific]). After insertion into each nostril for at least 5 s per side, the swab was returned to the microbiology lab. Swabs were run on the GeneXpert Infinity 48s instrument using the Xpert MRSA nasal cartridge (Cepheid). This instrument uses real-time PCR detection of Staphylococcus aureus and mecA-specific DNA targets. Once received, the turnaround time was less than 4 h from time of receipt (hands-on time was less than 5 min and approximately 1 h on-board the instrument). If there was interference with the assay cartridge, a repeat was done via a second swab. If the repeated PCR assay failed, testing was continued via culture on the chromID MRSA Chromagar (bioMérieux). The PCR tests were performed 7 days a week from 0400 to 2000 h.
Data collection and outcomes.
Baseline data collected included age, sex, past medical history, HCAP risk factors per IDSA guidelines (hospitalization in last 90 days, recent antibiotic use, residence in a long-term care facility, hemodialysis, home infusion therapy, or immunosuppression), type of pneumonia, admission to intensive care, requirement of mechanical ventilation, and vasopressor use. Severity of illness was assessed with the simplified acute physiology score (SAPS II) using values from the first 24 h of admission (18). Microbiological data in addition to the nasal MRSA PCR were also collected.
The primary endpoint evaluated was the length of vancomycin or linezolid therapy in hours. The secondary endpoints assessed included the number of vancomycin levels, mean total intravenous vancomycin doses, and number of vancomycin levels drawn for pharmacokinetic monitoring. Clinical secondary endpoints included the incidence of new acute kidney injury (AKI) during treatment (defined according to the RIFLE (risk, injury, failure, loss of function, and end-stage kidney disease) criteria as a doubling of serum creatinine or increase in serum creatinine of 0.5 mg/dl), time to clinical improvement (defined as the time to a white blood count [WBC] of <12,000 and afebrile for at least 24 h), length of hospital stay, and mortality after conclusion of the initial MRSA-targeted regimen (19).
Statistical analysis.
The hypothesis of this study was that the use of nasal MRSA PCR testing would reduce the mean duration of MRSA-targeted therapy by at least 1 day. A sample size of 24 patients in each group was necessary to detect a difference in duration of therapy of 1 day with 90% power, assuming a baseline duration of therapy of 4 ± 1.5 days. Data are reported as numbers and percentages or means with standard deviations where appropriate. Categorical data were analyzed with Fisher's exact test using a 2 × 2 contingency table. Unpaired student's t test was performed for continuous data. Ninety-five percent confidence intervals were constructed for these outcomes. All tests were two sided, and a P value of <0.05 was considered to be statistically significant. All statistical analysis was performed using JMP 7.0.1 (SAS Institute, Inc.).
ACKNOWLEDGMENT
We certify that we have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in the manuscript.
REFERENCES
- 1.Self WH, Wunderink RG, Williams DJ, Zhu Y, Anderson EJ, Balk RA, Fakhran SS, Chappell JD, Casimir G, Courtney DM, Trabue C, Waterer GW, Bramley A, Magill S, Jain S, Edwards KM, Grijalva CG. 2016. Staphylococcus aureus community-acquired pneumonia: prevalence, clinical characteristics, and outcomes. Clin Infect Dis 63:300–309. doi: 10.1093/cid/ciw300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones BE, Jones MM, Huttner B, Stoddard G, Brown KA, Stevens VW, Greene T, Sauer B, Madaras-Kelly K, Rubin M, Goetz MB, Samore M. 2015. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis 61:1403–1410. doi: 10.1093/cid/civ629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis SS, Walker VJ, Lee MS, Chen L, Moehring RW, Cox CE, Sexton DJ, Anderson DJ. 2014. Epidemiology of methicillin-resistant Staphylococcus aureus pneumonia in community hospitals. Infect Control Hosp Epidemiol 35:1452–1457. doi: 10.1086/678594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JH, Carratala J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Robert JA, Waterer GM, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Thoracic Society. 2005. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Bayer A, Cosgrove SE, Daum RB, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq015. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L. 2015. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill RL, Duckworth GJ, Casewell MW. 1988. Elimination of nasal carriage of methicillin-resistant Staphylococcus aureus with mupirocin during a hospital outbreak. J Antimicrob Chemother 22:377–384. doi: 10.1093/jac/22.3.377. [DOI] [PubMed] [Google Scholar]
- 9.French GL. 2009. Methods for screening for methicillin-resistant Staphylococcus aureus carriage. Clin Microbiol Infect 15(Suppl):S10–S16. [DOI] [PubMed] [Google Scholar]
- 10.Tilahun B, Faust AC, McCorstin P, Ortegon A. 2015. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care 24:8–12. doi: 10.4037/ajcc2015102. [DOI] [PubMed] [Google Scholar]
- 11.Langsjoen J, Brady C, Obenauf E, Kellie S. 2014. Nasal screening is useful in excluding methicillin-resistant Staphylococcus aureus in ventilator-associated pneumonia. Am J Infect Control 42:1014–1015. doi: 10.1016/j.ajic.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Dangerfield B, Chung A, Webb B, Seville MT. 2014. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother 58:859–864. doi: 10.1128/AAC.01805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JA, Wright ME, Sheperd LA, Musher DM, Dang BN. 2015. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J 19:34–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer E, Schwab F, Gastmeier P. 2010. Nosocomial methicillin resistant Staphylococcus aureus pneumonia—epidemiology and trends based on data of a network of 586 German ICUs (2005-2009). Eur J Med Res 15:514–524. doi: 10.1186/2047-783X-15-12-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GEN, Albrecht V, Limbago B, Talan DA. 2012. Prevalence of methicillin-resistant Staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis 54:1126–1133. doi: 10.1093/cid/cis022. [DOI] [PubMed] [Google Scholar]
- 16.Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, Lankiewicz J, Gombosev A, Terpstra L, Hartford F, Hayden MK, Jernigan JA, Weinstein RA, Fraser VJ, Haffenreffer K, Cui E, Kaganov RE, Lolans K, Perlin JB, Platt R. 2013. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith T, Philmon CL, Johnson GD, Ward WS, Rivers LL, Williamson SA, Goodman EL. 2014. Antimicrobial stewardship in a community hospital: attacking the more difficult problems. Hosp Pharm 49:839–846. doi: 10.1310/hpj4909-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F. 1993. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. 2004. Acute renal failure—definition, outcome measures, animal models, fluid therapy, and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]