Abstract
Background: Both an elevated brain-reward–region response to palatable food and elevated weight variability have been shown to predict future weight gain.
Objective: We examined whether the brain-reward response to food is related to future weight variability.
Design: A total of 162 healthy-weight adolescents, who were aged 14–18 y at baseline, were enrolled in the study and were assessed annually over a 3-y follow-up period with 127 participants completing the final 3-y follow-up assessment. With the use of functional magnetic resonance imaging, we tested whether the neural responses to a cue that signaled an impending milkshake receipt and the receipt of the milkshake predicted weight variability over the follow-up period. Weight variability was modeled with a root mean squared error method to reflect fluctuations in weight independent of the net weight change.
Results: Elevated activation in the medial prefrontal cortex and supplementary motor area, cingulate gyrus, cuneus and occipital gyrus, and insula in response to milkshake receipt predicted greater weight variability. Greater activation in the precuneus and middle temporal gyrus predicted lower weight variability.
Conclusions: From our study data, we suggest that the elevated activation of reward and emotional-regulation brain regions (medial prefrontal cortex, cingulate cortex, and insula) and lower activation in self-reference regions (precuneus) in response to milkshake receipt predict weight variability over 3 y of follow-up. The fact that the reward response in the current study emerged in response to high-calorie palatable food receipt suggests that weight variability may be a measure of propensity periods of a positive energy balance and should be examined in addition to measures of the net weight change. With our collective results, we suggest that weight variability and its brain correlates should be added to other variables that are predictive of weight gain to inform the design of obesity-preventive programs in adolescents. This trial was registered at clinicaltrials.gov as NCT01807572.
Keywords: body weight, fMRI, obesity, weight fluctuations, weight variability
INTRODUCTION
Because of the alarming prevalence of obesity and the lack of treatments that produce long-term weight loss, numerous studies have evaluated factors that predict future weight gain with the hope that this assessment will inform the design of more effective weight-control interventions. In most of these studies, the outcome of interest has been the net weight change between a starting point and one or more follow-up points. However, body weight varies to some extent within individuals over time. Over periods of months or years, a person’s body weight may increase, decrease, or show little absolute change, but the variability in body weight over that time period could vary independently of the degree of the overall change in body weight. When assessed repeatedly over time, these individual differences in body weight may, themselves, convey important information about people’s current or future ability to regulate their body weights (1). Therefore, it is important to understand the processes that predict weight variability independent of the total weight change.
There are 2 main reasons why individual differences in weight variability may be valuable in understanding the relation between body mass and long-term risk of health problems. First, there has been preliminary evidence that an elevated weight variability in healthy-weight individuals increases risk of future weight gain (1). Therefore, it may be important to understand individual differences in weight variability in healthy-weight individuals, because weight variability may predate an increase in weight and, ultimately, the development of overweight or obesity. Second, the extant literature that has examined the role of weight variability in adverse health problems has suggested that instability of body weight presents risks of cardiac events, coronary heart disease, and stroke above and beyond the average body weight or weight gain (2–6). Adolescence, in particular, is a vulnerable time period for weight gain and obesity onset. Because weight variability has been linked to both future weight gain and adverse health events in adults, it is useful to identify neural vulnerability factors that predict weight variability in adolescents.
Although no research, to our knowledge, has examined neural risk factors of future weight variability, several prospective studies have investigated neural predictors of future weight gain. Elevated responsivity in brain regions that are associated with reward, hedonic valuation, attention, and motivation (i.e., the striatum, insula, anterior cingulate cortex, and orbitofrontal cortex) has been shown to predict future weight gain. This relation has been observed in response to palatable food images (7), commercials for palatable foods (8), cues that predict the subsequent presentation of high-calorie, palatable food (9), cues that predict the impending receipt of high-calorie, palatable food (10), and the receipt of high-calorie, palatable food (11).
The current study tested the hypothesis that an elevated reward-region response to the receipt of high-calorie, palatable food and cues that signal an impending food receipt will predict greater future weight variability in healthy-weight adolescents.
METHODS
Participants
Participants included 82 female and 80 male adolescents (n = 162) aged 14–18 y (mean ± SD: 15.3 ± 1.06 y) who were recruited in a city in Oregon via advertisements for a 3-y prospective study (see Supplemental Figure 1 for a diagram of participant flow throughout the study). Adolescents were at or near a healthy weight at baseline (which was deemed according to BMI z scores that were roughly between the 27th and 75th percentiles) and were divided into children at low risk of weight gain with 2 healthy-weight parents [BMI (in kg/m2) <25] (n = 37) and children at high risk of weight gain with 2 obese or overweight parents (BMI >25) (n = 125). BMI for participants ranged from 16.6 to 26.1 (mean ± SD: 20.82 ± 1.93). A participant with BMI of 16.6 did not report any eating-disorder symptoms or differ from other participants in a systematic manner, and thus, this participant was included in the study. The sample was 80% Caucasian, 11% Hispanic, 5% American Indian or Alaskan Indian, 3% African American, and 2% Asian. In the current study, ethnicity was treated as a mutually exclusive variable rather than as orthogonal. Parents provided written informed consent, and adolescents provided written assent. Individuals were excluded if they did not fall within a healthy-weight BMI range, reported the current use of psychoactive medications or drugs >1 time/wk, were pregnant, had had a head injury with a loss of consciousness, or had a current Axis I psychiatric disorder (including anorexia nervosa, bulimia nervosa, or a binge-eating disorder).
Procedures
Participants were recruited for participation in a 3-y study to examine the neural response to food stimuli and future weight gain. Participants were asked to consume their regular meals on their scan day but to refrain from eating or drinking caffeinated beverages 4–6 h before their scans. At baseline, participants completed an fMRI scan. Most scans were conducted in the afternoon between 1600 and 1800. Some scans were completed on no-school days between 1200 and 1300. At baseline and annual follow-up assessments, participants’ heights and weights were directly measured while they wore light, indoor clothing without shoes. This method permitted the calculation of BMI scores. BMI is correlated with dual-energy X-ray absorptiometry–measured body fat (r = 0.80–0.90) and health measures such as adverse lipoprotein profiles (12). Air-displacement plethysmography was used to assess the percentage of body fat of participants at baseline and at all follow-ups with the use of a Bod Pod S/T device (COSMED USA Inc.) according to recommended procedures (13). The procedures followed were in accordance with the ethical standards of the Oregon Research Institute Institutional Committee on Human Experimentation.
fMRI paradigm
An anticipatory and consummatory food-reward paradigm was used in the current study [details and additional findings from the current study are available in Stice et al. (10)]. Visual stimuli for the paradigm were presented with a digital-project and reverse-screen display system to a screen at the back of the MRI-scanner bore. Stimuli were visible via a mirror that was mounted on the head coil.
The anticipatory and consummatory food-reward paradigm assessed responses to an anticipated receipt and the receipt of a chocolate milkshake. In the first portion, the stimuli consisted of 2 images (glasses of milkshake and glasses of water) that indicated the delivery of either 0.5 mL chocolate milkshake or a tasteless solution. Details on the preparation and ingredients for the milkshake and the tasteless solution have been described in Stice et al. (14). In 40% of these trials, neither the milkshake nor the tasteless solution was delivered (unpaired cues) so than trials of cues that signaled the impending receipt were not confounded by the actual receipt of a beverage. However, because no differences were observed in responses between paired and unpaired milkshake and tasteless-solution cues, paired and unpaired cues were combined for analyses to increase sensitivity. Cues were presented for 2 s and were followed by a jitter of 1–7 s during which time the screen was blank. After the jitter in the receipt trials, 0.5 mL of the associated beverage was delivered on the participant’s tongue over the course of 5 s. Taste receipt was followed by a second jitter of 1–7 s. Participants were instructed to keep the taste in their mouths until they saw the swallow cue. After the swallow cue, participants received a rinse (4 s), which was followed by another swallow cue (2 s). The next taste cue appeared 1–7 s after the swallow cue.
fMRI data acquisition, preprocessing, and statistical analyses
Scanning was performed with the use of a Siemens Allegra 3-tesla head-only scanner (Siemens) at the University of Oregon. Participants were familiarized with the fMRI paradigms before the imaging session. Foam padding, a vacuum pillow, and tape were used to minimize participant head movement. Tastes were delivered with the use of a gustometer (15). Functional scans used a T2*-weighted gradient echo-planar–imaging sequence (echo time: 30 ms; repetition time: 2000 ms; flip angle: 80°) with an in-plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192-mm2 field of view). A shorter echo time was used to improve the signal-to-noise ratio and to minimize distortion effects in more-subcortical regions of interest. To cover the whole brain, thirty-two 4-mm slices (interleaved acquisition without a skip) were acquired along the Anterior-Posterior Commissure transverse oblique plane as determined by the midsaggital section. High-resolution, T1-weighted, 3-dimensional structural scans (Magnetization Prepared Rapid Acquisition Gradient Echo) were also collected in the same orientation for alignment with the functional scans (repetition time: 2100 ms; echo time: 2.4 ms; flip angle: 15° matrix size: 256 × 256; slice thickness: 1 mm; slice number: 160).
Neuroimaging data were preprocessed with the use of SPM12 software (Functional Imaging Laboratory, University College of London) in the MATLAB platform (Mathworks Inc.). Before preprocessing, images were realigned manually to the Anterior-Posterior Commissure line in the SPM12 program and skull stripped with the use of the Brain Extraction Tool in FSL (FMRIB Analysis Group, Version 5.0), a neuroimaging analysis software program. Anatomic data were segmented and normalized with the use of DARTEL, an image registration toolbox in SPM, thereby providing a sample-specific template and individual-level deformation fields for use in normalization. Functional data were slice-time corrected, adjusted for variation in the magnetic field with the use of field maps, realigned to the mean functional scan, co-registered with the anatomical scan, and normalized stereotactic space using the Montreal Neurological Institute template with the use of the DARTEL template and deformation fields output. After normalization, data were smoothed with the use of a 6-mm Gaussian Full Width at Half Maximum. Data were assessed for spikes in the mean response and motion outliers with the use of the Artifact Detection Toolbox (ART; Gabrieli Laboratory, McGovern Institute for Brain Research, 2015). Motion variables were used as regressors in the design matrix in individual-level analysis. Image volumes, when z-normalized global brain activation exceeded 3 SDs from the mean of the run or showed >1 mm composite movement, were flagged as outliers and deweighted during the first-level model estimation.
The activation in response to milkshake receipt was assessed by contrasting the BOLD signal during receipt of the milkshake compared with during receipt of the tasteless solution; the activation in response to anticipated milkshake receipt was assessed by contrasting the BOLD signal during the milkshake cue compared with during the tasteless-solution cue. At the first-level, T maps were constructed for comparison of the activation within each participant for the previously mentioned contrasts (e.g., milkshake receipt > tasteless-solution receipt, where the BOLD signal in the specific brain region is higher during milkshake receipt than during tasteless-solution receipt.). These individual contrasts were entered into a second-level regression model with weight variability over follow-up. In the initial analysis, baseline BMIs, baseline weight variability (the difference between self-reported highest and lowest weights in the 12 mo before the baseline assessment), and self-reported hunger levels for each individual were entered as covariates for the full sample of 162 participants. The self-reported difference between highest and lowest weights was included as a covariate to control for past weight variability as an alternative explanation for any prediction of future weight variability by baseline brain activation. To test if regions of activation differed by sex, we ran independent-samples t tests with the use of sex and the extracted variable estimates from each brain region that emerges as significant in the primary analyses. To determine whether the addition of the net weight change as a covariate changed the relation between the response to the milkshake at baseline and weight variability, we included a second analysis that, in addition to other covariates, also controlled for the weight change from baseline to the 3-y follow-up. Because of missing weights at the 3-y follow-up, 127 total participants were analyzed in the supplementary analysis with the BMI change controlled for. BMI variability was selected as the outcome of interest in analyses because all of the extant literature on weight variability has used either BMI or raw weights (16, 17). However, some studies that used the same or similar paradigms used body fat as the outcome of interest (18). Thus, a third analysis examined the relation between brain activation in the milkshake task and the subsequent body fat variability with the baseline body fat and hunger level controlled for. This analysis was conducted to determine whether similar patterns of activation predicted BMI variability and body fat variability. Whole-brain analyses were conducted after the binarized DARTEL-derived, sample-specific, gray-matter mask was applied. A whole-brain α = 0.05 was corrected for multiple comparisons across the masked whole brain with 10,000 Monte Carlo simulations of random noise at 3 mm3 with the use of the 3DClustSim module of AFNI software (1996; Cox). Simulation results indicated that a threshold of P < 0.001 and a cluster (k) ≥15 would provide sufficient correction for multiple comparisons. Data were inspected to ensure that outliers did not drive significant effects. Effect sizes (r) were derived from the z values (z divided by the square root of n).
Weight and fat variability
Weight and body fat variability were calculated in R software (R Project for Statistical Computing, version 3.1.1, available from: http://www.r-project.org) with the use of 4 measured BMIs (or body fat values) in the study at baseline and 1, 2, and 3 y, respectively. BMI scores, which reflected body weight adjusted for height, were used instead of raw weights to ensure that any increases in height over time did not confound our analyses. In addition, age-adjusted BMIs were not used because research has suggested that within-subject raw-score changes are better for modeling than is the removal of the developmental variation with age-adjusted scores (19). A growth-curve analysis was used to calculate BMI-change trajectories over the 3 y. Linear regression curves were modeled by taking into account the between-subject trends in weight over time as well as within-subject trends. In accordance with established convention (1), the root mean square error of variation (RMSE)5 was calculated around each participant’s regression line for BMI. RMSE values that represented the degree of weight variability ranged from 0.09 to 2.32 (mean ± SD: 0.55 ± 0.36) with higher values being indicative of greater weight variability. RMSE values that represented the degree of fat variability ranged from 0.15 to 5.05 (mean ± SD: 1.84 ± 1.02). Hypotheses were also tested with missing data imputed, but because this method is robust to missing data, and results did not differ on variables of interest, we report the original data without imputation. Analyses tested whether the BOLD response to receipt and the anticipated receipt of the milkshake predicted future weight variability.
RESULTS
Sample characteristics and behavioral data
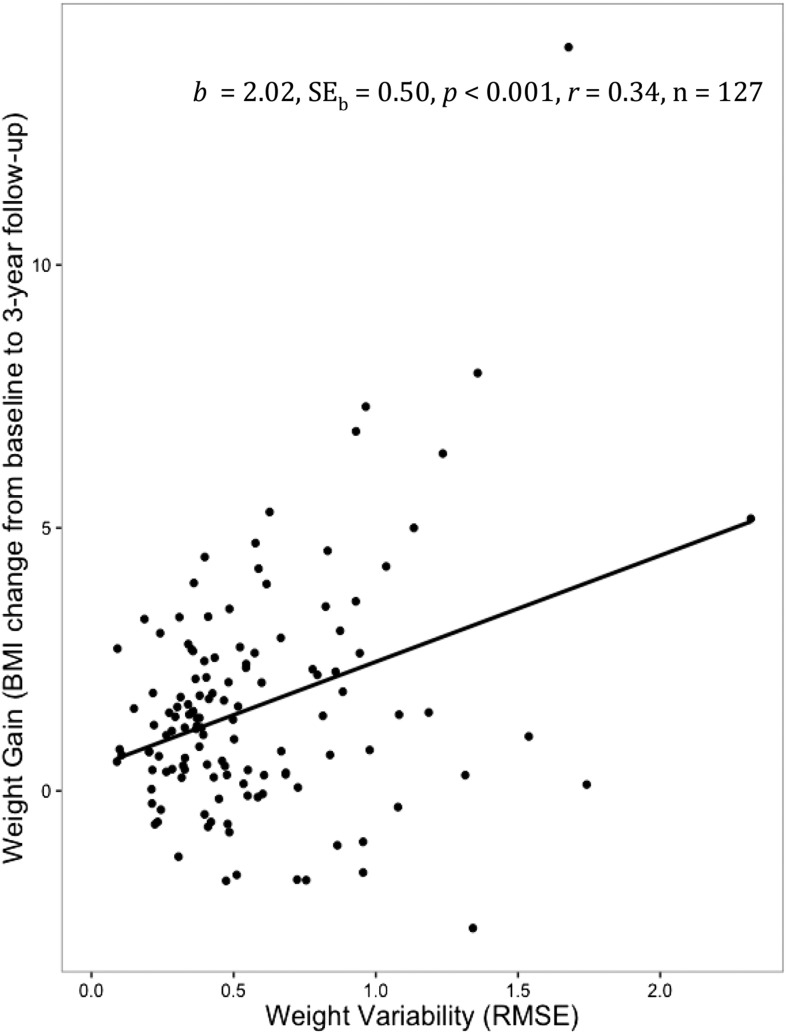
Table 1 shows BMI for the full sample at each assessment point (at baseline and the 1-, 2-, and 3-y follow-ups). The net change in BMI from baseline to the 3-y follow-up ranged from −2.61 to 14.14 (mean ± SD: 1.59 ± 2.2). Although the participant with a BMI change of 14.14 was a statistical outlier, results were identical with and without this participant, and thus, the results were included in the analyses. A simple linear regression analysis revealed that the weight variability over 3 y of follow-up (calculated with the use of BMIs) was correlated with the net weight gain over follow-up [F(1,125) = 16.24; b ± SE: 2.02 ± 0.50; P < 0.001; r = 0.34] (Figure 1). This finding was maintained when controlled for baseline BMI, prescan hunger level, sex, parental obesity status, and age (P < 0.001, r = 0.39). Results were also maintained with the exclusion of statistically determined outliers. Note that adolescents with obese or overweight parents showed significantly higher body weight variability (mean ± SD: 0.59 ± 0.39) than did adolescents with healthy-weight parents [mean ± SD: 0.43 ± 0.24; t(155) = −2.39; P = 0.02; 95% CI for weight-variability mean difference score: −0.29, −0.02; d = 0.53]. This result was maintained when controlling for the BMI change over follow-up [F(2,124) = 12.36; b ± SE: 0.20 ± 0.07; P = 0.007; r = 0.41]. Weight variability was significantly positively correlated with the cognitive restraint scale of the Three-Factor Eating Questionnaire (mean ± SD: 5.22 ± 3.22) but not with other subscales [r(160) = 0.16, P = 0.04]. However, this finding was not maintained when controlling for baseline BMI, prescan hunger level, sex, age, and parental obesity status (b ± SE: 0.01 ± 0.01; P = 0.20). In addition, the Three-Factor Eating Questionnaire and other self-reported measures of restraint were not correlated with objective measures of caloric intake, and thus, this result is not discussed (20). Sex was not significantly related to weight variability [t(160) = 0.42; P = 0.68; 95% CI: −0.09, 0.14; d = 0.07].
TABLE 1.
Summary statistics1
| Total | Boys | Girls | |
| Baseline (n = 162) | |||
| Age, y | 15.32 ± 1.06 | 15.43 ± 1.14 | 15.22 ± 0.97 |
| BMI, kg/m2 | 20.82 ± 1.93 | 20.71 ± 1.99 | 20.92 ± 1.88 |
| BMI, kg/m2 | |||
| At the 1-y follow-up (n = 141) | 21.49 ± 2.3 | 21.67 ± 2.64 | 21.34 ± 1.97 |
| At the 2-y follow-up (n = 152) | 21.84 ± 2.38 | 22.11 ± 2.56 | 21.59 ± 2.19 |
| At the 3-y follow-up (n = 127) | 22.42 ± 2.97 | 22.58 ± 3.04 | 22.29 ± 2.93 |
All values are means ± SDs.
FIGURE 1.
Relation between BMI change (from baseline to a 3-y follow-up) and weight variability. A linear regression analysis was run, and the estimate ± SE, significance value, effect size, and sample size are noted in the figure. RMSE, root mean squared error.
Relation of neural responsivity to weight variability over 3 y of follow-up
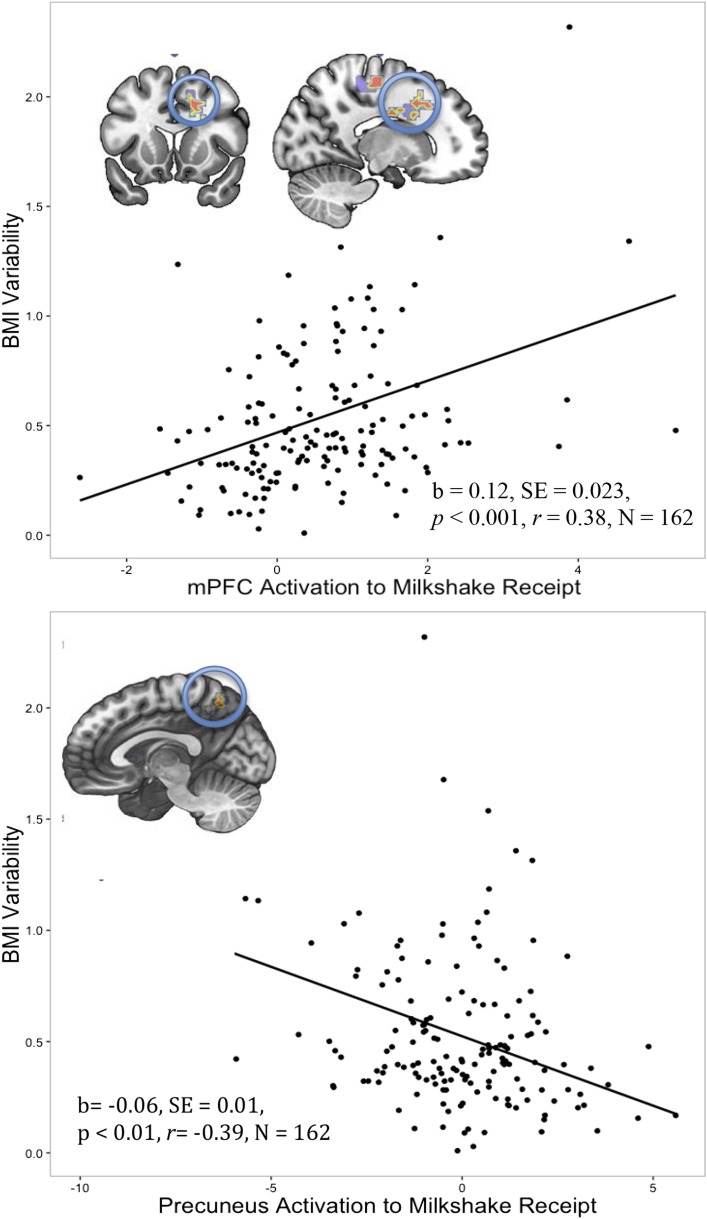
A greater BOLD response in the medial prefrontal cortex (mPFC) and supplementary motor area (k = 68, r = 0.40), cingulate gyrus (k = 63, r = 0.32), cuneus and occipital gyrus (k = 30, r = 0.31), and insula (k = 24, r = 0.29) to the contrast of milkshake receipt > tasteless-solution receipt predicted higher weight variability over follow-up (Figure 2). In contrast, the elevated BOLD response in the bilateral precuneus (k = 34, r = 0.38) and middle temporal gyrus (MTG) (k = 25, r = 0.30) to milkshake receipt > tasteless-solution receipt predicted lower weight variability over follow-up (Figure 2). Findings that are depicted in Figure 2 (P < 0.001) persisted with the removal of 1 highly weight-variable outlier. There was no significant relation between the BOLD response to anticipated milkshake receipt and future weight variability. Group-level analyses included the prescan hunger level, starting BMI, and baseline weight variability as covariates (BOLD results are shown in Table 2). Variable estimates in each significant brain region were evaluated for sex differences, and no significant differences emerged between boys and girls. In addition, significant brain responses did not differ by parental obesity status.
FIGURE 2.
Greater BOLD response in the right mPFC to milkshake receipt predicted future weight variability over 3 y of follow-up. The neural response (circled in blue) and a graph depicting the positive correlation between variable estimates from this region in response to milkshake receipt and weight variability are shown (areas of color indicate greater activation with red indicating the strongest region). A greater BOLD response in the precuneus to milkshake receipt predicted less weight variability over 3 y of follow-up. The neural response (circled in blue) and a graph depicting the negative correlation between variable estimates from this region in response to milkshake receipt and weight variability are shown. Linear regressions were run on the variable estimates in the regions, and estimates ± SEs, significance values, effect sizes, and sample sizes are noted in the figure. mPFC, medial prefrontal cortex.
TABLE 2.
Whole-brain analyses of correlations between BOLD activation to contrasts of milkshake receipt > tasteless-solution receipt and weight variability over 3 y of follow-up (n = 162)1
| MNI coordinates |
|||||||
| Milkshake receipt > tasteless-solution receipt | k | z | P | x | y | z | R2 |
| Positive relation with weight variability | |||||||
| mPFC, right | 68 | 5.12 | <0.001 | 21 | −31 | 55 | 0.40 |
| Anterior and middle cingulate, right | 63 | 4.03 | <0.001 | 18 | 14 | 40 | 0.32 |
| Cuneus, right | 30 | 3.97 | <0.001 | 30 | −73 | 1 | 0.31 |
| Insula, left | 24 | 3.69 | <0.001 | −33 | 5 | 19 | 0.29 |
| Negative relation with weight variability | |||||||
| Precuneus, bilateral | 34 | 4.81 | <0.001 | −6 | −61 | 58 | 0.38 |
| Middle temporal gyrus, left | 25 | 3.83 | <0.001 | −48 | −67 | 10 | 0.30 |
MNI, stereotactic space coordinates; mPFC, medial prefrontal cortex.
Effect sizes were derived from z values (z divided by the square root of n).
An additional analysis was conducted with the BMI change from baseline to the 3-y follow-up controlled for in addition to the above covariates to determine whether the neural effects of weight variability persisted above and beyond those of the net weight change. After controlling for the BMI change, larger clusters and a stronger activation to milkshake receipt > tasteless receipt predicted higher weight variability in the same regions as previously noted [the mPFC and supplementary motor area (k = 298, r = 0.43), cingulate gyrus (k = 232, r = 0.35), cuneus and occipital gyrus (k = 44, r = 0.41), and insula (k = 57, r = 0.35)]. However, in the condition of milkshake receipt > tasteless receipt, no brain regions significantly predicted lower weight variability with the inclusion of the BMI change as a covariate. No brain regions significantly predicted body fat variability in either the anticipation or receipt conditions. Scores of body weight variability and body fat variability were moderately correlated [r(160) = 0.47, P < 0.01]. Baseline BMI and the percentage of body fat were weakly correlated [r(160) = 0.28, P < 0.01], which was relatively low and might have been due to the restriction in ranges of BMI and body fat at baseline in this study than in previous studies that examined the agreement of these 2 measures in samples with a broader weight range (21). In addition, neural responsivity was not related to ratings of milkshake pleasantness or the frequency of milkshake consumption.
DISCUSSION
The current study provides behavioral evidence for the potential clinical relevance of weight variability. First, the current study observed a relation between weight variability and weight gain independent of the starting weight. Second, weight variability in the current sample was related to parental obesity status with adolescents with 2 overweight or obese parents exhibiting greater weight variability than that of their counterparts with 2 lean parents, which to our knowledge, is a novel finding. These results, in concert with neural evidence, shed light on the clinical relevance of weight variability both independently and in concert with a net weight gain.
Whole-brain analyses indicated that greater activation in the insula, cingulate gyrus, mPFC and supplementary motor area, and cuneus in response to milkshake receipt predicted greater weight variability over follow-up. The insula, which is instrumental in primary gustatory, secondary gustatory, and olfactory functioning (22), is also considered the part of an appetitive neural circuit that is specifically responsible for interoceptive awareness and hedonic valuation (22, 23). In healthy individuals, greater insula activity has been observed in response to milkshake receipt in frequent milkshake consumers (24) as well as to passive viewing of appetitive food images (25, 26), thereby suggesting that this region is related to the motivational salience of palatable foods. Greater insula activation to appetitive food images has predicted less-successful weight loss (27), whereas greater activation in response to food commercials in adolescents has predicted weight gain at 1 y of follow-up (8). Insular activation in the current study may suggest that there is a higher incentive salience of food that ultimately manifests in weight variability.
The dorsal and middle cingulate cortices receive projections from key dopaminergic regions of the brain and are considered to be integral in the processing of reward-related stimuli (22). Greater activation in these regions has been associated with the viewing of highly palatable food images when fasted (28), thinking about liked foods compared with not-liked foods (29), and subjective pleasantness ratings of food odors (30). The mPFC has not been as well represented in the food literature especially in healthy-weight individuals. However, the animal literature and drug research in humans have established the mPFC as a region of the mesocorticolimbic dopaminergic system, which is responsible for reward-related decision making and motivation (31, 32). One food-related study showed bilateral mPFC activation to high-calorie–food images compared with low-calorie–food images when participants were instructed to remember the images for a later recognition test (33). Finally, the occipital gyrus and cuneus are most-commonly associated with visual processing. Two studies showed greater activation in these regions to palatable compared with unpalatable foods (26, 34). Similarly, greater cuneus activation to food commercials than to nonfood commercials predicted weight gain over 1 y of follow-up (8). The activation in these regions that underlies attention, reward, and food-specific memory in response to food tastes predicted the subsequent variability in body weight.
In contrast, MTG and precuneus responses to milkshake receipt predicted less weight variability. Studies have shown that the MTG shows greater activation in response to food images in a sated state than in a hungry state (35), in the viewing of nonfood images than of food images when hungry (36), and in the viewing of low-calorie–food images than of high-calorie–food images (33), thereby suggesting that MTG activation is involved in some aspect of control over food intake. The precuneus appears to play a role in many functions, but it has been argued that this region is related to self-referential processes and appetite control (37). The precuneus has been further implicated in the valuation of the benefits of not eating compared with eating high-calorie palatable foods (38). Taken together, it appears that the activation of the MTG and precuneus on palatable taste receipt may be protective against weight variability. However, more work is necessary to further elucidate this relation.
In sum, findings of milkshake > tasteless solution suggest that activations in regions that have been previously associated with hedonic valuation and incentive salience of food are related to future weight variability. Although these regions are not considered to be traditional reward regions, the regions that predicted future weight variability are involved in incentive salience and have been related to eating motivation, palatable food viewing, and weight gain across other studies, thereby suggesting the relevance of these regions to eating-related outcomes. These activation patterns persist even when the net BMI change is controlled, which bolsters the claim that weight variability assesses a unique weight characteristic that is distinct from weight gain. However, the regions that predicted less weight variability over follow-up disappeared with the inclusion of net BMI change. This result suggests that, at high levels of weight variability, the BMI change serves as a suppressor variable, thereby improving the unique predictive power of weight variability when the BMI change is included in the model. However, in conditions of low-weight variability, the BMI change and weight variability seem to be more conflated, and the predictive nature of weight variability is unknown. Together, these findings underscore the importance of examining weight variability and the net weight change in concert as a marker of vulnerability to the overconsumption of food. These factors may be able to predict who is most vulnerable to overconsumption, which, in excess, has been shown to impair inhibitory control and may ultimately lead to unopposed weight gain (39).
Despite the relation of a number of brain regions to body weight variability, the current study showed that no regions predicted body fat variability in the current sample in response to milkshake receipt. Body fat variability only accounted for 22% of the variance in BMI variability. This value was far lower than the 50–80% of variance in BMI that was explained by body fat in studies of adults (40, 41). In addition to the fact that our participants were still developing physically, it is possible that the variance accounted for in this sample was smaller because the design of the study called for a restriction in body weight at baseline; BMI tends to be correlated more highly with body fat in larger children and adolescents (21). It is also possible that, because this was an adolescent sample, body weight variability captured fluctuations in lean muscle mass, bone density, and other factors beyond body fat. Finally, mixed findings have suggested that air-displacement plethysmography may not be a reliable method of body fat assessment in this adolescent age group (42). In sum, this pattern of findings implies that the variation in muscle mass and bone density may play a more critical role in driving the variation in BMI during adolescence than during adulthood. It is possible that brain activity is more predictive of gross fluctuations in body weight than of isolated changes in body fat mass or that methodologic limitations drove this pattern of results.
In the current study, all significant activation emerged as a result of receipt of the milkshake rather than from the cue that signaled impending receipt. Although there was not an a priori reason to suspect this pattern of results, the finding strengthen the argument for examining the relation of the neural response to the actual taste of palatable food to future weight variability. Converse to the current findings, neural regions that are related to fat gain in the same sample were largely observed in response to the milkshake cue (10). This result bolsters the argument that weight variability and the net weight or fat change are somewhat distinct and potentially complementary constructs.
To our knowledge, this is the first study to directly relate brain activation in response to any food stimulus to weight variability. Additional strengths of the current study include the large sample, the inclusion of large samples of both male and female adolescents in an imaging study, the prospective design, and the manipulation that involved brain responses to both food cues signaling subsequent taste and actual tastes of a palatable food. Moreover, the inclusion of healthy-weight adolescents allowed for the examination of neural correlates of weight variability that were not confounded by existing overweight. Finally, the study was able to both establish a link between weight variability and a family history of overweight or obesity and showed that the findings were preserved when this familial association was included in the statistical model. Limitations include the fact that all participants were adolescents, which implied that the results may not be generalizable to other developmental periods. In addition, because 80% of the sample was Caucasian, the results may not be generalizable to ethnic minority groups. Baseline weight variability was controlled for with the use of self-reported weights before study enrollment. It is possible that these self-reported weights were not accurate. In addition, BMI was only assessed annually throughout the course of the study, which limited the ability to precisely model the degree of weight variability that was shown by participants. It is possible that 4 BMI measurements are not sufficient for modeling the RMSE; however, the significant findings that related brain activity to future BMI variability suggested that there was sufficient sensitivity with only the 4 measurements. Finally, findings were not related to subjective ratings of milkshake pleasantness or the frequency of milkshake consumption.
In conclusion, the findings from the current study underscore the importance of examining weight variability for 2 major reasons. First, increased weight variability might represent some sort of early marker that is ultimately associated with a chronic positive energy balance and weight gain (1). The magnitude of the neural response that predicts weight variability in the current study reflects the potential utility of weight variability in addition to weight gain in the understanding of eating and weight regulation. Second, research has linked weight variability to a number of adverse health events. Although much more work needs to be done to fully understand the genesis of weight variability and its downstream implications, it is possible that weight variability in and of itself is important from a clinical and public health perspective.
Acknowledgments
The authors would like to thank the staff of the Lewis Center for Neuroimaging at the University of Oregon for their assistance in data collection for this project.
The authors’ responsibilities were as follows—SRW: analyzed the data, performed the statistical analysis, and wrote the manuscript; SY: designed and conducted the research and wrote the manuscript; ES: designed and conducted the research and edited the manuscript; KO: analyzed the data; MRL: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: mPFC, medial prefrontal cortex; MTG, middle temporal gyrus; RMSE, root mean squared error.
REFERENCES
- 1.Lowe MR, Feig EH, Winter SR, Stice E. Short-term variability in body weight predicts long-term weight gain. Am J Clin Nutr 2015;102:995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lissner L. Health consequences of weight cycling? An open question. Nutr Bull 1996;21:95–101. [Google Scholar]
- 3.Diaz VA, Mainous AG, Everett CJ. The association between weight fluctuation and mortality: results from a population-based cohort study. J Community Health 2005;30:153–65. [DOI] [PubMed] [Google Scholar]
- 4.French S, Folsom A, Jeffery R, Zheng W, Mink P, Baxter J.. Weight variability and incident disease in older women: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord 1997;21:217–23. [DOI] [PubMed] [Google Scholar]
- 5.Folsom A, French S, Zheng W, Baxter J, Jeffery R. Weight variability and mortality: the Iowa women’s health study. Int J Obes Relat Metab Disord 1996;20:704–9. [PubMed] [Google Scholar]
- 6.Muls E, Kempen K, Vansant G, Saris W. Is weight cycling detrimental to health? A review of the literature in humans. Int J Obes Relat Metab Disord 1995;19 Suppl 3:S46–50. [PubMed] [Google Scholar]
- 7.Demos KE, Kelley WM, Heatherton TF. Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. J Cogn Neurosci 2011;23:1952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring) 2014;22:2544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 2011;19:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stice E, Burger KS, Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J Neurosci 2015;35:10316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geha PY, Aschenbrenner K, Felsted J, O’Malley SS, Small DM. Altered hypothalamic response to food in smokers. Am J Clin Nutr 2013;97:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr 1998;132:204–10. [DOI] [PubMed] [Google Scholar]
- 13.Lohman TG, Hingle M, Going SB. Body composition in children. Pediatr Exerc Sci 2013;25:573–90. [DOI] [PubMed] [Google Scholar]
- 14.Stice E, Spoor S, Bohon C, Small D. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008;322:449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses 2007;32:569–81. [DOI] [PubMed] [Google Scholar]
- 16.Lowe MR, Feig EH, Winter SR, Stice E. Short-term variability in body weight predicts long-term weight gain. Am J Clin Nutr 2015;102:995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller C, Siegrist M. Ambivalence toward palatable food and emotional eating predict weight fluctuations. Results of a longitudinal study with four waves. Appetite 2015;85:138–45. [DOI] [PubMed] [Google Scholar]
- 18.Stice E, Yokum S. Gain in body fat is associated with increased striatal response to palatable food cues, whereas body fat stability is associated with decreased striatal response. J Neurosci 2016;36:6949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Ann Epidemiol 2007;17:44–50. [DOI] [PubMed] [Google Scholar]
- 20.Stice E, Sysko R, Roberto CA, Allison S. Are dietary restraint scales valid measures of dietary restriction? Additional objective behavioral and biological data suggest not. Appetite 2010;54:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics 2009;124 Suppl 1:S23–34. [DOI] [PubMed] [Google Scholar]
- 22.Asmaro D, Liotti M. High-caloric and chocolate stimuli processing in healthy humans: an integration of functional imaging and electrophysiological findings. Nutrients 2014;6:319–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav 2012;106:317–24. [DOI] [PubMed] [Google Scholar]
- 24.Stice E, Burger KS. Neurobiology of overeating. Chichester (United Kingdom): John Wiley & Sons Ltd.; 2012. [Google Scholar]
- 25.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate. Brain 2001;124:1720–33. [DOI] [PubMed] [Google Scholar]
- 26.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis 2004;23:39–53. [DOI] [PubMed] [Google Scholar]
- 27.Murdaugh DL, Cox JE, Cook EW, Weller RE. fMRI reactivity to high-calorie food pictures predicts short-and long-term outcome in a weight-loss program. Neuroimage 2012;59:2709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroemer NB, Krebs L, Kobiella A, Grimm O, Vollstädt‐Klein S, Wolfensteller U, Kling R, Bidlingmaier M, Zimmermann US, Smolka MN. (Still) longing for food: insulin reactivity modulates response to food pictures. Hum Brain Mapp 2013;34:2367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage 2004;23:1486–93. [DOI] [PubMed] [Google Scholar]
- 30.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav 2005;85:45–56. [DOI] [PubMed] [Google Scholar]
- 31.Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids 2000;19:211–9. [DOI] [PubMed] [Google Scholar]
- 32.Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol 2005;493:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high-versus low-calorie foods. Neuroimage 2003;19:1381–94. [DOI] [PubMed] [Google Scholar]
- 34.van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 2011;55:296–303. [DOI] [PubMed] [Google Scholar]
- 35.Santel S, Baving L, Krauel K, Münte TF, Rotte M. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res 2006;1114:138–48. [DOI] [PubMed] [Google Scholar]
- 36.Führer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–50. [DOI] [PubMed] [Google Scholar]
- 37.Tuulari JJ, Karlsson HK, Hirvonen J, Salminen P, Nuutila P, Nummenmaa L. Neural circuits for cognitive appetite control in healthy and obese individuals: an fMRI study. PLoS One 2015;10:e0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokum S, Stice E. Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes (Lond) 2013;37:1565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkow ND, Wang G-J, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 2011;15:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deurenberg P, Yap M, Van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Metab Disord 1998;22:1164–71. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson R. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 1994;60:23–8. [DOI] [PubMed] [Google Scholar]
- 42.Radley D, Gately P, Cooke C, Carroll S, Oldroyd B, Truscott J. Estimates of percentage body fat in young adolescents: a comparison of dual-energy X-ray absorptiometry and air displacement plethysmography. Eur J Clin Nutr 2003;57:1402–10. [DOI] [PubMed] [Google Scholar]