ABSTRACT
Tenofovir disoproxil fumarate (TDF), a nucleotide reverse transcriptase inhibitor, after conversion to tenofovir (TFV), is mainly eliminated by glomerular filtration and active tubular secretion. The major adverse effect of tenofovir is nephrotoxicity; however, the exact mechanism remains poorly understood. In this study, the ATP-binding cassette subfamily C member 11 (ABCC11; multidrug resistance protein 8 [MRP8]) transporter, which is abundant in proximal tubular cells, was demonstrated to act as an efflux transporter of tenofovir. Real-time PCR (RT-PCR) and indirect immunofluorescence assays were used to determine MRP8 overexpression in a continuous cell line. Tenofovir accumulations were assessed by cytotoxicity, cellular transport, and vesicular uptake assays. Substrate specificity was confirmed using MK-571, an MRP-specific inhibitor, and methotrexate, which served as a known substrate. Intracellular and intravesicular concentrations of tenofovir were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The 50% cytotoxic concentration (CC50) of TDF in MRP8-overexpressing cells was 4.78 times higher than that of parental cells. Transport assays also showed that the intracellular accumulation of tenofovir in MRP8-overexpressing cells was 55 times lower than that in parental cells and was partly reversed by MK-571. Similarly, an “inside-out” vesicular uptake assay, using Sf9 inverted membrane vesicles to allow measuring of accumulation of the substrates into the vesicles, demonstrated a higher intravesicular concentration of tenofovir in MRP8-overexpressing vesicles than in Sf9 insect control vesicles. These effects were effectively reversed by increasing concentrations of the specific inhibitor MK-571. In conclusion, tenofovir is a new substrate of the MRP8 transporter. An alteration in the activity of this efflux pump may increase the intracellular accumulation of tenofovir in proximal renal tubular cells.
KEYWORDS: tenofovir, methotrexate, tenofovir disoproxil fumarate, ATP-binding cassette subfamily C11, ABCC11, MRP8 transporter, nephrotoxicity
INTRODUCTION
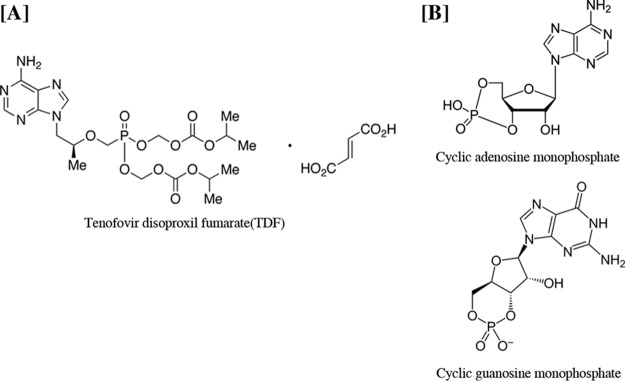
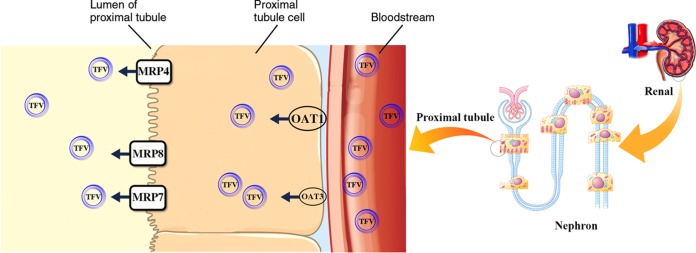
Tenofovir (TFV) disoproxil fumarate (TDF) is an orally bioavailable prodrug of tenofovir, an acyclic nucleotide analog reverse transcriptase inhibitor (1, 2). Tenofovir is widely used for effective treatment of HIV and hepatitis B infection (1, 2). Concerns regarding nephrotoxicity were initially raised because of the similarity of the chemical structures of tenofovir and other cyclic nucleotide analogs such as adefovir and cidofovir (Fig. 1). Use of adefovir and cidofovir was associated with proximal tubulopathy due to decreased mitochondrial DNA replication through inhibition of mitochondrial DNA polymerase γ (3). Furthermore, numerous clinical studies have indicated a significant association between tenofovir use and a decline in the estimated glomerular filtration rate (eGFR). The nephrotoxicity of tenofovir varied widely, ranging from less than a minimal effect to severe cases of renal Fanconi syndrome or acute kidney injury (4). The incidences of tubular dysfunction were demonstrated in 17 to 22% of the tenofovir-treated patients (1, 4). The risk factors for nephrotoxicity included long-term use, preexisting kidney diseases, increased age, lower CD4+ cell count, baseline elevation of serum creatinine, dose, concomitant nephrotoxic medications, and low body mass (1, 2, 4, 5). Mitochondria of the proximal tubular cells are the major target of tenofovir toxicity due to their complement of cell membrane transporters that favor tenofovir accumulation, but the exact mechanism of toxicity remains unclear (1, 4, 5). Tenofovir undergoes elimination unchanged in urine via the combination of glomerular filtration and active proximal tubular secretion (1, 2). Approximately 20 to 30% of tenofovir is actively transported into renal proximal tubular cells by the organic anion transporters at the basolateral membrane human OAT1 (hOAT1) and, to a lesser extent, hOAT3 (1, 4). Subsequently, the drug is secreted by the ATP-binding cassette subfamily C member 4 (ABCC4; multidrug resistance protein 4 [MRP4]) and ABCC10 (MRP7) (1, 4, 5) (Fig. 2). In addition, the existing reabsorption pathway of tenofovir at proximal tubular epithelial cells has never been reported (1), and the uptake transporters of this drug, hOAT1 and hOAT3 (1, 4), are located only on the basolateral membrane.
FIG 1.
Chemical structures of tenofovir disoproxil fumarate (TDF), an oral prodrug and acyclic nucleotide analog of AMP that inhibits HIV-1, and cyclic nucleotide analogs AMP and GMP, as indicated.
FIG 2.
The pathway of tenofovir (TFV) transport in proximal tubular epithelium cells (1, 4, 5). Approximately, 20 to 30% of tenofovir is actively transported into renal proximal tubular cells by the organic anion transporters hOAT1 and, to lesser extent, hOAT3 (1, 4) at the basolateral membrane. Subsequently, the drug is secreted by ABCC4 (MRP4), ABCC10 (MRP7) (1, 4, 5), and ABCC11 (MRP8) (this study).
Among the members of ABC transporter subfamily C, ABCC11, also called MRP8, encoded by the abcc11 gene belongs to a new class of MRP members (6). MRP8 expression is low in all normal human tissues except lung, fetal tissue, kidney, spleen, colon, and brain (7–12). At the kidney, MRP8 is highly expressed on the proximal region but is not found on glomeruli. MRP8 is able to transport a diverse range of lipophilic anions, including cyclic nucleotides, estradiol-17-beta-glucuronide, steroid sulfates such as dehydroepiandrosterone (DHEAS) and estrone sulfate [E (1)S], glutathione conjugates such as leukotriene C4 and dinitrophenyl-S-glutathione, and monoanionic bile acids (11, 13). The MRP8 transmembrane protein structure resembles the structures of MRP4 and MRP5 with respect to possessing only two membrane-spanning domains (13). An amino acid comparison indicates that MRP8 more closely resembles MRP5, and the substrate selectivity of MRP8 is more similar to that of MRP4 (14–16). Moreover, cyclic nucleotides are the only physiological transport substrates that MRP4, MRP5, and MRP8 are known to have in common (13, 17, 18), and tenofovir has a chemical structure related to the structures of cyclic nucleotide analogs (1, 2). With the abundance of ABCC11 in the kidney, in this study we hypothesized that ABCC11 plays a role in TDF transport in renal proximal tubular cells. Pig kidney epithelial parental cells (LLC-PK1) and cells overexpressing MRP8 (LLC-PK1-ABCC11) were selected as a suitable epithelium model to demonstrate the efflux transport of TDF of the proximal renal tubular region (19–22).
RESULTS
Characterization of cell lines.
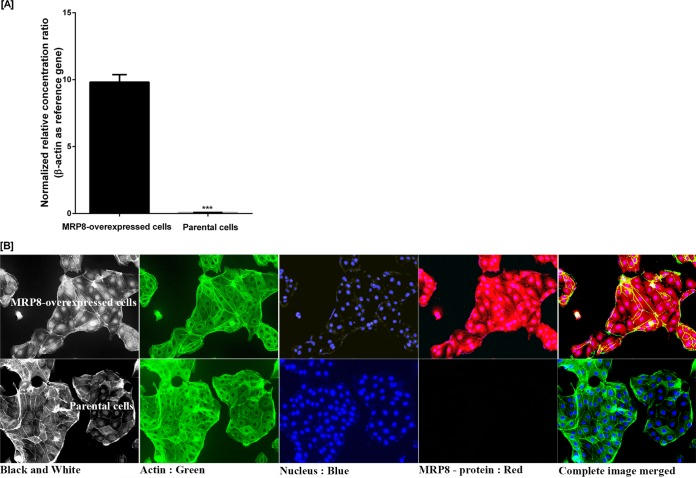
Human abcc11 mRNA levels in MRP8-overexpressing LLC-PK1 cells were higher than those in parental cells (Fig. 3A). Indirect immunofluorescence staining of MRP8 also showed that the transporter protein was highly expressed in MRP8-overexpressing LLC-PK1 cells whereas no signal was observed in parental cells by an EVOS-II imaging station (Fig. 3B, upper panel). The findings confirmed the suitable characteristics of the MRP8-overexpressing LLC-PK1 cells for further experimental assays.
FIG 3.
Expression of recombinant MRP8 in LLC-PK1 cells. (A) RNA expression of recombinant human ABCC11 gene in LLC-PK1 ABCC11-overexpressing cells was significantly higher (left bar) than that of the parental cells (LLC-PK1). Data are shown as the ratio of ABCC11 gene expression relative to that of beta-actin gene expression determined by real-time PCR as described in Materials and Methods. Values are the means ± standard deviations from three independent experiments. Error bars represent standard errors from three independent experiments. Statistical significance was assessed by a two-way ANOVA multiple comparison test assuming equal variance (***, P value < 0.0001). (B) Human overexpressed MRP8 protein in LLC-PK1-ABCC11 and LLC-PK1 parental cells. Immunofluorescence staining of MRP8 protein, β-actin, and DAPI in both cell types was described in Materials and Methods. Photos were taken under the EVOS-II imaging station at a magnification of ×1,000.
Cell viability and cytotoxicity assays.
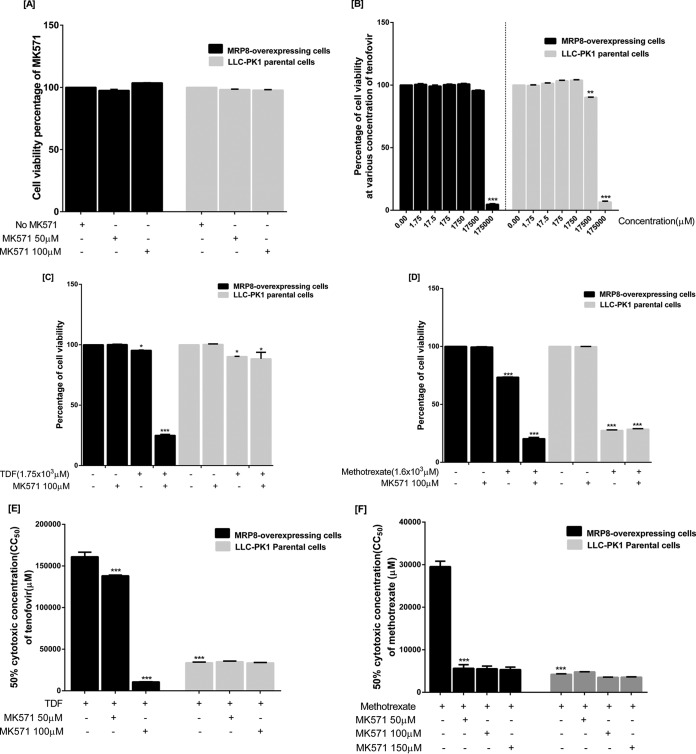
MK-571, an MRP-specific inhibitor, did not reduce MRP8-overexpressing and parental cell viability at the concentrations used (Fig. 4A). At 17,500 μM, TDF alone reduced a significant proportion of parental cell viability whereas no effect was seen on MRP8-overexpressing cells (Fig. 4B). When MK-571 was added, TDF significantly reduced viability of only MRP8-overexpressing cells (Fig. 4C). Methotrexate (MTX) was, however, more cytotoxic to both cells. Similarly, MTX toxicity was markedly increased when MK-571 was added in MRP8-overexpressing cells only (Fig. 4D). When 10 serial concentrations of TDF were used to determine 50% cytotoxic concentrations (CC50s) in both cell lines, TDF was found to be more toxic to parental cells. However, the CC50 of TDF was significantly reduced in the presence of MK-571 only in MRP8-overexpressing cells (Table 1 and Fig. 4E). Similarly, the CC50 of MTX was also dramatically reduced when MK-571 concentrations were increased only in MRP8-overexpressing cells (Fig. 4F).
FIG 4.
Cell viability assays with TDF and methotrexate in the presence and absence of the specific inhibitor MK-571 (A) Specific inhibitor MK-571 at various concentrations did not reduce MRP8-overexpressing and parental cell viability. (B) Cytotoxic effects of TDF on MRP8-overexpressing and parental cells. (C) MK-571 further reduced viability of the MRP8-overexpressing cells, but not parental cells, treated with TDF. (D) MK-571 also enhanced cytotoxicity of methotrexate only in MRP8-overexpressing cells. (E and F) Cytotoxicity assays showing methotrexate and TDF concentrations that reduced cell viability by 50% (CC50) in MRP8-overexpressing LLC-PK1 or parental cells with or without the specific inhibitor MK-571. Statistical significance was analyzed by a two-way ANOVA multiple comparison assuming equal variance (*, P < 0.01; **, P < 0.001; ***, P < 0.0001). All values are the means ± standard deviations from five independent experiments.
TABLE 1.
Effect of MRP8 overexpression on cytotoxicity of tenofovir in LLC-PK1 cells
| Compound | CC50 (μM) in:a |
Fold change (CC50MRP8/CC50WT)c | |
|---|---|---|---|
| WT cellsb | MRP8-overexpressing cells | ||
| Tenofovir | 33,694 ± 839 | 161,076 ± 5,478 | 4.78 |
| Tenofovir + 50 μM MK-571 | 34,938 ± 770 | 138,115 ± 976 | 3.95 |
| Tenofovir + 100 μM MK-571 | 33,530 ± 466 | 10,713 ± 132 | 0.32 |
Values represent the means ± standard deviations of five independent experiments. The calculation was fitted to Richard's five-parameter dose-response curve (45) (asymmetric sigmoidal, with robust fit). See Materials and Methods for details.
WT, wild type, LLC-PK1 parental cells.
For all compounds there was a significant (P < 0.0001) decrease in toxicity due to MRP8 overexpression based on two-way ANOVA multiple comparison tests assuming equal variance.
Tenofovir transport assays.
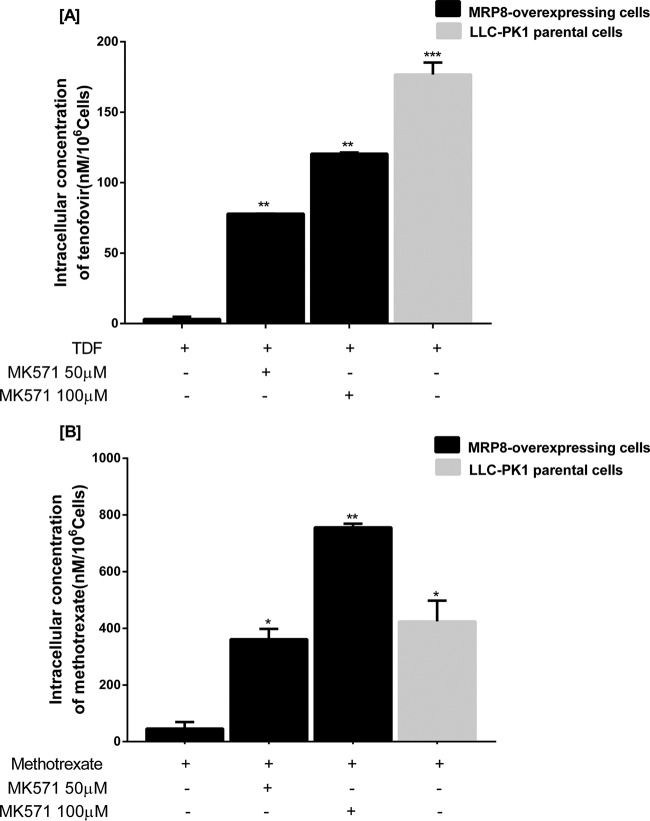
A transport assay was performed by measuring the intracellular accumulations of TDF and MTX after they entered the cells. The intracellular concentration was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) quantification. After conversion to TFV, TDF was found to be present in only small amounts. Only the area under the concentration-time curves of tenofovir (m/z 208) and methotrexate (m/z 455) were used for the graphs shown in Fig. 5. Compared to parental cells, MRP8-overexpressing cells had significantly reduced levels of intracellular accumulation of tenofovir (Fig. 5A) and methotrexate (Fig. 5B). The reduced accumulation of both substrates was reversed by increasing concentrations of MRP8-specific inhibitor MK-571.
FIG 5.
Intracellular accumulation of tenofovir (A) and methotrexate (B) with and without the specific MRP8-inhibitor MK-571 in a cellular transport assay. Error bars represent standard errors from duplicate independent experiments. Statistical significance was assessed by a two-way ANOVA multiple comparison test assuming equal variance. *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
Vesicular uptake assays.
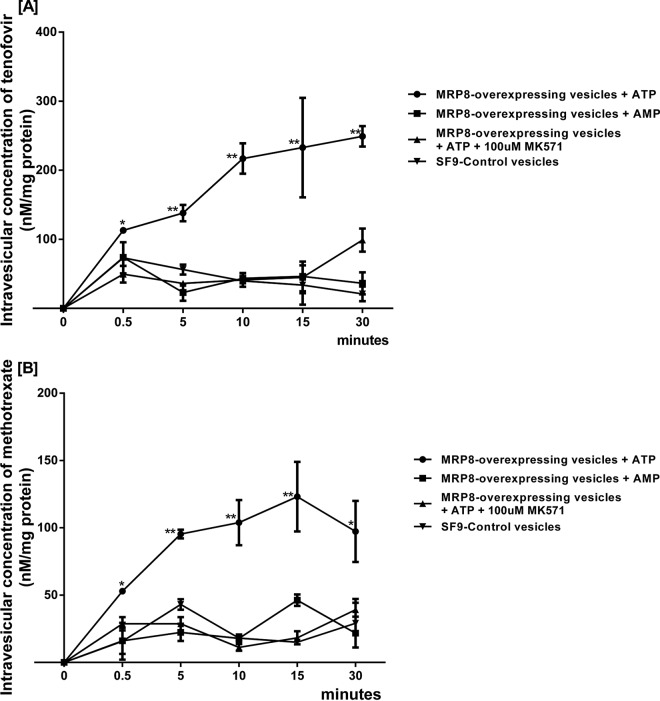
Incubating substrates in the presence of the inverted membrane vesicles overexpressing the respective efflux transporter and ATP allowed direct measurement of substrate accumulation into the vesicles. A vesicular uptake assay was designed by incubation of tenofovir, not TDF, and MTX with ATP or AMP in the presence and absence of MK-571 in MRP8-overexpressing and “inside-out” Sf9 control membrane vesicles. Addition of ATP, but not AMP, stimulated the uptake of tenofovir (Fig. 6A) and methotrexate (Fig. 6B) into MRP8-overexpressing vesicles. Accordingly, ATP-dependent intravesicular accumulation of tenofovir and MTX in MRP8-overexpressing vesicles was diminished with the MRP8-specific inhibitor. Significant differences were seen as early as the 5-min time point and maintained throughout the 30-min experiment. A vesicular uptake assay of a known substrate (methotrexate) also showed similar results (Fig. 6B).
FIG 6.
Time course for uptake of tenofovir and methotrexate by inside-out vesicles from Sf9 insect cells. The uptake of tenofovir and methotrexate into MRP8-overexpressing cells was compared to that of parental membrane vesicles derived from Sf9 insect cells. (A) Addition of ATP, but not AMP, stimulated the uptake of tenofovir and methotrexate into MRP8-overexpressing vesicles. ATP-dependent intravesicular accumulation of tenofovir in MRP8-overexpressing vesicles was diminished with the specific MRP8 inhibitor. (B) Intravesicular concentrations of methotrexate also showed similar results. Error bars represent standard errors from duplicate independent experiments. Statistical significance was assessed by repeated two-way ANOVA with Turkey's post hoc analysis and unpaired t test comparisons (*, P < 0.01; **, P < 0.001).
DISCUSSION
The known substrate of MRP8, TDF, is less cytotoxic to both LLC-PK1 ABCC11-overexpressing (LLC-PK1-ABCC11) and LLC-PK1 parental cells than methotrexate. This may be due to the fact that tenofovir has a very high selectivity index (SI of 324.8) for the viral reverse transcriptase enzyme (23) and, therefore, has lower cytotoxicity than methotrexate. However, in the presence of the specific inhibitor MK-571, the cytotoxicity of tenofovir in MRP8-overexpressing cells increased almost 15-fold. Although TDF and MTX were not tested concomitantly in cytotoxicity assays in our study, it may be assumed that intracellular accumulations of tenofovir due to combination with a drug known as the substrate or inhibitor of the MRP8 transporter may contribute to its increased cytotoxicity.
Results of cellular transport assays also indicated that the intracellular tenofovir concentration in MRP8-overexpressing cells was noticeably and significantly lower (approximately 55-fold) than that of parental cells. As expected, intracellular tenofovir accumulations were increased as the cells were exposed to increasing concentrations of the specific MRP8 inhibitor. In addition, the data were also consistent with those from MRP8 and Sf9 inside-out vesicles. A statistically significant increase in intravesicular accumulation of tenofovir in an ATP-dependent manner was observed at all time points (0.5, 5, 10, 15, and 30 min) compared to levels in controls (AMP and parental Sf9 vesicles). MK-571 was able to specifically reverse the intravesicular accumulation of tenofovir. Our study is the first to demonstrate that the human MRP8 transporter protein at proximal tubular cells mediates the efflux transport of tenofovir. It can be concluded that tenofovir is a new substrate of the MRP8 transporter protein. Therefore, alteration of the physiologic functions of this efflux pump may influence the accumulation of drug in proximal renal tubular cells and may contribute to developing nephrotoxicity. Since renal elimination is the major pathway of tenofovir clearance, these findings are very important to expand the basic knowledge of the molecular pharmacology of this drug. Proximal tubular cells are uniquely susceptible to tenofovir toxicity because there exists a complement of transporters that increase intracellular concentrations of the drug. The inhibition properties of mitochondrial DNA (mtDNA) polymerase γ encoded by the POLG gene has been proposed to play a central role in tenofovir-induced mitochondrial toxicity, which may contribute to tenofovir nephropathy (1, 4, 24, 25). Although tenofovir has not yet been studied, we note that a similar theory was raised in support of the idea that this drug might also induce proximal tubular apoptosis through caspase-9 activation (1, 18, 26), as previously described for other nucleotide analog reverse transcriptase inhibitors such as adefovir and cidofovir (1). Whether or not this may have a role in tenofovir-induced renal tubular cell injury will deserve further study.
Genetic variants in a number of transporter proteins involved in tenofovir excretion have not been clearly associated with renal damage. In fact, correlations between genetic variations of genes encoding other ABCC transporters and renal proximal tubulopathy had been shown. Polymorphisms of the ABCC4 gene at several positions were identified, but their correlation with intracellular accumulation and kidney damage yielded conflicting results (27–30). Studies of various single nucleotide polymorphisms (SNPs) at both intronic and coding regions of the ABCC10 (31, 32) and ABCC2 genes also showed a discrepancy in their correlations with renal proximal tubulopathy (33). However, the role of MRP2 as a renal efflux transporter of TDF has now been challenged and is questionable (1, 4, 5, 32). Therefore, which genetic variants may predispose renal cells to TDF toxicity remains controversial. Since multiple efflux transporters exist, it might be difficult to find significant proteins whose polymorphisms could be of significance in tenofovir nephrotoxicity. It is also possible that other compensated efflux mechanisms via other MRPs located in the renal proximal tubular region may play a role. Furthermore, there exist over 48 distinct members of multidrug resistance proteins encoded by abcc genes that belong to the ATP-binding cassette (ABC) transporter superfamily in the renal proximal tubular region (34–40).
Conclusion.
This in vitro study is the first to demonstrate the role of MRP8 as an efflux transporter of the antiretroviral tenofovir. This transporter protein is highly expressed in the proximal renal tubular region. Genetic polymorphism or concomitant drugs that diminish the physiologic function of the MRP8 transporter may contribute to tenofovir intracellular accumulation and, consequently, tenofovir nephrotoxicity.
MATERIALS AND METHODS
Reagents and chemicals.
Diethylpyrocarbonate (DEPC)-treated water was purchased from Thermo Fisher Scientific (Waltham, MA). A one-step quantitative RT-PCR (qRT-PCR) SuperScript III Platinum Sybr Green kit, a PureLink RNA Mini purification kit, M199 medium, fetal bovine serum (FBS), sodium pyruvate, penicillin, streptomycin, 0.25% trypsin-EDTA, Alexa Fluor 488-phalloidin, 4′,6′-diamidino-2-phenylindole (DAPI), Dulbecco's phosphate-buffered saline (DPBS) with magnesium and phosphate, DPBS without magnesium and phosphate, ABCC11 antibody, Alexa Fluor 488-conjugated goat anti-mouse serum IgM, puromycin dihydrochloride, synthetic ABCC11 primary antibody, an MRP-BCRP (breast cancer resistance protein) vesicular transport assay reagent set (catalog number GM3010), PrestoBlue reagent, and mouse serum were purchased from Life Technologies Corporation (Grand Island, NY). Triton X-100, paraformaldehyde, vinblastine, methotrexate, and methanol were purchased from Sigma-Aldrich (St. Louis, MO). Tenofovir disoproxil fumarate and tenofovir were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). MK-571 was purchased from Merck Millipore, Inc. (Darmstadt, Germany).
Cells and vesicles.
LLC-PK1 ABCC11-overexpressing(LLC-PK1-ABCC11) cells and LLC-PK1 parental cells (ATCC 123546) were purchased from GenScript, Inc. (Piscataway, NJ). Human ABCC11-overexpressing inside-out vesicles, control ABC transporter vesicles, and primers were purchased from Life Technologies Corporation (Grand Island, NY). LLC-PK1-ABCC11 and parental cells were grown under recommended conditions in M199 medium with 3% heat-inactivated fetal bovine serum, 100 μg/ml penicillin-streptomycin, and 2 μg/ml puromycin dihydrochloride. Cells were passaged twice a week. Gene expression and protein expression were characterized by real-time PCR and indirect immunofluorescence assay, respectively.
mRNA isolation and RT-PCR.
To evaluate human abcc11 mRNA levels in MRP8-overexpressing LLC-PK1 cells compared with the levels in parental cells, relative quantification was determined by real-time PCR (RT-PCR). Briefly, cells were seeded in T75 cell culture flasks until they reached 80 to 95% confluence. For RNA extraction, PureLink reagent was added into cells, and mRNA was isolated according to the manufacturer's protocol. The ABCC11 primers (forward primer, AGTATGATGCTGCCTTGA; reverse primer, GGTGAGGTAGGAGAACAG) and β-actin primers (forward primer, AACTACCTTCAACTCCATCA; reverse primer, ATCTCCTTCTGCATCCTG) were purchased from Life Technologies Corporation (Grand Island, NY). A one-step SuperScript III Platinum Sybr Green qRT-PCR kit was used for quantification of mRNA expression under the following conditions: 50°C for a 3-min hold (cDNA synthesis), followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 40°C for 1 min. Data were quantified as relative expression levels using βactin as a reference gene.
Immunofluorescence staining.
To assess in situ MRP8 protein expression, an immunofluorescence technique using an anti-MRP8 antibody was performed in MRP8-overexpressing and parental LLC-PK1 cells. This method was adopted from Robillard et al. (41). Cells were seeded in 24-well tissue culture plates at a density of 5,000 cells/well. Cells were incubated at 37°C in 5% CO2 overnight. The experiments were duplicated. Briefly, cells were fixed with 4% paraformaldehyde for 20 min. Cells were permeabilized by addition of a solution of 0.3% (vol/vol) Triton X for 5 min at 37°C. Thereafter, 5% (vol/vol) goat serum diluted in DPBS solution was added to cells, and samples were incubated for 60 min at room temperature. Cells were incubated at 4°C overnight with primary mouse anti-MRP8 antibody (10 μg/ml). Then, cells were washed three times with phosphate-buffered saline (PBS) and incubated with an Alexa Fluor 488-conjugated goat anti-mouse antibody (10 μg/ml) for 60 min at room temperature. For staining of actin and nuclei, Alexa Fluor 488-phalloidin (10 μg/ml) and DAPI solution (3 ng/ml), respectively, were used according to the manufacturer's protocol. Photos of selected areas of cells were taken under the EVOS-II imaging station at a magnification of ×1,000.
Cytotoxic assays.
A modified MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay was performed to determine cell viability and CC50s between MRP8-overexpressing and parental cells in the presence of various concentrations of TDF with or without the specific inhibitor MK-571. Methotrexate was used as a positive control. This method was adopted from Ray et al. (13) Cells were seeded in 96-well tissue culture plates at an approximate density of 5,000 cells/well in 100 μl of M199 medium. Twenty-four hours later, both cell types were preincubated at 37°C in 5% CO2 with various concentrations of specific inhibitor (50, 100, or 150 μM MK-571) for 1 h. Serially diluted test drugs or methotrexate was then added in triplicate and mixed well. Following 4 days (96 h) of incubation, cell viability was determined using a PrestoBlue assay kit (purchased from Life Technologies Corporation, Grand Island, NY) according to the manufacturer's protocol. After 2 h of continuous incubation, the luminescence signal was measured at an excitation wavelength of 550 nm using a microplate reader (M-965+; Metertech, Taiwan), and the percentage of cell viability was calculated.
Drug transport assays.
To compare the intracellular accumulation of tenofovir and methotrexate between MRP8-overexpressing and parental cells, a cellular transport assay with or without MK-571 was performed as previously described (13, 31, 42). LLC-PK1-ABCC11 and parental cells were seeded at an approximate density of 300,000 cells/well into 12-well tissue culture plates and then incubated at 37°C in 5% CO2 overnight. Cells were preincubated with 50 μM or 100 μM MK-571 solution at 37°C in 5% CO2 for 1 h, followed by addition of 200 μM tenofovir disoproxil fumarate (TDF) or 160 μM methotrexate (MTX). After cells were incubated for 1 h with shaking at 37°C in 5% CO2, reactions were stopped by three washes with ice-cold phosphate-buffered saline to remove extracellular drug. Cells were harvested by the addition of 70% (vol/vol) ice-cold methanol, followed by overnight incubation at −20°C. Cellular debris was removed by centrifugation at 10,000 × g for 15 min. Supernatants were collected, and intracellular concentrations of tenofovir and methotrexate were determined by validated liquid chromatography coupled with tandem mass spectrometry as described previously (14–17, 43). The reference method was validated (14, 17, 43, 44), the lower limits of quantification were determined, and the calibration curves demonstrated linearity, with average correlation coefficients greater than 0.99 for both drugs. Chromatographic separation of tenofovir and methotrexate was achieved by using a mobile phase of acetonitrile–1 mM ammonium acetate buffer in water at pH 6.5 ± 0.3 (50:50, vol/vol) and acetonitrile–1 mM ammonium formate containing 0.1% formic acid (18:82, vol/vol), respectively. The delivered flow rate was 0.4 ml/min through an analytical column (C18 Zorbax Eclipse XDB; Agilent, USA). The column temperatures were maintained at 10°C for tenofovir and at 35°C for methotrexate.
Uptake assays.
To demonstrate active transport of tenofovir and methotrexate through MRP8, a time course of the uptake of tenofovir and methotrexate was performed by inside-out Sf9 vesicles to compare the intravesicular accumulations of drugs in MRP8 (ABCC11)-overexpressing vesicles and Sf9 vesicles with or without MK-571. This assay was adopted from Ray et al. (13). Briefly, membrane vesicles from Sf9 insect cells overexpressing MRP8 (ABCC11) protein and control vesicles were purchased (Life Technologies Corporation, NY). They were confirmed by the company to contain no other protein transporters. The vesicle transport assays were performed with a combination of (i) transport buffer obtained from an MRP-BCRP vesicular transport assay reagent kit (GM3010; Life Technologies Corporation, NY), (ii) 100 μM MK-571 (Merck Millipore, Inc., Germany), (iii) 200 μM tenofovir (Santa Cruz Biotechnology, Inc., TX) or 160 μM methotrexate (Sigma-Aldrich, MO), and (iv) vesicles at a total protein concentration of 500 μg/ml. The total reaction volume was 1,000 μl. After samples were incubated for 1 h at 37°C, 160-μl reaction mixture aliquots were collected at various time points (0, 0.5, 5, 10, 15, and 30 min). They were diluted into 1 ml of ice-cold stop buffer and passed to vacuum filters through 1-μm-pore-size 96-well glass filter plates (Pall Corporation, Port Washington, NY). Filters were washed five times with 200 μl of ice-cold wash buffer. Vesicles were harvested by addition of 70% ice-cold methanol, followed by incubation at −20°C overnight. Cellular debris was removed by centrifugation at 10,000 × g for 15 min. Supernatants were collected, and intracellular concentrations of tenofovir and methotrexate were determined as previously described (14–17). To determine the transporter-specific uptake of the substrates, MRP8-overexpressing vesicles were assayed side by side with the control vesicles and specific inhibitor. Accumulation of substrates in vesicles was expressed in nanomoles per milligram of total protein.
Calculations and statistical tests.
The significance of the results was determined by two-way analysis of variance (ANOVA) multiple comparison and unpaired t tests, assuming equal variance, with the Prism program, version 6 (GraphPad, San Diego, CA). The concentrations rendering 50% cell viability (CC50) were calculated and fitted to Richard's five-parameter logistical dose-response curve (45) [asymmetric sigmoidal with robust fit; logXb = logEC50 + (1/Hill slope) × log(21/S − 1), where EC50 is the 50% effective concentration, X is the concentration, S is the asymmetry parameter, and Xb is the inflection point; denominator = (1 + 10(logXb − X) × Hill slope)S, with an initial Hill slope value of 1 and S of 0.5] using Prism 6 (GraphPad, San Diego, CA). Untreated cells and cells treated with 100 μM vinblastine were used as a reference of cell viability for 100% and 0%, respectively.
ACKNOWLEDGMENTS
We thank Sindhchai Kaewkittichai, Faculty of Pharmaceutical Sciences, Burapha University, Thailand, for expert help in discussion. The statistical analysis was supported by Titinun Auamnoy, Faculty of Pharmaceutical Sciences, Burapha University. We also thank Manat Pongchaidecha from the Department of Pharmacy, Faculty of Pharmacy, Silpakorn University, for his comments and help in statistical analyses.
This work was supported by the National Research Council of Thailand, Faculty of Pharmacy, Silpakorn University, and Faculty of Pharmaceutical Sciences, Burapha University.
REFERENCES
- 1.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Nino MD, Izquierdo MC, Poveda J, Sainz-Prestel V, Ortiz-Martin N, Parra-Rodriguez A, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A. 2011. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat 2011:354908. doi: 10.1155/2011/354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallant JE, Deresinski S. 2003. Tenofovir disoproxil fumarate. Clin Infect Dis 37:944–950. doi: 10.1086/378068. [DOI] [PubMed] [Google Scholar]
- 3.Birkus G, Hitchcock MJ, Cihlar T. 2002. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 46:716–723. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AM. 2013. Update on tenofovir toxicity in the kidney. Pediatr Nephrol 28:1011–1023. doi: 10.1007/s00467-012-2269-7. [DOI] [PubMed] [Google Scholar]
- 5.Jafari A, Khalili H, Dashti-Khavidaki S. 2014. Tenofovir-induced nephrotoxicity: incidence, mechanism, risk factors, prognosis and proposed agents for prevention. Eur J Clin Pharmacol 70:1029–1040. doi: 10.1007/s00228-014-1712-z. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZS, Guo Y, Belinsky MG, Kotova E, Kruh GD. 2005. Transport of bile acids, sulfated steroids, estradiol 17-beta-d-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11). Mol Pharmacol 67:545–557. [DOI] [PubMed] [Google Scholar]
- 7.Bera TK, Lee S, Salvatore G, Lee B, Pastan I. 2001. MRP8, a new member of ABC transporter superfamily, identified by EST database mining and gene prediction program, is highly expressed in breast cancer. Mol Med 7:509–516. [PMC free article] [PubMed] [Google Scholar]
- 8.Tammur J, Prades C, Arnould I, Rzhetsky A, Hutchinson A, Adachi M, Schuetz JD, Swoboda KJ, Ptacek LJ, Rosier M, Dean M, Allikmets R. 2001. Two new genes from the human ATP-binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene 273:89–96. doi: 10.1016/S0378-1119(01)00572-8. [DOI] [PubMed] [Google Scholar]
- 9.Yabuuchi H, Shimizu H, Takayanagi S, Ishikawa T. 2001. Multiple splicing variants of two new human ATP-binding cassette transporters, ABCC11 and ABCC12. Biochem Biophys Res Commun 288:933–939. doi: 10.1006/bbrc.2001.5865. [DOI] [PubMed] [Google Scholar]
- 10.Jedlitschky G, Burchell B, Keppler D. 2000. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZS, Lee K, Kruh GD. 2001. Transport of cyclic nucleotides and estradiol 17-beta-d-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem 276:33747–33754. [DOI] [PubMed] [Google Scholar]
- 12.van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. 2002. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol 13:595–603. [DOI] [PubMed] [Google Scholar]
- 13.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother 50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delahunty T, Bushman L, Robbins B, Fletcher CV. 2009. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci 877:1907–1914. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valluru RK, Reddy BP, Sumanth SK, Kumar VP, Kilaru NB. 2013. High throughput LC-MS/MS method for simultaneous determination of tenofovir, lamivudine and nevirapine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 931:117–126. doi: 10.1016/j.jchromb.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Meng F, Li L, Zhong B, Zhao Y. 2011. Development and validation of an LC/MS/MS method for the determination of tenofovir in monkey plasma. Biol Pharm Bull 34:877–882. doi: 10.1248/bpb.34.877. [DOI] [PubMed] [Google Scholar]
- 17.Guo P, Wang X, Liu L, Belinsky MG, Kruh GD, Gallo JM. 2007. Determination of methotrexate and its major metabolite 7-hydroxymethotrexate in mouse plasma and brain tissue by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 43:1789–1795. doi: 10.1016/j.jpba.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallant JE, Parish MA, Keruly JC, Moore RD. 2005. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis 40:1194–1198. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 19.Hull RN, Cherry WR, Weaver GW. 1976. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro 12:670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- 20.Takakura Y, Morita T, Fujikawa M, Hayashi M, Sezaki H, Hashida M, Borchardt RT. 1995. Characterization of LLC-PK1 kidney epithelial cells as an in vitro model for studying renal tubular reabsorption of protein drugs. Pharm Res 12:1968–1972. doi: 10.1023/A:1016256325921. [DOI] [PubMed] [Google Scholar]
- 21.Heussner AH, Dietrich DR. 2013. Primary porcine proximal tubular cells as an alternative to human primary renal cells in vitro: an initial characterization. BMC Cell Biol 14:55. doi: 10.1186/1471-2121-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlatter P, Gutmann H, Drewe J. 2006. Primary porcine proximal tubular cells as a model for transepithelial drug transport in human kidney. Eur J Pharm Sci 28:141–154. doi: 10.1016/j.ejps.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Balestrieri E, Sciortino MT, Mastino A, Macchi B. 2005. Protective effect of the acyclic nucleoside phosphonate tenofovir toward human T-cell leukemia/lymphotropic virus type 1 infection of human peripheral blood mononuclear cells in vitro. Antiviral Res 68:154–162. doi: 10.1016/j.antiviral.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Bendele RA, Richardson FC. 2002. Adefovir nephrotoxicity and mitochondrial DNA depletion. Hum Pathol 33:574. doi: 10.1053/hupa.2002.124012. [DOI] [PubMed] [Google Scholar]
- 25.Gitman MD, Hirschwerk D, Baskin CH, Singhal PC. 2007. Tenofovir-induced kidney injury. Expert Opin Drug Saf 6:155–164. doi: 10.1517/14740338.6.2.155. [DOI] [PubMed] [Google Scholar]
- 26.Hall AM, Hendry BM, Nitsch D, Connolly JO. 2011. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis 57:773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. 2008. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr 47:298–303. doi: 10.1097/QAI.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
- 28.Izzedine H, Hulot JS, Villard E, Goyenvalle C, Dominguez S, Ghosn J, Valantin MA, Lechat P, Deray AG. 2006. Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis 194:1481–1491. doi: 10.1086/508546. [DOI] [PubMed] [Google Scholar]
- 29.Likanonsakul S, Suntisuklappon B, Nitiyanontakij R, Prasithsirikul W, Nakayama EE, Shioda T, Sangsajja C. 2016. A single-nucleotide polymorphism in ABCC4 is associated with tenofovir-related beta2-microglobulinuria in Thai patients with HIV-1 infection. PLoS One 11:e0147724. doi: 10.1371/journal.pone.0147724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rungtivasuwan K, Avihingsanon A, Thammajaruk N, Mitruk S, Burger DM, Ruxrungtham K, Punyawudho B, Pengsuparp T. 2015. Influence of ABCC2 and ABCC4 polymorphisms on tenofovir plasma concentrations in Thai HIV-infected patients. Antimicrob Agents Chemother 59:3240–3245. doi: 10.1128/AAC.04930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pushpakom SP, Liptrott NJ, Rodriguez-Novoa S, Labarga P, Soriano V, Albalater M, Hopper-Borge E, Bonora S, Di Perri G, Back DJ, Khoo S, Pirmohamed M, Owen A. 2011. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis 204:145–153. doi: 10.1093/infdis/jir215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishijima T, Komatsu H, Higasa K, Takano M, Tsuchiya K, Hayashida T, Oka S, Gatanaga H. 2012. Single nucleotide polymorphisms in ABCC2 associate with tenofovir-induced kidney tubular dysfunction in Japanese patients with HIV-1 infection: a pharmacogenetic study. Clin Infect Dis 55:1558–1567. doi: 10.1093/cid/cis772. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Novoa S, Labarga P, Soriano V, Egan D, Albalater M, Morello J, Cuenca L, Gonzalez-Pardo G, Khoo S, Back D, Owen A. 2009. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis 48:e108–116. doi: 10.1086/598507. [DOI] [PubMed] [Google Scholar]
- 34.Toyoda Y, Hagiya Y, Adachi T, Hoshijima K, Kuo MT, Ishikawa T. 2008. MRP class of human ATP binding cassette (ABC) transporters: historical background and new research directions. Xenobiotica 38:833–862. doi: 10.1080/00498250701883514. [DOI] [PubMed] [Google Scholar]
- 35.Gillet JP, Efferth T, Remacle J. 2007. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim Biophys Acta 1775:237–262. [DOI] [PubMed] [Google Scholar]
- 36.Han HK. 2011. Role of transporters in drug interactions. Arch Pharm Res 34:1865–1877. doi: 10.1007/s12272-011-1107-y. [DOI] [PubMed] [Google Scholar]
- 37.Russel FG, Masereeuw R, van Aubel RA. 2002. Molecular aspects of renal anionic drug transport. Annu Rev Physiol 64:563–594. doi: 10.1146/annurev.physiol.64.081501.155913. [DOI] [PubMed] [Google Scholar]
- 38.Kruh GD, Belinsky MG. 2003. The MRP family of drug efflux pumps. Oncogene 22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 39.Kruh GD, Guo Y, Hopper-Borge E, Belinsky MG, Chen ZS. 2007. ABCC10, ABCC11, and ABCC12. Pflugers Arch 453:675–684. doi: 10.1007/s00424-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 40.Kruh GD, Zeng H, Rea PA, Liu G, Chen ZS, Lee K, Belinsky MG. 2001. MRP subfamily transporters and resistance to anticancer agents. J Bioenerg Biomembr 33:493–501. doi: 10.1023/A:1012827221844. [DOI] [PubMed] [Google Scholar]
- 41.Robillard KR, Hoque T, Bendayan R. 2012. Expression of ATP-binding cassette membrane transporters in rodent and human Sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther 340:96–108. doi: 10.1124/jpet.111.186916. [DOI] [PubMed] [Google Scholar]
- 42.Cihlar T, Laflamme G, Fisher R, Carey AC, Vela JE, Mackman R, Ray AS. 2009. Novel nucleotide human immunodeficiency virus reverse transcriptase inhibitor GS-9148 with a low nephrotoxic potential: characterization of renal transport and accumulation. Antimicrob Agents Chemother 53:150–156. doi: 10.1128/AAC.01183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Avolio A, Sciandra M, Siccardi M, Baietto L, Gonzalez de Requena D, Bonora S, Di Perri G. 2008. A new assay based on solid-phase extraction procedure with LC-MS to measure plasmatic concentrations of tenofovir and emtricitabine in HIV infected patients. J Chromatogr Sci 46:524–528. doi: 10.1093/chromsci/46.6.524. [DOI] [PubMed] [Google Scholar]
- 44.Rodin I, Braun A, Stavrianidi A, Shpigun O. 2013. A validated LC-MS/MS method for rapid determination of methotrexate in human saliva and its application to an excretion evaluation study. J Chromatogr B Analyt Technol Biomed Life Sci 937:1–6. doi: 10.1016/j.jchromb.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Giraldo J, Vivas NM, Vila E, Badia A. 2002. Assessing the (a)symmetry of concentration-effect curves: empirical versus mechanistic models. Pharmacol Ther 95:21–45. doi: 10.1016/S0163-7258(02)00223-1. [DOI] [PubMed] [Google Scholar]