Abstract
Background: The potential confounding effect of different amounts and proportions of macronutrients across eating patterns on meal or dietary glycemic index (GI) and glycemic load (GL) value determinations has remained partially unaddressed.
Objective: The study aimed to determine the effects of different amounts of macronutrients and fiber on measured meal GI and GL values.
Design: Four studies were conducted during which participants [n = 20–22; women: 50%; age: 50–80 y; body mass index (in kg/m2): 25–30)] received food challenges containing different amounts of the variable nutrient in a random order. Added to the standard 50 g available carbohydrate from white bread was 12.5, 25, or 50 g carbohydrate; 12.5, 25, or 50 g protein; and 5.6, 11.1, or 22.2 g fat from rice cereal, tuna, and unsalted butter, respectively, and 4.8 or 9.6 g fiber from oat cereal. Arterialized venous blood was sampled for 2 h, and measured meal GI and GL and insulin index (II) values were calculated by using the incremental area under the curve (AUCi) method.
Results: Adding carbohydrate to the standard white-bread challenge increased glucose AUCi (P < 0.0001), measured meal GI (P = 0.0066), and mean GL (P < 0.0001). Adding protein (50 g only) decreased glucose AUCi (P = 0.0026), measured meal GI (P = 0.0139), and meal GL (P = 0.0140). Adding fat or fiber had no significant effect on these variables. Adding carbohydrate (50 g), protein (50 g), and fat (11.1 g) increased the insulin AUCi or II; fiber had no effect.
Conclusions: These data indicate that uncertainty in the determination of meal GI and GL values is introduced when carbohydrate-containing foods are consumed concurrently with protein (equal amount of carbohydrate challenge) but not with carbohydrate-, fat-, or fiber-containing foods. Future studies are needed to evaluate whether this uncertainty also influences the prediction of average dietary GI and GL values for eating patterns. This trial was registered at clinicaltrials.gov as NCT01023646.
Keywords: glycemic index, glycemic load, healthy participants, macronutrients and fiber, variability
INTRODUCTION
Carbohydrate-containing foods differ in their effects on blood glucose concentrations. The concept of the glycemic index (GI)4 was introduced to differentiate foods on the basis of the blood glucose response to 50 g available carbohydrate during a 2-h period (1). The concept of the glycemic load (GL) was introduced to adjust for serving sizes (2, 3). A considerable body of work has assessed the association between average dietary GI and GL values, which were calculated with the use of dietary questionnaire data, and chronic-disease risk (4–17). Low-GI or low-GL diets have sometimes been associated with a reduced risk of cardiovascular disease (4–6) and diabetes (7–11) but not consistently (12–17). The inconsistency may have been introduced by the variability in average dietary GI and GL values that were calculated from dietary questionnaires or caused by unaccounted-for compositional, methodologic, or physiologic factors (18, 19).
The average meal or dietary GI value, which is defined as the average sum of all foods eaten daily, or the average meal or dietary GL value, which is defined as the average GI value that is corrected for the carbohydrate content of each item by the serving size, has been calculated with the use of data that was derived from instruments that were designed to estimate overall daily food intake (7–9). However, eating occasions usually contain multiple foods and beverages, thereby resulting in different absolute amounts of carbohydrate and combinations of carbohydrate with fat, protein, or fiber. Thus, the accuracy of average meal or dietary GI and GL values may be dependent on the GI value of individual foods that comprise daily intake as well as on unaccounted-for factors that are imposed by the combination of different foods on different eating occasions. Although some studies have concluded that individual GI and GL values can be used to accurately calculate meal or dietary GI and GL values (2, 4, 7, 20–22), other studies have suggested that individual GI and GL values do not accurately reflect meal or dietary GI and GL values (23–29). Dietary protein, fat, and fiber can modify the gastrointestinal transit time (30–34), which, in turn, can alter rates of glucose absorption; and some fatty acids and amino acids can stimulate insulin and glucagon secretions (35–38), thereby influencing blood glucose homeostasis.
Our objective was to assess the effects of different amounts of macronutrients (carbohydrate, protein, and fat) and fiber added to a standard test food (50 g available carbohydrate from white bread) on measures of glucose homeostasis, meal GI and GL values, and serum lipid concentrations. Our overall hypothesis was that different amounts of additional macronutrients or fiber would affect the postprandial glycemic response and, hence, alter measured meal GI and GL values and concentrations of serum insulin, HDL cholesterol, LDL cholesterol, triacylglycerol, and nonesterified fatty acids (NEFAs).
METHODS
Study population
Study participants [n = 20–22/study; women: 50% (all women were postmenopausal); age: 50–80 y; BMI (in kg/m2): 25–35] were recruited from the Greater Boston area. Exclusion criteria were as follows: fasting glucose concentrations ≥7 mmol/L; known chronic diseases (cardiovascular disease, diabetes, kidney, thyroid, and liver diseases); untreated hypertension; irritable bowel syndrome or malabsorptive disorder; the use of medications known to affect glucose or lipid metabolism; smoking; abnormal blood chemistry; poor venous access; alcohol consumption >7 drinks/wk; weight gain or loss ≥5 kg within the past 6 mo; and unwillingness to adhere to the study protocol. The current study was conducted according to the Declaration of Helsinki guidelines, and all procedures were approved by the Institutional Review Board of Tufts University/Tufts Medical Center. Written informed consent was obtained from all the study participants. This trial was registered at clinicaltrials.gov as NCT01023646 on 30 November 2009. The studies reported here were conducted between 2011 and 2015.
Sample size estimation
With the use of an SD of 22 for a mean GI value of 71 of white bread from our earlier study (18), a sample size of 20 in each study was estimated to provide 90% power and 80% power to detect differences in groups in mean GI values of 22.0 and 17.6, respectively. A sample size of 20 participants/group had 80% power to detect differences of 25 with SDs ≤31. The calculations were based on preliminary data (18), and α was set at 0.05 for all calculations.
Recruitment and screening
Any volunteer who responded to the study advertisements via e-mail or a telephone call was contacted to determine further interest in study participation. Volunteers were provided with information about the study design and, if interested, a first-screening telephone questionnaire was administered to determine general eligibility. If eligibility was established, a packet that included protocol-specific and medical-history questionnaires was sent to potential participants. For volunteers who returned the packet and were potentially eligible, a prescreening appointment was scheduled to acquaint them with Metabolic Research Unit (MRU) procedures and to collect screening data. If a potential participant’s characteristics fell within the predetermined criteria, the individual was invited for a second full health screening and asked to complete a physical-activity assessment form. This trial comprised 4 studies. Participant-flow diagrams of all studies are presented in Supplemental Figures 1–4. In study 1 (Supplemental Figure 1), 77 volunteers were screened, and 22 participants were enrolled in the study. One volunteer did not complete the study because of poor venous access, and one volunteer did not complete the study because of noncompliance with study procedures. In study 2 (Supplemental Figure 2), 55 volunteers were screened, and 23 participants were enrolled in the study. One volunteer was withdrawn because of noncompliance with study procedures. In study 3 (Supplemental Figure 3), 77 volunteers were screened, and 23 participants were enrolled into the study. Three volunteers did not complete the study because of the following reasons: poor venous access (n = 1), no longer interested (n = 1), and noncompliance with study procedures (n = 1). In study 4 (Supplemental Figure 4), 43 volunteers were screened, and 22 participants were enrolled in the study. Two of the volunteers did not complete the study because of a change in medical status.
Study design and interventions
Within each study, all foods or food combinations were referred to as food challenges. Detailed information about food challenges in all studies is presented in Supplemental Tables 1 and 2. In all studies, white bread (Pepperidge Farm Original White Bread; Pepperidge Farm Inc.) containing 50 g available carbohydrate was used as the test carbohydrate challenge to which the macronutrient-containing foods or fiber-containing food was added. Food combinations in study 1 (Supplemental Table 1) included white bread alone or with rice cereal (General Mills Rice Chex cereal; General Mills Inc.) that contained 12.5, 25, or 50 g available carbohydrate. Food combinations in study 2 (Supplemental Table 1) were white bread alone or with drained light tuna that was packed in water that contained 12.5, 25, or 50 g protein. Food combinations in study 3 (Supplemental Table 1) were white bread alone or with unsalted butter that contained 5.6, 11.1, or 22.2 g fat. In studies 1–3 (Supplemental Table 1), the amounts of carbohydrate, protein, and fat were calculated to provide an additional 50, 100, or 200 kcal. Food combinations in study 4 (Supplemental Table 2) were white bread alone or with oat cereal (General Mills Cheerios; General Mills Inc.) that contained an additional 4.8 or 9.6 g fiber. Because oat cereal also contains carbohydrate, the amount of oat cereal was adjusted so that the total available carbohydrate from white bread or oat cereal was 50 g. The test foods were chosen because they are commonly consumed and are relatively high in the macronutrient of interest and contained no additional carbohydrate or fiber that could confound the results. Chemical analyses of single foods as well as of food combinations were completed before the start of the studies, and the carbohydrate content was determined by differences (Covance Laboratories Inc.). Available carbohydrate (total carbohydrate by difference minus dietary fiber) was used in this study for GI and GL determinations. These data were cross-checked with the use of the USDA National Nutrient Database (https://ndb.nal.usda.gov/ndb/) before study initiation. Macronutrient and fiber contents of foods in all studies are presented in Supplemental Table 3. Sufficient food from a single lot was purchased to complete the studies. For all studies, a 500-mL glucose drink (100 g/L; 50 g were available as carbohydrate) was used as the reference food for GI calculations and was tested in each individual study. When a solid food constituted the food challenge, an equivalent volume of water (500 mL) to that of the glucose drink was provided. Within each study, all glucose or food challenges were tested in each study participant in a random order. The randomization sequence was generated by the statistician (LMA) before the start of the study with the use of a block design as described previously (19), and assignment was based on the enrollment date and time. HM, NRM, AHL, and all laboratory personnel were blinded to the testing order.
Participants underwent 5 sessions in the MRU in studies 1–3 and underwent 4 sessions in study 4. Sessions took place 1–2 times/wk with a maximum of 12 wk to complete all sessions. In the study period, participants were requested to maintain their habitual diets and physical activity patterns. Subjects were instructed not to engage in strenuous physical activities or consume alcohol 72 h before the test day and were also instructed to fast for 12 h before arrival at the MRU. Blood pressure and body weight were measured at each visit. Height, waist circumference, and hip circumference were measured at the first and last visits. Fifteen minutes before the intervention, a retrograde intravenous cannula was inserted into the lower cephalic or superficial dorsal veins of the hand for the collection of arterialized venous blood. A continuous normal-saline infusion was used to maintain the blood sampling line during the course of the challenge. In addition, 15 min before each blood-drawing time point, each volunteer was asked to place a hand in a moderately heated box (44–46°C). This technique avoided the inconsistencies that are associated with temperature control when heated pads are used. Immediately after the fasting blood sample was collected, participants were provided with the food challenge and were instructed to consume the food within 10 min under observation of an MRU staff member. Additional blood samples were collected at 15, 30, 45, 60, 90, and 120 min thereafter. During the test period, participants were requested to remain in the MRU under observation and were restricted to engaging in sedentary activities.
GI and GL calculations
Measured meal GI and GL values were used to represent GI and GL values of food challenges that were generated with the use of the incremental AUC (AUCi) that was measured in the study, whereas calculated meal GI and GL values were used to represent GI and GL values of food challenges that were calculated on the basis of a formula with the use of a weighted sum of GIs of individual contributing foods (21). Measured meal GI values were calculated by dividing the AUCi for blood glucose that was obtained after food challenges with 50 g available carbohydrate from the test food (white bread) with additional amounts of carbohydrate-, protein-, fat-, or fiber-containing foods by the AUCi that was obtained in response to the reference (glucose drink), which contained an equivalent amount of available carbohydrate (50 g glucose) over a 2-h period and multiplied by 100 (1, 39). The AUCi was calculated with the use of the geometric sums of the areas of the triangles and trapezoids above the fasting glucose concentration over a 2-h period as previously described (21). Per the recommended calculation method, the AUC that fell beneath the initial fasting glucose concentration was not included in the calculation. The glucose drink was used as the reference food for all calculations of measured meal GI and GL values. Similar calculations were used to obtain the insulin AUCi and insulin index (II).
In study 1, because the total amount of available carbohydrate of the food challenges in the 3 groups was >50 g, 2 different approaches were used for the data analysis. In the first approach (presented in Online Supplemental Material and Supplemental Figure 5), the GI was calculated with the use of the standard formula noted previously, and the amount of available carbohydrate in the glucose drink remained at 50 g regardless of the total amount of available carbohydrate in the food challenges. This approach aimed to keep the reference group consistent so that the only variable was the amount of available carbohydrate in the food challenges. In the second approach (presented in Results), glucose AUCi values in response to the glucose drink in different groups was adjusted to match the amount of available carbohydrate in the corresponding food challenges with the use of a previously published formula (20, 40). The relative glucose response (RGR) of the glucose drinks that contained 50, 62.5, 75, and 100 g available carbohydrate was calculated with the use of the GI value of a glucose drink with 50 g available carbohydrate (which was equal to 100) and the corresponding amount of available carbohydrate in the glucose drink (20, 40). Estimated glucose AUCi values were calculated by dividing the calculated RGR of the glucose drink that contained different amounts of available carbohydrate by the calculated RGR of the glucose drink that contained 50 g available carbohydrate, and this value was multiplied by the glucose AUCi of the glucose drink that contained 50 g available carbohydrate as measured in our study. The measured meal GI value in study 1 was calculated by dividing the glucose AUCi of food challenges by the predicted glucose AUCi of the glucose drink with same amount of available carbohydrate as in the food challenges, and this value was multiplied by 100. The II in study 1 was calculated with the use of the same method that was used for GI values and with a previously published formula (20, 40).
Calculated meal GI values were the weighted sum of GI contributions of each carbohydrate-containing foods, which were obtained by multiplying the GI value (measured in the current study) of an individual food by the percentage of available carbohydrate in each food relative to the total available carbohydrate in the food challenges (21). The measured and calculated meal GLs were calculated by adjusting the corresponding meal GI values by the available carbohydrate content per serving (2, 3).
Biochemical measures
In every food challenge, serum concentrations of glucose, insulin, HDL cholesterol, LDL cholesterol, triacylglycerol, and NEFA were monitored throughout the 2-h study period. Blood samples were allowed to clot at room temperature for 30 min, and serum was immediately separated by centrifugation at 1500 × g at 4°C for 20 min. Serum glucose concentrations were determined with the use of an enzymatic method (assay CV <2%; Roche Diagnostics). Serum insulin concentrations were measured with the use of an ELISA kit (assay CV <5%; ALPCO Diagnostics). Serum HDL-cholesterol, LDL-cholesterol, and triacylglycerol concentrations were measured with the use of an automated analyzer (Hitachi 911; Roche Diagnostics) with the use of enzymatic reagents, and serum NEFA was determined with the use of an in vitro enzymatic method (Wako Chemicals). The lipid assays were standardized through the Lipid Standardization Program of the CDC. High-sensitivity C-reactive protein was measured with the use of the Tina-quant CRP (Latex) High Sensitive immunoturbidimetric assay (Roche Diagnostics). Glycated hemoglobin and fructosamine were measured with the use of immunoturbidimetric and enzymatic assays, respectively (Roche Diagnostics). The HOMA score was calculated according to the following formula (41):
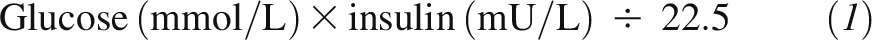 |
Habitual physical activity assessment
Physical activity levels were assessed with the use of Community Healthy Activities Model Program for Seniors questionnaire. The physical activity questionnaire was scored with the use of the coding algorithm that was developed by the Community Healthy Activities Model Program for Seniors (42), and the average weekly physical activity energy expenditure was expressed as kcal/wk.
Statistical analyses
Data were analyzed with the use of SAS for Windows software (version 9.4; SAS Institute). Descriptive statistics and graphs (PROC UNIVARIATE; SAS Institute) were used to summarize the distributions of outcome measures. In all studies, comparisons were made only in food challenges with white bread. A 2-factor mixed ANOVA (PROC MIXED; SAS Institute) with the main effects of food challenges and time and the food challenges × time interaction with repeated measures for participants was carried out to determine differences in serum glucose, insulin, HDL-cholesterol, LDL-cholesterol, triacylglycerol, and NEFA concentrations in food challenges over the 2-h study period. When a food challenge × time interaction was significant at P < 0.05, multiple comparisons at each time point were carried out with the use of the Tukey-Kramer method. The mixed-design ANOVA model (PROC MIXED) was used to test the differences in glucose and insulin AUCi, measured meal GI and GL, calculated meal GI and GL, and II values in food challenges in each individual study. Outcomes were modeled as repeated measures with a compound symmetry covariance matrix. Participant was designated as a random effect, and the food challenge was a fixed effect. For all outcomes, model selection was based on optimizing fit statistics (evaluated as the lowest Bayesian information criterion), and α was set at 0.05 for all tests. The Tukey-Kramer method was used for post hoc analyses. Differences in calculated meal GI and GL values in study 1 were compared with the use of Friedman’s chi-square test with Dunn’s multiple comparison test as post hoc analyses because the data were not under normal distribution. Differences between measured and calculated meal GI and GL values within each food-challenge group were compared with the use of paired t test when the data were under normal distribution, and Wilcoxon’s signed rank sum test when the data were not under normal distribution. In all studies, analyses with glucose AUCi, insulin AUCi, measured meal GI and GL, and II values, with the exclusion of a possible outlier (which was defined as any data point beyond 2 SDs from the mean value), did not alter results, and therefore, the data for all subjects who completed all interventions are reported. Statistical significance was accepted at P ≤ 0.05. Graphs were plotted with the use of GraphPad Prism 5 software.
RESULTS
Baseline characteristics of study participants
Study participants were healthy, older adults (62–64 y), and 50% of the participants were women (Table 1). Mean BMI was within the overweight range, and blood pressure, waist circumference, and high-sensitive C-reactive protein, fasting serum glucose, insulin, glycated hemoglobin, fructosamine, NEFA, and lipoprotein concentrations were within optimal or near-optimal values. The mean weekly physical activity energy expenditure (on the basis of self-reported responses) was 3500–4800 kcal/wk.
TABLE 1.
Baseline characteristics of study participants1
| Variable | CHO plus additional CHO (n = 20) | CHO plus protein (n = 22) | CHO plus fat (n = 20) | CHO plus fiber (n = 20) |
| Age, y | 63 ± 92 | 64 ± 8 | 62 ± 7 | 63 ± 8 |
| Women, n (%) | 10 (50) | 11 (50) | 10 (50) | 10 (50) |
| Systolic blood pressure, mm Hg | 122 ± 11 | 123 ± 11 | 118 ± 9 | 121 ± 10 |
| Diastolic blood pressure, mm Hg | 78 ± 7 | 79 ± 6 | 75 ± 7 | 77 ± 9 |
| BMI, kg/m2 | 29.1 ± 1.9 | 29.3 ± 2.6 | 29.5 ± 2.2 | 29.3 ± 3.0 |
| Waist circumference, cm | 95.4 ± 8.8 | 96.6 ± 8.8 | 94.7 ± 9.1 | 95.9 ± 10.5 |
| Hip circumference, cm | 106.1 ± 4.8 | 104.8 ± 5.1 | 106.0 ± 4.7 | 106.3 ± 6.4 |
| Waist:hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| hsCRP, mg/L | 3.0 ± 3.4 | 4.0 ± 4.4 | 2.2 ± 2.0 | 3.1 ± 3.0 |
| Glucose, mmol/L | 5.1 ± 0.5 | 5.2 ± 0.5 | 5.4 ± 0.6 | 5.2 ± 0.6 |
| Insulin, mU/L | 13.0 ± 3.8 | 13.0 ± 5.2 | 13.3 ± 6.1 | 12.9 ± 6.8 |
| HOMA | 52.2 ± 16.2 | 54.0 ± 25.2 | 57.6 ± 28.8 | 55.8 ± 36.0 |
| HbA1c, % | 5.8 ± 0.4 | 5.7 ± 0.4 | 6.0 ± 0.9 | 5.6 ± 0.4 |
| Fructosamine, μmol/L | 279 ± 36 | 281 ± 29 | 279 ± 41 | 264 ± 41 |
| NEFA, mmol/L | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.3 |
| Serum lipids, mmol/L | ||||
| Total cholesterol | 4.9 ± 0.7 | 4.9 ± 1.1 | 4.4 ± 0.9 | 4.4 ± 1.0 |
| VLDL cholesterol | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.5 ± 0.3 |
| LDL cholesterol | 3.1 ± 0.5 | 3.0 ± 0.9 | 2.8 ± 0.8 | 2.7 ± 0.8 |
| Non-HDL cholesterol | 3.6 ± 0.6 | 3.5 ± 1.0 | 3.2 ± 0.7 | 3.2 ± 0.9 |
| HDL cholesterol | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.3 ± 0.3 | 1.2 ± 0.2 |
| Triacylglycerol | 1.1 ± 0.5 | 1.2 ± 0.7 | 0.9 ± 0.4 | 1.1 ± 0.6 |
| Total cholesterol:HDL cholesterol ratio | 3.8 ± 0.8 | 3.7 ± 0.9 | 3.6 ± 0.7 | 3.8 ± 0.7 |
| Physical activity, 1000 kcal/wk | 3.5 ± 2.3 | 4.8 ± 2.6 | 4.2 ± 3.0 | 4.3 ± 2.8 |
CHO, carbohydrate; HbA1c, glycated hemoglobin; hsCRP, high sensitive C-reactive protein; NEFA, nonesterified fatty acid.
Mean ± SD (all such values).
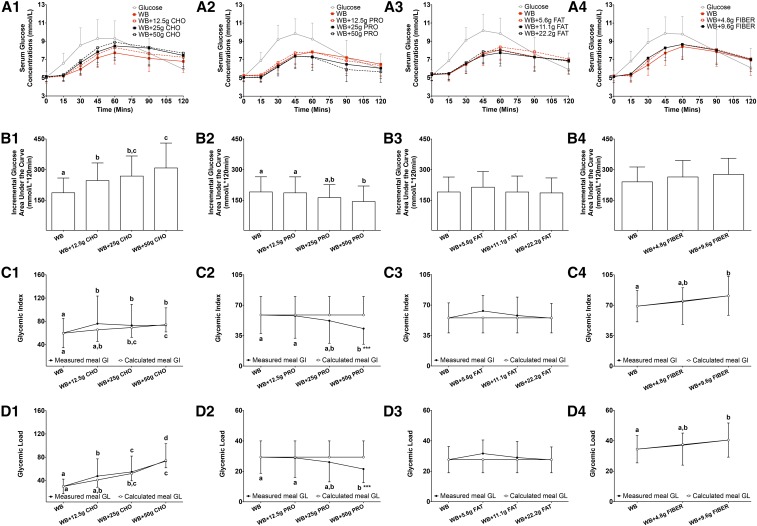
Addition of increasing amounts of carbohydrate to white bread altered postprandial glucose and insulin AUCi and measured meal GI and GL values
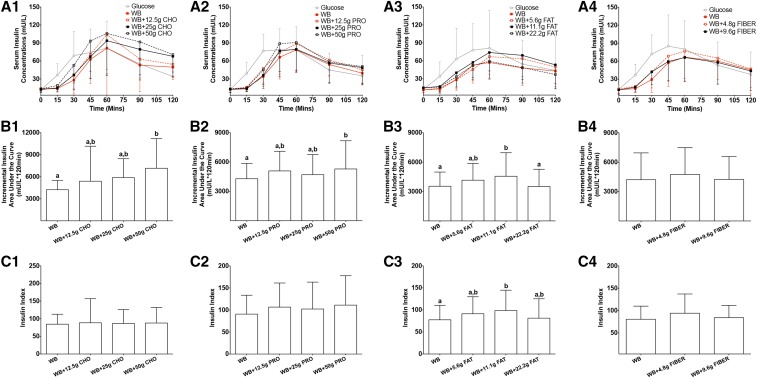
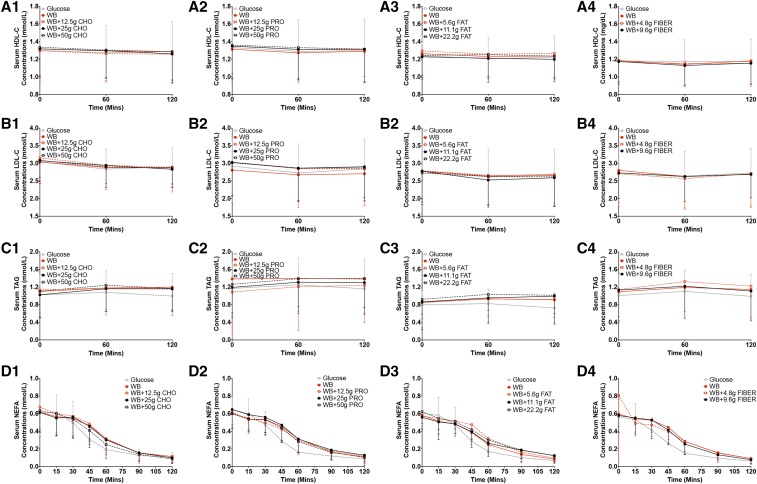
During the 2-h test period, increasing the carbohydrate content of the food challenge did not significantly affect serum glucose concentrations at each blood sampling time point (P = 0.98) (Figure 1A1, Table 2). However, differences in response to an additional 12.5, 25, and 50 g carbohydrate to white bread resulted in a higher glucose AUCi (by 32%, 44%, and 65%, respectively; P < 0.0001) (Figure 1B1), higher measured meal GI values (27%, 22% and 23%, respectively; P = 0.0066) (Figure 1C1; the measured GI value of white bread alone was 60, and the GI value of rice cereal alone was 89), and higher measured meal GL values (59%, 84% and 146%, respectively; P < 0.0001) (Figure 1D1). The addition of 25 and 50 g carbohydrate to white bread also resulted in higher calculated meal GI values (by 16% and 24%, respectively; P < 0.0001) (Figure 1C1) and higher calculated meal GL values (by 73% and 148%, respectively; P < 0.0001) (Figure 1D1), and the changes were similar to measured meal GI (Figure 1C1) and meal GL (Figure 1D1) values. Serum insulin concentrations during the 2-h test period were not significantly different at the blood sampling time points (P = 0.78) (Figure 2A1, Table 3). The addition of the highest amount of carbohydrate (50 g) resulted in a higher insulin AUCi (by 69%; P = 0.0019) (Figure 2B1). There was no significant effect of the additional carbohydrate on II values (P = 0.43) (Figure 2C1) or postprandial HDL-cholesterol (P = 0.88) (Figure 3A1), LDL-cholesterol (P = 0.95) (Figure 3B1), triacylglycerol (P = 1.00) (Figure 3C1), or NEFA (P = 0.98) (Figure 3D1) concentrations.
FIGURE 1.
Mean ± SD glycemic response and measured and calculated meal GI and GL after the consumption of a glucose reference and WB with different amounts of additional macronutrients or fiber. Serum postprandial glucose concentrations (A1–A4), incremental glucose AUC (B1–B4), measured and calculated meal GI (C1–C4), and measured and calculated meal GL (D1–D4) after the food challenges or consumption of the glucose reference drink during the 2-h test period are presented for studies 1–4. Differences in serum postprandial glucose concentrations (A1–A4) in food challenges over the 2-h test period were determined with the use of 2-factor mixed ANOVA with the main effects of food challenges and time and the food challenges × time interaction with repeated measures. Differences in the incremental glucose AUC (B1−B4), measured and calculated meal GI (C1–C4) and measured and calculated meal GL (D1–D4) values in food challenges over the 2-h test period in each individual study were determined with the use of mixed-design ANOVA model with the participant as a random effect and the food challenge as a fixed effect. The Tukey-Kramer method was used for the post hoc analyses. Differences in calculated meal GI and GL values in study 1 were compared with the use of Friedman’s chi-square test with Dunn’s multiple-comparison test as post hoc analyses. Differences between measured and calculated meal GI and GL values within each food-challenge group were compared with the use of a paired t test or Wilcoxon’s signed rank sum test depending on the data distribution. The statistical analysis was performed only with food challenges that contained WB and did not include the glucose reference drink. Statistical analyses with measured meal GI and GL values and calculated meal GI and GL values were performed separately. Significance was accepted at P ≤ 0.05. Means denoted by different lowercase letters were significantly different from each other. ***Significantly different from calculated meal GI or GL values within the same food-challenge group, P < 0.001. Sample sizes for studies 1–4 were 20, 22, 20, and 20, respectively. CHO, carbohydrate; GI, glycemic index; GL, glycemic load; PRO, protein; WB, white bread.
TABLE 2.
Glycemic response after consumption of a glucose reference and WB with different amounts of additional macronutrients or fiber1
| Time, min |
||||||||
| Serum glucose | 0 | 15 | 30 | 45 | 60 | 90 | 120 | P-time × food |
| CHO plus additional CHO (n = 20), mmol/L | 0.98 | |||||||
| Glucose | 5.0 ± 0.6 | 6.6 ± 1.1 | 8.6 ± 1.9 | 9.3 ± 2.1 | 9.3 ± 1.9 | 7.7 ± 1.6 | 5.9 ± 1.7 | |
| WB | 5.1 ± 0.6 | 5.2 ± 0.5 | 5.9 ± 0.7 | 7.2 ± 1.1 | 7.7 ± 1.2 | 7.1 ± 1.4 | 6.8 ± 1.2 | |
| WB + 12.5 g CHO | 5.0 ± 0.6 | 5.2 ± 0.6 | 6.3 ± 1.1 | 7.6 ± 1.2 | 8.3 ± 1.3 | 7.6 ± 1.4 | 7.2 ± 1.2 | |
| WB + 25 g CHO | 5.1 ± 0.2 | 5.2 ± 0.5 | 6.6 ± 1.2 | 7.8 ± 1.6 | 8.5 ± 1.6 | 8.2 ± 1.9 | 7.4 ± 1.2 | |
| WB + 50 g CHO | 5.0 ± 0.5 | 5.3 ± 0.7 | 6.9 ± 1.5 | 8.3 ± 1.6 | 8.9 ± 1.7 | 8.3 ± 1.9 | 7.7 ± 1.7 | |
| CHO plus protein (n = 22), mmol/L | 0.09 | |||||||
| Glucose | 5.1 ± 0.6 | 6.9 ± 0.9 | 9.2 ± 1.6 | 9.8 ± 1.6 | 9.2 ± 1.8 | 7.2 ± 1.9 | 6.0 ± 1.6 | |
| WB | 5.2 ± 0.5 | 5.2 ± 0.6 | 6.4 ± 1.0 | 7.5 ± 1.0 | 7.8 ± 1.3 | 7.2 ± 1.5 | 6.5 ± 1.3 | |
| WB + 12.5 g protein | 5.3 ± 0.6 | 5.3 ± 0.5 | 6.7 ± 0.8 | 7.7 ± 1.2 | 7.9 ± 1.4 | 6.9 ± 1.4 | 6.3 ± 1.2 | |
| WB + 25 g protein | 5.1 ± 0.5 | 5.0 ± 0.4 | 6.2 ± 0.8 | 7.4 ± 1.2 | 7.3 ± 1.1 | 6.5 ± 1.2 | 6.1 ± 1.1 | |
| WB + 50 g protein | 5.0 ± 0.5 | 5.1 ± 0.6 | 6.3 ± 1.1 | 7.4 ± 1.4 | 7.3 ± 1.5 | 5.9 ± 1.3 | 5.7 ± 1.2 | |
| CHO plus fat (n = 20), mmol/L | 0.71 | |||||||
| Glucose | 5.3 ± 0.6 | 6.9 ± 0.9 | 9.1 ± 1.4 | 10.2 ± 1.8 | 9.8 ± 1.7 | 7.6 ± 1.8 | 5.9 ± 1.8 | |
| WB | 5.4 ± 0.6 | 5.4 ± 0.6 | 6.5 ± 1.0 | 7.6 ± 1.1 | 8.1 ± 1.1 | 7.3 ± 1.2 | 6.9 ± 1.4 | |
| WB + 5.6 g fat | 5.4 ± 0.7 | 5.4 ± 0.8 | 6.5 ± 1.0 | 7.6 ± 1.3 | 8.4 ± 1.3 | 7.9 ± 1.6 | 7.1 ± 1.4 | |
| WB + 11.1 g fat | 5.3 ± 0.7 | 5.5 ± 0.7 | 6.6 ± 1.1 | 7.4 ± 0.9 | 7.7 ± 1.5 | 7.3 ± 1.6 | 6.9 ± 1.1 | |
| WB + 22.2 g fat | 5.5 ± 0.8 | 5.5 ± 0.8 | 6.7 ± 0.9 | 7.9 ± 1.2 | 8.0 ± 1.4 | 7.3 ± 1.5 | 6.8 ± 1.4 | |
| CHO plus fiber (n = 20), mmol/L | 0.35 | |||||||
| Glucose | 5.2 ± 0.7 | 6.8 ± 1.1 | 8.9 ± 1.5 | 9.9 ± 1.8 | 9.8 ± 1.9 | 7.9 ± 2.4 | 6.1 ± 2.1 | |
| WB | 5.2 ± 0.7 | 5.3 ± 0.6 | 6.4 ± 1.1 | 7.7 ± 1.3 | 8.4 ± 1.4 | 7.9 ± 1.6 | 7.0 ± 1.7 | |
| WB + 4.8 g fiber | 5.2 ± 0.6 | 5.2 ± 0.7 | 6.9 ± 0.9 | 8.3 ± 1.0 | 8.7 ± 1.4 | 8.1 ± 1.5 | 7.1 ± 1.1 | |
| WB + 9.6 g fiber | 5.1 ± 0.6 | 5.4 ± 0.8 | 7.2 ± 1.1 | 8.3 ± 1.4 | 8.7 ± 1.6 | 8.0 ± 1.6 | 7.0 ± 1.3 | |
All values are means ± SDs. The statistical analysis was performed only with food challenges that contained WB and did not include the glucose reference drink. A 2-factor mixed ANOVA with the main effects of food challenges and time and the food challenges × time interaction with repeated measures for participants was carried out to determine differences in serum glucose concentrations in food challenges over a 2-h study period. The food challenges × time interaction did not reach significance at P ≤ 0.05 in any study. CHO, carbohydrate; WB, white bread.
FIGURE 2.
Mean ± SD insulin response and insulin index after consumption of a glucose reference and WB with different amounts of additional macronutrients or fiber. Serum postprandial insulin concentrations (A1–A4), incremental insulin AUC (B1–B4), and insulin index (C1–C4) after food challenges and consumption of the glucose reference drink during the 2-h test period are presented for studies 1–4. Differences in serum postprandial insulin concentrations (A1–A4) in food challenges over the 2-h test period were determined with the use of a 2-factor mixed ANOVA with the main effects of food challenges and time and the food challenges × time interaction with repeated measures. Differences in incremental insulin AUC (B1–B4) and insulin index (C1–C4) values in food challenges over the 2-h test period in each individual study were determined with the use of a mixed-design ANOVA model with the participant as a random effect and the food challenge as a fixed effect. The Tukey-Kramer method was used for post hoc analyses. The statistical analysis was performed only with food challenges that contained WB and did not include the glucose reference drink. Significance was accepted at P ≤ 0.05. Means denoted by different lowercase letters were significantly different from each other. Sample sizes for studies 1–4 were 20, 22, 20, and 20, respectively. CHO, carbohydrate; PRO, protein; WB, white bread.
TABLE 3.
Insulin response after consumption of a glucose reference and WB with different amounts of additional macronutrients or fiber1
| Time, min |
||||||||
| Serum insulin | 0 | 15 | 30 | 45 | 60 | 90 | 120 | P-time × food |
| CHO plus additional CHO (n = 20), mU/L | 0.78 | |||||||
| Glucose | 12.0 ± 4.1 | 36.5 ± 19.1 | 68.9 ± 40.3 | 74.1 ± 39.2 | 81.7 ± 44.2 | 55.0 ± 26.2 | 33.9 ± 21.1 | |
| WB | 13.2 ± 4.8 | 14.0 ± 5.6 | 28.1 ± 14.8 | 62.7 ± 31.3 | 81.4 ± 35.1 | 53.1 ± 21.6 | 49.6 ± 20.4 | |
| WB + 12.5 g CHO | 13.6 ± 4.9 | 15.1 ± 6.9 | 36.3 ± 31.3 | 70.8 ± 48.1 | 104.1 ± 108.6 | 63.2 ± 54.3 | 54.4 ± 21.9 | |
| WB + 25 g CHO | 12.5 ± 4.8 | 14.9 ± 5.1 | 36.9 ± 25.1 | 67.0 ± 36.7 | 93.9 ± 58.0 | 79.3 ± 42.5 | 67.8 ± 33.6 | |
| WB + 50 g CHO | 13.5 ± 5.2 | 19.0 ± 6.1 | 52.8 ± 29.5 | 93.3 ± 50.3 | 106.0 ± 67.7 | 91.8 ± 63.4 | 70.7 ± 29.1 | |
| CHO plus protein (n = 22), mU/L | 0.73 | |||||||
| Glucose | 12.8 ± 5.5 | 39.4 ± 17.7 | 76.7 ± 27.4 | 78.5 ± 35.4 | 78.5 ± 38.7 | 45.0 ± 27.9 | 35.2 ± 34.1 | |
| WB | 12.7 ± 5.7 | 14.9 ± 5.7 | 34.1 ± 16.7 | 66.4 ± 36.5 | 78.5 ± 33.6 | 54.1 ± 19.1 | 39.7 ± 19.8 | |
| WB + 12.5 g protein | 13.4 ± 5.2 | 16.3 ± 6.2 | 47.5 ± 24.2 | 74.3 ± 36.1 | 88.6 ± 44.5 | 61.7 ± 26.4 | 45.2 ± 21.9 | |
| WB + 25 g protein | 12.7 ± 5.7 | 13.6 ± 4.6 | 36.2 ± 21.9 | 76.8 ± 43.2 | 79.6 ± 38.4 | 56.6 ± 33.0 | 49.0 ± 28.1 | |
| WB + 50 g protein | 13.5 ± 6.3 | 15.1 ± 6.4 | 45.1 ± 24.8 | 88.9 ± 46.6 | 90.8 ± 48.9 | 58.4 ± 35.4 | 50.7 ± 28.4 | |
| CHO plus fat (n = 20), mU/L | 0.33 | |||||||
| Glucose | 12.5 ± 7.0 | 34.2 ± 24.0 | 63.2 ± 51.3 | 77.9 ± 44.1 | 81.3 ± 62.8 | 54.7 ± 40.0 | 44.3 ± 55.8 | |
| WB | 11.7 ± 4.5 | 14.1 ± 5.6 | 29.0 ± 15.8 | 52.7 ± 25.8 | 57.4 ± 26.1 | 48.4 ± 29.5 | 43.0 ± 28.2 | |
| WB + 5.6 g fat | 11.7 ± 4.9 | 12.5 ± 4.9 | 28.5 ± 17.4 | 45.7 ± 22.6 | 67.1 ± 29.2 | 63.7 ± 30.2 | 49.8 ± 32.4 | |
| WB + 11.1 g fat | 15.3 ± 12.6 | 17.2 ± 8.4 | 40.2 ± 23.8 | 57.5 ± 25.2 | 74.0 ± 30.8 | 69.3 ± 40.2 | 53.4 ± 31.6 | |
| WB + 22.2 g fat | 15.2 ± 16.3 | 16.3 ± 10.3 | 33.7 ± 17.3 | 52.4 ± 23.2 | 59.2 ± 29.5 | 48.9 ± 25.9 | 37.2 ± 23.9 | |
| CHO plus fiber (n = 20), mU/L | 0.54 | |||||||
| Glucose | 13.2 ± 7.1 | 36.6 ± 18.2 | 72.1 ± 31.8 | 85.1 ± 52.2 | 80.1 ± 47.4 | 53.3 ± 38.6 | 37.8 ± 37.4 | |
| WB | 12.5 ± 6.3 | 14.1 ± 6.2 | 29.5 ± 21.6 | 57.2 ± 39.1 | 66.8 ± 41.1 | 60.1 ± 38.5 | 45.8 ± 40.7 | |
| WB + 4.8 g fiber | 13.7 ± 8.0 | 17.9 ± 9.8 | 42.4 ± 27.0 | 68.5 ± 42.8 | 76.7 ± 45.2 | 65.0 ± 35.0 | 47.0 ± 30.5 | |
| WB + 9.6 g fiber | 12.2 ± 7.2 | 16.7 ± 8.0 | 42.3 ± 21.6 | 59.4 ± 31.8 | 66.2 ± 39.3 | 57.8 ± 36.0 | 43.6 ± 32.4 | |
All values are means ± SDs. The statistical analysis was performed only with food challenges that contained WB and did not include the glucose reference drink. A 2-factor mixed ANOVA with the main effects of food challenges and time and the food challenges × time interaction with repeated measures for participants was carried out to determine differences in serum insulin concentrations in food challenges over a 2-h study period. The food challenges × time interaction did not reach significance at P ≤ 0.05 in any study. CHO, carbohydrate; WB, white bread.
FIGURE 3.
Mean ± SD postprandial serum HDL-C, LDL-C, TAG, and NEFA responses to challenges with a glucose reference and WB with different amounts of additional macronutrients or fiber. Serum HDL-C (A1–A4), LDL-C (B1–B4), TAG (C1–C4), and NEFA (D1–D4) concentrations after food challenges and consumption of the glucose reference drink during the 2-h test period are presented for studies 1–4. Differences in serum HDL-C (A1–A4), LDL-C (B1–B4), TAG (C1–C4), and NEFA (D1–D4) concentrations in food challenges over the 2-h test period were determined with the use of 2-factor mixed ANOVA with the main effects of food challenges and time and the food challenges × time interaction with repeated measures. The statistical analysis was performed only with food challenges that contained WB and did not include the glucose reference drink. Significance was accepted at P ≤ 0.05. Sample sizes for studies 1–4 were 20, 22, 20, and 20, respectively. CHO, carbohydrate; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; NEFA, nonesterified fatty acid; PRO, protein; TAG, triacylglycerol; WB, white bread.
Addition of increasing amounts of protein to white bread altered postprandial glucose and insulin AUCi and measured meal GI and GL values
In the 2-h test period, the addition of increasing amounts of protein to the carbohydrate challenge had no significant effect on serum glucose concentrations at any of the blood sampling time points (P = 0.09) (Figure 1A2, Table 2). However, the addition of the highest amount of protein (50 g) to white bread resulted in a lower glucose AUCi (by 25%; P = 0.0026) (Figure 1B2), a lower measured meal GI value (by 27%; P = 0.0139) (Figure 1C2), and a lower meal GL value (by 27%; P = 0.0140) (Figure 1D2). In contrast, the calculated meal GI (Figure 1C2) and meal GL (Figure 1D2) values remained constant with the addition of increasing amounts of protein to the carbohydrate challenge and were significantly smaller than the measured meal GI (Figure 1C2; P = 0.0006) and meal GL (P = 0.0006) (Figure 1D2) values with the addition of 50 g protein. There was no significant effect of the addition of protein to the white-bread challenge on insulin concentrations at any time point (P = 0.73) (Figure 2A2, Table 3). The addition of 50 g protein to the white-bread challenge resulted in a higher insulin AUCi (by 23%; P = 0.0386) (Figure 2B2) but had no significant effect on II values (P = 0.14) (Figure 2C2). The addition of different amounts of protein to the white-bread challenge had no significant effect on postprandial HDL-cholesterol (P = 0.94) (Figure 3A2), LDL-cholesterol (P = 0.99) (Figure 3B2), triacylglycerol (P = 1.00) (Figure 3C2), or NEFA (P = 1.00) (Figure 3D2) concentrations.
Addition of increasing amounts of fat to white bread altered postprandial insulin AUCi and II values but had no significant effect on glucose AUCi or measured meal GI or GL values
Similar to what was observed for additional carbohydrate and the addition of protein to the white-bread challenge, in the 2-h test period, the addition of increasing amounts of fat to the carbohydrate challenge had no significant effect on serum glucose concentrations at each blood sampling time point (P = 0.71) (Figure 1A3, Table 2). The addition of fat to the white-bread challenge had no significant effect on the glucose AUCi (P = 0.23) (Figure 1B3), measured meal GI (P = 0.31) (Figure 1C3) and measured meal GL (P = 0.31) (Figure 1D3) values, calculated meal GI (Figure 1C3) and calculated meal GL (Figure 1D3) values, or serum insulin concentrations (P = 0.33) (Figure 2A3, Table 3). The addition of 11.1 g fat to white bread resulted in a higher insulin AUCi (P = 0.0112) (by 29%; Figure 2B3) and II value (P = 0.0205 (by 27%; Figure 2C3), but additional fat had no significant effect on postprandial HDL-cholesterol (P = 0.93) (Figure 3A3), LDL-cholesterol (P = 0.99) (Figure 3B3), triacylglycerol (P = 0.98) (Figure 3C3), or NEFA (P = 0.10) (Figure 3D3) concentrations.
Addition of increasing amounts of fiber to 50 g available carbohydrate from white bread had no significant effect on glucose and insulin AUCi, measured meal GI and GL values, or II values
The addition of fiber (4.8 and 9.6 g) to 50 g available carbohydrate from white bread had no significant effect on serum glucose concentrations at any time point (P = 0.35) (Figure 1A4, Table 2). Similarly, the addition of fiber had no significant effect on the glucose AUCi (P = 0.15) (Figure 1B4), measured meal GI (the GI value of oat cereal was 81; P = 0.17) (Figure 1C4) and measured meal GL (P = 0.16) (Figure 1D4) values, or serum insulin concentrations (P = 0.54) (Figure 2A4, Table 3). The addition of 9.6 g fiber to the white-bread challenge increased calculated meal GI (P = 0.0360) (Figure 1C4) and calculated meal GL (P = 0.0321) (Figure 1D4) values although calculated meal GI and GL values were similar to the measured values. There was no significant effect of additional fiber on the insulin AUCi (P = 0.40) (Figure 2B4), II (P = 0.23) (Figure 2C4), or postprandial HDL-cholesterol (P = 0.76) (Figure 3A4), LDL-cholesterol (P = 0.96) (Figure 3B4), triacylglycerol (P = 0.83) (Figure 3C4), or NEFA (P = 1.00) (Figure 3D4) concentrations.
DISCUSSION
Foods are rarely eaten alone or in 50-g available carbohydrate servings, and the relative and absolute macronutrient contents of meals and snacks can differ considerably throughout the day. These issues raise the question of how to predict a GI value for an eating occasion or average food intakes that are estimated from food-frequency questionnaires and other dietary intake data. The approach to estimating such values is to calculate the weighted sum of the GI contribution of each carbohydrate-containing food by multiplying the GI value of an individual food by the percentage of available carbohydrate in each food relative to the total available carbohydrate in the meal or total diet (19, 21, 39). The estimation does not adjust for foods that contain other macronutrients or fiber that are consumed concurrently with carbohydrate-containing foods. Our study was designed to address this issue by investigating the effect of different amounts of carbohydrate or combinations of carbohydrate with other macronutrients or fiber on measured meal GI and GL values of food combinations.
Results indicated that increasing the available carbohydrate content of the standard food challenge by 25%, 50% and 100% with the addition of a high carbohydrate breakfast cereal resulted in glucose AUCi values that were 32%, 44%, and 65%, respectively, higher than standard-challenge values. Concomitant differences in measured meal GI and GL values were also observed. Consistent with these findings, it has been previously reported that the glycemic response rises with an increasing amount of available carbohydrate after being challenged with a wide range of foods (43). Stepwise increases in the GL have been reported to be positively correlated with the glucose AUC (2, 44). We also showed that an additional 50 g carbohydrate to the standard carbohydrate challenge increased insulin AUCi values, which was consistent with results that have been reported previously (2). In addition, with increasing amounts of available carbohydrate in the food challenges, the calculated meal GI and GL values that were obtained on the basis of the published formula (21) were increased and were similar to the increased values in measured meal GI and GL values. These findings suggest that calculated GI and GL values of a mixed meal using individual GI values match directly measured meal GI and GL values. However, it is unclear if this association also applies to the calculation of average GI and GL values of the whole diet from daily food-intake data with the use of individual GI values.
In contrast to the results observed for additional carbohydrate, the combination of the highest amount of protein tested (50 g) with the standard carbohydrate challenge resulted in glucose AUCi values that were 25% lower, which resulted in lower measured meal GI and GL values. These changes were different from calculated meal GI and GL values, which did not alter with increasing amounts of protein added to the standard carbohydrate challenge. This result was not surprising because the formula that was used to predict meal or dietary GI and GL values has no adjustment for the amount of protein as well as other nutrients other than carbohydrate. The effect of protein blunting the glycemic response within the context of a standard 50-g carbohydrate challenge has been reported for a wide range of protein-rich foods and amounts of protein (20–90 g) (25, 26, 45–53). This effect has been attributed in part to the slowing of gastric-emptying rates that are mediated by the stimulatory effect of protein on gut hormones, including cholecystokinin, gastric inhibitory polypeptides, and glucagon like peptide-1 (48, 52, 54–56). Protein may also blunt rises in blood glucose concentrations by enhancing the insulin response, which is an effect that has been attributed to the stimulation of insulin secretion from pancreatic β cells by some amino acids (26, 38, 45, 48, 49, 51–53).
The addition of fat in an amount ≤100% of the energy in the standard carbohydrate challenge had no significant effect on glucose AUCi, measured meal GI or GL values with the exception of insulin AUCi and II values. Because this result was observed for only the intermediate amount of fat, we could not exclude the possibility that this observation was spurious. The finding of no significant effect was somewhat unexpected because fat has been previously reported to delay gastric-emptying rates (32, 54, 57) via the stimulation of the release of gut hormones including cholecystokinin, gastric inhibitory polypeptides, and glucagon like peptide-1 (54, 57, 58). The majority of previous studies have reported that the co-ingestion of carbohydrate with fat from various sources resulted in a significant reduction in the glucose AUCi (26, 32, 58, 59) or GI value (60). Most of these studies used a much greater amount of fat (30–50 g) than was used in our study (≤22.2 g). However, one study that added a similar amount of fat (22.8 g) from the same source reported lower glucose AUCi and GL values (27). The reason for the discordant results is not obvious.
Because bread is the most frequently consumed source of available carbohydrate, many strategies have been used to reduce the postprandial glucose and insulin responses to bread. One of the main strategies has been to replace breads that are made from refined gains with whole grains or by increasing fiber intake from other sources (61). Notwithstanding the benefits of fiber with respect to gastrointestinal function, the data to support benefits for the glycemic response have been inconsistent. Some studies have reported that fiber-rich foods, either soluble or insoluble fiber, resulted in lower glucose AUCi (28, 29, 62–67), insulin AUCi (62, 64, 67, 68), and GI or GL (28, 29, 65, 66, 68, 69) values. This effect has been attributed to the ability of fiber to increase the gastrointestinal content viscosity, to decrease the gastric-emptying rate, and to slow glucose-absorption rates (61, 62). In contrast, other studies have reported that the fiber content in foods, regardless of the type, has no significant effect on the postprandial glucose response (22, 62, 70–73), insulin response (62, 70, 71), or GI or GL values (73). Our results are consistent with these latter observations. Cheerios cereal is made from oats, which contain primarily soluble fiber in the form of β-glucan. The ability of β-glucan to attenuate postprandial glycemic and insulin responses has been attributed to its viscous nature (61, 62, 66). However, the effect of food processing on β-glucan during the manufacturing of Cheerios cereal is unknown; hence, the possibility that the β-glucan is degraded cannot be ruled out. Note that, during food preparation, fiber-containing foods can differ in many ways because of physical disruption and heat exposure (61). Thus, it is difficult to attribute the results solely to the presence or absence of dietary fiber. Although the values were similar to measured meal GI and GL values, the calculated meal GI and GL values significantly increased with the addition of 9.6 g fiber to the standard carbohydrate challenge. This result may have been due to the smaller variance in the calculated values than in the measured values. This possibility is of concern because calculated values may mask the large interindividual variability in the determination of GI and GL values.
None of the assessed interventions altered serum postprandial HDL-cholesterol, LDL-cholesterol, triacylglycerol, or NEFA concentrations during the 2-h study period. These data are consistent with previously reported findings (59, 74, 75).
The strength of this series of studies is the controlled nature of the food challenges in terms of the environment, subject characteristics, physical activity intensity, the time of day that the tests were conducted, and the standardization of the actual test foods.
In conclusion, glycemic responses are reduced and measured meal GI and GL are lower compared with calculated meal GI and GL values when carbohydrate-containing foods are consumed in combination with protein (an equal amount of carbohydrate challenge) but not with carbohydrate-, fat-, or fiber-containing foods. These findings suggest that the use of individual GI values to calculate GI and GL values of meals containing equal amounts of carbohydrate and protein on the basis of the previously published formula (21) may overestimate the actual effect and, thus, may provide uncertainty regarding the prediction of meal GI and GL values. Future studies are needed to evaluate whether this uncertainty also influences the prediction of average dietary GI and GL values and, hence, the interpretation of the associations between diet and chronic-disease risk.
Acknowledgments
We thank the study coordinators, Janey Ronxhi and Jean Galluccio, and the Metabolic Research Unit and Nutrition Evaluation Laboratory for assistance.
The authors’ responsibilities were as follows—HM: analyzed the data, performed the statistical analysis, and wrote the initial draft of the manuscript; NRM, LMA, and AHL: designed and conducted the research; AHL: had primary responsibility for the final content of the manuscript; and all authors: contributed to the critical review of the manuscript and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AUCi, incremental AUC; GI, glycemic index; GL, glycemic load; II, insulin index; MRU, Metabolic Research Unit; NEFA, nonesterified fatty acid; RGR, relative glucose response.
REFERENCES
- 1.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6. [DOI] [PubMed] [Google Scholar]
- 2.Brand-Miller JC, Thomas M, Swan V, Ahmad ZI, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr 2003;133:2728–32. [DOI] [PubMed] [Google Scholar]
- 3.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr 2000;71:1455–61. [DOI] [PubMed] [Google Scholar]
- 5.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk–a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins DJ, Kendall CW, Vuksan V, Faulkner D, Augustin LS, Mitchell S, Ireland C, Srichaikul K, Mirrahimi A, Chiavaroli L, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care 2014;37:1806–14. [DOI] [PubMed] [Google Scholar]
- 7.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50. [DOI] [PubMed] [Google Scholar]
- 8.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7. [DOI] [PubMed] [Google Scholar]
- 9.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 2004;80:348–56. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006;29:2223–30. [DOI] [PubMed] [Google Scholar]
- 11.Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr 2014;100:218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer KA, Kushi LH, Jacobs DR Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- 13.Stevens J, Ahn K, Juhaeri, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care 2002;25:1715–21. [DOI] [PubMed] [Google Scholar]
- 14.van Woudenbergh GJ, Kuijsten A, Sijbrands EJ, Hofman A, Witteman JC, Feskens EJ. Glycemic index and glycemic load and their association with C-reactive protein and incident type 2 diabetes. J Nutr Metab 2011;2011:623076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sluijs I, Beulens JW, van der Schouw YT, van der ADL, Buckland G, Kuijsten A, Schulze MB, Amiano P, Ardanaz E, Balkau B, et al. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J Nutr 2013;143:93–9. [DOI] [PubMed] [Google Scholar]
- 16.van Dam RM, Visscher AW, Feskens EJ, Verhoef P, Kromhout D. Dietary glycemic index in relation to metabolic risk factors and incidence of coronary heart disease: the Zutphen Elderly Study. Eur J Clin Nutr 2000;54:726–31. [DOI] [PubMed] [Google Scholar]
- 17.Levitan EB, Mittleman MA, Hakansson N, Wolk A. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. Am J Clin Nutr 2007;85:1521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega-López S, Ausman LM, Griffith JL, Lichtenstein AH. Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care 2007;30:1412–7. [DOI] [PubMed] [Google Scholar]
- 19.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev 2005;18:145–71. [DOI] [PubMed] [Google Scholar]
- 20.Wolever TM, Yang M, Zeng XY, Atkinson F, Brand-Miller JC. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am J Clin Nutr 2006;83:1306–12. [DOI] [PubMed] [Google Scholar]
- 21.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 1986;43:167–72. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins DJ, Wolever TM, Jenkins AL, Lee R, Wong GS, Josse R. Glycemic response to wheat products: reduced response to pasta but no effect of fiber. Diabetes Care 1983;6:155–9. [DOI] [PubMed] [Google Scholar]
- 23.Dodd H, Williams S, Brown R, Venn B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index. Am J Clin Nutr 2011;94:992–6. [DOI] [PubMed] [Google Scholar]
- 24.Monro J. Redefining the glycemic index for dietary management of postprandial glycemia. J Nutr 2003;133:4256–8. [DOI] [PubMed] [Google Scholar]
- 25.Moghaddam E, Vogt JA, Wolever TM. The effects of fat and protein on glycemic responses in nondiabetic humans vary with waist circumference, fasting plasma insulin, and dietary fiber intake. J Nutr 2006;136:2506–11. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Ranawana DV, Leow MK, Henry CJ. Effect of chicken, fat and vegetable on glycaemia and insulinaemia to a white rice-based meal in healthy adults. Eur J Nutr 2014;53:1719–26. [DOI] [PubMed] [Google Scholar]
- 27.Henry CJ, Lightowler HJ, Newens KJ, Pata N. The influence of adding fats of varying saturation on the glycaemic response of white bread. Int J Food Sci Nutr 2008;59:61–9. [DOI] [PubMed] [Google Scholar]
- 28.Marangoni F, Poli A. The glycemic index of bread and biscuits is markedly reduced by the addition of a proprietary fiber mixture to the ingredients. Nutr Metab Cardiovasc Dis 2008;18:602–5. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins AL, Kacinik V, Lyon M, Wolever TM. Effect of adding the novel fiber, PGX, to commonly consumed foods on glycemic response, glycemic index and GRIP: a simple and effective strategy for reducing post prandial blood glucose levels–a randomized, controlled trial. Nutr J 2010;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beglinger C, Degen L. Fat in the intestine as a regulator of appetite–role of CCK. Physiol Behav 2004;83:617–21. [DOI] [PubMed] [Google Scholar]
- 31.O’Donovan D, Horowitz M, Russo A, Feinle-Bisset C, Murolo N, Gentilcore D, Wishart JM, Morris HA, Jones KL. Effects of lipase inhibition on gastric emptying of, and on the glycaemic, insulin and cardiovascular responses to, a high-fat/carbohydrate meal in type 2 diabetes. Diabetologia 2004;47:2208–14. [DOI] [PubMed] [Google Scholar]
- 32.Welch IM, Bruce C, Hill SE, Read NW. Duodenal and ileal lipid suppresses postprandial blood glucose and insulin responses in man: possible implications for the dietary management of diabetes mellitus. Clin Sci (Lond) 1987;72:209–16. [DOI] [PubMed] [Google Scholar]
- 33.Lang V, Bellisle F, Alamowitch C, Craplet C, Bornet FR, Slama G, Guy-Grand B. Varying the protein source in mixed meal modifies glucose, insulin and glucagon kinetics in healthy men, has weak effects on subjective satiety and fails to affect food intake. Eur J Clin Nutr 1999;53:959–65. [DOI] [PubMed] [Google Scholar]
- 34.Gray DS. The clinical uses of dietary fiber. Am Fam Physician 1995;51:419–26. [PubMed] [Google Scholar]
- 35.Zaloga GP, Siddiqui RA. Biologically active dietary peptides. Mini Rev Med Chem 2004;4:815–21. [DOI] [PubMed] [Google Scholar]
- 36.Burrin DG, Davis TA. Proteins and amino acids in enteral nutrition. Curr Opin Clin Nutr Metab Care 2004;7:79–87. [DOI] [PubMed] [Google Scholar]
- 37.Beysen C, Karpe F, Fielding BA, Clark A, Levy JC, Frayn KN. Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia 2002;45:1533–41. [DOI] [PubMed] [Google Scholar]
- 38.van Loon LJ, Saris WH, Verhagen H, Wagenmakers AJ. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr 2000;72:96–105. [DOI] [PubMed] [Google Scholar]
- 39.Food and Agriculture Organization. Carbohydrates in human nutrition: a summary of the Joint FAO/WHO Expert Consultation, 1997. Rome (Italy): Food and Agriculture Organization (United Nations); 1997.
- 40.Wolever TM, Bolognesi C. Source and amount of carbohydrate affect postprandial glucose and insulin in normal subjects. J Nutr 1996;126:2798–806. [DOI] [PubMed] [Google Scholar]
- 41.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 42.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001;33:1126–41. [DOI] [PubMed] [Google Scholar]
- 43.Venn BJ, Wallace AJ, Monro JA, Perry T, Brown R, Frampton C, Green TJ. The glycemic load estimated from the glycemic index does not differ greatly from that measured using a standard curve in healthy volunteers. J Nutr 2006;136:1377–81. [DOI] [PubMed] [Google Scholar]
- 44.Liu P, Perry T, Monro JA. Glycaemic glucose equivalent: validation as a predictor of the relative glycaemic effect of foods. Eur J Clin Nutr 2003;57:1141–9. [DOI] [PubMed] [Google Scholar]
- 45.Roberts S, Desbrow B, Grant G, Anoopkumar-Dukie S, Leveritt M. Glycemic response to carbohydrate and the effects of exercise and protein. Nutrition 2013;29:881–5. [DOI] [PubMed] [Google Scholar]
- 46.Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V. A whey protein supplement decreases post-prandial glycemia. Nutr J 2009;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva Ton WT, das Gracas de Almeida C, de Morais Cardoso L, Marvila Girondoli Y, Feliciano Pereira P, Viana Gomes Schitini JK, Galvão Cândido F, Marques Arbex P, de Cássia Gonçalves Alfenas R. Effect of different protein types on second meal postprandial glycaemia in normal weight and normoglycemic subjects. Nutr Hosp 2014;29:553–8. [DOI] [PubMed] [Google Scholar]
- 48.Hutchison AT, Piscitelli D, Horowitz M, Jones KL, Clifton PM, Standfield S, Hausken T, Feinle-Bisset C, Luscombe-Marsh ND. Acute load-dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men. Am J Clin Nutr 2015;102:1574–84. [DOI] [PubMed] [Google Scholar]
- 49.Gunnerud UJ, Ostman EM, Bjorck IM. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose-response study. Eur J Clin Nutr 2013;67:749–53. [DOI] [PubMed] [Google Scholar]
- 50.Gunnerud UJ, Heinzle C, Holst JJ, Ostman EM, Bjorck IM. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS One 2012;7:e44731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984;7:465–70. [DOI] [PubMed] [Google Scholar]
- 52.Lan-Pidhainy X, Wolever TM. The hypoglycemic effect of fat and protein is not attenuated by insulin resistance. Am J Clin Nutr 2010;91:98–105. [DOI] [PubMed] [Google Scholar]
- 53.Gannon MC, Nuttall FQ, Neil BJ, Westphal SA. The insulin and glucose responses to meals of glucose plus various proteins in type II diabetic subjects. Metabolism 1988;37:1081–8. [DOI] [PubMed] [Google Scholar]
- 54.Panahi S, El Khoury D, Kubant R, Akhavan T, Luhovyy BL, Goff HD, Anderson GH. Mechanism of action of whole milk and its components on glycemic control in healthy young men. J Nutr Biochem 2014;25:1124–31. [DOI] [PubMed] [Google Scholar]
- 55.Ishikawa Y, Hira T, Inoue D, Harada Y, Hashimoto H, Fujii M, Kadowaki M, Hara H. Rice protein hydrolysates stimulate GLP-1 secretion, reduce GLP-1 degradation, and lower the glycemic response in rats. Food Funct 2015;6:2525–34. [DOI] [PubMed] [Google Scholar]
- 56.Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G, Hendriks HF. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr 2006;83:211–20. [DOI] [PubMed] [Google Scholar]
- 57.Gentilcore D, Chaikomin R, Jones KL, Russo A, Feinle-Bisset C, Wishart JM, Rayner CK, Horowitz M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab 2006;91:2062–7. [DOI] [PubMed] [Google Scholar]
- 58.Collier G, McLean A, O’Dea K. Effect of co-ingestion of fat on the metabolic responses to slowly and rapidly absorbed carbohydrates. Diabetologia 1984;26:50–4. [DOI] [PubMed] [Google Scholar]
- 59.Ercan N, Gannon MC, Nuttall FQ. Effect of added fat on the plasma glucose and insulin response to ingested potato given in various combinations as two meals in normal individuals. Diabetes Care 1994;17:1453–9. [DOI] [PubMed] [Google Scholar]
- 60.MacIntosh CG, Holt SH, Brand-Miller JC. The degree of fat saturation does not alter glycemic, insulinemic or satiety responses to a starchy staple in healthy men. J Nutr 2003;133:2577–80. [DOI] [PubMed] [Google Scholar]
- 61.Scazzina F, Siebenhandl-Ehn S, Pellegrini N. The effect of dietary fibre on reducing the glycaemic index of bread. Br J Nutr 2013;109:1163–74. [DOI] [PubMed] [Google Scholar]
- 62.Poppitt SD, van Drunen JD, McGill AT, Mulvey TB, Leahy FE. Supplementation of a high-carbohydrate breakfast with barley beta-glucan improves postprandial glycaemic response for meals but not beverages. Asia Pac J Clin Nutr 2007;16:16–24. [PubMed] [Google Scholar]
- 63.Lightowler HJ, Henry CJ. Glycemic response of mashed potato containing high-viscocity hydroxypropylmethylcellulose. Nutr Res 2009;29:551–7. [DOI] [PubMed] [Google Scholar]
- 64.Keogh JB, Lau CW, Noakes M, Bowen J, Clifton PM. Effects of meals with high soluble fibre, high amylose barley variant on glucose, insulin, satiety and thermic effect of food in healthy lean women. Eur J Clin Nutr 2007;61:597–604. [DOI] [PubMed] [Google Scholar]
- 65.Panlasigui LN, Thompson LU. Blood glucose lowering effects of brown rice in normal and diabetic subjects. Int J Food Sci Nutr 2006;57:151–8. [DOI] [PubMed] [Google Scholar]
- 66.De Angelis M, Rizzello CG, Alfonsi G, Arnault P, Cappelle S, Di Cagno R, Gobbetti M. Use of sourdough lactobacilli and oat fibre to decrease the glycaemic index of white wheat bread. Br J Nutr 2007;98:1196–205. [DOI] [PubMed] [Google Scholar]
- 67.Ames N, Blewett H, Storsley J, Thandapilly SJ, Zahradka P, Taylor C. A double-blind randomised controlled trial testing the effect of a barley product containing varying amounts and types of fibre on the postprandial glucose response of healthy volunteers. Br J Nutr 2015;113:1373–83. [DOI] [PubMed] [Google Scholar]
- 68.Casiraghi MC, Garsetti M, Testolin G, Brighenti F. Post-prandial responses to cereal products enriched with barley beta-glucan. J Am Coll Nutr 2006;25:313–20. [DOI] [PubMed] [Google Scholar]
- 69.Capriles VD, Areas JA. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct 2013;4:104–10. [DOI] [PubMed] [Google Scholar]
- 70.Linderborg KM, Jarvinen R, Lehtonen HM, Viitanen M, Kallio HP. The fiber and/or polyphenols present in lingonberries null the glycemic effect of the sugars present in the berries when consumed together with added glucose in healthy human volunteers. Nutr Res 2012;32:471–8. [DOI] [PubMed] [Google Scholar]
- 71.Juntunen KS, Laaksonen DE, Autio K, Niskanen LK, Holst JJ, Savolainen KE, Liukkonen KH, Poutanen KS, Mykkanen HM. Structural differences between rye and wheat breads but not total fiber content may explain the lower postprandial insulin response to rye bread. Am J Clin Nutr 2003;78:957–64. [DOI] [PubMed] [Google Scholar]
- 72.Jenkins DJ, Wolever TM, Taylor RH, Barker HM, Fielden H, Gassull MA. Lack of effect of refining on the glycemic response to cereals. Diabetes Care 1981;4:509–13. [DOI] [PubMed] [Google Scholar]
- 73.Thondre PS, Wang K, Rosenthal AJ, Henry CJ. Glycaemic response to barley porridge varying in dietary fibre content. Br J Nutr 2012;107:719–24. [DOI] [PubMed] [Google Scholar]
- 74.Vega-López S, Ausman LM, Matthan NR, Lichtenstein AH. Postprandial lipid responses to standard carbohydrates used to determine glycaemic index values. Br J Nutr 2013;110:1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turner KM, Keogh JB, Clifton PM. Acute effect of red meat and dairy on glucose and insulin: a randomized crossover study. Am J Clin Nutr 2016;103:71–6. [DOI] [PubMed] [Google Scholar]