ABSTRACT
The fungal pathogen Candida albicans causes a variety of oral infections, including denture stomatitis, which is characterized by inflammation of the oral mucosa in direct contact with dentures and affects a significant number of otherwise healthy denture wearers. While antifungal treatment reduces symptoms, infections are often recurrent. One strategy to address this problem is to incorporate compounds with fungicidal activities into denture materials to prevent colonization. Our laboratory synthesized novel derivatives of 1,4-diazabicyclo[2.2.2]octane (DABCO), which is an organic compound typically used as a catalyst in polymerization reactions. DABCO derivatives with different aliphatic chain lengths (DC16, DC16F, DC18, and C6DC16), as well as methacrylate monomers conjugated to DABCO compounds (DC11MAF and C2DC11MAF), were synthesized and tested for antimicrobial activity. All the compounds exhibited fungicidal activity against several Candida species at concentrations ranging between 2 and 4 μg/ml. Moreover, acrylic denture base resins fabricated to contain 1, 2, or 4 wt% DABCO compounds inhibited surface C. albicans biofilm formation, as well as fungal growth, in disc diffusion assays. Remarkably, discs (4 wt%) aged for 2 months also exhibited approximately 100% growth-inhibitory activity. While some DABCO compounds exerted intermediate to high cytotoxicity against mammalian oral cell types, DC11MAF and denture base resin discs containing 2 or 4 wt% C2DC11MAF exhibited relatively low cytotoxicity against periodontal ligament (PDL) cell and gingival fibroblast (GF) lines, as well as primary oral epithelial cells. These studies demonstrate that DABCO derivatives can be incorporated into denture materials and exert fungicidal activity with minimal cytotoxicity to mammalian cells. DC11MAF and C2DC11MAF are considered strong candidates as therapeutic or preventive alternatives against Candida-associated denture stomatitis.
KEYWORDS: Candida albicans, antifungal agents, antifungal material, antifungal susceptibility testing, denture stomatitis
INTRODUCTION
Denture stomatitis, most frequently caused by the fungal pathogen Candida albicans, is the most common form of oral candidiasis, affecting 30 to 75% of otherwise healthy denture wearers (reviewed in reference 1). Denture stomatitis causes inflammation of the oral mucosa in direct contact with the prosthetic surface, most commonly of the maxillary denture, and often results from poor oral hygiene and failure to adhere to nightly denture removal (2). Clinical presentation of symptoms can range from mild (localized palatal inflammation and erythema) to severe (diffuse erythema and edema and nodular papillary hyperplasia) (2). The ability of C. albicans to form biofilms is thought to be a key requirement in the pathogenesis of denture stomatitis (3). C. albicans can readily form biofilms on denture materials in both in vitro and in vivo models (4–6). Biofilm formation contributes to increased drug resistance and protection against host antifungal defenses (4, 7–9).
Treatment strategies for denture stomatitis include denture cleaning and disinfection, antimicrobial mouth rinses, proper denture wearing habits, use of tissue conditioners or liners, and administration of topical (lozenges, cream, or powder) or systemic antifungal drugs (2). While these strategies are effective at reducing symptoms, the treatment regimens often fail to fully eradicate the infection, leading to recurrent/chronic infections (10). This is due to several factors, including variable drug concentrations in vivo, static activity of some drugs, patient compliance, and possible drug resistance (reviewed in reference 11).
An alternative approach is to develop dental biomaterials with antifungal activity. There are two main types of antimicrobial materials: materials that can be loaded with drugs postfabrication and materials that have drugs incorporated prefabrication. Challenges with drug loading include variable drug absorption and high drug release rates, resulting in lack of sustained antimicrobial activity. There are several reports using a novel denture material, prepared by grafting poly(N-vinyl-2-pyrrolidinone) (PNVP) onto poly(methylmethacrylate) (PMMA), that binds to antifungal drugs through ionic interactions (12–14). This novel material provides controlled, sustained release of antifungal drugs over several weeks (12–14). Direct incorporation of drugs into biomaterials would eliminate problems with drug absorption and rapid release but also poses challenges. First, drugs must be solubilized or biochemically linked to a biomaterial component and withstand the polymerization process. Second, drugs should not negatively impact biomaterial polymerization, heat curing, or physical and mechanical properties. Finally, drugs should be released at levels that are antimicrobial for sustained periods with limited cytotoxicity. Alternatively, drugs covalently linked to denture base materials could provide sustained contact-dependent antimicrobial activity with low drug release, limiting cytotoxicity. For these reasons, drug incorporation has been restricted to soft, thin, temporary-use materials, such as tissue conditioners and relines. Studies have demonstrated up to 100% inhibition of fungal surface growth on denture relines with several antifungal drugs incorporated (15). However, there are limited reports of the efficacy of antifungal drugs incorporated into solid denture materials. There is also concern that diffused drug concentration levels exert fungistatic activity and may lead to drug resistance. Therefore, development of biomaterials with stable, sustained fungicidal activity would represent a significant advance.
A group of novel antimicrobial compounds containing 1,4-diazabicyclo[2.2.2]octane (DABCO) with different long aliphatic chain or methacrylate groups have been recently developed at the Louisiana State University Health Sciences Center (LSUHSC) School of Dentistry (patent pending), with projected use in oral devices and/or restorative materials (X. Xu and S. Costin, antimicrobial monomers for use in dental polymers, U.S. provisional patent application 61/810,136, 2014) (17–20). These DABCO derivatives are positively charged compounds that can withstand heat curing of denture materials. Several DABCO derivatives have been previously shown to have antibacterial and antifungal activities, but their application in denture base materials and cytotoxicity to mammalian oral cells have not been reported (21). The objective of this study was to evaluate the antifungal activities and cytotoxicities of these novel DABCO compounds, DABCO-conjugated monomers, and denture base resins containing these compounds. The studies will provide a solid foundation for the development and further optimization of novel antimicrobial biomaterials for use in oral applications.
RESULTS
Synthesis and fabrication of DABCO derivatives.
Four long-chain DABCO compounds (DC16, DC16F, DC18, and C6DC16) and two DABCO-conjugated methacrylate monomers (DC11MAF and C2DC11MAF) were synthesized using the reactions shown in Fig. S1 in the supplemental material. In the initial pilot study, it was found that the heat-cured materials containing bromide forms of DABCO compounds (DC16, C6DC16, and DC18) were soft and showed yellow or brownish colors (Fig. 1). This was likely caused by the oxidation of the bromide to bromine (iodide to iodine in C6DC16) during the heat-curing process. The molecular bromine (or iodine) not only gave the material undesirable colors, but also interfered with the polymerization process (consuming free radicals) and generated soft materials. To solve this problem, DABCO compound DC16 was converted to fluoride (see Fig. S1 in the supplemental material, reactions 1 to 4), which generated a denture base resin with a pink color (Fig. 1). Therefore, fluoride-based DABCO methacrylate monomers were also synthesized (see Fig. S1 in the supplemental material, reactions 5 and 6), producing similar pink resins (Fig. 1). The fluoride modification had a positive impact on both the physical and mechanical properties of the denture base resin (unpublished data); therefore, these compounds were chosen for further testing.
FIG 1.
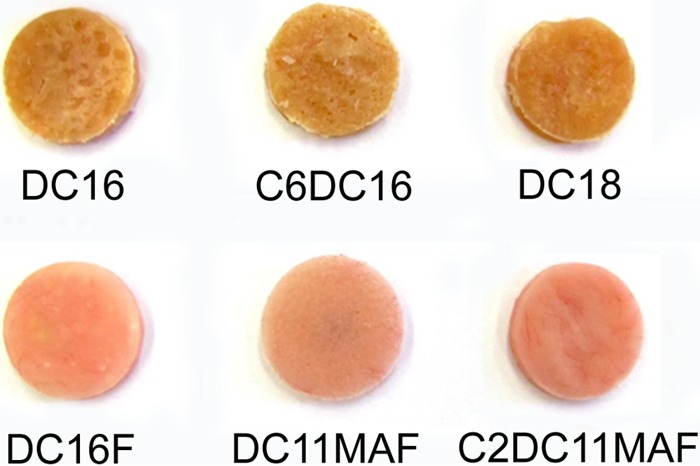
Heat-cured experimental antifungal denture base resin discs. The resins containing DC16, C6DC16, and DC18 appear discolored (yellowish) and soft. Those containing DABCO-fluoride are hard and have the same pink color as the control.
Antifungal activities of DABCO derivatives.
Standard MIC assays were performed to measure the antifungal activities of LSUHSC DABCO derivatives against C. albicans according to CLSI guidelines. The MIC90 values of the DABCO derivatives ranged from 2 to 4 μg/ml (Table 1). No growth was observed from plated samples at the MIC level or above, indicating that all the derivatives exhibit fungicidal activity. One DABCO derivative, DC16, was chosen to investigate antifungal activity against several Candida species, including C. albicans, C. dubliniensis, C. glabrata, C. parapsilosis, and C. tropicalis, and to compare the activity with that of traditional antifungal azoles, clotrimazole and fluconazole (Table 2). DC16 exhibited antifungal activity against all Candida species, with a MIC90 range of 2 to 4 μg/ml, similar to that of fluconazole. While, as expected, clotrimazole MIC values were lower, both azole drugs were confirmed as static inhibitors of growth, while DC16 exhibited fungicidal activity.
TABLE 1.
In vitro susceptibility of C. albicans to DABCO compounds
Range, 0.008 to 8.0 μg/ml.
MFC, minimum fungicidal concentration resulting in 99.9% killing.
TABLE 2.
In vitro susceptibility of non-albicans Candida (NAC) species to DABCO compound DC16
| Candida species | MIC90 (μg/ml)a |
DC16b MFC (μg/ml)c | |
|---|---|---|---|
| Clotrimazolec | Fluconazolec | ||
| C. albicans | 0.5 | 4 | 4 |
| C. dubliniensis | 0.004 | 1 | 4 |
| C. glabrata | 2 | 16 | 4 |
| C. parapsilosis | 0.125 | 1 | 4 |
| C. tropicalis | 0.125 | 8 | 2 |
MIC90, MIC resulting in 90% growth inhibition.
Range, 0.004 to 4.0 μg/ml.
MFC, minimum fungicidal concentration resulting in 99.9% killing.
Inhibition of C. albicans biofilm formation by DABCO-containing denture base resins.
To investigate the surface antimicrobial activity of DABCO-containing denture base resins, discs containing 4 wt% DC16F, DC11MAF, and C2DC11MAF were fabricated and tested in C. albicans biofilm growth assays. The discs were inoculated with C. albicans to allow adhesion, transferred to RPMI, and incubated at 37°C for 24 h to facilitate biofilm formation. Biofilm growth was measured using the XTT reduction assay, which measures metabolic activity. Compared with control denture base resin discs (no drug), discs containing DABCO compounds exhibited almost complete inhibition of C. albicans biofilm growth/viability (P < 0.0001) (Fig. 2). We next conducted dose-response studies using discs containing 1, 2, or 4 wt% drug. No significant differences in antifungal activity were observed for discs containing DC16F or C2DC11MAF regardless of the drug concentration (Fig. 3). Reduced antifungal activity was observed with discs containing 1 wt% C2DC11MAF compared with 2- or 4-wt% discs. To investigate the stability and/or longevity of antifungal activity of DABCO-containing denture material, discs were aged by immersion in deionized water at 37°C for 2 months. Antifungal activity was reduced by approximately 40% after aging discs containing DC16F, indicating that drug may leach out of the material over time and/or lose activity. However, no reduction was observed with aged DC11MAF and C2DC11MAF discs (4 wt%), which retained approximately 100% growth inhibition (Fig. 3).
FIG 2.
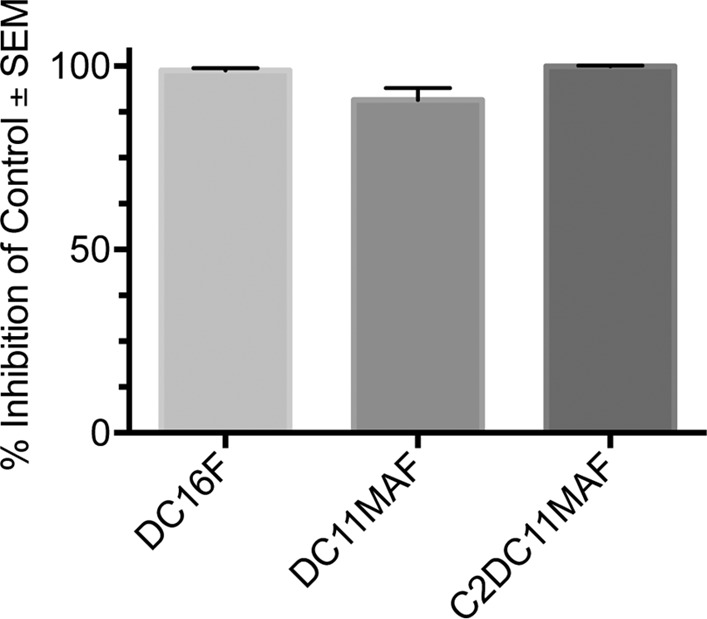
Inhibition of C. albicans biofilm formation by DABCO-containing denture base resin discs. Denture discs containing 4 wt% DABCO derivatives were fabricated and inoculated with 1 × 106 CFU of C. albicans for 2 h to allow fungal adherence. The discs were washed, transferred to fresh medium, and incubated overnight at 37°C to allow biofilm growth. Cell viability was assessed using an XTT reduction assay. The data are presented as percent inhibition compared to the drug-free control and represent cumulative data from 3 repeats. SEM, standard error of the mean.
FIG 3.
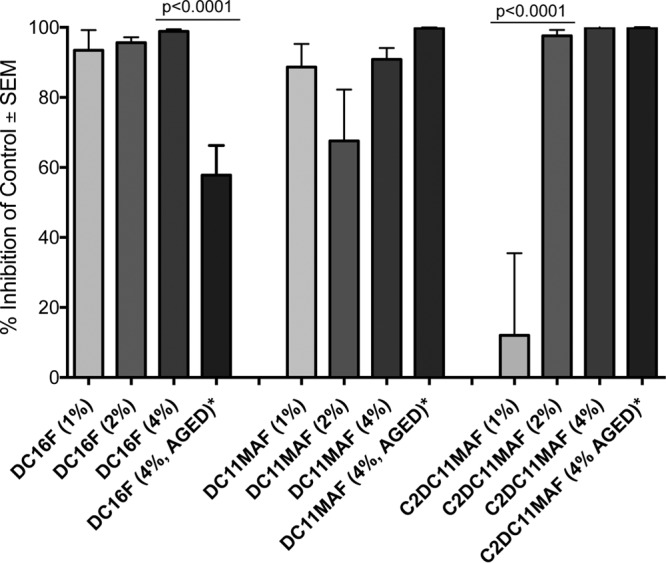
Effects of drug concentration and aging of DABCO-containing denture base resin discs on C. albicans biofilm formation. Control denture base resin discs (no drug) and discs containing 1 wt%, 2 wt%, 4 wt%, and 4 wt% aged (2 months) DABCO derivatives were fabricated and used in biofilm growth assays as described in the legend to Fig. 2. The data are represented as percent inhibition compared to the drug-free control discs and are representative of the results of 3 to 5 repeat experiments. Statistics included one-way ANOVA for each group of compounds followed by Tukey post hoc tests and unpaired Student t tests between 4 wt% and 4 wt% aged samples.
Drug diffusion from DABCO-containing denture base resin.
To evaluate the initial release of DABCO compounds incorporated into denture base material over 24 h, disc diffusion assays were performed. Control discs (no drug) and discs containing DC16F, DC11MAF, or C2DC11MAF (4 wt%) were placed on agar plates inoculated confluently with C. albicans. All three DABCO compound discs resulted in visible zones of inhibition compared to the control discs, which did not inhibit fungal growth (Fig. 4A). Among the DABCO compounds, discs containing DC11MAF and C2DC11MAF produced significantly larger zones of inhibition than DC16F (Fig. 4B), which may indicate either a higher rate of early diffusion or greater activity in agar.
FIG 4.
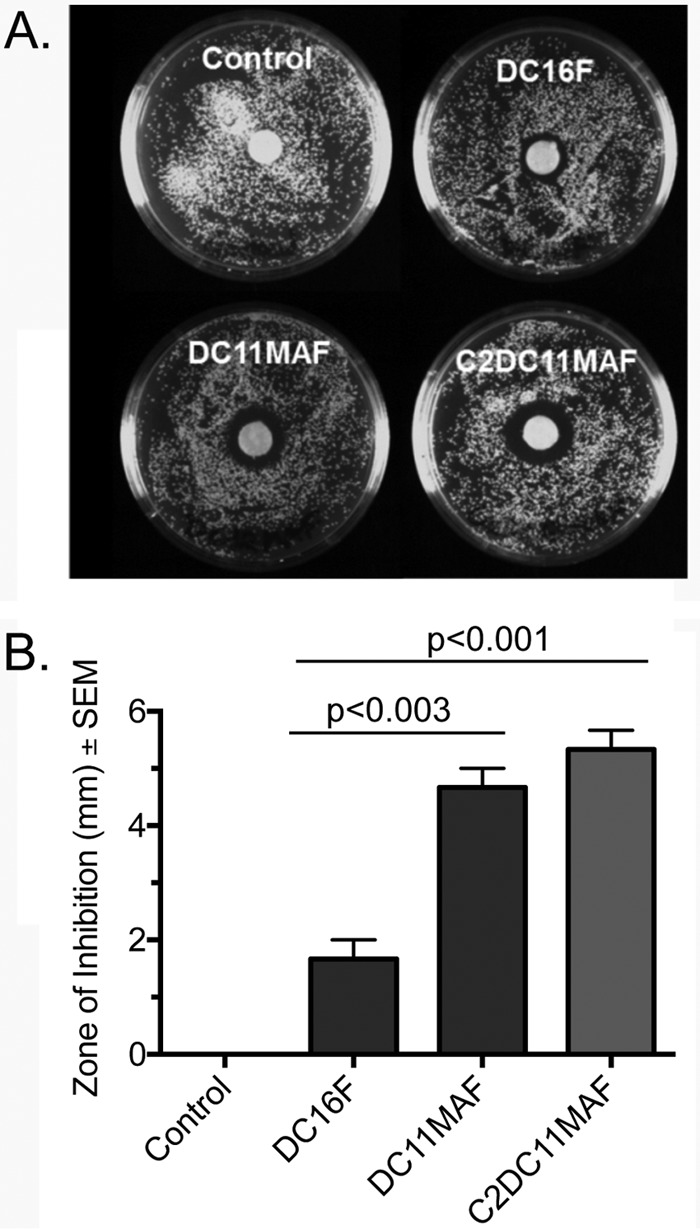
Disc diffusion assays with DABCO-containing denture base resin discs. A standardized suspension of C. albicans containing 1 × 104 CFU was spread onto the surfaces of YPD agar plates to form fungal lawns. Control denture discs or those containing DABCO derivatives DC16F, DC11MAF, and C2DC11MAF were placed in the centers of the plates and incubated at 37°C for 24 h. The plates were photographed using a CCD imager. (A) Zones of inhibition surrounding the discs. (B) Quantitative assessment of zones. The data are representative of the results of 3 repeat experimentss. Statistics included unpaired Student t tests.
Cytotoxicities of DABCO derivatives on mammalian oral cells.
DABCO derivatives were tested for cytotoxicity against periodontal ligament (PDL) cells, gingival fibroblasts (GF), or primary oral epithelial (EPI) cells. Cell cultures were exposed to DABCO derivatives or a standard cytotoxicity control compound, BisGMA (bisphenol A-glycidyl methacrylate; Polysciences, Inc.), for 24 h and assessed for cell survival. DC11MAF exhibited the lowest toxicity against PDL cells and GF (Fig. 5A and B), with survival severely affected only at 32 μg/ml, the highest concentration tested. C2DC11MAF and DC16F exhibited toxicity similar to or greater than that of the BisGMA positive control, reaching 50% cytotoxicity in the range of 2.0 to 8.0 μg/ml, depending on the compound and cell type (Fig. 5A and B). All the DABCO compounds showed little to no cytotoxicity against oral epithelial cells at concentrations up to 8 μg/ml (Fig. 5C). The exception was DC16F at 32 μg/ml (P = 0.0188). The cytotoxicities of DABCO compounds incorporated into denture base resin were also evaluated. Oral cell cultures were exposed to denture discs containing 2 wt% or 4 wt% DABCO derivatives or control discs for 24 h, and survival was similarly assessed. DC16F and DC11MAF exhibited moderate (∼50% survival) to severe (<10% survival) cytotoxicity against PDL cells and GF at 2 and 4 wt%, while C2C11MAF produced only mild toxicity (70 to 90% survival) at similar concentrations (Fig. 6A and B). Similar to observations with free compounds, DABCO compounds incorporated into denture discs produced little to no cytotoxicity for oral epithelial cells (Fig. 6C).
FIG 5.
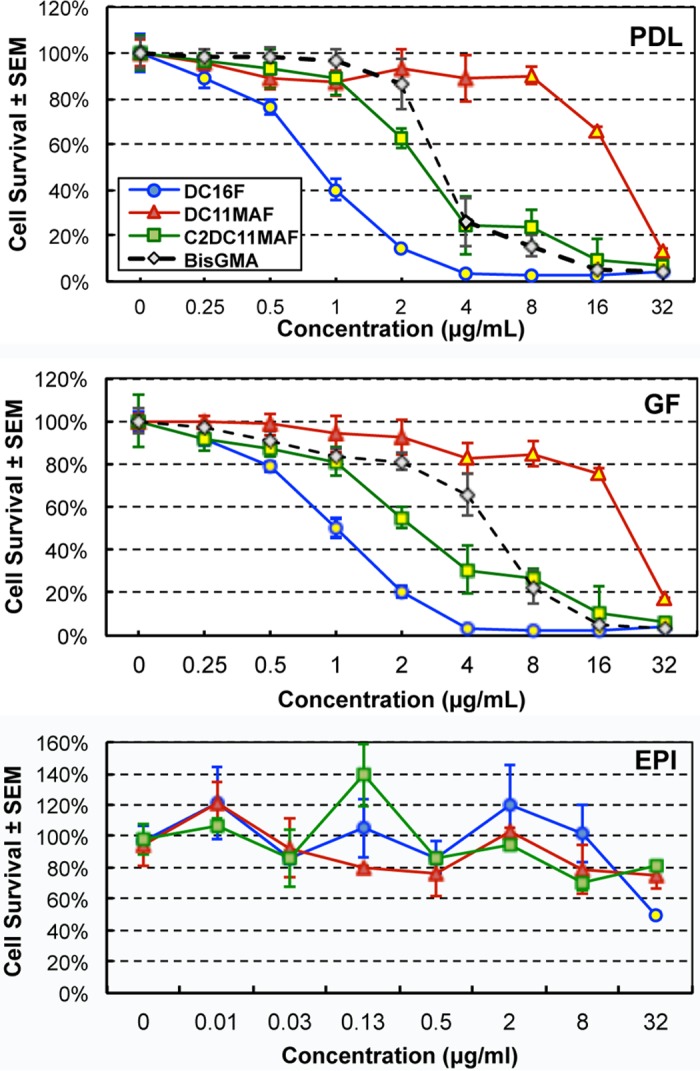
Cytotoxicity of DABCO derivatives on mammalian oral cells. PDL cells, GF, or primary EPI cells were exposed to BisGMA (positive-control compound) or DABCO compounds dissolved in RPMI medium at concentrations ranging from 0 to 32.0 μg/ml. Cytotoxicity was evaluated at 24 h. The data are representative of the results of 2 repeat experiments with 8 replicates/concentration. The yellow filled symbols indicate the lowest drug concentrations with significantly reduced cell survival compared to no drug (P < 0.05 using the unpaired Student t test).
FIG 6.
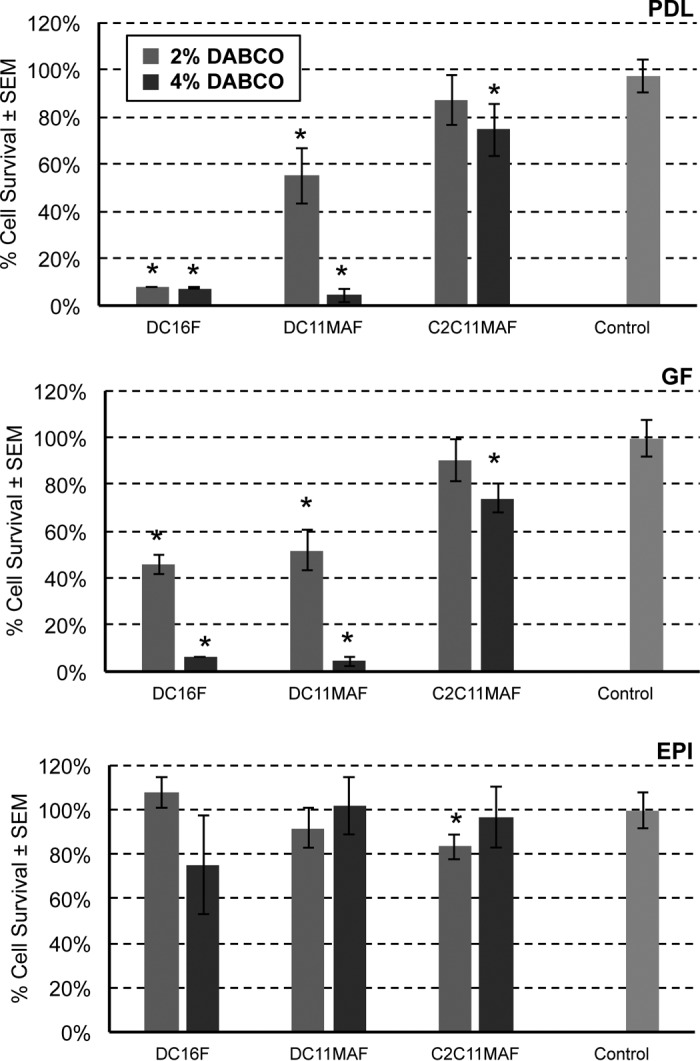
Cytotoxicities of DABCO-containing denture base resin discs. PDL cell and GF lines or primary EPI cells were exposed to denture resin base discs containing 2% or 4% DABCO compounds or control discs and incubated for 24 h. Cytotoxicity was evaluated at 24 h. The data are representative of the results of 2 repeat experiments with 4 replicates/disc. *, statistical significance versus control discs using an unpaired Student t test. Significance was defined as a P value of <0.05.
DISCUSSION
Candida-associated denture stomatitis is a prevalent oral fungal infection with high rates of recurrence despite treatment with antifungal drugs. In this study, we evaluated novel DABCO derivatives for antifungal activity against various Candida species and whether these compounds could be effectively incorporated into denture base resins and retain antifungal activity. The six original DABCO derivatives all exhibited significant fungicidal activity against C. albicans. The preliminary tests showed that DC16, DC18, and C6DC16 had similar fungicidal activities, but DC18 and C6DC16 were more difficult and expensive to synthesize than DC16. Therefore, DC16 was chosen for further testing compared to conventional antifungal drugs (azoles) and exhibited similar antifungal activity against several pathogenic Candida species, indicating broad efficacy.
Despite strong fungicidal activity, the bromide form of DC16 was found to interfere with the heat-curing process, decreased the mechanical properties of denture base resin significantly (resulting in a softer material), and resulted in a color change (yellow or brownish). Conversion of DC16 to a fluoride salt (DC16F) eliminated these effects on the denture material's mechanical and physical properties. However, there were concerns that DC16F might readily diffuse out of the cured denture base resin, resulting in diminished antimicrobial activity over time, as demonstrated by the aged samples. There was also concern that the aliphatic chain length may promote considerable toxicity of mammalian tissues/cells. Therefore, DC11MAF and C2DC11MAF, which contain a polymerizable methacrylate group, were synthesized to allow copolymerization with methyl methacrylate (MMA) in the denture base resin. The aliphatic chain length was also reduced from 16 to 11 in an effort to sustain antimicrobial activity while reducing potential mammalian cell cytotoxicity. This strategy was predicted to produce denture base resins with long-lasting surface antimicrobial activity and reduced potential for biological cytotoxicity. Hence, denture base resins containing DC16F, DC11MAF, or C2DC11MAF were carried forward for further testing.
Each of the DABCO-containing denture base resins produced significant antifungal activity, inhibiting C. albicans biofilm surface growth nearly 100%. Interestingly, both DABCO monomer (DC11MAF and C2DC11MAF) denture discs produced larger zones of growth inhibition around the discs than DC16F after 24 h. These results indicate either increased early rates of diffusion of the DABCO monomer drugs out of the denture material or greater diffusion or activity of the drugs within the agar. Overall, these data demonstrate that the DABCO compounds incorporated into denture base resins are capable of diffusing out of the material and exerting more than just surface-associated antifungal activity. Perhaps most important, several drugs in denture discs aged for up to 2 months retained significant contact-dependent antifungal activity. DABCO monomers DC11MAF and C2DC11MAF retained nearly 100% activity, indicating that early initial drug diffusion observed with disc diffusion assays does not continue over a 2-month period, likely due to retention of drug linked to the resin. In contrast, DC16F lost approximately 40% of antifungal activity after 2 months, consistent with the predicted increased chronic leakage from the resin, resulting in reduced surface antimicrobial activity over time. This indicates that synthesizing polymerizable denture base resin monomers linked with antimicrobial drugs is an effective strategy to retain long-term surface antimicrobial activity.
The cytotoxicities of DABCO derivatives were evaluated both for free compounds and for DABCO-containing denture base resins. Free DABCO compounds exhibited varying levels of cytotoxicity for mammalian oral cells, including PDL cells, GF, and oral epithelial cells. DC16F had the highest toxicity. A possible reason may be that DC16F has a longer aliphatic chain (C16) and thus higher lipophilicity than DC11MAF and C2DC11MAF. Nevertheless, the DABCO derivatives compared favorably to the control, BisGMA, which is used as a standard comparative compound for oral cell cytotoxicity (22). BisGMA, which is FDA approved, showed some cytotoxicity against PDL cells and GF at high concentrations. Derivatives with similar or lower cytotoxicity toward the same cells would be considered favorable for clinical use. Of the three free derivatives tested against PDL cells and GF, DC16F and C2DC11MAF were shown to be more toxic than BisGMA while DC11MAF was less toxic, suggesting that DC11MAF would have the highest potential for use in oral applications. However, different results were observed with DABCO-containing denture base resins. Again, varying levels of cytotoxicity against PDL cells and GF were evident, with C2DC11MAF exhibiting the lowest cytotoxicity at 2 and 4 wt%. In general, the denture base resin disc containing 2% DABCO derivatives resulted in higher oral cell survival (less cytotoxicity) than those containing the same compounds at 4%. These results demonstrate the importance of cytotoxicity testing using drug-containing materials.
Interestingly, none of the free DABCO derivatives, with the exception of DC16F, showed cytotoxicity toward oral epithelial cells at the highest concentration tested (32 μg/ml). Similarly, none of the derivatives in disc form showed any toxicity to oral epithelial cells. The oral epithelial cells used in these assays were collected from saliva. Thus, the cells were predominantly in a fully differentiated state with little to no metabolic activity. An alternative would be to use immortalized cell lines or reconstituted epithelium, which may not accurately reflect primary cell responses. Another alternative is preparing primary epithelial cell cultures from biopsy specimens, but this poses ethical concerns and may lead to variability. However, it is expected that the superficial layers of epithelium, dominated by fully differentiated, metabolically inactive cells, would receive the most exposure to any dental materials. Hence, use of these primary cells for cytotoxicity studies is justified, and the low cytotoxicity of DABCO compounds toward these cells is a clinically relevant observation.
Overall, considering the MIC assays, denture disc antifungal activity, and free-drug and drug disc cytotoxicity, DC11MAF and C2DC11MAF monomers are the most effective compounds with the strongest antifungal activity and the least mammalian cell cytotoxicity. Although DC11MAF had the lowest cytotoxicity in free form, C2DC11MAF had the lowest cytotoxicity in discs. DC11MAF is clearly effective against Candida at concentrations lower than that which caused up to 20% toxicity to PDL cells and GF (4 versus 8 μg/ml). On the other hand, C2DC11MAF is toxic to PDL cells and GF at the MIC90 (4 μg/ml) but only moderately toxic to the same cells in disc form (<25% toxicity) at the highest wt% tested (4%). Since the exposure of mammalian tissue to the discs will likely not reach the MIC90, C2DC11MAF remains a viable candidate. In contrast, DC16F had the least antifungal activity overall and the highest cytotoxicity. More importantly, the methacrylate monomers DC11MAF and C2DC11MAF are copolymerized with MMA in the denture base materials during the heat cure process to provide the materials with antimicrobial activity upon contact, with minor short-term leakage and long-term stability otherwise. As a result, the concentration of free monomers that leached out from the cured denture materials that allowed a zone of inhibition in agar was minimal compared to those of other free DABCO compounds. Therefore, they are expected to have lower cytotoxicity than other free DABCO compounds. Based on these findings, DC11MAF and C2DC11MAF would be strong candidates for further testing for efficacy and cytotoxicity in animal models. We established a contemporary rat model of Candida-associated denture stomatitis using a custom-fitted two-part denture system with fixed and removable portions (3, 5). Colonization and tissue damage can be monitored longitudinally in this model; hence, the model is ideal for evaluating the effects of DABCO derivatives in denture material on infection, as well as the host. The efficacy of aged discs containing both DC11MAF and C2DC11MAF suggests that long-term antifungal effects might be expected.
Recently, another group of DABCO derivatives were reported to have antimicrobial activity (21). Light-activated phenylene ethylene (PE)-DABCO polymers and oligomers were shown to have activity against Gram-positive and -negative bacteria, as well as C. albicans. The oligomer form of PE-DABCO had less activity against C. glabrata when tested along with C. albicans and C. parapsilosis (21). The DABCO derivatives tested in the present study (LSUHSC DABCO) do not require light activation and are equally effective against several Candida species either in the form of free compounds or incorporated into denture base resin polymers. Nearly all denture base materials are heat cured (100°C under nitrogen at 6 × 106 Pa), and light curing is used only for repairing damaged dentures. Therefore, the LSUHSC DABCO derivatives are more suitable for applications in antifungal denture base materials than the PE-DABCO derivatives. Cytotoxicity studies were not conducted with the PE-DABCO compounds; however, the polymer form apparently had no direct cytotoxic effect on HEK-293 cells, which suggests potentially low cytotoxicity (21). The polymer form also caused unmasking of β-glucan in the cell wall of C. albicans, which promoted Dectin-1 mediated phagocytosis in a light-independent manner. It will be interesting to determine if similar properties are identified for DC16F, DC11MAF, and C2DC11MAF. The mechanism of antimicrobial action of the LSUHSC DABCO derivatives is likely cell membrane disruption through the interaction between the positively charged DABCO molecule and the negatively charged fungal membrane, similar to the PE-DABCO polymers and oligomers (21). The antimicrobial activities generally increase with the increasing length of the aliphatic chain (and thus the lipophilicity).
Several other experimental antimicrobial denture materials have been developed, including a denture base resin containing dimethylaminododecyl methacrylate (DMADDM) (23). DMADDM is a quaternary ammonium salt (QAS) that can be copolymerized with acrylic denture base resin. Acrylic denture bases containing 1.65%, 3.3%, and 6.6% DMADDM reduced C. albicans CFU by 53%, 80 to 90%, and 97%, respectively. Biofilm biomass was also reduced; however, even at the level of 6.6% DMADDM, significant amounts of microbial mass remained (106 C. albicans CFU/ml and ∼108 total CFU), indicating limited inhibitory activity against fungal biofilms. In contrast, the DABCO derivatives developed and tested in these studies resulted in nearly 100% inhibition against C. albicans at 2 wt% or 4 wt%. The reason for the apparently higher antimicrobial effects of DABCO derivatives may result from the structure of DABCO (containing two nitrogens), which results in two covalent quaternary cations (e.g., in C2DC11MAF) or one covalent quaternary cation plus a hydrogen-bonded quaternary cation through the protonation of the tertiary amine at the terminus. This higher charge density resulting from doubly charged DABCO derivatives would be expected to have stronger antimicrobial efficacy than DMADDM or other QAS that are singly charged.
Overall, this study represents the first evidence of antifungal activity of DABCO derivatives in denture materials. Preliminary studies have also indicated broad-spectrum activity against cariogenic oral bacterial species, including Streptococcus mutans and Lactobacillus casei (Z. Wen, personal communication). Future studies will address more specifically the mechanism of action and whether drug resistance is a potential issue. DC11MAF and C2DC11MAF are the strongest DABCO candidates to move forward into additional biological testing. If the results of further testing are favorable, the hope is that these compounds can be tested in clinical trials and can ultimately be used as preventive or therapeutic agents against Candida-associated denture stomatitis.
MATERIALS AND METHODS
Synthesis of DABCO derivatives.
DC16 and DC18 were synthesized by reaction of DABCO (5.695 g; 51 mmol) with cetyl bromide (21.3738 g; 70 mmol) and octodecyl bromide (23.3373 g; 60 mol) (see Fig. S1 in the supplemental material, reactions 1 and 2, respectively). Ethyl acetate was used as a solvent, and the mixture was stirred at room temperature for 24 h, filtered, washed, and dried (24). C6DC16 was synthesized by reaction of DC16 with 1-bromohexane (see Fig. S1 in the supplemental material, reaction 3) (25, 26). DC16 and DC18 were further converted to fluoride (DC16F and DC18F) by reaction with aqueous silver(I) fluoride (see Fig. S1 in the supplemental material, reaction 4).
DC11MAF was synthesized as follows (see Fig. S1 in the supplemental material, reaction 5). A 250-ml round-bottom flask equipped with a magnetic stirring bar was charged with 11-bromoundecan-1-ol (10 g; 40 mmol), DABCO (10 g; 40 mmol), and ethyl acetate (120 ml). The reaction mixture was stirred at room temperature overnight. The resulting white solid was precipitated, washed with cold ethyl acetate, and dried in a vacuum oven to give 9.9 g (34 mmol), providing an 87.5% yield. ES-MS (electrospray-mass spectrometry) analysis (in MeOH; positive ions) was as follows: m/z = 283.0, (M+H)+, calculated 283.2749. The intermediate synthesized as described above (8.49 g; 30 mmol) and dichloromethane (100 ml) were added to a 250-ml round flask and cooled at 0°C for 15 min. Methacryloyl chloride (31 mmol) was added dropwise via a syringe over 10 min. The reaction mixture was stirred at 0°C for 2 h and at room temperature overnight. The reaction was quenched by adding saturated aqueous K2CO3 (150 ml). The aqueous layer was extracted with dichloromethane (30 ml, 3 times). The combined organic extract was washed sequentially with saturated aqueous NaHCO3 (60 ml, 2 times) and brine (100 ml, 2 times), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure with a rotary evaporator. The crude product was purified on a silica gel column using a 3:1 ratio of CH3COOC2H5 and CH3OH as a mobile phase. The purified product (10.75 g; 25 mmol) represented a yield of 83%. ES-MS analysis (in MeOH; positive ions) was as follows: m/z = 351.0, calculated 351.3006. DC11MABr was converted to DC11MAF by reaction with AgF (aq).
C2DC11MAF was synthesized by reaction of ethyliodide with DC11MABr using acetonitrile as a solvent, reflexed for 3 to 5 days until the reaction was finished, and confirmed by thin-layer chromatography (TLC) (see Fig. S1 in the supplemental material, reaction 6). The product was purified using the method described above. ES-MS analysis (in MeOH, positive ions) was as follows: m/z = 379.2, calculated 380.34. The C2DC11MABr was further reacted with AgF (aq) to be converted to C2DC11MAF.
Fabrication of denture base resin discs.
Denture base resins were prepared by adding 1 wt%, 2 wt%, or 4 wt% DABCO derivatives to the liquid monomer component of the denture base material (Ivocap High Impact; Ivoclar Vivadent) and then mixing it with the powder component. The disc samples (11.0-mm diameter; 2.0 mm thick) of the experimental denture base resins were fabricated by injection of the mixed liquid and powder into a flask containing a gypsum mold with negative replicas of the discs (n = 12) and heat cured (under 100°C; nitrogen at 6 × 106 Pa) for 45 min. After cooling to room temperature, the discs were removed from the flask and polished with 600 grit SiC papers. The discs were sterilized by UV irradiation (302 nm for 2 min on each side) without losing antimicrobial activity because the UV irradiation did not change the composition of the material.
Fungal strains and handling.
The Candida isolates tested included C. albicans, C dubliniensis, C. glabrata, C. parapsilosis, and C. tropicalis. C. albicans strain DAY185 is a prototrophic control strain derived from the parent strain SC5314. C. dubliniensis strain 962926 and C. glabrata strain LF574.92 (also referred to as BG2) are vaginal isolates from a recurrent vulvovaginal candidiasis (VVC) patient and were provided by J. Sobel at Wayne State University School of Medicine. C. tropicalis and C. parapsilosis were obtained from the American Type Culture Collection (ATCC) (C. tropicalis, ATCC 44508; C. parapsilosis, ATCC 90018). Frozen stocks of fungal strains were kept at −80°C and streaked onto Sabouraud dextrose agar (SDA) (Becton, Dickinson and Company). A single colony was transferred to 5 ml of yeast extract-peptone-dextrose (YPD) broth and incubated with shaking at 30°C for 24 h. All the isolates were washed 3 times in sterile phosphate-buffered saline (PBS), enumerated using a hemocytometer, and diluted to final working concentrations as described for each assay.
MIC/MFC assay.
The CLSI reference method for broth dilution antifungal susceptibility testing of yeasts (M27-A3) was used to determine the MIC (27). Isolates were diluted in sterile RPMI 1640 medium to a final concentration of 2.5 × 103 CFU/ml, and 100-μl aliquots were added to wells of sterile round-bottom 96-well plates. Stock solutions of DABCO compounds were diluted in sterile RPMI 1640 prior to adding them to the wells. Drug concentrations ranged from 0.008 to 8 μg/ml and were tested in triplicate. The plates were incubated at 37°C for 24 h and examined for growth. Fungicidal or fungistatic activity was assessed by plating MIC and near-MIC wells on YPD agar at 37°C for 48 h and assessing growth. Controls included clotrimazole (0.004 to 4 μg/ml) and fluconazole (0.062 to 64 μg/ml). Minimal fungicidal concentrations (MFCs) were determined according to the method of Canton et al. (28). Briefly, the MFCs were evaluated by transferring 50 μl from all the clear MIC wells (with no growth seen in microdilution trays) onto SDA plates and incubating them at 37°C for 72 h. The MFC was the lowest drug concentration that killed ≥99.9% of the cells.
XTT assay.
The XTT reduction assay was used to determine the metabolic activity (viability) of fungal biofilms grown on denture discs, as previously described (29). Conversion of the XTT substrate to a soluble colored formazan product correlates with cell viability. Isolates were diluted in sterile RPMI 1640 medium to a final concentration of 1 × 106 CFU/ml. Denture base resin discs with and without DABCO were inoculated with 1 ml C. albicans for 2 h to allow fungal adherence. The discs were washed in 1× PBS to remove nonadherent fungi, transferred to fresh RPMI 1640 broth, and incubated overnight at 37°C to allow biofilm growth. After gentle washing in 1× PBS, the denture discs were aseptically transferred to a 24-well plate, and 750 μl of freshly prepared XTT solution (0.5 mg/ml XTT, 1 μM menadione) was added to each well. The plate was covered and allowed to incubate for 45 min at 37°C. Following incubation, 100 μl of the reaction solution was added to a fresh 96-well plate, and the absorbance was measured at 490 nm using a microplate reader (VersaMax; Molecular Devices). The data are represented as percent inhibition compared to the drug-free control.
Disc diffusion assay.
The disc diffusion assay was used to measure antifungal drug diffusion from denture base resin discs. Isolates were diluted in sterile PBS to a final concentration of 1 × 106 CFU/ml, and 100 μl of the cell suspension was spread onto the surfaces of YPD agar plates to produce confluent fungal growth. The plates were incubated at room temperature for approximately 1 h to dry sufficiently. Control denture discs or those containing DABCO derivatives DC16F, DC11MAF, and C2DC11MAF (n = 3) were placed in the centers of the agar plates and incubated at 37°C for 24 h. Following incubation, the plates were photographed using a charge-coupled-device (CCD) imager, and the clear zones of inhibition surrounding the discs were measured to the nearest whole millimeter and averaged for three replicates.
Cell cytotoxicity assay.
PDL cells and GF were originally established from patients with healthy gingiva who underwent oral surgery at the Louisiana State University School of Dentistry. In all cases, tissues were obtained from subjects following informed consent as prescribed in an approved institutional review board protocol. The cells were maintained in minimum essential medium alpha (MEMα) containing 10% fetal calf serum (FCS), 200 units/ml of penicillin, and 200 μg/ml streptomycin (Gibco, Thermo Fisher Scientific), and frozen stocks were prepared. Cells from frozen stocks were used between the 12th and 18th passages. Oral epithelial cells were collected from healthy volunteers following informed consent and in accordance with the guidance of the institutional review board at LSUHSC. Unstimulated saliva (10 ml) was expectorated into sterile polypropylene test tubes and centrifuged. The cell pellet was passed over a 20-μm sterile nylon membrane, and the cells retained on the membrane were collected. Enriched oral epithelial cells were enumerated by trypan blue dye exclusion.
For the cytotoxicity assay, cells (5 × 104) were seeded, allowed to adhere to 24-well plates for 24 h, and exposed to compounds (DC16F, DC11MAF, and C2DC11MAF) in solution for 24 h. BisGMA, which is cytotoxic but is also a component of many commercially available dental materials due to its reduced cytotoxicity upon polymerization, was used as a comparative control compound (22). Alternatively, discs containing compounds were added to the surfaces of cell monolayers and incubated for 24 h. After exposure to the cells for 24 h, the discs were manually removed, and the remaining cells attached to the wells were assayed for survival. In the case of epithelial cells, the cultures were performed in 15-ml tubes with discs (n = 3). All cultures were performed in MEMα containing 10% FCS and 1% penicillin-streptomycin. After exposure, the discs were removed (if applicable), and cell survival was assessed using calcein AM (a nonfluorescent esterase substrate readily taken up by live cells; cleaved by endogenous esterases and rendered fluorescent) for 1 h. In the case of epithelial cell cultures, cell suspensions were harvested, washed, and resuspended in 1 ml of PBS containing calcein AM. Cell survival was quantified using a BioTek Synergy2 fluorescent multiwell plate reader and expressed as percent survival compared to untreated cells/discs. For both cultured cell lines (PDL cells and GF) and primary cells (oral epithelial cells), the fluorescence values for untreated cells ranged from 20,000 to 25,000 (with a background of 800). Those for treated cells with the highest cell death at the highest concentrations were ∼1,200. Sensitivity was set at 70 (in a range from 25 to 255), which is moderate to low sensitivity.
Statistical analysis of data.
All statistics were performed using GraphPad Prism software. Multiple groups were analyzed by one-way analysis of variance (ANOVA) plus post hoc tests (Tukey). Where two groups were tested (control versus experimental), an unpaired Student t test was used. Significance was defined as a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the financial support of NIH/NIDCR grants R01DE019203 (X. Xu) and R01DE022069 (M. Noverr).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02575-16.
REFERENCES
- 1.Gleiznys A, Zdanaviciene E, Zilinskas J. 2015. Candida albicans importance to denture wearers. A literature review. Stomatologija 17:54–66. [PubMed] [Google Scholar]
- 2.Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. 1998. Candida-associated denture stomatitis. Aetiology and management: a review. Part 3. Treatment of oral candidosis. Aust Dent J 43:244–249. [DOI] [PubMed] [Google Scholar]
- 3.Yano J, Yu A, Fidel PL Jr, Noverr MC. 2016. Transcription factors Efg1 and Bcr1 regulate biofilm formation and virulence during Candida albicans-associated denture stomatitis. PLoS One 11:e0159692. doi: 10.1371/journal.pone.0159692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res 80:903–908. doi: 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CC, Yu A, Lee H, Fidel PL Jr, Noverr MC. 2012. Development of a contemporary animal model of Candida albicans-associated denture stomatitis using a novel intraoral denture system. Infect Immun 80:1736–1743. doi: 10.1128/IAI.00019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nett JE, Marchillo K, Spiegel CA, Andes DR. 2010. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun 78:3650–3659. doi: 10.1128/IAI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwivedi P, Thompson A, Xie Z, Kashleva H, Ganguly S, Mitchell AP, Dongari-Bagtzoglou A. 2011. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS One 6:e16218. doi: 10.1371/journal.pone.0016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katragkou A, Kruhlak MJ, Simitsopoulou M, Chatzimoschou A, Taparkou A, Cotten CJ, Paliogianni F, Diza-Mataftsi E, Tsantali C, Walsh TJ, Roilides E. 2010. Interactions between human phagocytes and Candida albicans biofilms alone and in combination with antifungal agents. J Infect Dis 201:1941–1949. doi: 10.1086/652783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katragkou A, Simitsopoulou M, Chatzimoschou A, Georgiadou E, Walsh TJ, Roilides E. 2011. Effects of interferon-gamma and granulocyte colony-stimulating factor on antifungal activity of human polymorphonuclear neutrophils against Candida albicans grown as biofilms or planktonic cells. Cytokine 55:330–334. doi: 10.1016/j.cyto.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Bissell V, Felix DH, Wray D. 1993. Comparative trial of fluconazole and amphotericin in the treatment of denture stomatitis. Oral Surg Oral Med Oral Pathol 76:35–39. doi: 10.1016/0030-4220(93)90290-K. [DOI] [PubMed] [Google Scholar]
- 11.Ellepola AN, Samaranayake LP. 2000. Oral candidal infections and antimycotics. Crit Rev Oral Biol Med 11:172–198. doi: 10.1177/10454411000110020301. [DOI] [PubMed] [Google Scholar]
- 12.Malakhov A, Wen J, Zhang BX, Wang H, Geng H, Chen XD, Sun Y, Yeh CK. 2016. Rechargeable anticandidal denture material with sustained release in saliva. Oral Dis 22:391–398. doi: 10.1111/odi.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villar CC, Lin AL, Cao Z, Zhao XR, Wu LA, Chen S, Sun Y, Yeh CK. 2013. Anticandidal activity and biocompatibility of a rechargeable antifungal denture material. Oral Dis 19:287–295. doi: 10.1111/odi.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Z, Sun X, Yeh CK, Sun Y. 2010. Rechargeable infection-responsive antifungal denture materials. J Dent Res 89:1517–1521. doi: 10.1177/0022034510379604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueno MG, Urban VM, Barberio GS, da Silva WJ, Porto VC, Pinto L, Neppelenbroek KH. 2015. Effect of antimicrobial agents incorporated into resilient denture relies on the Candida albicans biofilm. Oral Dis 21:57–65. doi: 10.1111/odi.12207. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Xu X, Wang Y, Zhang J-F, Hamdan S, Peters BM, Fidel PL Jr, Noverr MC. 2015. Synthesis and characterization of antifungal compounds and denture base materials. J Dent Res 94 (special issue A):2818. [Google Scholar]
- 18.Herman JL, Wang Y, Lallier TE, Hamdan S, Xu X, Fidel PL Jr, Noverr MC. 2016. Antifungal activity of novel synthesized antimicrobial compounds solubilized and incorporated into denture base resins, abstr 153, p 187 13th ASM Conference on Candida and Candidiasis, Seattle, WA. [Google Scholar]
- 19.Herman JL, Wang Y, Peters BM, Hamdan S, Xu X, Fidel PL Jr, Noverr MC. 2015. Antifungal properties of experimental antimicrobial compounds and denture resins. J Dent Res 94 (special Issue A):2157. [Google Scholar]
- 20.Xu X, Wang Y, Herman JL, Maldonado K, Hamdan S, Fidel PL Jr, Noverr MC. 2016. Formulation and characterization of novel antimicrobial denture base materials, abstr 2746, p 2657 10th World Biomaterials Congress, Montreal, Canada. [Google Scholar]
- 21.Pappas HC, Sylejmani R, Graus MS, Donabedian PL, Whitten DG, Neumann AK. 2016. Antifungal properties of cationic phenylene ethynylenes and their impact on beta-glucan exposure. Antimicrob Agents Chemother 60:4519–4529. doi: 10.1128/AAC.00317-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soderholm KJ, Mariotti A. 1999. BIS-GMA-based resins in dentistry: are they safe? J Am Dent Assoc 130:201–209. doi: 10.14219/jada.archive.1999.0169. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Ren B, Zhou X, Xu HH, Chen Y, Han Q, Li B, Weir MD, Li M, Feng M, Cheng L. 2016. Effect of antimicrobial denture base resin on multi-species biofilm formation. Int J Mol Sci 17:E1033. doi: 10.3390/ijms17071033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dizman B, Elasri MO, Mathias LJ. 2005. Synthesis, characterization, and antibacterial activities of novel methacrylate polymers containing norfloxacin. Biomacromolecules 6:514–520. doi: 10.1021/bm049383+. [DOI] [PubMed] [Google Scholar]
- 25.Engel R, Rizzo JI, Rivera C, Ramirez M, Huang ML, Montenegro D, Copodiferro C, Behaj V, Thomas M, Klaritch-Vrana B, Engel JF. 2009. Polycations. 18. The synthesis of polycationic lipid materials based on the diamine 1,4-diazabicyclo[222]octane. Chem Phys Lipids 158:61–69. doi: 10.1016/j.chemphyslip.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Thomas M, Montenegro D, Castano A, Friedman L, Leb J, Huang ML, Rothman L, Lee H, Capodiferro C, Ambinder D, Cere E, Galante J, Rizzo J, Melkonian K, Engel R. 2009. Polycations. 17. Synthesis and properties of polycationic derivatives of carbohydrates. Carbohydr Res 344:1620–1627. doi: 10.1016/j.carres.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 27.CLSI. 2008. Reference methods for broth dilution antifungal susceptibility testing of yeast; approved standard, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Canton E, Peman J, Gobernado M, Viudes A, Espinel-Ingroff A. 2004. Patterns of amphotericin B killing kinetics against seven Candida species. Antimicrob Agents Chemother 48:2477–2482. doi: 10.1128/AAC.48.7.2477-2482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun 70:878–888. doi: 10.1128/IAI.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


