ABSTRACT
Although obesity is prevalent among children in the United States, pharmacokinetic (PK) data for obese children are limited. Clindamycin is a commonly used antibiotic that may require dose adjustment in obese children due to its lipophilic properties. We performed a clindamycin population PK analysis using data from three separate trials. A total of 420 samples from 220 children, 76 of whom had a body mass index greater than or equal to the 95th percentile for age, were included in the analysis. Compared to other metrics, total body weight (TBW) was the most robust measure of body size. The final model included TBW and a sigmoidal maturation relationship between postmenstrual age (PMA) and clearance (CL): CL (liters/hour) = 13.8 × (TBW/70)0.75 × [PMA2.83/(39.52.83+PMA2.83)]; volume of distribution (V) was associated with TBW, albumin (ALB), and alpha-1 acid glycoprotein (AAG): V (liters) = 63.6 × (TBW/70) × (ALB/3.3)−0.83 × (AAG/2.4)−0.25. After accounting for differences in TBW, obesity status did not explain additional interindividual variability in model parameters. Our findings support TBW-based dosing for obese and nonobese children.
KEYWORDS: clindamycin, pharmacokinetics, children, obesity, antibiotics
INTRODUCTION
Childhood obesity has reached epidemic proportions in the United States. Over 17% of U.S. children meet the Centers for Disease Control and Prevention definition of obesity, which is a body mass index (BMI) greater than or equal to the 95th percentile for age (1). Obesity is associated with increased health care use and multiple adverse health outcomes, including increased prevalence and severity of infections (2).
The relationship between obesity and infection remains to be fully elucidated. However, one potential explanation for poor outcomes after infection may be suboptimal antimicrobial dosing. Obesity results in important physiologic changes that may have an impact on drug absorption, distribution, metabolism, and elimination. Specifically, volume of distribution (V) may be affected by distribution of drug into excess adipose tissue, while clearance (CL) may be affected by changes in renal function and drug metabolism.
Despite the prevalence of childhood obesity, pharmacokinetic (PK) data for obese children are limited. A recently published systematic review of PK studies that included obese children identified only 20 studies of 21 medications published between 1970 and 2012 (3). Of these 20 studies, only four focused on antimicrobials. This review identified clinically important PK changes in obese children compared to nonobese children for over half of the studied drugs. However, a drug's physicochemical properties (lipophilicity and Biopharmaceutical Drug Disposition Classification System class) did not predict the PK changes, highlighting the need for prospective PK studies with obese children. Because antibiotics are the most common medication class prescribed to children, additional studies of antimicrobials in obese pediatric patients are urgently needed.
Clindamycin is particularly well suited for study in this population. It is a lipophilic molecule that may require dose adjustment in obese patients. From a clinical perspective, clindamycin is widely used in the pediatric inpatient setting to treat invasive infections for which optimal dosing is critical (4). From 1999 to 2008, 3% of all admissions to freestanding children's hospitals in the United States were due to Staphylococcus aureus infections. Overall, two-thirds of these children received clindamycin, and 90% of children with skin and soft tissue infections (SSTIs), to which obese children are predisposed, received clindamycin.
It is essential to identify the optimal dosing for antibiotics, both to maximize cure and to reduce the development of antimicrobial resistance. Because of its extensive use in the pediatric population, specifically for conditions commonly associated with obesity, clindamycin is a priority drug for dose optimization in obese children. Therefore, the objectives of this study were to describe the PK of clindamycin in obese children compared to nonobese children using data from three prospective PK studies.
RESULTS
Participant characteristics.
Four hundred twenty samples from 220 children were included in the analysis. These included 89 samples from 21 children who received intravenous clindamycin in the Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile trial (CLIN01; NICHD-2012-CLN01, ClinicalTrials.gov NCT01744730; IND 115,396), 267 samples from 178 children in the Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care study (PTN POPS; NICHD-2011-POP01, ClinicalTrials.gov NCT01431326; IND 113,645), and 64 samples from 21 children in the Pharmacokinetics of Antistaphylococcal Antibiotics in Infants study (Staph Trio; NICHD-2012-STA01, ClinicalTrials.gov NCT01728363; IND 115,396). Demographic and clinical characteristics are summarized in Table 1. In total, 76 children had a BMI greater than or equal to the 95th percentile for age: 13 from CLIN01 and 63 from PTN POPS. Thirty participants had a BMI greater than the 99th percentile for age. The remaining 144 children (8 from CLIN01, 115 from PTN POPS, and 21 from Staph Trio) were not obese but were included in the analysis to create a robust model that accounted for variations in PK across age and BMI spectra.
TABLE 1.
Demographic and clinical characteristics for participants in the CLIN01, PTN POPS, and Staph Trio trialsa
| Covariate | Value in study |
||
|---|---|---|---|
| CLIN01 (n = 21) | PTN POPS (n = 178) | Staph Trio (n = 21) | |
| Ageb | 13.0 (6.5–17.4) | 5 (0.01–20.5) | 23 (5–65) |
| Wt (kg) | 69.5 (27.9–224) | 23.0 (0.5–139.8) | 1.0 (0.5–3.0) |
| BMI (kg/m2) | 27.1 (18.6–74) | 24.8 (13.7–46.7) | NA |
| SCR (mg/dl) | 0.6 (0.3–1.5) | 0.4 (0.1–3.4) | 0.7 (0.2–1.5) |
| AST (U/liter) | 25 (8–151) | 35.5 (8–389) | 25 (15–116) |
| ALT (U/liter) | 25 (10–114) | 31.5 (5–266) | 11 (6–55) |
| TBIL (mg/dl) | 0.5 (0.2–3.8) | 1.1 (0–11) | 4.7 (0.5–8.2) |
| Albumin (g/dl) | 3.7 (2.1–4.6) | 3.3 (1.9–4.5) | 2.3 (1.3–2.8) |
| AAG (mg/ml) | 2.0 (0.5–3.8) | 2.5 (0.5–6.3) | 0.8 (0.4–1.8) |
Data are medians (ranges). Descriptive statistics are calculated based on values at the time of first recorded dose. PTN POPS, Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care; CLIN01, Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile; Staph Trio, Pharmacokinetics of Antistaphylococcal Antibiotics in Infants; BMI, body mass index; SCR, serum creatinine; AST, aspartate aminotransferase; ALT, alanine transaminase; TBIL, total bilirubin; AAG, alpha-1 acid glycoprotein; NA, not available.
Age is reported in years for CLIN01 and PTN POPS and days for Staph Trio.
Population PK (popPK) model development and evaluation.
Plasma clindamycin concentrations over time are presented in Fig. 1. Consistent with previous studies, a one-compartment PK model described the clindamycin concentration-versus-time data well (5, 6). Body weight was assumed to be a significant predictor of CL and V and was incorporated into the base model before evaluation of other covariates. Because the optimal dosing weight for clindamycin in obese children was uncertain, total body weight (TBW) was compared with normal fat mass (NFM), free fat mass (FFM), and lean body weight (LBW). Height was missing for all 21 neonates from Staph Trio and 6/178 (3%) children from PTN POPS, so NFM, FFM, and LBW were not calculated. TBW resulted in the lowest objective function value (OFV) (7,157.9 for TBW, 7,173.0 for FFM, 7,173.0 for NFM, and 7,164.8 for LBW); therefore, all additional covariate models were evaluated after accounting for TBW (see Table S5 in the supplemental material).
FIG 1.
Clindamycin concentration versus time for merged data set of the Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile (CLN01), Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care (POP01), and Pharmacokinetics of Antistaphylococcal Antibiotics in Infants (Staph Trio [STA01]) trials (A) and the CLN01 trial only (B).
After accounting for TBW, use of a sigmoidal maturation function for CL with postmenstrual age (PMA) resulted in the largest drop in the OFV. On univariable analysis, total bilirubin, serum creatinine (SCR), albumin (ALB), and alpha-1 acid glycoprotein (AAG) on CL and AAG, ALB, and obesity (BMI > 95%) on V resulted in a significant drop in OFV and were included in multivariable analysis. On multivariable analysis, forward addition of SCR on CL and ALB and AAG on V resulted in a significant drop in the OFV, but only ALB and AAG on V were retained after backward elimination (Table 2). Although backward elimination of SCR reached statistical significance, it was not included in the model, as this significance was due to one individual participant with SCR of 3.4 mg/dl. The following relationships characterized the typical values for CL and V in the final model:
Maturation reached 50% adult CL values at ∼40 weeks PMA, with near complete maturation by 2 years of age. Diagnostic plots for the final model are shown in Fig. S1 in the supplemental material. Visual predictive checks including all data and stratified by study are shown in Fig. S2. Shrinkage estimates were <30% for all random effect parameters. In sensitivity analysis, after exclusion of data from children <2 years of age and the maturational function between CL and PMA, there were clinically insignificant differences (<15%) in fixed effects parameters with the final model using the reduced data set (Table S6).
TABLE 2.
Population pharmacokinetic parameter estimates for the final modela
| Parameter | Final model |
Bootstrap (n = 1,000) |
|||
|---|---|---|---|---|---|
| Estimate | RSE (%) | 2.5th percentile | Median | 97.5th percentile | |
| CL70kg (liters/h) | 13.8 | 6.2 | 12.3 | 13.8 | 15.7 |
| V70kg (liters) | 63.6 | 5.0 | 59.0 | 64.1 | 71.4 |
| TM50 (wks) | 39.5 | 12.1 | 32.5 | 39.4 | 53.4 |
| HILL | 2.8 | 33.7 | 1.5 | 3.0 | 5.1 |
| Albumin on V exponent | −0.83 | 27.9 | −1.29 | −0.87 | −0.43 |
| Alpha-1 acid glycoprotein on V exponent | −0.25 | 44.0 | −0.41 | −0.25 | −0.03 |
| IIV (CL) (%) | 58.5 | 11.5 | 52.1 | 58.4 | 64.8 |
| IIV (V) (%) | 11.6 | 145.5 | 5.9 | 15.7 | 27.9 |
| ρ CL-V | 0.8 | 64.5 | 0 | 0.7 | 0.8 |
| Prop., PTN POPS (%) | 33.6 | 16.5 | 26.7 | 32.7 | 38.0 |
| Prop., Staph Trio (%) | 32.1 | 28.8 | 19.3 | 31.1 | 40.1 |
| Prop., CLIN01 (%) | 20.3 | 30.4 | 13.0 | 18.7 | 25.0 |
RSE, relative standard error; CL70kg, population clearance estimate scaled to a 70-kg adult; V70kg, population volume of distribution estimate scaled to a 70-kg adult; TM50, maturation half-life calculated as a function of PMA; HILL, Hill coefficient in sigmoidal maturation function; IIV (CL), interindividual variability in drug clearance; IIV (V), interindividual variability in volume; PTN POPS, Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care; Staph Trio, Pharmacokinetics of Antistaphylococcal Antibiotics in Infants; CLIN01, Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile; Prop., proportional residual error.
Empirical Bayesian estimates for children ≥2 years of age were obtained from the final model and stratified by obesity status and age (Table 3). For the >6- to 12-year and >12-year age categories, statistically significant differences were observed in the absolute (i.e., non-weight normalized) V estimates (P < 0.001). Half-life of elimination was also significantly different, but only for the >6- to 12-year age group (P = 0.01). No other statistically significant differences were observed between obese and nonobese children. Parameter estimates from the sensitivity analysis were within 15% of those from the full model (Table S7), with the same qualitative results; absolute (not weight-normalized) V in children >6 years and half-life of elimination in the >6- to 12-year age group were the only significant differences between obese and nonobese children.
TABLE 3.
Comparison of empirical Bayesian estimates for the final model using total body weight to correct for body size
| Parameter | Median (range) for age categorya |
|||||
|---|---|---|---|---|---|---|
| >2–6 yrsb |
>6–12 yrs |
>12 yrsc |
||||
| Nonobese (n = 8) | Obese (n = 12) | Nonobese (n = 15) | Obese (n = 20) | Nonobese (n = 26) | Obese (n = 44) | |
| CL (liters/h) | 4.17 (0.90–9.10) | 5.69 (1.84–8.27) | 12.50 (3.55–34.40) | 10.70 (4.71–26.70) | 14.25 (5.56–37.40) | 19.15 (3.92–33.70) |
| CL (liters/h/kg) | 0.23 (0.082–0.78) | 0.28 (0.11–0.40) | 0.33 (0.13–0.78) | 0.22 (0.092–0.64) | 0.23 (0.064–0.53) | 0.18 (0.041–0.65) |
| CL (liters/h/70 kg) | 10.64 (3.59–35.03) | 14.83 (5.49–20.32) | 20.67 (7.08–48.48) | 14.69 (5.94–39.16) | 15.82 (4.72–34.68) | 13.97 (3.12–37.89) |
| V (liters) | 15.25 (7.58–19.70) | 17.60 (8.39–25.20) | 29.0 (17.50–57.40) | 46.90 (32.90–85.80)** | 60.10 (22.50–94.60) | 85.75 (28.50–160.0)** |
| V (liters/kg) | 0.81 (0.69–1.26) | 0.86 (0.66–1.03) | 0.90 (0.67–1.06) | 1.03 (0.66–1.29) | 0.89 (0.70–1.34) | 0.89 (0.62–1.58) |
| Half-life (h) | 2.41 (1.12–5.85) | 2.15 (1.53–4.36) | 2.15 (0.95–5.77) | 3.03 (1.19–6.26)** | 2.84 (1.17–7.60) | 3.55 (0.90–11.30) |
Statistically significant differences (**) were observed using a rank sum test.
Three participants with missing height (and BMI) were not included in this parameter summary.
Ten participants (6 nonobese and 4 obese) who were >18 years of age were included in the parameter summary.
Dosing simulations.
The median simulated total drug steady-state areas under the curve from 0 to 8 h (AUC0–8,SS) when stratified by age-based dosing were 44.2 μg · h/ml for ages 2 to 6 years (12 mg/kg every 8 h), 44.8 μg · h/ml for ages >6 to 12 years (10 mg/kg every 8 h), and 48.6 μg · h/ml for ages >12 years (10 mg/kg every 8 h). The median exposures of these dosing regimens were within 25% of the median observed in a 70-kg simulated adult receiving 600 mg intravenously every 8 h: 44.7 μg · h/ml. A box plot stratified by age and obesity status is shown in Fig. 2.
FIG 2.
Box plot of simulated total drug steady-state area under the curve (AUC) from 0 to 8 h following age-based clindamycin dosing and stratified by obesity status. Dosing was 12 mg/kg every 8 h for ages 2 to 6 years and 10 mg/kg every 8 h for ages >6 years, with a maximum dosage of 900 mg every 8 h. The upper and lower whiskers extend to the highest and lowest points that are within 1.5 times the interquartile range.
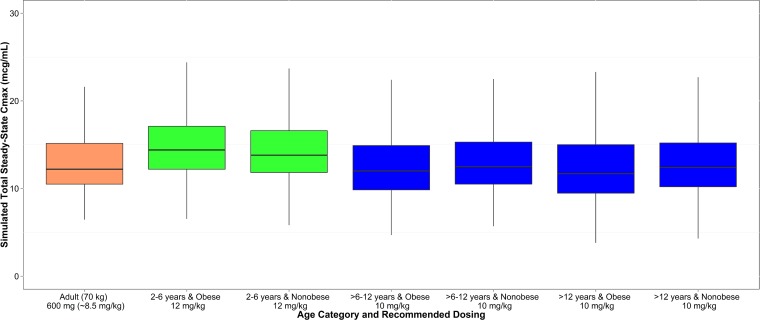
Simulated maximal drug concentrations at steady state (Cmax,SS) were also within 20% of that simulated for a 70-kg adult receiving 600 mg every 8 h (12.2 μg/ml): 14.1 μg/ml for ages 2 to 6 years (12 mg/kg every 8 h), 12.2 μg/ml for ages >6 to 12 years (10 mg/kg every 8 h), and 12.2 μg/ml for ages >12 years (10 mg/kg every 8 h). A box plot stratified by age and obesity status is shown in Fig. 3. After correcting for protein binding and using the optimal dosing regimens described above, the simulated unbound, steady-state clindamycin concentrations were above an MIC of 0.12 μg/ml for at least half the dosing interval in >80% of participants across age groups.
FIG 3.
Box plot of simulated maximal drug concentration at steady state (Cmax,SS) following age-based clindamycin dosing and stratified by obesity status. Dosing was 12 mg/kg every 8 h for ages 2 to 6 years and 10 mg/kg every 8 h for ages >6 years, with a maximum dose of 900 mg every 8 h. The upper and lower whiskers extend to the highest and lowest points that are within 1.5 times the interquartile range.
Safety.
In obese children in the CLIN01 study, clindamycin was very well tolerated. Three adverse events, including one serious event, were reported for 2 (9%) CLIN01 participants; none of the adverse events were considered related to the study drug. The serious adverse event consisted of an apneic event that occurred and resolved after consent but before administration of the study drug. One other participant experienced 2 adverse events: moderate worsening of rash and development of a moderate right internal jugular vein thrombus.
DISCUSSION
This is the first study to assess the effect of obesity on clindamycin PK; our model supports clindamycin dosing based on TBW for obese and nonobese children.
Multiple methods have been suggested for dosing antimicrobials in obese patients, but there is no clear consensus. Adult studies suggest that the most appropriate metric for dosing weight varies by specific drug and differs even among medications with similar physicochemical profiles. There are very limited data for obese children, making choice of a dosing metric difficult. We evaluated alternative measurements of body composition, including FFM, NFM, and LBW, compared with the use of TBW. Each of these measures increased the objective function relative to use of TBW. This suggests that TBW is the most robust measure of accounting for variation in CL and V for clindamycin.
After accounting for TBW, the additional effect of obesity status on clindamycin PK was nonsignificant. The limited impact of obesity on clindamycin PK was verified in the empirical Bayesian estimates derived from the final model. Although there were statistically significant differences between absolute V in obese compared to nonobese children, these differences did not persist after adjusting for TBW. This suggests that clindamycin distributes into excess weight as would be expected given its lipophilic profile. Additionally, simulations were performed using the final model to assess dosing in obese and nonobese children using recommended age-based intravenous dosing. Simulated doses were comparable to those for a 70-kg adult receiving standard clindamycin dosing regardless of obesity status.
In the final model, V was also associated with ALB and AAG. This is not surprising, as clindamycin is highly bound to serum proteins (7). Although clindamycin primarily binds to AAG, it also binds to ALB (8). In the final model, ALB was a more significant predictor of V. This may have occurred because AAG is present in serum in much smaller concentrations than ALB. Although clindamycin has a higher affinity for AAG, binding to AAG may reach saturation at lower drug concentrations, allowing for changes in serum ALB to account for greater variability in V (8). The only other covariate that was included in the final model was PMA, which was included in the analysis to account for expected maturational changes in infants but did not impact CL estimates in children ≥2 years of age.
There is a paucity of PK studies of antimicrobials in obese children. In the only other published prospective PK study for this population, five obese children (BMI > 95th percentile) were administered single doses of cefazolin and tobramycin (9). The tobramycin V adjusted for TBW was significantly lower in obese than in nonobese children; there were no differences for tobramycin CL or either parameter for cefazolin. Similarly, retrospective studies of gentamicin (10) and vancomycin (11, 12) concentrations collected as part of clinical care found that obese children had significantly higher drug concentrations than nonobese controls, suggesting that alternate measures of body weight, such as LBW, may be needed for dosing of these drugs in obese children.
Vancomycin, cefazolin, and the aminoglycosides are hydrophilic molecules. In contrast, clindamycin is lipophilic. Therefore, it might be expected to have a higher absolute V in obese patients. Few studies have reported clindamycin PK in the setting of obesity. Appropriate dosing is particularly important in bone and joint infections, for which adequate tissue concentrations are needed to achieve clinical cure. An adult study used a population PK model to assess adequate dosing in 50 patients being treated for osteomyelitis, 4 of whom were obese (BMI > 30 kg/m2) (6). Assuming a Staphylococcus aureus MIC of 0.125 mg/liter and clindamycin bone penetration of 30%, the researchers concluded that the standard adult dosage of 600 mg intravenously every 8 h achieved adequate serum concentrations in adults up to 75 kg but that 900 mg intravenously every 8 h should be used in adults weighing >75 kg. This 2.7-g/day dosage coincides with the maximum labeled dose for clindamycin used in clinical practice and was the maximum study dose in the current analysis.
Our data suggest that TBW-based clindamycin dosing should achieve sufficient bone concentrations for the treatment of osteomyelitis in obese children. Regardless of obesity status, simulated doses using TBW achieved clindamycin exposures comparable to those in adults receiving 600 mg intravenously every 8 h, the dosage recommended for osteomyelitis in national guidelines (13) and supported by popPK modeling in adults (6). Further, clindamycin bone concentrations obtained from adults undergoing total hip replacement demonstrate that doses as low as 300 mg, given either intramuscularly (14) or intramuscularly and intravenously (15), achieve concentrations well above the staphylococcal MIC for clindamycin.
Because of small sample sizes, previous PK studies of obese children may have been underpowered to detect differences in PK parameters in obese compared to nonobese children. A strength of the present analysis is that it leveraged PK samples from three separate trials to create a robust popPK model. This allowed us to create the largest cohort of obese children included in a prospective PK analysis to date. However, this approach does have some limitations. The inclusion of different study designs and populations likely introduces additional variability into the model. For this reason, separate proportional residual errors were used for each study. Laboratory data (particularly aspartate transaminase [AST], alanine transaminase [ALT], total bilirubin, and ALB) were missing for many of the participants in PTN POPS. Missing values were imputed using median values for the sample, which might have underestimated the impact of these variables on clindamycin PK. Measurements of height and weight were required study procedures for CLIN01, but height and weight were abstracted from the medical record for PTN POPS. This may have resulted in incorrect BMI assessments and misclassification of obesity status for some participants. Additionally, while BMI is a commonly used metric to determine obesity, it is not a direct measure of adiposity. Athletic children with increased muscle mass would have met our BMI-based criteria for obesity without being truly obese. Use of skinfold assessments might provide a useful alternative measure of adiposity for PK studies in obese children moving forward. We did not model clindamycin dosing based on body surface area (BSA). Although the current label provides dosing based on BSA as an alternative to dosing by body weight, this is not commonly used in clinical practice. National guidelines for MRSA bone/joint infections and skin and SSTIs (13), community-acquired pneumonia (16), and sinusitis (17) all recommend weight-based clindamycin dosing for children. In post hoc analyses, the OFVs obtained using BSA were higher than those obtained using TBW, so we did not pursue this strategy. Finally, we cannot comment on the optimal dosing for children weighing >90 kg whose TBW-based dose exceeds the labeled maximum adult dosage of 2.7 g/day. The CLIN01 protocol allowed dosing >2.7 g/day only if it was being used as part of standard-of-care treatment. However, only one adolescent received a dosage of >900 mg every 8 h.
Conclusion.
Data from three separate trials were combined to create a popPK model to assess the impact of obesity on clindamycin PK. Compared to FFM, NFM, and LBW, TBW was the most appropriate metric for clindamycin dosing in obese and nonobese children.
MATERIALS AND METHODS
Patient population.
PK and safety data were obtained from patients receiving intravenous clindamycin in the Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile trial (CLIN01; NICHD-2012-CLN01, ClinicalTrials.gov NCT01744730; IND 115,396). CLIN01 was a prospective, multicenter (n = 6), open-label, multiple-dose PK and safety study of intravenous and oral clindamycin. Pediatric participants were enrolled in the trial if they met the following inclusion criteria: age of 2 to <18 years at the time of first dose of study drug, suspected or confirmed infection or receiving intravenous clindamycin per standard of care, negative pregnancy test, BMI greater than or equal to the 85th percentile for age and sex based on Centers for Disease Control and Prevention recommendations, signed informed consent, and assent where required. Exclusion criteria included exposure to current potent CYP3A4 inducers and inhibitors. Patients who were post-cardiac bypass (within 24 h) or receiving inotropes/vasopressors were also excluded. For participants not receiving clindamycin per standard of care, additional exclusion criteria included history of hypersensitivity or allergic reaction to clindamycin or lincomycin, history of Clostridium difficile colitis with previous administration of clindamycin, aspartate transaminase (AST) of >120 U/liter, alanine transaminase (ALT) of >210 U/liter, total bilirubin of >3 mg/dl, and receipt of a neuromuscular blocker as part of their therapy. Data from two additional trials were included in the popPK model development: the Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care (PTN POPS; NICHD-2011-POP01, ClinicalTrials.gov NCT01431326; IND 113,645) study and the Pharmacokinetics of Antistaphylococcal Antibiotics in Infants (Staph Trio; NICHD-2012-STA01, ClinicalTrials.gov NCT01728363; IND 115,396) study. Full details of these two studies have been previously published (5, 18). Briefly, PTN POPS was a multicenter (n = 27), prospective PK and safety study of understudied drugs (including clindamycin) administered to children (obese and nonobese, <21 years of age) per standard of care. Staph Trio was a multicenter (n = 8), prospective, multiple-dose PK and safety study of clindamycin, rifampin, and ticarcillin-clavulanate in premature infants. PK samples from neonates and nonobese children in these additional studies were included in the main analysis to develop a robust population PK model designed to describe clindamycin disposition across all pediatric age groups and body types. However, a sensitivity analysis was performed excluding all data from Staph Trio and data from children less than 2 years of age in PTN POPS.
Drug dosing and sample collection.
In the CLIN01 trial, intravenous clindamycin was prescribed at a dosage of 30 to 40 mg/kg/day based on total body weight (TBW) every 6 or 8 h. Optimal PK sampling times are presented in Table S1 in the supplemental material.
For the PTN POPS study, dosing information was collected for up to eight doses prior to the sampling dose (last dose before first biological sample collection). Due to the opportunistic study design, the timing of blood sample collection was dependent on standard-of-care laboratory assessments. However, parents/guardians were also given the option to allow sample collection for research purposes only (Table S2).
In the Staph Trio trial, all infants received clindamycin at 10 mg/kg every 6, 8, or 12 h unless prescribed clindamycin per standard of care, in which case dosing was at the discretion of the treating caregiver. The dosing interval and sampling schedule were stratified by gestational age (GA) and postnatal age (PNA) (Tables S3 and S4). Up to eight PK samples were collected based on the predetermined sampling window.
Analytical methods.
For all studies, blood was collected (200 μl) in an EDTA-K2 Microtainer and was processed immediately prior to freezing at the study sites. PK samples were sent to the Pediatric Trials Network central laboratory (OpAns, LLC, Durham, NC) for storage and analysis. Clindamycin concentrations collected from the three trials were quantified using a validated liquid chromatography-tandem spectrometry assay. The chromatography system and mass spectrometer used for sample analysis were the Agilent 1200 series high-performance liquid chromatography system and an Agilent 6400 series triple quadrupole system, respectively. The Pursuit XRS Ultra C18 column (50 by 2 mm; inside diameter [i.d.], 2.8 μm; Agilent) and a gradient mobile phase (water containing 0.5% [vol/vol] formic acid; methanol containing 0.1% [vol/vol] formic acid) were used. The validation range for the assay was 50 to 50,000 ng/ml. Quality control samples included the following nominal concentrations: 50, 150, 4,000, and 40,000 ng/ml. The lower limit of quantification was 50 ng/ml. Accuracy and precision assessed using five determinations at theoretical levels 50, 150, 4,000, and 40,000 ng/ml were within the Food and Drug Administration bioanalytical assay validation criteria (e.g., ±15%).
Model development.
Clindamycin plasma PK data collected following intravenous administration were analyzed with a nonlinear mixed effects modeling approach using the software NONMEM (version 7.2; Icon Development Solutions, Ellicott City, MD). The first-order conditional estimation method with interaction was used for all model runs. Run management was performed using Pirana (version 2.8.1) (19). Visual predictive checks and bootstrap methods were performed with Perl-speaks-NONMEM (version 3.6.2) (20). Data manipulation and visualization were performed using the software R (version 3.0.2; R Foundation for Statistical Computing, Vienna, Austria) and RStudio (version 0.97.551; RStudio, Boston, MA), with the packages lattice, Xpose, and ggplot2 used for the latter (21–23).
Based on our previously published popPK model, a one-compartment model was used in the analyses described herein (5). Between-subject variability was assessed for PK model parameters using an exponential relationship (equation 1):
| (1) |
where Pij is the estimate of parameter j in the ith individual, θPop,j is the population value for parameter j, and ηij is the deviation from the average population value for parameter j in the ith individual with mean zero and variance ω2. The correlation between random effect parameters was calculated according to equation 2, and a proportional error model was used to estimate the intraindividual variability (equation 3):
| (2) |
| (3) |
where ωCL,V is the off-diagonal element between CL and V, Cobs,ij is the jth observed clindamycin concentration in the ith individual, Cpred,ij is the jth predicted concentration in the ith individual, and εprop,ij is a random variable with mean zero and variance σprop,ij2. Because data were combined from the three trials, separate proportional residual errors were used for each study.
The final popPK model included a relationship between TBW and postmenstrual age (PMA) with CL and V as depicted in equations 4 and 5 (5):
| (4) |
| (5) |
where CLstd and Vstd represent population estimates of CL and V in a 70-kg adult, TBWi is total body weight for the ith subject, HILL is a slope parameter for the sigmoidal maturation model, and TM50 is the value of PMA (weeks) when 50% adult clearance is reached.
Alternative measures of body composition.
To assess whether use of other measures of body composition—normal fat mass (NFM), fat free mass (FFM), and lean body weight (LBW)—resulted in superior model performance, we tested these first in place of TBW and in the absence of accounting for PMA.
(i) FFM.
FFM is a sex-specific estimate of body weight that excludes weight due to fat. FFM was estimated using the following equation (24):
| (6) |
WHSmax and WHS50 are sex-specific values: WHSmax = 42.92 kg/m2 and WHS50 = 30.93 kg/m2 for men; WHSmax = 37.99 kg/m2 and WHS50 = 35.98 kg/m2 for women. If height was missing, TBW was used in place of FFM.
(ii) NFM.
NFM accounts for the differential impact of fat mass and FFM on PK. NFM was estimated using the following equation (25):
| (7) |
The parameter Ffat is not constant but is estimated from the observed data for a given drug and accounts for different contributions of fat for each PK parameter (e.g., CL and V). If height was missing, NFM was assumed to be 90% TBW.
(iii) LBW.
LBW measures body weight that is not due to water. We applied an approach whereby estimated extracellular fluid volume (eECV) is calculated from TBW and height (H) (26):
| (8) |
| (9) |
If height was missing, TBW was used.
Covariate analysis.
Following assessment of the ideal measure of weight, additional covariates were tested for model inclusion, beginning with PMA, which was in the originally published model. Determination of which covariates to test for model inclusion was made by visual inspection of scatter and box plots (continuous and categorical variables, respectively) of the individual deviations from the population-typical value PK parameters (ETAs) against covariates. The following covariates were explored: PMA (years), ALT (units/liter), AST (units/liter), serum creatinine (SCR; milligrams/deciliter), bilirubin (total and direct, milligrams/deciliter), albumin (ALB; grams/deciliter), alpha-1 acid glycoprotein (AAG; milligrams/milliliter), BMI, obese status (BMI ≥ 95th percentile for age), ethnicity, and sex. A forward inclusion (P < 0.05 and Δ in objective function value [ΔOFV] of >3.8) and backward elimination (P < 0.001 and ΔOFV > 10.8) approach was used to evaluate statistical significance of all covariates.
Population PK model evaluation and validation.
During assessment of covariates and measures of weight, standard model diagnostic methods were used and included successful minimization, diagnostic plots, plausibility and precision of parameter estimates, and objective function and shrinkage values. Diagnostic plots generated included individual predictions and population predictions versus observations, conditional weighted residuals versus population predictions and time, and individual weighted residuals versus individual predictions. Parameter precision for the final popPK model was evaluated using nonparametric bootstrapping (1,000 replicates) to generate the 95% confidence intervals for parameter estimates. Visual predictive checks were performed whereby the base and final models were used to generate 1,000 Monte Carlo simulation replicates per time point of clindamycin exposure, and simulated results were compared with those observed in the study. The dosing and covariate values used to generate the simulations in the visual predictive check were the same as those used in the study population.
A sensitivity analysis was performed excluding all children younger than 2 years of age. Because such children cannot by definition be obese, their inclusion may have biased our parameter estimates. Population and individual estimates were first derived from the final model excluding children <2 years old. Subsequently, the maturational function between PMA and CL was dropped from the model, as neonates and infants were excluded from this limited data set.
Model-based simulations to assess pediatric dosing.
The final model parameter estimates were used to perform dosing simulations. Age-based dosing regimens of 12 mg/kg every 8 h for children ≤6 years old and 10 mg/kg every 8 h for children >6 years old were chosen because they were previously shown to match adult (70 kg) clindamycin exposure of 600 mg every 8 h (5). The following parameters were calculated for each virtual subject: steady-state area under the curve from 0 to 8 h (AUC0–8,SS), maximal drug concentrations at steady state (Cmax,SS), and minimum concentration at steady state (Cmin,SS). Model equations are shown in equations 10 to 12:
| (10) |
| (11) |
| (12) |
where kel is the first-order elimination rate constant, calculated as CL/V; DUR is the infusion duration (0.5 h for all dosing simulations); and τ is the dosing interval (8 h for all dosing simulations).
The covariate values for virtual patients were the same as those in the study population used for model development. Using PK parameter estimates from the final population model, 200 concentration-versus-time profiles were simulated for each virtual patient and then related to adult simulated exposure. Patients who would receive >900 mg following weight-based dosing (>75 kg for 12 mg/kg and >90 kg for 10 mg/kg) received a maximum dose of 900 mg. Unbound, steady-state clindamycin concentrations at half the dosing interval (fC50,SS) were calculated assuming a fraction unbound of 6% (7, 27). The proportion of virtual participants with an fC50,SS greater than a MIC of 0.12 μg/ml (MIC90 for Staphylococcus aureus) following optimal dosing was calculated (28).
Safety.
Participants in CLIN01 were monitored for safety. All participants who received at least one dose of study drug were included in the safety analysis. Participants were followed for adverse events during study drug administration through 3 days after discontinuation of study drug. Additionally, laboratory assessments (hematology and biochemistry) were obtained within 72 h prior to the first study dose and within 24 h of last study dose on all participants.
Statistical analysis.
Using the value at the time of first recorded dose, the median and range were calculated for demographic and clinical variables. We tested the equality of distribution of each PK parameter of interest among the obese and nonobese population using the nonparametric Wilcoxon rank sum test and stratified the analysis by participant age: 2 to 6 years, >6 to 12 years, and >12 years. With the exception of the PK modeling, all statistical analyses were performed using the software R (version 3.0.2; R Foundation for Statistical Computing, Vienna, Austria) or Stata (version 13.1; College Station, TX).
Supplementary Material
ACKNOWLEDGMENTS
The Best Pharmaceuticals for Children Act—Pediatric Trials Network Steering Committee includes Gregory L. Kearns, Arkansas Children's Hospital, Little Rock, AR; Matthew M. Laughon, University of North Carolina, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; John van den Anker, George Washington University School of Medicine and Health, Washington, DC; and Kelly Wade, Children's Hospital of Philadelphia, Philadelphia, PA. The committee also includes, from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, David Siegel, Perdita Taylor-Zapata, Anne Zajicek, Zhaoxia Ren, Ekaterini Tsilou, and Alice Pagan, and from the EMMES Corporation (Data Coordinating Center), Ravinder Anand, Traci Clemons, and Gina Simone.
This work was funded under National Institute of Child Health and Human Development (NICHD) contract HHSN27500018 (Kevin Watt) for the Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile study (protocol NICHD-2012-CLN01). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Daniel Gonzalez is funded by K23HD083465 from the National Institute for Child Health and Human Development (NICHD) and by the nonprofit Thrasher Research Fund (https://www.thrasherresearch.org). Daniel K. Benjamin, Jr., receives support from the National Institutes of Health (NIH) (award 2K24HD058735-06, National Center for Advancing Translational Sciences award UL1TR001117, NICHD contract HHSN275201000003I, and National Institute of Allergy and Infectious Diseases [NIAID] contract HHSN272201500006I); he also receives research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) and industry for neonatal and pediatric drug development (https://www.dcri.duke.edu/research/coi.jsp). P. Brian Smith receives salary support for research from the NIH and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the NICHD (HHSN275201000003I and 1R01-HD081044-01), and the Food and Drug Administration (FDA) (1R18-FD005292-01); he also receives research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and Shionogi and industry for neonatal and pediatric drug development (https://www.dcri.duke.edu/research/coi.jsp). Michael Cohen-Wolkowiez receives support for research from the NIH (1R01-HD076676-01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the NIAID (HHSN272201500006I and HHSN272201300017I), the NICHD (HHSN275201000003I), the FDA (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (HHSO100201300009C), the nonprofit Thrasher Research Fund, and industry (CardioDx and Durata Therapeutics) for drug development in adults and children (https://www.dcri.duke.edu/research/coi.jsp). Kevin Watt receives support from the NICHD (1K23HD075891, 5K12HD047349) and the Thrasher Research Fund for his work in pediatric clinical pharmacology. The remaining authors have no disclosures.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
M. J. Smith, D. Gonzalez, P. B. Smith, M. Cohen-Wolkowiez, and K. Watt wrote the manuscript; M. J. Smith, D. Gonzalez, J. E. Sullivan, M. D. Reed, R. Anand, K. Martz, K. Berezny, D. K. Benjamin, Jr., P. B. Smith, M. Cohen-Wolkowiez, and K. Watt designed the research; M. J. Smith, J. L. Goldman, R. Yogev, J. E. Sullivan, and M. D. Reed performed the research; and D. Gonzalez analyzed the data.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02014-16.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. 2014. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Kompoti M. 2006. Obesity and infection. Lancet Infect Dis 6:438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 3.Harskamp-van Ginkel MW, Hill KD, Becker KC, Testoni D, Cohen-Wolkowiez M, Gonzalez D, Barrett JS, Benjamin DK Jr, Siegel DA, Banks P, Watt KM; Best Pharmaceuticals for Children Act—Pediatric Trials Network Administrative Core Committee. 2015. Drug dosing and pharmacokinetics in children with obesity: a systematic review. JAMA Pediatr 169:678–685. doi: 10.1001/jamapediatrics.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herigon JC, Hersh AL, Gerber JS, Zaoutis TE, Newland JG. 2010. Antibiotic management of Staphylococcus aureus infections in US children's hospitals, 1999–2008. Pediatrics 125:e1294–e1300. doi: 10.1542/peds.2009-2867. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez D, Melloni C, Yogev R, Poindexter BB, Mendley SR, Delmore P, Sullivan JE, Autmizguine J, Lewandowski A, Harper B, Watt KM, Lewis KC, Capparelli EV, Benjamin DK Jr, Cohen-Wolkowiez M; Best Pharmaceuticals for Children Act—Pediatric Trials Network Administrative Core Committee. 2014. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther 96:429–437. doi: 10.1038/clpt.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouazza N, Pestre V, Jullien V, Curis E, Urien S, Salmon D, Tréluyer JM. 2012. Population pharmacokinetics of clindamycin orally and intravenously administered in patients with osteomyelitis. Br J Clin Pharmacol 74:971–977. doi: 10.1111/j.1365-2125.2012.04292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon RC, Regamey C, Kirby WM. 1973. Serum protein binding of erythromycin, lincomycin, and clindamycin. J Pharmaceut Sci 62:1074–1077. doi: 10.1002/jps.2600620704. [DOI] [PubMed] [Google Scholar]
- 8.Craig WA, Suh B. 1991. Protein binding and the antimicrobial effects: methods for determination of protein binding, p 367–402. In Lorian V. (ed), Antibiotics in laboratory medicine, 3rd ed Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 9.Koshida R, Nakashima E, Taniguchi N, Tsuji A, Benet LZ, Ichimura F. 1989. Prediction of the distribution volumes of cefazolin and tobramycin in obese children based on physiological pharmacokinetic concepts. Pharm Res 6:486–491. doi: 10.1023/A:1015968407226. [DOI] [PubMed] [Google Scholar]
- 10.Choi JJ, Moffett BS, McDade EJ, Palazzi DL. 2011. Altered gentamicin serum concentrations in obese pediatric patients. Pediatr Infect Dis J 30:347–349. doi: 10.1097/INF.0b013e3181ff023e. [DOI] [PubMed] [Google Scholar]
- 11.Miller M, Miller JL, Hagemann TM, Harrison D, Chavez-Bueno S, Johnson PN. 2011. Vancomycin dosage in overweight and obese children. Am J Health Syst Pharm 68:2062–2068. doi: 10.2146/ajhp110107. [DOI] [PubMed] [Google Scholar]
- 12.Heble DE Jr, McPherson C, Nelson MP, Hunstad DA. 2013. Vancomycin trough concentrations in overweight or obese pediatric patients. Pharmacotherapy 33:1273–1277. doi: 10.1002/phar.1321. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak M, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 14.Nicholas P, Meyers BR, Levy RN, Hirschman SZ. 1975. Concentration of clindamycin in human bone. Antimicrob Agents Chemother 8:220–221. doi: 10.1128/AAC.8.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baird P, Hughes S, Sullivan M, Willmot I. 1978. Penetration into bone and tissues of clindamycin phosphate. Postgrad Med J 54:65–67. doi: 10.1136/pgmj.54.628.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT. 2011. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 53:e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, Pankey GA, Seleznick M, Volturo G, Wald ER, File TM Jr. 2012. IDSA—clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 54:e72–e112. doi: 10.1093/cid/cis370. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez D, Delmore P, Bloom BT, Cotten CM, Poindexter BB, McGowan E, Shattuck K, Bradford KK, Smith PB, Cohen-Wolkowiez M, Morris M, Yin W, Benjamin DK Jr, Laughon MM. 2016. Clindamycin pharmacokinetics and safety in preterm and term infants. Antimicrob Agents Chemother 60:2888–2894. doi: 10.1128/AAC.03086-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. 2011. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed 101:72–79. doi: 10.1016/j.cmpb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Lindbom L, Pihlgren P, Jonsson EN. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson EN, Karlsson MO. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64. [DOI] [PubMed] [Google Scholar]
- 22.Wickham H. 2009. ggplot2: elegant graphics for data analysis. Springer, New York, NY. [Google Scholar]
- 23.Sarkar D. 2008. Lattice: multivariate data visualization with R. Springer, New York, NY. [Google Scholar]
- 24.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. 2005. Quantification of lean bodyweight. Clin Pharmacokinet 44:1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 25.Anderson BJ, Holford NH. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 26.Peters AM, Snelling HL, Glass DM, Bird NJ. 2011. Estimation of lean body mass in children. Br J Anaesth 106:719–723. doi: 10.1093/bja/aer057. [DOI] [PubMed] [Google Scholar]
- 27.Burian A, Wagner C, Stanek J, Manafi M, Böhmdorfer M, Jäger W, Zeitlinger M. 2011. Plasma protein binding may reduce antimicrobial activity by preventing intra-bacterial uptake of antibiotics, for example clindamycin. J Antimicrob Chemother 66:134–137. doi: 10.1093/jac/dkq400. [DOI] [PubMed] [Google Scholar]
- 28.Reeves DS, Holt HA, Phillips I, King A, Miles RS, Paton R, Wise R, Andrews JM. 1991. Activity of clindamycin against Staphylococcus aureus and Staphylococcus epidermidis from four UK centres. J Antimicrob Chemother 27:469–474. doi: 10.1093/jac/27.4.469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.