ABSTRACT
Nephrotoxicity is the primary adverse effect of the polymyxins. The relative rates of toxicity of polymyxin B and colistin have not been fully elucidated, especially in patients with cystic fibrosis (CF). A retrospective cohort study of adults treated with polymyxin B or colistin for at least 48 h was conducted. The primary endpoint was the incidence of kidney injury assessed by RIFLE (i.e., risk, injury, failure, loss, end-stage renal disease) criteria. Risk factors for kidney injury were evaluated using multivariate Cox regression. A total of 414 patients were evaluated, 220 of whom had CF. In patients without CF, there was no difference in kidney injury with polymyxin B and colistin (42.9% versus 50.3%, P = 0.46). Loop diuretic exposure was a risk factor for kidney injury (adjusted hazard ratio [aHR], 1.82; 95% confidence interval [CI], 1.16 to 2.83) in this population. In patients with CF, polymyxin B and colistin were associated with similar rates of kidney injury (34.5% versus 29.8%, P = 0.77). Diabetes (aHR, 2.68; 95% CI, 1.01 to 7.11), loop diuretics (aHR, 3.02; 95% CI, 1.36 to 6.73), and progressive care unit admission (aHR, 8.21; 95% CI, 2.55 to 26.46) were risk factors for kidney injury, while higher baseline serum creatinine levels (per 1 mg/dl) were protective (aHR, 0.08; 95% CI, 0.01 to 0.48). Total unadjusted kidney injury in polymyxin-treated patients was less frequent in those who had CF (30.5% versus 48.5%, P < 0.001). Polymyxin B and colistin are associated with a high incidence of kidney injury; cystic fibrosis may be protective against polymyxin nephrotoxicity, but further investigation is needed to confirm this conjecture.
KEYWORDS: clinical therapeutics, multidrug resistance, pharmacokinetics, pharmacology, toxicity
INTRODUCTION
The increasing prevalence of multidrug-resistant (MDR) Gram-negative bacteria coupled with the lack of novel agents in the drug development pipeline has left clinicians with a rapidly dwindling armamentarium of effective antimicrobials (1, 2). Despite clinical use beginning over half a century ago, the polymyxin class of antibiotics retains activity against many MDR pathogens—namely, carbapenem-resistant Enterobacteriaceae (CRE), Pseudomonas aeruginosa, and Acinetobacter baumannii (3).
There are two systemically available agents in the polymyxin class: colistin (polymyxin E) and polymyxin B (PMB). While the pharmacologically active moieties are structurally similar, colistin is administered intravenously as a prodrug—colistimethate sodium (CMS) (3). Recent pharmacokinetic data suggest that conversion from colistimethate to pharmacologically active colistin exhibits high interpatient variability and is affected by the proportion of prodrug lost to renal elimination prior to activation (4–7). Polymyxin B, on the other hand, is administered directly as an active drug and undergoes only minimal renal elimination. These properties provide a compelling pharmacokinetic rationale for the use of PMB over CMS in the absence of differential toxicity (8).
For the antimicrobials which fell out of favor largely due to high rates of kidney injury, the issue of the relative levels of toxicity of the two agents has been investigated only recently. Historical rates of nephrotoxicity are confounded by variable and insensitive definitions of acute kidney injury (AKI) as well as by inadequate dosing by current standards. Recent toxicity studies suggested a higher incidence of nephrotoxicity with CMS than with PMB; however, this has not been consistently demonstrated (9–12).
The effect of underlying diseases which may alter the pharmacokinetic properties of the polymyxins has also been poorly investigated. Patients with cystic fibrosis (CF) have a high burden of MDR Gram-negative infections and may require treatment with polymyxins, and yet there are a paucity of pharmacokinetic and toxicity data in this patient population (13, 14). Phe and colleagues found CF to be a factor that is protective against the development of nephrotoxicity with CMS but were precluded from conducting a similar analysis with PMB due to a lack of baseline CF in the PMB cohort (12). Additionally, to our knowledge the relative rates of toxicity of the two systemic polymyxins have not been investigated in this patient population. We hypothesized that CMS would be more nephrotoxic than PMB in patients without cystic fibrosis and that this relationship would be reversed in patients with CF owing to differences in drug elimination. The objective of this study was to determine the incidence of acute kidney injury in patients with and without cystic fibrosis treated with colistin or polymyxin B.
RESULTS
Non-cystic fibrosis patients. (i) Patient characteristics.
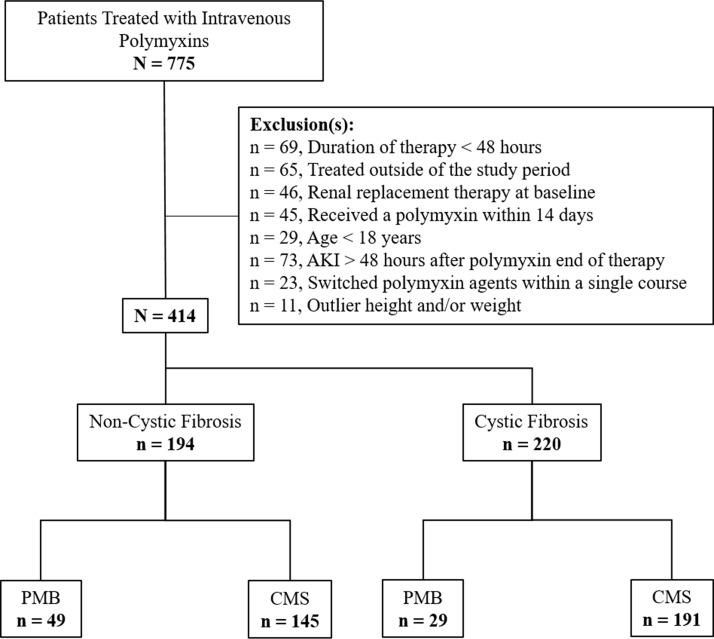
In total, 194 patients were included in the non-cystic fibrosis cohort (Fig. 1), 49 (25.3%) of whom received PMB and 145 (74.7%) of whom received CMS. Patients treated with PMB were older (57 [interquartile range {IQR}, 50 to 67] versus 51 [IQR, 37 to 62] years of age, P = 0.006) and more likely to be Caucasian (98.0% versus 84.1%, P = 0.02) than those treated with CMS. The median baseline serum creatinine levels were 1.3 mg/dl (IQR, 0.7 to 2.3 mg/dl) and 1.1 mg/dl (IQR, 0.7 to 2.0 mg/dl; P = 0.33) in the PMB and CMS groups, respectively. Additional baseline characteristics are summarized in Table 1.
FIG 1.
Enrollment and exclusion. Abbreviations: AKI, acute kidney injury; PMB, polymyxin B; CMS, colistimethate sodium.
TABLE 1.
Baseline characteristics of all patientsa
| Parameter | Value(s) |
|||||
|---|---|---|---|---|---|---|
| Non-cystic fibrosis patients |
Cystic fibrosis patients |
|||||
| PMB (n = 49) |
CMS (n = 145) |
P | PMB (n = 29) |
CMS (n = 191) |
P | |
| Demographics | ||||||
| Age (yrs) | 57 (50–67) | 51 (37–62) | 0.006 | 27 (22–31) | 23 (20–30) | 0.03 |
| Male sex | 31 (63.3%) | 92 (63.5%) | 1.00 | 13 (44.8%) | 82 (42.9%) | 1.00 |
| Caucasian | 48 (98.0%) | 122 (84.1%) | 0.02 | 29 (100.0%) | 191 (100.0%) | |
| CCI | 5 (3–7) | 5 (3–8) | 0.70 | 4 (2–6) | 4 (3–5) | 0.61 |
| Hypertension | 33 (67.4%) | 93 (64.1%) | 0.82 | 15 (51.7%) | 73 (38.2%) | 0.24 |
| Diabetes | 27 (55.1%) | 68 (46.9%) | 0.41 | 20 (69.0%) | 152 (79.6%) | 0.29 |
| ABW (kg) | 83.0 (74.8–99.3) | 80.3 (64.2–99.6) | 0.25 | 55.0 (50.7–59.0) | 51.3 (45.3–56.2) | 0.02 |
| IBW (kg) | 66.1 (57.0–75.3) | 68.4 (54.7–75.3) | 0.73 | 59.3 (50.1–70.7) | 57.0 (49.5–66.1) | 0.32 |
| Site of care | ||||||
| ICU | 19 (38.8%) | 44 (30.3%) | 3 (10.3%) | 8 (4.2%) | ||
| PCU | 10 (20.4%) | 51 (35.2%) | 0.15 | 2 (6.9%) | 14 (7.3%) | 0.33 |
| Acute | 20 (40.8%) | 50 (34.5%) | 24 (82.8%) | 169 (88.5%) | ||
| Clinical baseline | ||||||
| Scr (mg/dl) | 1.3 (0.7–2.3) | 1.1 (0.7–2.0) | 0.33 | 0.8 (0.6–0.9) | 0.7 (0.6–0.8) | 0.01 |
| CrCL (ml/min)b | 63 (27–100) | 74 (37–125) | 0.16 | 135 (114–158) | 158 (128–188) | 0.01 |
| Serum albumin (mg/dl) | 2.1 (1.7–2.6) | 2.3 (1.7–2.8) | 0.27 | 2.8 (2.4–3.1) | 2.9 (2.6–3.2) | 0.34 |
| Mechanical ventilation | 22 (44.9%) | 82 (56.6%) | 0.19 | 2 (6.9%) | 1 (0.5%) | 0.046 |
| Concurrent nephrotoxinsc | ||||||
| Aminoglycosides | 12 (24.5%) | 50 (34.5%) | 0.26 | 12 (41.4%) | 34 (17.8%) | 0.008 |
| Amphotericin B | 1 (2.0%) | 8 (5.5%) | 0.45 | |||
| Vancomycin | 24 (49.0%) | 96 (66.2%) | 0.048 | 10 (34.5%) | 50 (26.2%) | 0.48 |
| Calcineurin inhibitors | 3 (6.1%) | 15 (10.3%) | 0.57 | 9 (31.0%) | 13 (6.8%) | <0.001 |
| Intravenous contrast | 4 (8.2%) | 3 (2.1%) | 0.07 | 1 (3.5%) | 2 (1.1%) | 0.35 |
| Loop diuretics | 21 (42.9%) | 70 (48.3%) | 0.62 | 7 (24.1%) | 19 (10.0%) | 0.06 |
| NSAIDS | 1 (2.04%) | 18 (12.4%) | 0.048 | 3 (10.3%) | 22 (11.5%) | 1.00 |
| Vasopressors | 11 (22.5%) | 41 (28.3%) | 0.54 | 3 (10.3%) | 0 (0%) | 0.002 |
Abbreviations: PMB, polymyxin B; CMS, colistimethate sodium; CCI, Charlson comorbidity index; ABW, actual body weight; IBW, ideal body weight; ICU, intensive care unit; PCU, progressive care unit; Scr, serum creatinine; CrCL, creatinine clearance; NSIADs, nonsteroidal anti-inflammatory drugs. Values (other than P values) are presented as mean (± standard deviation [SD]), n (%), or median (IQR).
Data were calculated using the modified Cockroft-Gault equation: CrCL = [(140 − age in years)/(Scr value)] (× 0.85 for female subjects).
Concurrent use is defined as administration within 24 h prior to the first polymyxin dose through the end of polymyxin therapy.
(ii) Characteristics of polymyxin therapy.
Characteristics of therapy with PMB and CMS are summarized in Table 2. Patients were much more likely to receive a loading dose with PMB than with CMS (73.5% versus 13.8%, P < 0.001). Concurrent antibiotic exposure was seen in all patients in both treatment arms. The median durations of polymyxin therapy were 5 days (IQR, 4 to 10) with PMB and 7 days (IQR, 3 to 13) with CMS (P = 0.44).
TABLE 2.
Characteristics of polymyxin therapy in all patientsa
| Parameter | Value(s) |
|||||
|---|---|---|---|---|---|---|
| Non-cystic fibrosis patients |
Cystic fibrosis patients |
|||||
| PMB (n = 49) |
CMS (n = 145) |
P | PMB (n = 29) |
CMS (n = 191) |
P | |
| Loading dose used | 36 (73.5%) | 20 (13.8%) | <0.001 | 11 (37.9%) | 4 (2.1%) | <0.0001 |
| Loading dose (mg/kg)bc | 1.9 (±0.5) | 3.5 (±1.0) | 1.6 (±0.3) | 2.9 (±1.5) | ||
| Total daily dose (mg/day)bc | 200.9 (±81.3) | 226.4 (±106.1) | 124.4 (±34.2) | 213.5 (±67.5) | ||
| Total daily dose (mg/kg/day)bc | 2.3 (±0.7) | 3.4 (±1.6) | 2.2 (±0.4) | 4.1 (±1.1) | ||
| Duration of therapy (days) | 5 (4–10) | 7 (3–13) | 0.44 | 8 (5–21) | 8 (6–13) | 0.53 |
| Concurrent antibioticsd | 49 (100.0%) | 145 (100.0%) | 29 (100%) | 191 (100%) | ||
Abbreviations: PMB, polymyxin B; CMS, colistimethate sodium. Values (other than P values) are presented as mean (± SD), n (%), or median (IQR).
Dosing data per unit body weight was calculated using actual body weight (ABW) for PMB and ideal body weight (IBW) for CMS. ABW was used for CMS if ABW was less than IBW.
CMS doses are expressed as milligrams of colistin base activity (CBA).
Concurrent use is defined as administration within 24 h prior to the first polymyxin dose through the end of polymyxin therapy.
(iii) Outcomes.
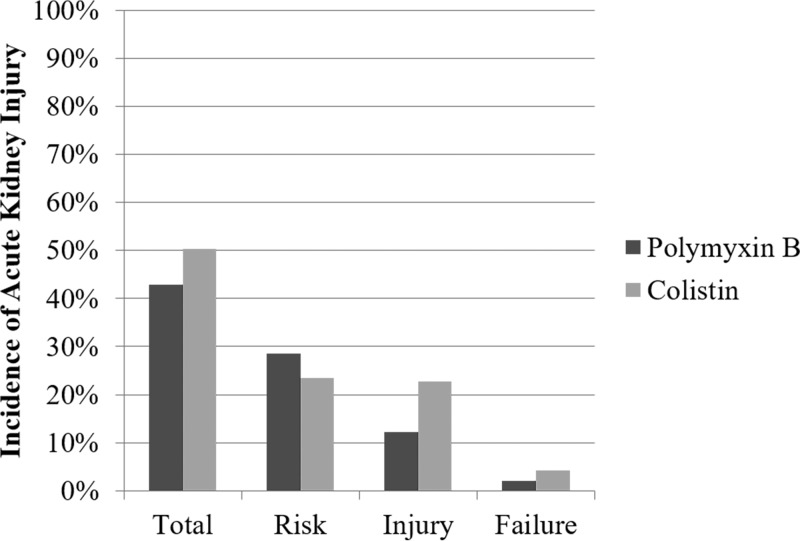
Acute kidney injury occurred in 21/49 (42.9%) of patients treated with PMB compared to 73/145 (50.3%) treated with CMS (P = 0.46; Fig. 2). There were no statistically significant differences between groups for each individual RIFLE stage. The median time to peak serum creatinine was 3 days (IQR, 2 to 6 days) with PMB compared to 6 days (IQR, 3 to 10 days) with CMS (P = 0.03), with no difference in the maximum serum creatinine values obtained (Table 3). Renal recovery occurred in 13/21 (61.9%) of polymyxin B-treated patients and 45/145 (61.6%) of colistin-treated patients by hospital discharge (P = 1.00).
FIG 2.
Acute kidney injury by RIFLE stage in non-cystic fibrosis patients treated with polymyxin B versus colistin. P values were nonsignificant (≥0.05) for all comparisons. Relative number of patients per outcome stratum: Total, PMB = 21/49, CMS = 73/145; Risk, PMB = 14/49, CMS = 34/145; Injury, PMB = 6/49, CMS = 33/145; Failure, PMB = 1/49, CMS = 6/145.
TABLE 3.
Outcomes in all patientsa
| Parameter | Value(s) |
|||||
|---|---|---|---|---|---|---|
| Non-cystic fibrosis patients |
Cystic fibrosis patients |
|||||
| PMB (n = 49) |
CMS (n = 145) |
P | PMB (n = 29) |
CMS (n = 191) |
P | |
| Primary outcome | ||||||
| Acute kidney injury | 21 (42.9%) | 73 (50.3%) | 0.46 | 10 (34.5%) | 57 (29.8%) | 0.77 |
| Secondary outcomes | ||||||
| Toxicity | ||||||
| RIFLE stageb | ||||||
| Risk | 14 (28.6%) | 34 (23.5%) | 0.60 | 7 (24.1%) | 50 (26.2%) | 1.00 |
| Injury | 6 (12.2%) | 33 (22.8%) | 0.17 | 3 (10.3%) | 7 (3.7%) | 0.13 |
| Failure | 1 (2.0%) | 6 (4.1%) | 0.68 | 0 (0%) | 0 (0%) | |
| Time course | ||||||
| Time to peak Scr (days) | 3 (2–6) | 6 (3–10) | 0.03 | 5 (4–15) | 5 (4–9) | 0.56 |
| Peak Scr (mg/dl) | 1.5 (0.9–3.1) | 1.7 (0.9–3.2) | 0.96 | 1.0 (0.7–1.7) | 0.7 (0.6–0.9) | 0.002 |
| Renal recoveryc | 13 (61.9%) | 45 (61.6%) | 1.00 | 9 (90.0%) | 52 (91.2%) | 1.00 |
| Efficacy | ||||||
| In-hospital mortality | 3 (6.1%) | 18 (12.4%) | 0.34 | 1 (3.5%) | 1 (0.5%) | 0.25 |
| 30-day mortality | 6 (12.2%) | 27 (18.6%) | 0.42 | 1 (3.5%) | 2 (1.1%) | 0.35 |
| LOS (days) | 24 (12–35) | 23 (12–51) | 0.65 | 14 (7–28) | 9 (6–14) | 0.008 |
Abbreviations: PMB, polymyxin B; CMS, colistimethate sodium; RIFLE, risk, injury, failure, loss, end-stage renal disease; Scr, serum creatinine; LOS, length of stay. Values (other than P values) are presented as mean (±SD), n (%), or median (IQR).
The stages of the RIFLE criteria are defined as follows: risk (≥25% decrease in CrCL), injury (≥50% decrease in CrCL), and failure (≥75% decrease in CrCL).
Renal recovery was assessed as return to baseline CrCL strata (i.e., ≥80 ml/min, 50 to 79 ml/min, 30 to 49 ml/min, and 10 to 29 ml/min).
There were no statistically significant differences between PMB and CMS in any of the secondary efficacy endpoints (Table 3).
(iv) Risk factors.
In bivariate analysis, factors associated with the development of AKI at a P value of ≤0.2 were high polymyxin dose, loading dose, aminoglycosides, intravenous contrast, loop diuretics, and nonsteroidal anti-inflammatory drugs (NSAIDs). In the multivariate Cox proportional hazard model, concurrent loop diuretic exposure (adjusted hazard ratio [aHR], 1.82 [95% confidence interval {CI}, 1.16 to 2.83]) remained independently predictive of AKI. The choice of polymyxin (PMB versus CMS) was not predictive of AKI (Table 4).
TABLE 4.
Risk factors for the development of acute kidney injury in non-cystic fibrosis patients treated with polymyxin B or colistin: bivariate and multivariate analysesa
| Variable | Value(s) |
|||||
|---|---|---|---|---|---|---|
| Bivariate analysis |
Multivariate analysis |
|||||
| HR | 95% CI | P | aHR | 95% CI | P | |
| Polymyxin formulation | ||||||
| Colistin | Reference value | Reference value | ||||
| Polymyxin B | 0.74 | 0.42–1.31 | 0.31 | 1.07 | 0.56–2.50 | 0.83 |
| Characteristics of polymyxin therapy | ||||||
| High doseb | 2.11 | 1.34–3.33 | 0.001 | 1.26 | 0.80–1.97 | 0.31 |
| Loading dose | 0.67 | 0.39–1.17 | 0.16 | 0.78 | 0.42–1.46 | 0.44 |
| Duration > 10 days | 1.38 | 0.85–2.24 | 0.20 | |||
| Demographics | ||||||
| Age > 60 yrs | 1.30 | 0.80–2.09 | 0.29 | 1.43 | 0.91–2.52 | 0.12 |
| CCI | 1.00 | 0.94–1.07 | 0.97 | |||
| Caucasian | 1.38 | 0.72–2.68 | 0.33 | |||
| Hypertension | 0.80 | 0.50–1.28 | 0.35 | |||
| Diabetes | 1.11 | 0.70–1.74 | 0.66 | |||
| Site of care | ||||||
| Acute | Reference value | |||||
| ICU | 1.11 | 0.63–1.94 | 0.72 | |||
| PCU | 0.56 | 0.20–1.53 | 0.26 | |||
| Concurrent nephrotoxinsc | ||||||
| Aminoglycosides | 1.50 | 0.94–2.37 | 0.09 | 1.22 | 0.80–1.87 | 0.35 |
| Amphotericin B | 1.05 | 0.38–2.90 | 0.92 | |||
| Vancomycin | 0.82 | 0.51–1.30 | 0.40 | 0.72 | 0.45–1.13 | 0.15 |
| Calcineurin inhibitors | 0.89 | 0.40–1.95 | 0.76 | |||
| Intravenous contrast | 0.27 | 0.04–1.97 | 0.20 | 0.25 | 0.03–1.86 | 0.18 |
| Loop diuretics | 2.25 | 1.39–3.65 | <0.001 | 1.81 | 1.16–2.83 | 0.008 |
| NSAIDS | 1.60 | 0.82–3.12 | 0.17 | 1.50 | 0.78–2.90 | 0.23 |
| Vasopressors | 1.35 | 0.84−2.18 | 0.22 | |||
| Laboratory abnormalities | ||||||
| Baseline Scr > 1.5 | 1.08 | 0.66–1.74 | 0.77 | |||
| Albumin | ||||||
| ≥3 g/dl | Reference value | |||||
| <3 g/dl | 0.74 | 0.43–1.28 | 0.28 | |||
| No albumin level | 0.85 | 0.29–2.54 | 0.77 | |||
| Total bilirubin | ||||||
| ≤3 mg/dl | Reference value | |||||
| >3 mg/dl | 0.84 | 0.36–1.96 | 0.69 | |||
| No total bilirubin level | 1.10 | 0.47–2.54 | 0.83 | |||
Abbreviations: PMB, polymyxin B; CMS, colistimethate sodium; OR, odds ratio; CI, confidence interval; aOR, adjusted odds ratio; CCI, Charlson comorbidity index; ICU, intensive care unit; PCU, progressive care unit; NSAIDS, nonsteroidal anti-inflammatory drugs; Scr, serum creatinine. The multivariate Cox proportional hazard model included all variables meeting a significance level of a P value of ≤0.20 in bivariate analysis or that were significantly different between the PMB and CMS cohorts at baseline. Data for polymyxin B relative to CMS were forced into the model. Independent predictors of kidney injury are highlighted in bold.
High polymyxin doses are defined as PMB doses of ≥200 mg/day and CMS doses of ≥270 mg colistin base activity/day.
Concurrent use is defined as administration within 24 h prior to the first polymyxin dose through the end of polymyxin therapy.
Cystic fibrosis patients. (i) Patient characteristics.
Of the 220 patients included in the CF cohort (Fig. 1), 29 (13.2%) were treated with PMB and 191 (86.8%) with CMS. The patients treated with PMB had a higher median actual body weight (ABW) (55.0 kg [IQR, 50.7 to 59.0 kg]) than the patients treated with CMS (51.3 kg [IQR, 45.3 to 56.2 kg]) (P = 0.02) despite similar median ideal body weights (Table 1). Median baseline serum creatinine levels were 0.8 mg/dl (IQR, 0.6 to 0.9 mg/dl) with PMB and 0.7 mg/dl (IQR, 0.6 to 0.8 mg/dl; P = 0.01) with CMS, corresponding to baseline creatinine clearance (CrCL) levels of 135 ml/min (IQR, 114 to 158 ml/min) and 158 ml/min (IQR, 128 to 188 ml/min; P = 0.01), respectively.
(ii) Characteristics of polymyxin therapy.
Table 2 summarizes factors associated with polymyxin therapy in patients with CF. As in the non-cystic fibrosis cohort, loading doses were more commonly employed with PMB (37.9%) than with CMS (2.1%; P < 0.001). The median duration of therapy was 8 days in both groups (P = 0.53), and concurrent antibiotics were used in all patients.
(iii) Outcomes.
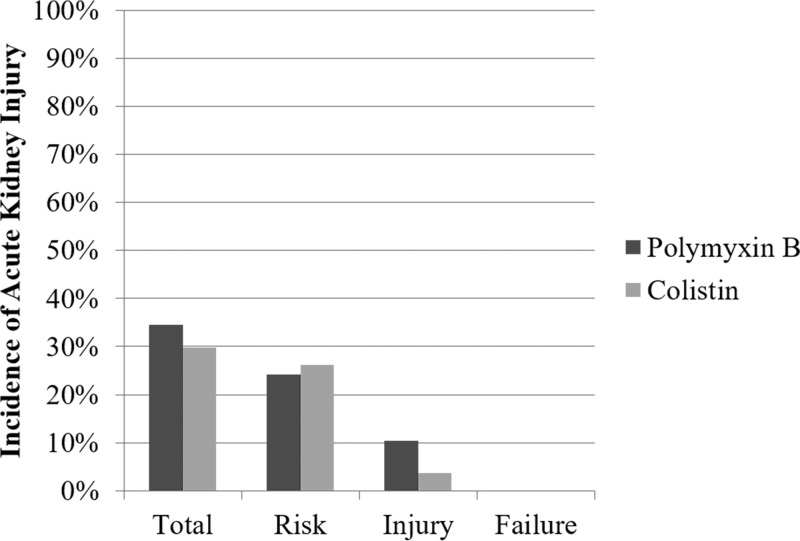
For the primary outcome, 10/29 (34.5%) of patients treated with PMB and 57/191 (29.8%) of patients treated with CMS developed AKI (P = 0.77; Fig. 3). Polymyxin B and CMS had statistically similar rates of patients meeting the risk (24.1% versus 26.2%; P = 1.00) and injury (10.3% versus 3.7%; P = 0.13) categories of RIFLE. No patient in either arm met the criteria for failure (≥75% decrease in CrCL from baseline). The median peak serum creatinine level was significantly higher with PMB (1.0 mg/dl; IQR, 0.7 to 1.7 mg/dl) than with CMS (0.7 mg/dl; IQR, 0.6 to 0.9 mg/dl; P = 0.002). The majority of patients, 90.0% with PMB and 91.2% with CMS, had recovered to their baseline CrCL strata by hospital discharge (P = 1.00).
FIG 3.
Acute kidney injury by RIFLE stage in cystic fibrosis patients treated with polymyxin B versus colistin. P values were nonsignificant (≥0.05) for all comparisons. Relative number of patients per outcome stratum: Total, PMB = 10/29, CMS = 57/191; Risk, PMB = 7/29, CMS = 50/191; Injury, PMB = 3/29, CMS = 7/191; Failure, PMB = 0/29, CMS = 0/191.
There were no statistically significant differences between PMB and CMS for in-hospital mortality (3.5% versus 0.5%, P = 0.25) or 30-day mortality (3.5% versus 1.1%, P = 0.35). The median hospital length of stay, however, was longer with PMB (14 days; IQR, 7 to 28 days) than with CMS (9 days; IQR, 6 to 14 days; P = 0.008) (Table 3).
(iv) Risk factors.
Risk factors associated with the development of AKI in bivariate analysis (P ≤ 0.2) were Charlson comorbidity index (CCI), diabetes, progressive care unit (PCU) admission, duration of therapy, vancomycin, loop diuretics, NSAIDs, and baseline albumin. The multivariate Cox proportional hazard model found diabetes (aHR, 2.68 [95% CI, 1.01 to 7.11]), PCU admission (aHR, 8.21 [95% CI, 2.55 to 26.46]), and concurrent loop diuretics (aHR, 3.02 [95% CI, 1.36 to 6.73]) to be independent predictors of kidney injury, while elevated baseline serum creatinine levels (per 1 mg/dl) were found to be protective (aHR, 0.08 [95% CI, 0.01 to 0.48]). Consistent with the non-cystic fibrosis cohort results, the polymyxin used (PMB versus CMS) was not predictive of AKI. Complete model results are presented in Table 5.
TABLE 5.
Risk factors for the development of acute kidney injury in cystic fibrosis patients treated with polymyxin B or colistin: bivariate and multivariate analysesa
| Variable | Value(s) |
|||||
|---|---|---|---|---|---|---|
| Bivariate analysis |
Multivariate analysis |
|||||
| HR | 95% CI | P | aHR | 95% CI | P | |
| Polymyxin formulation | ||||||
| Colistin | Reference value | Reference value | ||||
| Polymyxin B | 1.08 | 0.54–2.15 | 0.82 | 1.94 | 0.78–4.84 | 0.15 |
| Characteristics of polymyxin therapy | ||||||
| High doseb | 0.74 | 0.38–1.41 | 0.35 | |||
| Loading dose | 1.04 | 0.42–2.59 | 0.94 | 0.59 | 0.20–1.77 | 0.35 |
| Duration > 10 days | 1.43 | 0.87–2.34 | 0.16 | 1.13 | 0.63–2.02 | 0.69 |
| Demographics | ||||||
| Age (per additional yr) | 1.02 | 0.99–1.04 | 0.21 | |||
| CCI | 1.15 | 1.05–1.27 | 0.004 | 0.97 | 0.83–1.15 | 0.76 |
| Hypertension | 1.29 | 0.80–2.09 | 0.30 | |||
| Diabetes | 2.18 | 1.04–4.56 | 0.04 | 2.68 | 1.01–7.11 | 0.049 |
| Wt (per additional kg) | 1.00 | 0.98–1.03 | 0.82 | 1.00 | 0.98–1.03 | 0.82 |
| Site of care | ||||||
| Acute | Reference value | Reference value | ||||
| ICU | 1.27 | 0.60–2.68 | 0.53 | 0.78 | 0.33–1.81 | 0.56 |
| PCU | 3.45 | 1.38–8.63 | 0.008 | 8.21 | 2.55–26.46 | <0.001 |
| Concurrent nephrotoxinsc | ||||||
| Aminoglycosides | 1.19 | 0.68–2.10 | 0.54 | |||
| Amphotericin B | ||||||
| Vancomycin | 1.67 | 1.02–2.75 | 0.04 | 1.80 | 0.96–3.35 | 0.07 |
| Calcineurin inhibitors | 0.95 | 0.43–2.10 | 0.90 | 0.84 | 0.29–2.45 | 0.75 |
| Intravenous contrast | 1.89 | 0.43–2.10 | 0.38 | |||
| Loop diuretics | 2.38 | 1.32–4.27 | 0.004 | 3.02 | 1.36–6.73 | 0.006 |
| NSAIDS | 1.61 | 0.84–3.08 | 0.15 | 1.57 | 0.70–3.50 | 0.27 |
| Vasopressors | 1.19 | 0.26–5.45 | 0.82 | 0.31 | 0.04–2.20 | 0.24 |
| Laboratory abnormalities | ||||||
| Baseline Scr (per additional 1 mg/dl)d | 0.08 | 0.01–0.48 | 0.006 | |||
| Albumin | ||||||
| ≥3 g/dl | Reference value | Reference value | ||||
| <3 g/dl | 1.42 | 0.84–2.38 | 0.19 | 1.05 | 0.56–1.99 | 0.87 |
| No albumin level | 0.72 | 0.28–1.91 | 0.51 | 0.49 | 0.17–1.42 | 0.19 |
| Total bilirubin | ||||||
| Total bilirubin > 3 mg/dle | ||||||
Abbreviations: PMB, polymyxin B; CMS, colistimethate sodium; OR, odds ratio; CI, confidence interval; CCI, Charlson comorbidity index; kg, kilogram; ICU, intensive care unit; PCU, progressive care unit; NSAIDS, nonsteroidal anti-inflammatory drugs; Scr, serum creatinine. The multivariate Cox proportional hazard model included all variables meeting a significance level of a P value of ≤0.20 in bivariate analysis or that were significantly different between the PMB and CMS cohorts at baseline. Data for polymyxin B relative to CMS were forced into the model. Independent predictors of kidney injury are highlighted in bold.
High polymyxin doses are defined as PMB doses of ≥200 mg/day and CMS doses of ≥270 mg colistin base activity/day.
Concurrent use is defined as administration within 24 h prior to the first polymyxin dose through the end of polymyxin therapy.
No patient with cystic fibrosis had a baseline serum creatinine value greater than 1.5; therefore, baseline Scr (per 1 mg/dl increase) was included in the model due a significant baseline difference between PMB and CMS.
No patient with cystic fibrosis had a total bilirubin level of >3 mg/dl.
DISCUSSION
The rates of AKI did not differ between polymyxin B and colistin in patients with (34.5% versus 29.8%, P = 0.77) or without (42.9% versus 50.3%, P = 0.46) cystic fibrosis. Although there was no difference between PMB and CMS in rates of toxicity, it is notable that AKI developed in a significant proportion of patients treated with either polymyxin.
Our results are in conflict with recent studies showing higher rates of nephrotoxicity with CMS than with PMB (10, 12, 15). One explanation for this may be that the differences in the rates were due to institutional differences in polymyxin dosing. Patients in the non-cystic fibrosis cohort received a mean daily dose of 2.3 mg/kg of body weight/day (200.9 mg/day) of polymyxin B by actual body weight, which is concordant with a contemporary study of polymyxin B pharmacokinetics as well as with the doses used in comparator toxicity studies (1.7 to 2.4 mg/kg/day) (8, 10, 12, 15). In contrast, patients treated with CMS in our investigation received an average of 3.4 mg colistin base activity (CBA)/kg/day (226.4 mg CBA/day) by ideal body weight compared to 3.9 to 4.6 mg CBA/kg/day (275 to 300 mg CBA/day) in previous investigations. The colistin dosing used in our study is in line with current United States Food and Drug Administration (FDA) recommendations for patient baseline renal creatinine clearance of 50 to 80 ml/min (2.5 to 3.8 mg/kg/day) but falls short of the dosing recommended by the European Medicines Association (EMA) and supported by pharmacokinetic data (4–6, 16). A recent study by Nation and colleagues found FDA-labeled dosing to be inferior to the EMA-labeled dosing in attaining target colistin serum levels (16). In recent years, our institutional practice has shifted to PMB as the preferred agent; thus, much of the CMS dosing reflects historical practice prior to the publication of recent pharmacokinetic studies and dosing recommendations. This temporal effect of polymyxin use may have biased the results toward lesser toxicity with CMS due to suboptimal dosing by current standards. Similarly, loading doses have become the standard of care for polymyxin dosing at our institution only recently and the lower frequency of loading doses seen with CMS than with PMB may be reflective of historical practice.
The existing body of evidence suggests that either CMS has greater nephrotoxicity than PMB or there is no difference in the toxicity levels of these agents (9–12, 15). There are, however, clear pharmacokinetic advantages to PMB—namely, less interpatient variability and the lack of a significant renal component to drug elimination (4–8). In the absence of greater toxicity with PMB than with CMS, we assert that the pharmacokinetic argument is sufficient to recommend PMB as the first-line polymyxin for extraurinary infections due to MDR Gram-negative pathogens. Furthermore, it is unlikely that additional research into comparative nephrotoxicity will yield results that are greatly contradictory to the existing body of evidence. Therefore, resources should be focused on novel strategies to mitigate polymyxin toxicity (nephroprotective agents, synergistic dose sparing, etc.) or to minimize the use of polymyxins altogether (i.e., aminoglycoside use, novel combination therapy, drug development, etc.) (17–19). In this investigation, nephrotoxicity was reversible in only about 60% of non-cystic fibrosis patients by hospital discharge, highlighting the significant effect of this toxicity on patient outcomes.
The sum of the polymyxin pharmacokinetic and toxicity data in patients with CF is much less conclusive than in the general population. In our study, there were no statistically significant differences in the overall rates of AKI with PMB versus CMS (34.5% versus 29.8%, P = 0.77) or in the proportion of patients with recovery of renal function by hospital discharge (90.0% versus 91.2%, P = 1.00) in the CF cohort. Interestingly, the unadjusted rate of kidney injury in CF patients treated with polymyxins was 30.5% compared to 48.4% in those without the disease (P < 0.001). Unfortunately, the significant bias toward the use of CMS for patients with CF precludes the use of Cox regression to elucidate whether the disease itself is nephroprotective or if baseline differences in the patient populations are influencing the observed results.
In a study by Phe and colleagues, CF was found to be independently protective against the development of AKI with CMS in multivariate logistic regression analysis (odds ratio [OR], 0.03; 95% CI, 0.001 to 0.79; P = 0.04) (12). Unfortunately, a parallel analysis in patients treated with PMB was precluded by the complete lack of CF patients in the PMB therapy arm. Given that CMS undergoes significant renal elimination, a potential mechanism of this effect could be that of enhanced elimination of the prodrug before activation to the more toxic parent molecule in patients with an augmented renal baseline. A pharmacokinetic study of CMS in CF patients found mean steady-state terminal half-lives of 2.1 and 4.2 h for colistimethate and colistin, respectively, relative to 4.6 and 9.1 h in patients without CF in a study by Garonzik and colleagues (4, 14). This theory of reduced toxicity by augmented clearance is concerning because reduced toxicity may then be a marker of reduced efficacy due to an increase in the fraction of the colistimethate dose eliminated and a corresponding decrease in the fraction hydrolyzed to active colistin.
Multivariate Cox proportional hazard analysis identified differing predictors of AKI in patients with and without cystic fibrosis treated with polymyxins. Notably, loop diuretic exposure was an independent risk factor for AKI in both cohorts and was the only exposure significantly associated with AKI in patients without cystic fibrosis. In the CF cohort, diabetes and progressive care unit admission were independently associated with AKI whereas a higher baseline serum creatinine level was protective. The association between higher serum creatinine values and decreased risk for AKI in patients with CF may be due to inadequate dosing of colistin or polymyxin B in patients with renal dysfunction (20). It is notable that the use of loading doses, which are integral to the rapid attainment of bactericidal drug levels, did not appear to impact toxicity (5, 6, 8, 21).
There are several limitations which must be acknowledged. First, this was a retrospective evaluation and therefore subject to selection and chronology biases. All study outcomes were chosen as objective measures to limit any bias from misinterpretation of retrospective data; however, polymyxin use was not evenly distributed across the study period. Colistin-treated patients were enrolled earlier on average than PMB-treated patients, which likely accounts for the differences in dosing intensity and loading dose prescribing seen in this investigation. Additionally, there was disproportionate use of colistin and polymyxin B, with significantly fewer patients receiving PMB in both cohorts. This may have decreased the statistical power of the Cox models. There is also the potential for confounding factors contributing to AKI that were not quantified in our analysis. We controlled for multiple factors in the Cox proportional hazard model, including severity of illness, site of care, comorbidities, renal baseline, and concurrent nephrotoxins, in order to minimize confounding. Finally, we did not assess clinical outcomes of polymyxin therapy for treating infection or attempt to correlate serum drug levels with the development of toxicity.
Conclusions.
There was no difference between polymyxin B and colistin in the incidence of AKI in patients with and without CF; however, the overall rate of kidney injury was significantly lower in patients with CF. In both cohorts, loop diuretic use was independently associated with AKI. Diabetes and progressive care unit admission were associated with increased risk of AKI in patients with CF, while elevated baseline serum creatinine was protective against AKI in this patient population. Further investigation is warranted to determine if cystic fibrosis provides differential nephroprotective effects between colistin and polymyxin B and to confirm whether the effects, if any, exist independently of cofounding baseline differences in patient populations. Alternative therapeutic options and novel strategies to mitigate polymyxin nephrotoxicity are desperately needed.
MATERIALS AND METHODS
Design.
This was a retrospective cohort study conducted at the University of Kentucky Albert B. Chandler and Good Samaritan Hospitals between 1 July 2006 and 30 September 2015. Patients were enrolled retrospectively from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust database. Institutional review board (IRB) approval was obtained from the University of Kentucky.
Adult patients (≥18 years old) who received treatment with intravenous PMB or CMS for at least 48 h were eligible for inclusion and were stratified by baseline CF status into two cohorts. Patients receiving renal replacement therapy at baseline were excluded. Patients who had received either systemic CMS or PMB within the previous 14 days or those who had been switched from one agent to the other within a single course of therapy were also excluded. Additionally, we excluded patients with reported height and/or weight data points greater than four standard deviations from the mean. Polymyxin agent selection and dosing were at the discretion of the primary care team at the time of therapy, and no protocols or guidelines were available at either institution during the study period.
Study outcomes.
Data collected included demographics (i.e., age, race, sex), admission and discharge dates, site of care, comorbidities, Charlson comorbidity index (CCI), laboratory markers (i.e., serum creatinine, blood urea nitrogen, albumin, etc.), polymyxin information (i.e., loading dose used, total daily dose, duration), concomitant anti-infectives, and concomitant nephrotoxins (i.e., aminoglycosides, loop diuretics, vasopressors, contrast dye, etc.).
The primary endpoint was the overall incidence of AKI, defined using the RIFLE (risk, injury, failure, loss, end stage) criteria, in patients treated with systemic PMB or CMS (22). This endpoint was met if creatinine clearance (CrCL) decreased by 25% or greater from baseline at any time between treatment initiation and 48 h after the end of polymyxin therapy. Creatinine clearance was determined using the modified Cockroft-Gault equation (23). Multiple variables were evaluated as risk factors for AKI, including age, CCI, admission to an intensive care unit (ICU) or progressive care unit (PCU), polymyxin agent used (PMB versus CMS), loading dose, high polymyxin dose (CMS, ≥270 mg/day colistin base activity [CBA]; PMB, ≥200 mg/day), duration of therapy (>10 days), concurrent nephrotoxins (aminoglycosides, amphotericin B, vancomycin, calcineurin inhibitors, intravenous contrast, loop diuretics, nonsteroidal anti-inflammatory drugs [NSAIDs], vasopressors), elevated baseline serum creatinine (>1.5 mg/dl), elevated total bilirubin (>3 mg/dl), and hypoalbuminemia (<3 g/dl) (10, 11).
Multiple secondary toxicity and efficacy endpoints were investigated. Secondary toxicity outcomes included the proportion of patients meeting each stage of the RIFLE criteria, peak serum creatinine, and the proportion of patients with return to renal baseline. Return to renal baseline was assessed as the proportion of patients returning to their baseline CrCL strata (i.e., >80 ml/min, 50 to 70 ml/min, 30 to 49 ml/min, 10 to 29 ml/min) by hospital discharge. Secondary efficacy endpoints included in-hospital mortality, 30-day mortality, and length of stay.
Statistics.
Descriptive statistics were calculated as follows. Categorical variables were analyzed using the chi-square test or Fisher's exact test as appropriate. Continuous variables were compared using the Student t test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. Normality was assessed using the Shapiro-Wilk test. Risk factors for the development of AKI were assessed using bivariate Cox regression to generate hazard ratios (HR) and 95% confidence intervals (CI). Variables with a P value of ≤0.2 in bivariate analysis and those which differed between CMS and PMB at baseline were included in the final Cox proportional hazard model. The polymyxin antimicrobial used (PMB versus CMS) was forced into the final model regardless of bivariate association, as the toxicity difference between these agents was the primary outcome of interest. A power calculation was not performed due to the retrospective nature of this investigation. All statistical tests were performed in R (version 3.1.2) and were two-tailed at a significance level of 0.05.
ACKNOWLEDGMENTS
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR000117 and UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D; Infectious Diseases Society of America. 2013. 10 × '20 Progress–development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim LM, Ly N, Anderson D, Yang JC, Macander L, Jarkowski A III, Forrest A, Bulitta JB, Tsuji BT. 2010. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30:1279–1291. doi: 10.1592/phco.30.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 56:4241–4249. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karaiskos I, Friberg LE, Pontikis K, Ioannidis K, Tsagkari V, Galani L, Kostakou E, Baziaka F, Paskalis C, Koutsoukou A, Giamarellou H. 2015. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother 59:7240–7248. doi: 10.1128/AAC.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. 2009. Polymyxin B and colistimethate are comparable as to efficacy and renal toxicity. Diagn Microbiol Infect Dis 65:431–434. doi: 10.1016/j.diagmicrobio.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. 2013. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis 57:1300–1303. doi: 10.1093/cid/cit453. [DOI] [PubMed] [Google Scholar]
- 11.Tuon FF, Rigatto MH, Lopes CK, Kamei LK, Rocha JL, Zavascki AP. 2014. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int J Antimicrob Agents 43:349–352. doi: 10.1016/j.ijantimicag.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, Musick WL, Cottreau JM, Hu M, Tam VH. 2014. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother 58:2740–2746. doi: 10.1128/AAC.02476-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed MD, Stern RC, O'Riordan MA, Blumer JL. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J Clin Pharmacol 41:645–654. doi: 10.1177/00912700122010537. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J. 2003. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother 52:987–992. doi: 10.1093/jac/dkg468. [DOI] [PubMed] [Google Scholar]
- 15.Rigatto MH, Oliveira MS, Perdigao-Neto LV, Levin AS, Carrilho CM, Tanita MT, Tuon FF, Cardoso DE, Lopes NT, Falci DR, Zavascki AP. 8 February 2016. Renal failure in patients treated with colistin versus polymyxin B: a multicenter prospective cohort study. Antimicrob Agents Chemother. doi: 10.1128/AAC.02634-15. [DOI] [PMC free article] [PubMed]
- 16.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Turnidge JD, Forrest A, Silveira FP. 2016. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 62:552–558. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirijatuphat R, Limmahakhun S, Sirivatanauksorn V, Nation RL, Li J, Thamlikitkul V. 2015. Preliminary clinical study of the effect of ascorbic acid on colistin-associated nephrotoxicity. Antimicrob Agents Chemother 59:3224–3232. doi: 10.1128/AAC.00280-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalfino L, Puntillo F, Ondok MJ, Mosca A, Monno R, Coppolecchia S, Spada ML, Bruno F, Brienza N. 2015. Colistin-associated acute kidney injury in severely ill patients: a step toward a better renal care? A prospective cohort study. Clin Infect Dis 61:1771–1777. [DOI] [PubMed] [Google Scholar]
- 19.Yousef JM, Chen G, Hill PA, Nation RL, Li J. 2012. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother 67:452–459. doi: 10.1093/jac/dkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiaccadori E, Antonucci E, Morabito S, d'Avolio A, Maggiore U, Regolisti G. 2016. Colistin use in patients with reduced kidney function. Am J Kidney Dis 68:296–306. doi: 10.1053/j.ajkd.2016.03.421. [DOI] [PubMed] [Google Scholar]
- 21.Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N. 2012. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 54:1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. 2004. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelm SM, Kale-Pradhan PB. 2011. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy 31:658–664. doi: 10.1592/phco.31.7.658. [DOI] [PubMed] [Google Scholar]