ABSTRACT
Despite dose-limiting nephrotoxic potentials, polymyxin B has reemerged as the last line of therapy against multidrug-resistant Gram-negative bacterial infections. However, the handling of polymyxin B by the kidneys is still not thoroughly understood. The objectives of this study were to evaluate the impact of renal polymyxin B exposure on nephrotoxicity and to explore the role of megalin in renal drug accumulation. Sprague-Dawley rats (225 to 250 g) were divided into three dosing groups, and polymyxin B was administered (5 mg/kg, 10 mg/kg, and 20 mg/kg) subcutaneously once daily. The onset of nephrotoxicity over 7 days and renal drug concentrations 24 h after the first dose were assessed. The effects of sodium maleate (400 mg/kg intraperitoneally) on megalin homeostasis were evaluated by determining the urinary megalin concentration and electron microscopic study of renal tissue. The serum/renal pharmacokinetics of polymyxin B were assessed in megalin-shedding rats. The onset of nephrotoxicity was correlated with the daily dose of polymyxin B. Renal polymyxin B concentrations were found to be 3.6 ± 0.4 μg/g, 9.9 ± 1.5 μg/g, and 21.7 ± 4.8 μg/g in the 5-mg/kg, 10-mg/kg, and 20-mg/kg dosing groups, respectively. In megalin-shedding rats, the serum pharmacokinetics of polymyxin B remained unchanged, but the renal exposure was attenuated by 40% compared to that of control rats. The onset of polymyxin B-induced nephrotoxicity is correlated with the renal drug exposure. In addition, megalin appears to play a pivotal role in the renal accumulation of polymyxin B, which might contribute to nephrotoxicity.
KEYWORDS: Polymyxin B, renal drug concentration, megalin, polymyxins
INTRODUCTION
There is a renewed interest in the clinical use of polymyxins due to the increased prevalence of infections caused by multidrug-resistant Gram-negative bacteria (1). Many currently available antibiotics are no longer effective against these resistant bacterial strains. Additionally, the situation is further exacerbated by the limited number of new antibacterial agents in the advanced drug development pipeline. Consequently, the polymyxins have emerged as the last treatment resort against these life-threatening infections.
Polymyxins (polymyxin B and polymyxin E [colistin]) are cyclic polypeptide antibiotics which were available for clinical use in the 1950s. However, the clinical use of polymyxins was considerably reduced in the early 1970s due to concerns about nephrotoxicity (2–4). Despite being available for decades, the correlation between the pharmacokinetics and toxicodynamic profiles of polymyxin B is still not thoroughly understood. This knowledge gap often hinders the optimal clinical use of polymyxin B. There is evidence suggesting that polymyxin B is not eliminated through the renal route (5, 6). However, pharmacokinetic studies have reported preferential accumulation and prolonged residence of polymyxin B in rat kidneys (7, 8). Several studies have also reported a daily dose of polymyxin B as an independent risk factor associated with drug nephrotoxicity (9–11). Nevertheless, these reports did not provide a mechanistic framework correlating renal drug exposure with the onset of polymyxin B nephrotoxicity.
The underlying mechanism(s) of polymyxin B-induced nephrotoxicity has not been fully established. Many questions with respect to the intrarenal accumulation of polymyxin B and its contribution to nephrotoxicity remain unanswered. To date, there is very limited information on the mechanistic factors implicated in the preferential renal accumulation of polymyxin B. It was previously suggested that megalin might play a crucial role in renal handling of polymyxin B (12). Megalin is one of the members of the low-density-lipoprotein-related protein 2 (LRP2) receptor gene family, with a molecular size of approximately 600 kDa (13, 14). It is predominantly expressed in the microvilli of renal proximal tubular epithelium, labyrinth membrane of the inner ear, and retinal epithelium (15). Megalin functions as an endocytic receptor and is responsible for the internalization/uptake of a wide variety of endogenous molecules as well as xenobiotics (16). Several endogenous molecules, such as vitamin D, calcium, lipoprotein lipases, plasminogen activator inhibitor type-1 complex, and receptor-associated protein (RAP), are known ligands of megalin (17, 18). Polybasic drugs, such as polymyxin B, colistin, gentamicin, and amikacin, have a high binding affinity for megalin. It has been speculated that binding of these molecules to megalin leads to their internalization within the kidneys (12, 19, 20). Based on these relevant literature findings, we hypothesize that polymyxin B renal uptake might be mediated through megalin.
The objective of this study was to establish a correlation between the renal exposure of polymyxin B and the onset of nephrotoxicity, in conjunction with exploration of the role of megalin in the renal accumulation of polymyxin B. The outcomes from this research can be used for identifying the pharmacological target(s) and designing a future intervention(s) to mitigate polymyxin B-induced nephrotoxicity.
RESULTS
Correlation between onset of nephrotoxicity and renal tissue concentration of polymyxin B.
All of the animals (10 out of 10) that received 20 mg/kg of polymyxin B reached the predefined nephrotoxicity endpoint (i.e., a significant elevation in serum creatinine [≥2-fold the baseline level]). In contrast, none of the animals given 5 mg/kg of polymyxin B reached the endpoint by day 7. Furthermore, we observed a more gradual onset of nephrotoxicity in the 10-mg/kg group than in the 20-mg/kg dosing group, as shown in Fig. 1. More specifically, 8 out of 10 animals that received 20 mg/kg daily reached the predefined endpoint within the first 48 h of treatment. In contrast, only 1 out of 10 animals that received 10 mg/kg daily reached the endpoint within the first 48 h of treatment (P < 0.001). These findings suggest that a higher daily dose of polymyxin B is associated with a more rapid onset of nephrotoxicity.
FIG 1.
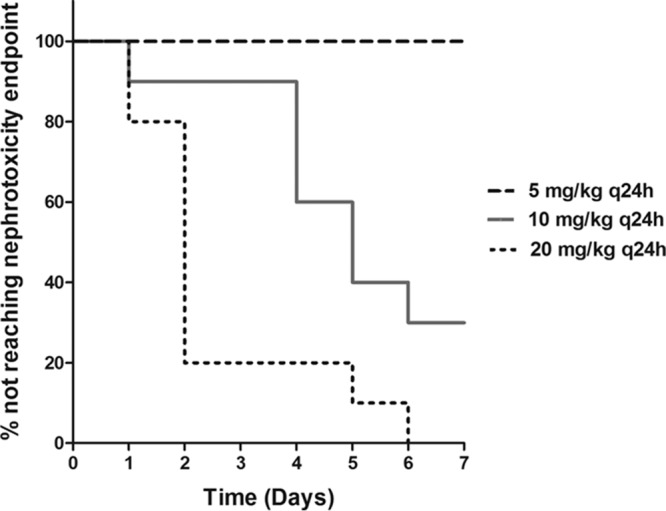
Comparison of times of onset of nephrotoxicity among different polymyxin B dosing groups (P < 0.001).
A similar trend was also observed in the renal tissue concentration of polymyxin B among the various dosing groups, as shown in Fig. 2. The observed concentrations of polymyxin B in renal tissue were 3.6 ± 0.4 μg/g, 9.9 ± 1.5 μg/g, and 21.7 ± 4.8 μg/g of renal tissue in the 5-mg/kg, 10-mg/kg, and 20-mg/kg dosing groups, respectively (P < 0.001). These data imply that the drug concentration observed in renal tissues is also correlated with the daily dose given.
FIG 2.
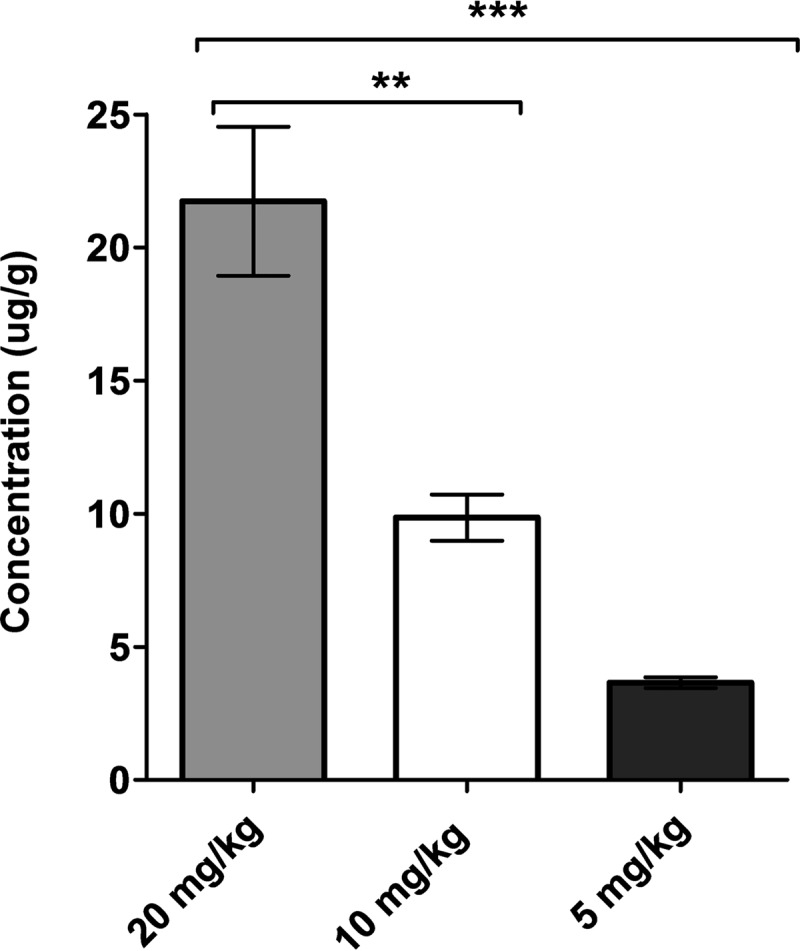
Renal tissue concentrations at escalating dose levels of polymyxin B. Vertical error bars represent the mean standard deviation within a group. The post hoc Tukey's test was used for multiple 2-way comparisons of means among different groups (***, P < 0.001; **, P < 0.01).
Effect of maleate administration on megalin homeostasis. (i) Urinary excretion of megalin.
There was a considerable elevation (approximately 20 times the baseline measurement) in the level of urinary megalin after maleate treatment. However, the urinary megalin levels reverted gradually to baseline approximately 11 days after treatment (data not shown).
(ii) Electron microscopy of maleate-treated kidney sections.
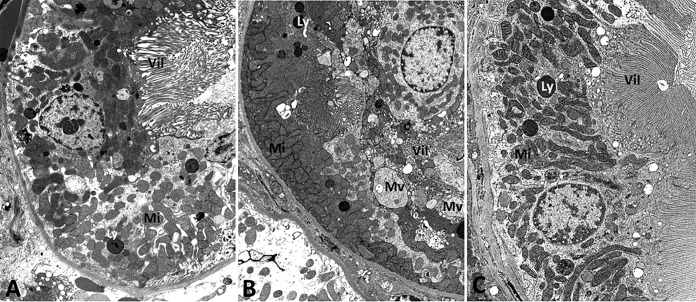
The electron microscopic images of control and maleate-treated kidney sections depict dramatic differences, as shown in Fig. 3. No significant ultrastructural changes were noticed in the control kidney section. Specifically, the proximal tubules in control rats revealed intact microvilli, mitochondria of normal size, shape, and density, and the presence of few microvesicles and lysosomes (Fig. 3A). In contrast, within 3 h, maleate pretreatment resulted in the appearance of morphological and ultrastructural changes in the proximal tubules, including multifocal disruption of microvilli and variations in size and shape of mitochondria. The presence of multiple dilated microvesicles and abundantly enlarged lysosomes were other notable changes seen in the ultrathin sections of the maleate-treated kidney, as shown in Fig. 3B. Of note, there were no noticeable changes seen in glomeruli, blood vessels, or any other type of renal tubules. Two weeks after maleate administration, the maleate-treated kidneys revealed no significant changes (Fig. 3C). Specifically, changes attributed to maleate administration were not seen. These findings suggest that the maleate-induced renal injuries are not long-lasting and are reversible.
FIG 3.
Electron microscopic images of ultrathin kidney sections: control, at 3 h (A); with maleate treatment, at 3 h (B); with maleate treatment, at 14 days (C). (A) No significant changes were noted in the control kidney. Specifically, the proximal tubular cells displayed intact microvilli (Vil) and mitochondria (Mi) of normal size, shape, and density. A few microvesicles/lysosomes (Ly) were noted. (B) Marked changes were noted in proximal tubular cells of an experimental animal, including degenerative changes of individual tubular cells (lower right), multifocal disruption or loss of microvilli (Vil), abundant mitochondria (Mi) of variable sizes and shapes, and multiple dilated microvesicles (Mv). There were no significant changes in glomeruli, blood vessels, or other types of renal tubules. (C) Proximal tubular cells displaying normal features, including intact microvilli (Vil), normal mitochondria (Mi), and few lysosomes (Ly). Original magnification, ×10,000 for all panels.
Polymyxin B pharmacokinetics in megalin-shedding rats.
In both groups (with or without maleate pretreatment), the overall model fits with the data were satisfactory. The coefficients of determination for serum concentration-time profiles were >0.94 and for renal concentration-time profiles were >0.87. The areas under the concentration-time curve from zero to infinity for serum (AUC0–∞, serum) for the control and experimental groups were comparable, i.e., 10.4 mg · h · liter−1 versus 11.1 mg · h · liter−1, respectively. In contrast, the AUC0–∞ values for renal tissue (AUC0–∞, renal tissue) differed by almost 2-fold, i.e., 211.9 mg · h · liter−1 and 121.0 mg · h · liter−1 for the control and experimental groups, respectively, as shown in Fig. 4. The AUC0–∞, renal tissue/AUC0–∞, serum ratios were 19.1 for the control group and 11.6 for the experimental group. These findings suggest that in megalin-shedding rats, the systemic exposure of polymyxin B remains unaltered but the renal exposure is reduced considerably after maleate administration.
FIG 4.
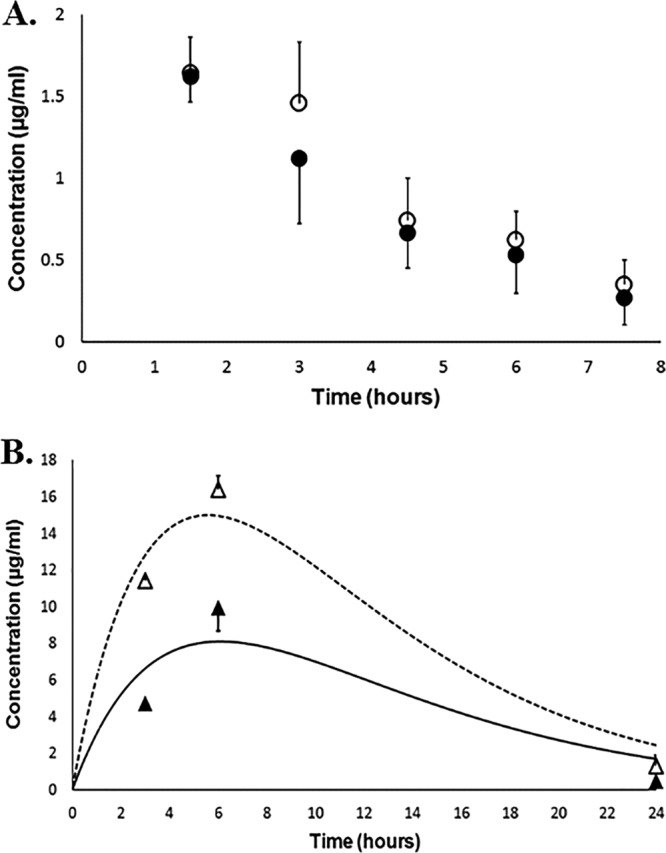
Mean polymyxin B concentrations in serum and renal tissues. Filled and open circles represent mean serum concentrations of polymyxin B in the experimental and control groups, respectively; filled and open triangles represent mean renal tissue concentrations of polymyxin B in the experimental and control groups, respectively. The dotted line is the best-fit line for the renal concentration-time profile in the control group, whereas the solid line is the best-fit line for the renal tissue concentration-time profile in the treatment group. Vertical error bars represent the standard deviation at each time point.
After 2 weeks of treatment with a single dose of maleate, our results showed that renal tissue polymyxin B concentrations (3 h postdosing) in sodium maleate-pretreated rats were comparable to those of control rats (11.08 μg/g versus 11.45 μg/g, P = 0.61). This finding suggests that the effect of sodium maleate on the renal accumulation of polymyxin B is also reversible in nature.
DISCUSSION
With the increasing prevalence of multidrug resistance in Gram-negative bacteria, the polymyxins are increasingly used as the last viable therapeutic option against life-threatening bacterial infections. However, limited understanding of polymyxin B pharmacokinetics and underlying mechanisms of nephrotoxicity is a major hindrance to the optimal clinical use of polymyxin B.
Over the past decades, several noteworthy attempts have been made to advance our understanding of nephrotoxicity associated with the polymyxins. Suzuki et al. reported significantly diminished renal concentrations of colistin in megalin-shedding rats, which suggested the involvement of megalin in renal drug accumulation (21). A recent study by Azad et al. investigated the underlying mechanism of polymyxin B nephrotoxicity in rat (NRK-52E) as well as human (HK-2) kidney proximal tubular cell lines. Cellular apoptosis was identified to be a potential mechanism of polymyxin B-induced nephrotoxicity. It was also reported that apoptosis was triggered via activation of the caspase pathway in a time- and concentration-dependent fashion (22).
In this study, we observed that the polymyxin B dose correlated with the renal drug concentration, and both correlated with the onset of nephrotoxicity. The higher daily dose of polymyxin B was associated with a greater degree of drug accumulation in the renal tissues, which subsequently manifested as a more rapid onset of nephrotoxicity (Fig. 1 and 2). These findings were consistent with the previous results from our laboratory demonstrating preferential renal accumulation and prolonged residence of polymyxin B in an animal model, thereby predisposing the kidneys to the toxic effect of polymyxin B (5, 7, 8).
Additional significant findings of this study involved delineation of the possible role of an endocytic receptor (megalin or low-density-lipoprotein-related protein 2 [Lrp2]) in the renal accumulation of polymyxin B. Maleate was reported to disrupt the association of megalin with the cell membrane along the microvilli in the brush border of epithelium in renal proximal tubular cells, resulting in loss of tissue megalin and urine excretion of megalin (i.e., megalin shedding) (21, 23). In this study, we have further verified megalin shedding by the quantitative recovery of megalin in urine and the morphological examination of maleate-treated kidney sections. The electron microscopic examination of kidney sections revealed marked ultrastructural changes in the proximal tubules after maleate treatment (Fig. 3). These results were consistent with those reported by Bergeron et al. (23). Maleate is also known to induce apical membrane-associated transport defects in the renal proximal epithelial cells similar to those observed in Fanconi syndrome. Maleate-induced ultrastructural changes transiently disrupt the apical endocytic and recycling apparatus, leading to accumulation of microvesicles in the proximal tubules (24). Therefore, the abundance of apical microvesicles in our electron microscopic study (Fig. 3) suggests that maleate might be involved in inhibition of the membrane recycling process, thereby inducing generalized transport defects similar to those observed in Fanconi syndrome. This finding corroborated the conclusion of Christensen et al., who previously demonstrated that maleate induced inhibition of lysozyme transport from the endocytic vacuoles to the lysosomes (25). On the basis of these collective findings, we hypothesize that megalin is involved in the internalization of polymyxin B by playing a crucial role in drug transport through the endocytic recycling apparatus. Subsequently, we wanted to ascertain whether the maleate-mediated changes and the altered megalin homeostasis were reversible. Fourteen days after treatment with maleate, the initial morphological findings (including megalin shedding in the urine) were reversed to the usual pattern seen in normal rats.
We further investigated the systemic/renal exposure of polymyxin B in megalin-shedding rats. Interestingly, our results indicated that polymyxin B exposure in renal tissue was attenuated by approximately 40% after pretreatment with maleate but the systemic drug exposure remained mostly unaltered in megalin-shedding rats (Fig. 4). This could be attributed primarily to the diminished availability of membrane-bound (i.e., functional) megalin after pretreatment with maleate. These findings suggest that preferential renal accumulation of polymyxin B might be reduced by a disrupting relevant mechanism(s) of drug uptake, leading to a possible delay in the onset of nephrotoxicity.
There are several limitations to this study. We could not directly demonstrate the delay in onset of nephrotoxicity in megalin-shedding rats. This was because maleate interfered with the nephrotoxicity endpoint detection by elevating serum creatinine levels. Therefore, the renal drug concentration was used as a surrogate marker, as an indirect approach of assessing the onset of nephrotoxicity. Also, our preliminary study established circumstantial evidence that megalin might be involved in renal accumulation of polymyxin B. However, we used a nonspecific approach (maleate administration) to facilitate disruption of membrane-bound megalin and induce transport defects. These findings should be validated with more specific methods. For future investigations, molecular tools (e.g., small interfering RNA [siRNA]-mediated gene silencing) to ascertain the role of megalin in renal accumulation of polymyxin B are warranted. In addition, we intend to examine the urinary concentration of polymyxin B in maleate-treated rats.
In conclusion, this study establishes a correlation between dose, renal drug exposure, and onset of polymyxin B-induced nephrotoxicity. This study also provides insights into the role of megalin in renal accumulation of polymyxin B. Megalin appears to be a promising target for designing future pharmacological interventions to alleviate polymyxin B-induced nephrotoxicity.
MATERIALS AND METHODS
Chemicals and reagents.
Polymyxin B sulfate (USP) powder was purchased from APP Pharmaceuticals LLC (lot number 6107834) (Schaumburg, IL) and Sigma-Aldrich (St. Louis, MO). Sodium maleate dibasic salt was obtained from Sigma-Aldrich. Liquid chromatography-mass spectrometry (LC-MS)-grade acetonitrile and water were obtained from Mallinckrodt Baker (Philipsburg, NJ); LC-MS-grade formic acid was purchased from Fluka Analytical (St. Louis, MO). The enzyme-linked immunosorbent assay (ELISA) kit for Lrp2/megalin was purchased from Cedarlane (Burlington, NC).
Animals.
Female Sprague-Dawley rats (225 to 250 g) (Harlan, Indianapolis, IN) were used. The rats received food and water ad libitum. All animals were cared for in accordance with the highest humane and ethical standards, as approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Houston. The jugular veins of selected animals were cannulated to facilitate intravenous drug administration.
Polymyxin B assay.
A validated ultraperformance liquid chromatography-tandem mass spectrometry (UPLC/MS-MS) method was modified to determine the concentrations of polymyxin B in rat serum and renal tissues, as previously described (8). The lower limit of quantification (LLOQ) was 50 ng/ml for all the major components of polymyxin B in serum as well as the renal tissue homogenate.
Correlation between onset of nephrotoxicity and renal tissue concentration of polymyxin B.
Prior to each experiment, polymyxin B for injection (USP) was reconstituted with sterile water for injection (USP) and diluted to achieve the desired concentration. The reconstituted drug solution was stored at −80°C in aliquots and thawed immediately before dosing.
To mimic a clinical course of polymyxin B treatment, three groups of rats (n = 13 each) were administered escalating dose levels of polymyxin B (5 mg/kg, 10 mg/kg, and 20 mg/kg, respectively) once daily subcutaneously for up to 7 days. Blood samples (approximately 200 μl) were drawn via the tail vein at baseline and on a daily basis (before dosing when applicable). Blood was allowed to clot on ice, and serum was separated by centrifugation at 4,000 × g for 10 min. Serum samples (100 μl) were further assayed for creatinine levels by use of a clinical chemistry analyzer (Piccolo Xpress; Abaxis, Inc., Union City, CA). A significant elevation in serum creatinine (≥2-fold the baseline level) was set as the endpoint for nephrotoxicity. Kaplan-Meier survival analysis and a log rank (Mantel-Cox) test were used to compare the times of onset of nephrotoxicity among various groups. Right censoring was used if the nephrotoxicity endpoint was not observed by day 7.
To determine the renal tissue concentration of polymyxin B, three rats from each dosing group were randomly selected and sacrificed at 24 h after the first dose; kidneys were harvested and homogenized. A fixed time point was selected to avoid any potential confounding due to different cumulative doses received when nephrotoxocity was observed. The concentrations of the major components of polymyxin B (polymyxin B1, polymyxin B2, polymyxin B3, and isoleucine polymyxin B1) were determined in serum samples as described above. For the purpose of drug quantification in the renal tissue homogenates, the estimated concentrations were divided by the total weight of the kidney and the final concentrations were expressed per gram of renal tissue (in micrograms/gram). The summed concentrations of individual components were used to estimate the overall renal drug exposure (26). One-way analysis of variance (ANOVA) and then post hoc Tukey's test were used to compare the mean renal tissue concentrations among the dosing groups, and P values of <0.05 were considered significant.
Effect of maleate administration on megalin homeostasis. (i) Urinary excretion of megalin.
Three rats were housed individually in separate metabolic cages 24 h prior to maleate pretreatment. A single dose of 400 mg/kg of sodium maleate was given intraperitoneally; this was previously reported to induce reversible ultrastructural modifications in the apical brush border membrane of proximal renal tubules (23). To study megalin urinary excretion, cumulative urine was collected daily from day −1 to day 15. The urine samples were spun down to remove particulate matter. Subsequently, the urine samples were aliquoted and stored at −80°C prior to analysis. The samples were thawed, and urinary Lrp2/megalin concentrations were quantitatively measured using a commercially available ELISA kit for megalin. For each animal, the daily megalin concentrations were expressed as a normalized ratio to the baseline megalin value.
(ii) Electron microscopy of maleate-treated kidney sections.
For morphological studies, one animal was sacrificed at 3 h and another at 14 days after maleate administration to collect the kidneys. The harvested kidneys were submitted for electron microscopic studies. Tissue samples from both renal cortical and medullary regions were fixed in 2% glutaraldehyde at 4°C overnight, followed by additional fixation in osmium tetroxide. The renal tissue was dehydrated and embedded in epoxy resin. Approximately 1-μm pilot sections were stained with toluidine for selection of the areas for further ultrastructural examination. Thin sections of these selected areas were cut and subjected to examination under a JEOL 300 electron microscope at 80 kV. The kidneys from two naive rats were harvested, processed in a similar fashion, and used as controls.
Polymyxin B pharmacokinetics in megalin-shedding rats.
To study the impact of sodium maleate pretreatment on systemic/renal exposure of polymyxin B, the rats were divided into two groups (n = 13 each). Animals in both control and experimental groups were administered a single intravenous dose of 3 mg/kg of polymyxin B sulfate (USP). The intravenous route of polymyxin B administration was preferred to avoid any interference with drug absorption at the injection site. In addition, the animals in the experimental group were given 400 mg/kg of sodium maleate intraperitoneally 3 to 6 h prior to polymyxin B administration. Serum samples were obtained at 1.5, 3, 4.5, 6, and 7.5 h after polymyxin B dosing. In addition, the rats were sacrificed to harvest the kidneys at 3, 6, and 24 h (n = 3 at each time point) postdosing. Polymyxin B concentrations in serum and renal tissue samples were assayed by the validated UPLC-MS/MS method detailed above.
The mean concentrations of polymyxin B in serum, as well as in renal tissue samples, at each time point were used. A modified two-compartment model (data not shown) was used to cofit the serum/renal tissue concentration-time profiles using ADAPT 5 (University of Southern California, Los Angeles, CA). Using the best-fit parameters, the AUC0–∞ was estimated by integrating instantaneous concentrations with respect to time. The AUC0–∞, renal tissue/AUC0–∞, serum ratio was used as an index to quantitatively assess preferential accumulation of polymyxin B in renal tissue.
To further examine whether the effect of sodium maleate on the renal accumulation of polymyxin B is transient, three additional rats were given a single dose of 400 mg/kg of sodium maleate intraperitoneally. Two weeks later, the animals were given a single dose of 3 mg/kg of polymyxin B intravenously. The rats were sacrificed at 3 h postdosing; kidneys were harvested and homogenized. Polymyxin B concentrations in renal tissue samples were assayed by the validated UPLC-MS/MS method.
ACKNOWLEDGMENT
No specific funding was received for this project.
We declare no conflicts of interest.
REFERENCES
- 1.Arnold TM, Forrest GN, Messmer KJ. 2007. Polymyxin antibiotics for gram-negative infections. Am J Health Syst Pharm 64:819–826. doi: 10.2146/ajhp060473. [DOI] [PubMed] [Google Scholar]
- 2.Nandha R, Sekhri K, Mandal AK. 2013. To study the clinical efficacy and nephrotoxicity along with the risk factors for acute kidney injury associated with parenteral polymyxin B. Indian J Crit Care Med 17:283–287. doi: 10.4103/0972-5229.120319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouderkirk JP, Nord JA, Turett GS, Kislak JW. 2003. Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrob Agents Chemother 47:2659–2662. doi: 10.1128/AAC.47.8.2659-2662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keirstead ND, Wagoner MP, Bentley P, Blais M, Brown C, Cheatham L, Ciaccio P, Dragan Y, Ferguson D, Fikes J, Galvin M, Gupta A, Hale M, Johnson N, Luo W, McGrath F, Pietras M, Price S, Sathe AG, Sasaki JC, Snow D, Walsky RL, Kern G. 2014. Early prediction of polymyxin-induced nephrotoxicity with next-generation urinary kidney injury biomarkers. Toxicol Sci 137:278–291. doi: 10.1093/toxsci/kft247. [DOI] [PubMed] [Google Scholar]
- 5.Abdelraouf K, Braggs KH, Yin T, Truong LD, Hu M, Tam VH. 2012. Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob Agents Chemother 56:4625–4629. doi: 10.1128/AAC.00280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. 2008. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 47:1298–1304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]
- 7.Abdelraouf K, He J, Ledesma KR, Hu M, Tam VH. 2012. Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob Agents Chemother 56:5724–5727. doi: 10.1128/AAC.01333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manchandani P, Zhou J, Ledesma KR, Truong LD, Chow DS, Eriksen JL, Tam VH. 2016. Characterization of polymyxin B biodistribution and disposition in an animal model. Antimicrob Agents Chemother 60:1029–1034. doi: 10.1128/AAC.02445-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendes CA, Cordeiro JA, Burdmann EA. 2009. Prevalence and risk factors for acute kidney injury associated with parenteral polymyxin B use. Ann Pharmacother 43:1948–1955. doi: 10.1345/aph.1M277. [DOI] [PubMed] [Google Scholar]
- 10.Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, Musick WL, Cottreau JM, Hu M, Tam VH. 2014. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother 58:2740–2746. doi: 10.1128/AAC.02476-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubrovskaya Y, Prasad N, Lee Y, Esaian D, Figueroa DA, Tam VH. 2015. Risk factors for nephrotoxicity onset associated with polymyxin B therapy. J Antimicrob Chemother 70:1903–1907. doi: 10.1093/jac/dkv014. [DOI] [PubMed] [Google Scholar]
- 12.Moestrup SK, Cui S, Vorum H, Bregengard C, Bjorn SE, Norris K, Gliemann J, Christensen EI. 1995. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest 96:1404–1413. doi: 10.1172/JCI118176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen EI, Nielsen R. 2007. Role of megalin and cubilin in renal physiology and pathophysiology. Rev Physiol Biochem Pharmacol 158:1–22. [DOI] [PubMed] [Google Scholar]
- 14.Marzolo MP, Farfan P. 2011. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res 44:89–105. doi: 10.4067/S0716-97602011000100012. [DOI] [PubMed] [Google Scholar]
- 15.Lundgren S, Carling T, Hjalm G, Juhlin C, Rastad J, Pihlgren U, Rask L, Akerstrom G, Hellman P. 1997. Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem 45:383–392. doi: 10.1177/002215549704500306. [DOI] [PubMed] [Google Scholar]
- 16.De S, Kuwahara S, Saito A. 2014. The endocytic receptor megalin and its associated proteins in proximal tubule epithelial cells. Membranes 4:333–355. doi: 10.3390/membranes4030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ternes SB, Rowling MJ. 2013. Vitamin D transport proteins megalin and disabled-2 are expressed in prostate and colon epithelial cells and are induced and activated by all-trans-retinoic acid. Nutr Cancer 65:900–907. doi: 10.1080/01635581.2013.805422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng G, Marino M, Zhao J, McCluskey RT. 1998. Megalin (gp330): a putative endocytic receptor for thyroglobulin (Tg). Endocrinology 139:1462–1465. doi: 10.1210/endo.139.3.5978. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz C, Hilpert J, Jacobsen C, Boensch C, Christensen EI, Luft FC, Willnow TE. 2002. Megalin deficiency offers protection from renal aminoglycoside accumulation. J Biol Chem 277:618–622. doi: 10.1074/jbc.M109959200. [DOI] [PubMed] [Google Scholar]
- 20.Dagil R, O'Shea C, Nykjaer A, Bonvin AM, Kragelund BB. 2013. Gentamicin binds to the megalin receptor as a competitive inhibitor using the common ligand binding motif of complement type repeats: insight from the nmr structure of the 10th complement type repeat domain alone and in complex with gentamicin. J Biol Chem 288:4424–4435. doi: 10.1074/jbc.M112.434159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki T, Yamaguchi H, Ogura J, Kobayashi M, Yamada T, Iseki K. 2013. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother 57:6319–6324. doi: 10.1128/AAC.00254-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azad MA, Finnin BA, Poudyal A, Davis K, Li J, Hill PA, Nation RL, Velkov T, Li J. 2013. Polymyxin B induces apoptosis in kidney proximal tubular cells. Antimicrob Agents Chemother 57:4329–4335. doi: 10.1128/AAC.02587-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergeron M, Mayers P, Brown D. 1996. Specific effect of maleate on an apical membrane glycoprotein (gp330) in proximal tubule of rat kidneys. Am J Physiol 271:F908–F916. [DOI] [PubMed] [Google Scholar]
- 24.Norden AG, Lapsley M, Igarashi T, Kelleher CL, Lee PJ, Matsuyama T, Scheinman SJ, Shiraga H, Sundin DP, Thakker RV, Unwin RJ, Verroust P, Moestrup SK. 2002. Urinary megalin deficiency implicates abnormal tubular endocytic function in Fanconi syndrome. J Am Soc Nephrol 13:125–133. [DOI] [PubMed] [Google Scholar]
- 25.Christensen EI, Gliemann J, Moestrup SK. 1992. Renal tubule gp330 is a calcium binding receptor for endocytic uptake of protein. J Histochem Cytochem 40:1481–1490. doi: 10.1177/40.10.1382088. [DOI] [PubMed] [Google Scholar]
- 26.Manchandani P, Dubrovskaya Y, Gao S, Tam VH. 2016. Comparative pharmacokinetic profiling of different polymyxin B components. Antimicrob Agents Chemother 60:6980–6982. doi: 10.1128/AAC.00702-16. [DOI] [PMC free article] [PubMed] [Google Scholar]