ABSTRACT
The protective efficacy of tedizolid phosphate, a novel oxazolidinone that potently inhibits bacterial protein synthesis, was compared to those of linezolid, vancomycin, and saline in a rabbit model of Staphylococcus aureus necrotizing pneumonia. Tedizolid phosphate was administered to rabbits at 6 mg/kg of body weight intravenously twice daily, which yielded values of the 24-h area under the concentration-time curve approximating those found in humans. The overall survival rate was 83% for rabbits treated with 6 mg/kg tedizolid phosphate twice daily and 83% for those treated with 50 mg/kg linezolid thrice daily (P = 0.66 by the log-rank test versus the results obtained with tedizolid phosphate). These survival rates were significantly greater than the survival rates of 17% for rabbits treated with 30 mg/kg vancomycin twice daily (P = 0.003) and 17% for rabbits treated with saline (P = 0.002). The bacterial count in the lungs of rabbits treated with tedizolid phosphate was significantly decreased compared to that in the lungs of rabbits treated with saline, although it was not significantly different from that in the lungs of rabbits treated with vancomycin or linezolid. The in vivo bacterial production of alpha-toxin and Panton-Valentine leukocidin, two key S. aureus-secreted toxins that play critical roles in the pathogenesis of necrotizing pneumonia, in the lungs of rabbits treated with tedizolid phosphate and linezolid was significantly inhibited compared to that in the lungs of rabbits treated with vancomycin or saline. Taken together, these results indicate that tedizolid phosphate is superior to vancomycin for the treatment of S. aureus necrotizing pneumonia because it inhibits the bacterial production of lung-damaging toxins at the site of infection.
KEYWORDS: PVL, alpha-toxin, linezolid, pneumonia, rabbit model, tedizolid, vancomycin
INTRODUCTION
Tedizolid phosphate (also known as TR-701 FA), a novel oxazolidinone prodrug, is rapidly converted in the bloodstream to microbiologically active tedizolid (TR-700). Tedizolid interacts with the 23S ribosome initiation complex to inhibit bacterial protein synthesis and is active against clinically relevant Gram-positive pathogens, including Staphylococcus aureus. Tedizolid phosphate is approved for use for the treatment of acute bacterial skin and skin structure infections (1, 2). Tedizolid and linezolid were recently shown to be comparable in their ability to inhibit in vitro toxin production at similar subinhibitory concentrations; because tedizolid inhibits S. aureus growth at a concentration 4- to 8-fold lower than that of linezolid, tedizolid could more potently inhibit toxin production at a lower overall drug concentration (3).
Although the clinical efficacy of tedizolid phosphate compared to that of linezolid for the treatment of methicillin-resistant S. aureus (MRSA) pneumonia remains to be established (ClinicalTrials.gov identifier NCT02019420), linezolid is already recommended in clinical practice guidelines as an acceptable alternative to vancomycin for this indication (4). In a preclinical rabbit model of necrotizing pneumonia caused by MRSA strain USA300, linezolid was shown to be superior to vancomycin, a cell wall synthesis inhibitor, in improving survival outcomes by inhibiting the production of two key lung-damaging staphylococcal toxins, Panton-Valentine leukocidin (PVL) and alpha-toxin (Hla) (5–7). MRSA strain USA300 is currently causing epidemic disease within communities and hospitals throughout the United States and other countries (8, 9) and is an important cause of difficult-to-treat and potentially lethal community-associated and hospital-associated pneumonia (10, 11).
In the study described here, we compared the protective efficacies of tedizolid phosphate, linezolid, and vancomycin for the treatment of necrotizing pneumonia caused by USA300 in a rabbit model, focusing on whether improved host survival outcomes are associated with the in vivo inhibition of staphylococcal toxin production. Rabbits were used in the present study because, like humans, this animal species is highly susceptible to the toxigenic effects of the PVL and Hla toxins (5, 12, 13). We found that tedizolid phosphate and linezolid, which exhibited similar protective efficacies, were superior to vancomycin in improving survival outcomes in the rabbit model of necrotizing pneumonia because they inhibit the bacterial production of the PVL and Hla toxins.
RESULTS
The MICs of the test drugs for USA300 clinical strain SF8300 were 0.5 μg/ml for tedizolid (TR-700), 2.0 μg/ml for linezolid, and 1.0 μg/ml for vancomycin. In uninfected rabbits, the tedizolid area under the concentration-time curve from 0 to 24 h (AUC0–24; mean ± standard deviation [SD]) was 5.7 ± 0.9 μg · h/ml for a single intravenous dose of 2.5 mg/kg of body weight tedizolid phosphate, 9.9 ± 1.5 μg · h/ml for 5 mg/kg tedizolid phosphate, and 14.9 ± 1.6 μg · h/ml for 6 mg/kg tedizolid phosphate (Table 1). Because the intravenous administration of 6 mg/kg tedizolid phosphate yielded AUC0–24 values similar to those found in humans (14), we selected this dosing regimen for use in the rabbits.
TABLE 1.
Pharmacokinetic parameters of tedizolid after administration of a single intravenous dose of tedizolid phosphate to uninfected New Zealand White rabbitsa
| Tedizolid phosphate dose (mg/kg) | Cmax (μg/ml) | t1/2 (h) | AUC0–24 (μg · h/ml) |
|---|---|---|---|
| 2.5 | 2.6 ± 0.3 | 5.8 ± 1.5 | 5.7 ± 0.9 |
| 5 | 6.1 ± 0.3 | 5.1 ± 0.7 | 9.9 ± 1.5 |
| 6 | 7.9 ± 0.8 | 5.7 ± 0.9 | 14.9 ± 1.6 |
Data are means ± SDs for three rabbits treated with each dose. Cmax, maximum concentration of drug in plasma; t1/2, half-life.
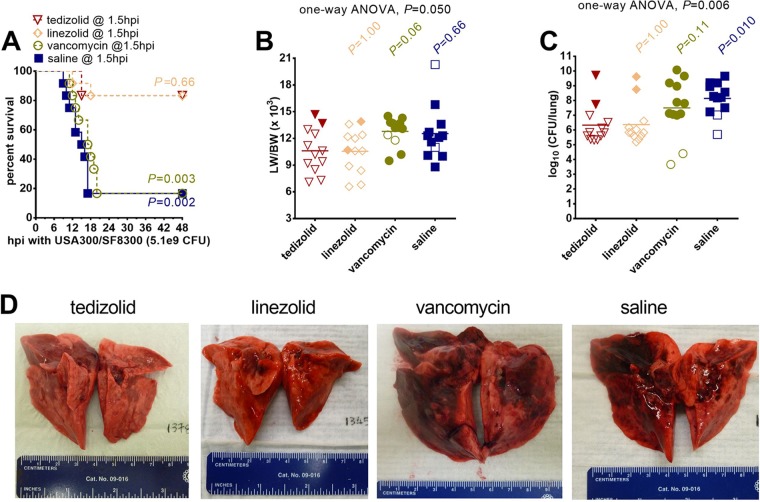
In the rabbit model of necrotizing pneumonia, the overall survival rate was 83% (10/12) for rabbits treated with 6 mg/kg tedizolid phosphate twice daily, whereas the overall survival rates were 83% (10/12) for those treated with 50 mg/kg linezolid thrice daily (P = 0.66 by the log-rank test versus tedizolid phosphate), 17% (2/12) for those treated with 30 mg/kg vancomycin twice daily (P = 0003 versus tedizolid phosphate), and 17% (2/12) for those treated with saline (P = 0.002 versus tedizolid phosphate) (Fig. 1A). The 50-mg/kg linezolid dosing regimen yielded mean ± SD peak serum concentration of 10.5 ± 2.3 μg/ml at 1 h postdosing, whereas 30 mg/kg vancomycin yielded 36.1 ± 4.2 μg/ml at 1 h postdosing, as determined in uninfected rabbits in a previous study (6).
FIG 1.
Treatment efficacy of tedizolid phosphate compared to that of linezolid, vancomycin, and saline in a rabbit model of USA300 necrotizing pneumonia. (A) Kaplan-Meier survival curves for animals treated with 6 mg/kg tedizolid phosphate intravenously at 1.5, 13, 25, and 37 h postinfection (hpi; n = 12 rabbits), 50 mg/kg linezolid subcutaneously at 1.5, 10, 18, and 26 h postinfection (n = 12 rabbits), 30 mg/kg vancomycin intravenously at 1.5, 13, 25, and 37 h postinfection (n = 12 rabbits), or saline intravenously at 1.5, 13, 25, and 37 h postinfection. A two-sided log-rank (Mantel-Cox) test was used to test the hypothesis that the rate of survival of animals treated with tedizolid phosphate is not different from the rate of survival of those treated with linezolid, vancomycin, or saline; a P value of <0.0167 (a significance level of 0.05 divided by 3 for three different comparisons), determined using the Bonferroni method to account for multiple comparisons, was considered statistically significant. (B, C) The lung weight-to-body weight (LW/BW [103]) ratio (B) and bacterial densities (in log10 number of CFU per lung) (C) for saline-treated animals were compared to those for animals in each of the other three treatment groups by a nonparametric one-way ANOVA with the Kruskal-Wallis test followed by Dunn's multiple-comparison test. Filled symbols, data from dead animals; open symbols, data from surviving animals that were euthanized at 48 h after infection. (D) Representative images of lungs from rabbits in the four treatment groups.
The lung weight-to-body weight (LW/BW) ratio, a marker of acute lung injury, was not significantly different among the groups (Fig. 1B). Although the bacterial count in the lungs of rabbits treated with tedizolid phosphate was significantly decreased compared to that in the lungs of rabbits treated with saline (P = 0.010), it was not significantly different from that in the lungs of rabbits treated with vancomycin or linezolid (Fig. 1C). Gross examination of rabbit lungs showed severe and extensive necrosis and hemorrhage in rabbits treated with vancomycin or saline, but comparatively less acute lung injury was observed in those treated with tedizolid phosphate or linezolid (Fig. 1D).
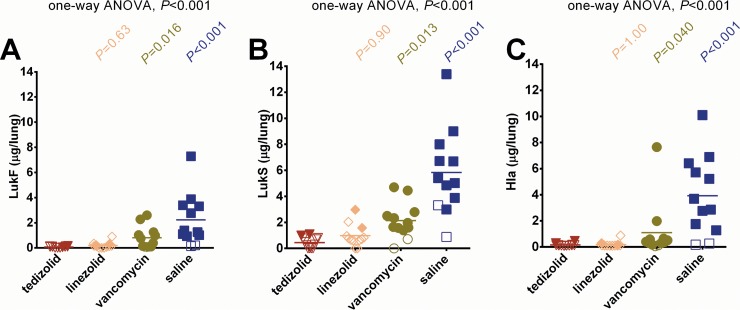
The improved survival outcomes of rabbits treated with tedizolid phosphate and linezolid were associated with the in vivo suppression of two key lung-damaging toxins in the lungs, the two-component Panton-Valentine leukocidin (LukF-PV and LukS-PV) and alpha-toxin (Hla) (5). The LukF-PV amount (mean ± SD) was 0.08 ± 0.05 μg/lungs for rabbits treated with tedizolid phosphate, whereas it was 0.20 ± 0.25 μg/lungs for those treated with linezolid (P = 0.63), 0.82 ± 0.87 μg/lungs for those treated with vancomycin (P = 0.016), and 2.22 ± 2.04 μg/lungs for those treated with saline (P < 0.001) (Fig. 2A). The LukS-PV amount was 0.45 ± 0.41 μg/lungs for rabbits treated with tedizolid phosphate, whereas it was 0.98 ± 0.84 μg/lungs for those treated with linezolid (P = 0.90), 2.14 ± 1.37 μg/lungs for those treated with vancomycin (P = 0.013), and 5.84 ± 3.27 μg/lungs for those treated with saline (P < 0.001) (Fig. 2B). The alpha-toxin amount was 0.16 ± 0.11 μg/lungs for rabbits treated with tedizolid phosphate, whereas it was 0.19 ± 0.22 μg/lungs for those treated with linezolid (P = 1.00), 1.09 ± 2.13 μg/lungs for those treated with vancomycin (P = 0.040), and 3.93 ± 3.01 μg/lungs for those treated with saline (P < 0.001) (Fig. 2C).
FIG 2.
Concentrations of the key lung-damaging toxins PVL and Hla in the lungs of rabbits. The LukF (A), LukS (B), and Hla (C) concentrations (in micrograms per lungs) of rabbits treated with tedizolid phosphate, linezolid, vancomycin, and saline were compared by a nonparametric one-way ANOVA with the Kruskal-Wallis test followed by Dunn's multiple-comparison test. Filled symbols, data from dead animals; open symbols, data from surviving animals that were euthanized at 48 h after infection.
DISCUSSION
Tedizolid phosphate demonstrated superiority over vancomycin but exhibited protective efficacy similar to that of linezolid for the treatment of MRSA USA300 necrotizing pneumonia in the rabbit model according to their effects on survival outcomes (Fig. 1). The enhanced protective efficacies of tedizolid phosphate and linezolid were associated with their inhibition of the bacterial production of alpha-toxin and PVL in the rabbit lungs (Fig. 2), a finding which is consistent with our previous findings on the mechanism of protection of linezolid in this rabbit model (6). These data demonstrate the potential clinical utility of tedizolid phosphate as an alternative to linezolid for the treatment of S. aureus necrotizing pneumonia. Tedizolid phosphate is also known to be active in vitro against clindamycin-resistant S. aureus strains (15) as well as linezolid-resistant S. aureus strains harboring the chloramphenicol-florfenicol resistance gene carried by a plasmid (16), thereby expanding potential therapeutic options.
Subinhibitory concentrations of protein synthesis inhibitors, including clindamycin, linezolid, and tedizolid phosphate, have previously been shown to inhibit the bacterial production of alpha-toxin and PVL (17–19). Both alpha-toxin and PVL have previously been shown to play critical roles in disease pathogenesis in a rabbit model of MRSA necrotizing pneumonia (5, 7). Preexposure prophylaxis and postexposure treatment with human monoclonal antibodies that neutralize alpha-toxin and PVL were previously shown to be sufficient for improving survival outcomes in the rabbit pneumonia model (12). More recently, we showed that combination treatment with human intravenous immunoglobulins—which are pools of polyvalent antibodies from thousands of donors which contain specific antibodies that neutralize both alpha-toxin and PVL—and linezolid provided even greater protection against community-associated MRSA necrotizing pneumonia than either one alone (7). Accordingly, antitoxin antibodies plus protein synthesis inhibitors, like linezolid and tedizolid phosphate, are expected to work together to further enhance survival outcomes by neutralizing preformed toxins and preventing their cytotoxic effects in the lungs, as well as inhibiting the bacterial production of these toxins.
The superior protective efficacy of tedizolid phosphate over that of vancomycin in the rabbit MRSA USA300 pneumonia model stands in contrast to the superior protective efficacy of vancomycin over that of tedizolid phosphate in the rabbit model of aortic valve endocarditis (20). One potential explanation for this discrepancy could be that the USA300/SF8300 clinical strain used in the rabbit pneumonia model is known to naturally hyperproduce alpha-toxin and PVL (21), which together play critical roles in the pathogenesis of necrotizing pneumonia (5, 7). In contrast, although alpha-toxin is known to contribute to the pathogenesis of aortic valve endocarditis (22), the MRSA strain used for evaluating the protective efficacies of tedizolid phosphate and vancomycin in the rabbit aortic valve endocarditis model, strain COL, produces little alpha-toxin and is largely nonhemolytic (23); COL also does not produce PVL because it lacks the horizontally acquired prophage encoding this toxin (24). Tedizolid phosphate may exert its protective effects in vivo through inhibition of bacterial toxin production, which could explain why tedizolid phosphate demonstrates greater protection against toxin-mediated pneumonia but is less effective against biofilm-centered endocarditis.
Our study has certain limitations. The protective efficacies of tedizolid phosphate and other antimicrobials against a single strain of community-associated MRSA USA300, strain SF8300, were evaluated in the rabbit model of necrotizing pneumonia. This strain is known to exhibit the upregulated expression of many toxins, including alpha-toxin and PVL (25). It remains to be determined whether tedizolid phosphate would be superior to vancomycin for the treatment of pneumonia caused by a less toxic hospital-associated MRSA strain. Moreover, tedizolid phosphate, linezolid, and vancomycin were administered at a single time point (1.5 h postinfection) in the present study. We have previously shown that although linezolid was protective when administered at 1.5 h postinfection, the delay of treatment to 4 to 9 h postinfection decreased or abolished its protective effects (6). It is not clear whether tedizolid phosphate could potentially extend the therapeutic window beyond 1.5 h postinfection in the rabbit model. It is also not clear how well our rabbit model mimics the full pathogenesis of S. aureus necrotizing pneumonia, since it is an end-stage disease model that requires a very high number of bacteria (i.e., 5 × 109 CFU) to induce lethal infection.
In conclusion, tedizolid phosphate is as effective as linezolid and superior to vancomycin in a rabbit model of MRSA necrotizing pneumonia. The mechanism of protection of tedizolid phosphate is similar to that of linezolid, in that they both suppress the in vivo production of S. aureus toxins.
MATERIALS AND METHODS
Bacterial strain.
Community-associated MRSA clinical isolate SF8300 (pulsed-field type USA300-0114, multilocus sequence type 8) was used to establish infection in the rabbit model of pneumonia (5, 6). SF8300 was cultured in tryptic soy broth at 37°C with shaking for 6 h to late exponential phase of growth (optical density at 600 nm, 1.2 to 1.5), harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), resuspended in PBS containing 10% glycerol to a concentration of 1 × 1010 to 2 × 1010 CFU/ml, aliquoted into individual cryovials, and immediately stored at −80°C. Frozen aliquots were thawed to room temperature immediately before each use and diluted with saline to approximately 3.5 × 109 to 4.0 × 109 CFU/ml, and the titer was determined by serial dilution and plating onto 5% sheep blood agar plates to determine the actual number of bacteria used for infection of rabbits.
In vitro susceptibility studies.
MIC determinations were performed in triplicate by microdilution methods according to CLSI recommendations (26).
Rabbit model of necrotizing pneumonia.
The protocol for the rabbit model of experimental pneumonia was reviewed and approved by the University of California, San Francisco, Institutional Animal Care and Use Committee. Outbred New Zealand White rabbits (age, 8 to 12 weeks; weight, 2.0 to 2.8 kg) were used. Necrotizing pneumonia was established by standard methods (5, 6). Briefly, a 1.5-ml inoculum containing approximately 5 × 109 CFU of SF8300 was delivered directly into the lungs of anesthetized rabbits through a 2.5-mm pediatric endotracheal tube, which was positioned 1 cm above the main stem bronchi and then removed after instillation of the bacterial inoculum. Antibiotic treatment was initiated at 1.5 h postinfection. Forty-eight infected rabbits were randomized into 1 of 4 experiment groups: (i) 12 control animals were treated intravenously with 5 ml saline at 1.5, 13, 25, and 37 h postinfection; (ii) 12 animals were treated intravenously with 30 mg/kg vancomycin (dissolved in saline) at 1.5, 13, 25, and 37 h postinfection; (iii) 12 animals were treated subcutaneously with 50 mg/kg linezolid (dissolved in 5% cyclodextrin in phosphate-buffered saline) at 1.5, 10, 18, and 26 h postinfection; and (iv) 12 animals were treated intravenously with 6 mg/kg tedizolid phosphate (TR-701 FA [Merck] dissolved in phosphate-buffered saline) at 1.5, 13, 25, and 37 h postinfection.
The rabbits were monitored every 2 h postinfection for the first 36 h postinfection, and survivors were euthanized at 48 h. Animals with profound pulmonary dysfunction (respiration rate of >75, cyanosis, and cough) were euthanized for humane reasons. Animals found dead and those euthanized for pulmonary dysfunction were counted similarly and in contrast to those that survived to 48 h postinfection. This is a model of acute infection in which mortality beyond the first 48 h is extremely rarely encountered.
The lungs were aseptically removed from the euthanized rabbits or those that were found dead. The lungs were cut into <0.5-cm pieces. Three lung pieces (weight, ∼0.2 to 0.3 g) were homogenized in 0.9% saline, and the titer was determined by plating serial dilutions on blood agar to determine the number of CFU.
Pharmacokinetics of tedizolid.
Nine uninfected rabbits were dosed intravenously with 2.5 mg/kg, 5 mg/kg, or 6 mg/kg of tedizolid phosphate (3 animals for each dose). Blood was drawn at approximately 5 min and 1 h, 2 h, 4 h, 8 h, and 24 h postdosing into Vacutainer Plus plastic K2 EDTA tubes (Becton Dickinson). Plasma was isolated in a refrigerated centrifuge and stored at −80°C until analysis of tedizolid using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) procedure (Charles River Laboratories). The pharmacokinetic parameters of tedizolid were determined using Phoenix WinNonlin (version 6.3) software (Certara, Princeton, NJ).
Quantification of LukS-PV, LukF-PV, and Hla concentrations in lung homogenates.
In brief, 4 g of lung pieces was added to 4 ml of PBS containing a protease inhibitor (PBS-PI; Thermo Fisher) and homogenized using a Tissue-Tearor homogenizer. The lung homogenate was centrifuged at 3,500 × g for 15 min at 4°C, and the supernatant was collected. The pellet was resuspended in another 4 ml of PBS-PI. The cell suspension was freeze-thawed (20 min at −80°C) and homogenized with the Tissue-Tearor homogenizer, and the supernatant was collected by centrifugation as described above. This extraction procedure was repeated to yield a total of 4 supernatants, which were pooled, aliquoted, and stored at −80°C until quantification by enzyme-linked immunosorbent assay (ELISA) of LukF-PV, LukS-PV, and Hla (5, 6).
Statistical analyses.
Survival curves were generated using the Kaplan-Meier method, and significance was assessed by a two-sided log-rank (Mantel-Cox) test with the Bonferroni correction for multiple comparisons (GraphPad software, version 6.0). A normal distribution was not assumed, so that the lung weight/body weight ratio, bacterial count, and concentrations of toxins were compared using one-way analysis of variance (ANOVA) with the Kruskal-Wallis test followed by Dunn's multiple-comparison post hoc test.
ACKNOWLEDGMENT
This study was funded by Cubist Pharmaceuticals, now part of Merck & Co. Inc.
REFERENCES
- 1.Prokocimer P, De Anda C, Fang E, Mehra P, Das A. 2013. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 309:559–569. doi: 10.1001/jama.2013.241. [DOI] [PubMed] [Google Scholar]
- 2.Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. 2014. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 14:696–705. doi: 10.1016/S1473-3099(14)70737-6. [DOI] [PubMed] [Google Scholar]
- 3.Bolz DD, Katahira EJ, Bryant AE, Stevens DL. 2016. Subinhibitory concentrations of tedizolid effectively inhibit extracellular toxin production by methicillin-sensitive and methicillin-resistant Staphylococcus aureus. Open Forum Infect Dis 3(suppl_1):2052. doi: 10.1093/ofid/ofw172.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 5.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis 208:75–82. doi: 10.1093/infdis/jit129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diep BA, Le VT, Badiou C, Le HN, Pinheiro MG, Duong AH, Wang X, Dip EC, Aguiar-Alves F, Basuino L, Marbach H, Mai TT, Sarda MN, Kajikawa O, Matute-Bello G, Tkaczyk C, Rasigade JP, Sellman BR, Chambers HF, Lina G. 2016. IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci Transl Med 8:357ra124. doi: 10.1126/scitranslmed.aag1153. [DOI] [PubMed] [Google Scholar]
- 8.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 9.Nimmo GR. 2012. USA300 abroad: global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 18:725–734. doi: 10.1111/j.1469-0691.2012.03822.x. [DOI] [PubMed] [Google Scholar]
- 10.Hidron AI, Low CE, Honig EG, Blumberg HM. 2009. Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis 9:384–392. doi: 10.1016/S1473-3099(09)70133-1. [DOI] [PubMed] [Google Scholar]
- 11.Hota B, Lyles R, Rim J, Popovich KJ, Rice T, Aroutcheva A, Weinstein RA. 2011. Predictors of clinical virulence in community-onset methicillin-resistant Staphylococcus aureus infections: the importance of USA300 and pneumonia. Clin Infect Dis 53:757–765. doi: 10.1093/cid/cir472. [DOI] [PubMed] [Google Scholar]
- 12.Diep BA, Le VT, Visram ZC, Rouha H, Stulik L, Dip EC, Nagy G, Nagy E. 2016. Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of leukocidins in addition to alpha-hemolysin. Antimicrob Agents Chemother 60:6333–6340. doi: 10.1128/AAC.01213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flanagan S, Passarell J, Lu Q, Fiedler-Kelly J, Ludwig E, Prokocimer P. 2014. Tedizolid population pharmacokinetics, exposure response, and target attainment. Antimicrob Agents Chemother 58:6462–6470. doi: 10.1128/AAC.03423-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson KS, Goering RV. 2013. Activity of tedizolid (TR-700) against well-characterized methicillin-resistant Staphylococcus aureus strains of diverse epidemiological origins. Antimicrob Agents Chemother 57:2892–2895. doi: 10.1128/AAC.00274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw KJ, Poppe S, Schaadt R, Brown-Driver V, Finn J, Pillar CM, Shinabarger D, Zurenko G. 2008. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob Agents Chemother 52:4442–4447. doi: 10.1128/AAC.00859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 18.Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, Hacker J. 1998. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 42:2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto MP, Martin E, Badiou C, Lebrun S, Bes M, Vandenesch F, Etienne J, Lina G, Dumitrescu O. 2013. Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 68:1524–1532. doi: 10.1093/jac/dkt073. [DOI] [PubMed] [Google Scholar]
- 20.Chan LC, Basuino L, Dip EC, Chambers HF. 2015. Comparative efficacies of tedizolid phosphate, vancomycin, and daptomycin in a rabbit model of methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 59:3252–3256. doi: 10.1128/AAC.04376-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, Daum RS. 2008. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis 198:561–570. doi: 10.1086/590157. [DOI] [PubMed] [Google Scholar]
- 22.Bayer AS, Ramos MD, Menzies BE, Yeaman MR, Shen AJ, Cheung AL. 1997. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun 65:4652–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbert S, Ziebandt AK, Ohlsen K, Schafer T, Hecker M, Albrecht D, Novick R, Gotz F. 2010. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect Immun 78:2877–2889. doi: 10.1128/IAI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol 187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. 2010. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis 202:1866–1876. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]