Abstract
Background: Fracture is a complex trait, affected by both genetic and environmental factors. A meta-analysis of genome-wide association studies (GWASs) identified multiple bone mineral density (BMD) and fracture-associated loci.
Objective: We conducted a study to evaluate whether fracture genetic risk score (Fx-GRS) and bone mineral density genetic risk score (BMD-GRS) modify the association between the intake of calcium with vitamin D (CaD) and fracture risk.
Design: Data from 5823 white postmenopausal women from the Women’s Health Initiative CaD randomized trial were included. Participants received 1000 mg elemental Ca with 400 IU vitamin D3/d or placebo (median follow-up: 6.5 y). Total fracture was defined as first fracture of any type. We computed the Fx-GRS with 16 fracture- and BMD-associated variants, and the BMD-GRS with 50 BMD-associated variants. We used Cox regression and a case-only approach to test for multiplicative interaction. Additive interaction was assessed with the relative excess risk due to interaction (RERI). We analyzed genetic risk score as a continuous variable and a categorical variable based on quartile (quartile 1, quartiles 2–3, and quartile 4).
Results: We observed no interaction between the Fx-GRS and CaD on fracture risk; however, we observed a significant multiplicative interaction between the BMD-GRS and CaD assignment (P-interaction = 0.01). In addition, there was a significant negative additive interaction between placebo assignment and higher BMD-GRS: quartiles 2–3, PRERI = 0.03; quartile 4, PRERI = 0.03. In a stratified analysis, the protective effect of CaD on fracture risk was observed in women in the lowest BMD-GRS quartile (HR: 0.60, 95% CI: 0.44, 0.81) but not in women with a higher BMD-GRS.
Conclusions: We observed significant effects of CaD intake on fracture risk only in women with the lowest genetic predisposition to low BMD. Future large-scale studies with functional characterization of GWAS findings are warranted to assess the utility of genetic risk score in analysis of risks and benefits of CaD for bone.
Keywords: fracture, bone mineral density, genetic risk score, calcium, vitamin D, postmenopausal women
See corresponding editorial on page 777.
INTRODUCTION
Hormonal and physiologic changes after menopause and age increase the risk of osteoporosis and fracture in postmenopausal women. In the United States, ∼40% of white women aged ≥50 y will experience a hip, spine, or wrist fracture in their later life (1). Because fracture in the elderly population is associated with increased comorbidity, mortality, and a substantial health care cost burden (1), it is an important public health concern that needs immediate attention.
Evidence supports the involvement of both genetic and environmental factors in systemic bone loss and fracture risk. Calcium and vitamin D intake are important environmental regulators of bone. Calcium is a major component of the skeleton, and ∼99% of total body calcium is present in bones and teeth. Vitamin D stimulates calcium absorption from the intestine and influences calcium homeostasis (2). In addition, vitamin D influences osteoblast proliferation, differentiation, and mineralization (3). The mechanisms of action of 1,25-dihydroxvitamin D3, the active form of vitamin D, include the regulation of expression of various bone cell genes (4).
Heritability estimates reported from family and twin studies are 25–54% for wrist fracture (5, 6), 3–68% for hip fracture (7), and 50–85% for bone mineral density (BMD)12 (8), suggesting that genetic factors play a role in fracture risk and BMD. In the last decade, multiple genetic variants associated with bone phenotypes have been identified via genome-wide association studies (GWASs). The largest meta-GWAS of BMD to date reported 56 genome-wide significant loci associated with BMD, and 14 of them are also associated with fracture risk (9). Previous studies have demonstrated that the aggregate effect of variants identified by the meta-GWAS can predict the risk of fracture in various populations (9, 10). However, no published studies have focused on the interaction between the cumulative effects of these variants and calcium and vitamin D intake on fracture risk in postmenopausal women. As for the main effects of calcium with vitamin D (CaD) in the Women’s Health Initiative (WHI) trial, supplementation improved hip bone density; however, there was no significant reduction in total fracture incidence with CaD intervention (11).
The WHI CaD randomized clinical trial (CT) provides a unique opportunity to evaluate gene-CaD interaction, because random assignment of CaD minimizes concerns about biases and confounding. While using this powerful study design, we examined the interaction between CaD supplementation and genetic risk scores (GRSs) based on established common variants from the meta-GWAS on BMD (9). We hypothesized that the protective effect of CaD supplementation on fracture risk would be stronger in participants with higher genetic risk. We further examined the interactions of the GRSs with CaD on fracture risk at different anatomical sites by grouping fracture into 3 categories (central body, lower limb, and upper limb).
METHODS
WHI CaD supplementation clinical trial
The WHI was initiated in 1992 to determine the risk factors and strategies for preventing cardiovascular disease, cancer, and osteoporotic fractures in postmenopausal women (12). A total of 161,808 postmenopausal women aged 50–79 y participated in the CTs or the observational study in the WHI. The WHI CT has 3 overlapping components [2 hormone therapy (HT) trials, a dietary modification trial, and a CaD trial] that enrolled 68,132 postmenopausal women. The CTs used a partial factorial design, and eligible participants were enrolled in 1, 2, or 3 CTs. All women were able to join the HT trials, dietary modification trial, or both at WHI baseline. In the HT trials, participants who had a hysterectomy before random assignment were randomly assigned to conjugated equine estrogen 0.625 mg/d or placebo (n = 10,739) (13), and those with an intact uterus at baseline were randomly assigned to conjugated equine estrogen 0.625 mg plus medroxyprogesterone acetate 2.5 mg/d or placebo (n = 16,608) (14). From those enrolled in the trials, participants were further recruited into the CaD trial at the first or second annual visit (11).
In total, 36,282 CT participants were randomly assigned to the CaD trial (33,070 in year 1 and 3212 in year 2) (11). Of those, 18,176 women were randomly assigned into the active CaD intervention group and 18,106 women were randomly assigned into the matching placebo group. The intervention group received 1000 mg elemental Ca as calcium carbonate plus vitamin D3 400 IU daily (11). The dose was divided to take one-half of the dose 2 times/d with meals. Participants were allowed to take their own calcium (≤1000 mg/d) and/or vitamin D supplementation (up to 600 IU/d initially, later raised to 1000 IU/d in 1999) (11). Participants were followed until loss to follow-up, death, or trial close-out (11). Details on the CaD trial design have been published elsewhere (11, 15).
Study participants
Of 30,153 self-reported white CaD trial participants, complete imputed GWAS data for fracture genetic risk score (Fx-GRS) and bone mineral density genetic risk score (BMD-GRS) were available for 5828 and 5827 women, respectively, through the Genomics and Randomized Trials Network (GARNET; n = 2459 for Fx-GRS and n = 2458 for BMD-GRS) GWAS or the WHIMS+ (Women’s Health Initiative Memory Study + n = 3369) GWAS. The WHI GARNET substudy aimed to identify genetic factors affecting myocardial infarction, stroke, venous thrombosis, and diabetes phenotypes via genome-wide analysis of response to HT treatment with the use of a nested case-control study design. The WHIMS (Women's Health Initiative Memory Study) was an ancillary study to the WHI HT trial that examined whether HT reduces the incidence of dementia in women aged ≤65 y (16). The initial WHIMS+ cohort was composed of WHIMS participants who were not in the GARNET (had GWAS already available), and it also recruited women who were neither in the WHIMS nor in the GARNET (17). All participants provided written informed consent. Institutional review board approval was obtained from the University at Buffalo Health Sciences Institutional Review Board.
Of the 5828 (5827 for BMD-GRS) self-reported white women with complete imputed single-nucleotide polymorphism (SNP) data available, an additional 5 participants were excluded for a lack of follow-up data, leaving 5823 (5822 for BMD-GRS analyses) women for the current analyses (Supplemental Figure 1). Of those, 2882 women were assigned to CaD intervention and 2041 women were assigned to matching placebo.
Genotyping, quality control, and imputation
GARNET sample genotyping was conducted with the Illumina HumanOmni1-Quad v1-0 B array (18). GARNET DNA samples were excluded before imputation for the following reasons: overall missingness ≥2% or sample-chromosome combinations with a chromosomal anomaly and/or with a missing call rate >5%. SNPs were excluded if they failed recommended quality control procedures or had an intensity-only technical failure by the genotyping center, minor allele frequency = 0, call rate <98%, >0 discordant call in duplicate pairs, or Hardy-Weinberg equilibrium P value <1.0 × 10−4. Genotype imputation in GARNET was performed with the use of the 1000 Genomes Version 3 reference panel (release date 20100804) sequence and alignment release with BEAGLE software (19).
WHIMS+ participants were genotyped with the use of the Illumina HumanOmniExpressExome-8v1_B array (18). WHIMS+ samples were removed before imputation for the following reasons: missingness >3% or 1 of a related pair. While following the Gene, Environment Association Studies Consortium protocol (20, 21), SNPs were removed for a missing call rate ≥2%, Hardy-Weinberg equilibrium P value <1.0 × 10−4, or minor allele frequency <0.01. Genotype imputation was conducted with Minimac with the use of the 1000 Genomes Version 3 (release date 23 November 2010).
GRSs
With the use of variants identified from the GEnetic Factors for OSteoporosis consortium (GEFOS) meta-GWAS (9), we derived 2 weighted GRSs. The Fx-GRS was constructed based on 16 BMD-associated SNPs that were also associated with any type of fracture at a Bonferroni-corrected significance level of P < 5 × 10−4. Weights for Fx-GRS were computed based on ORs for any low-trauma fracture reported in the GEFOS meta-GWAS (9). For each participant, the Fx-GRS was computed by summing the product of allelic dosage of each SNP (ranging from 0 to 2) and its relative effect sizes calculated as a log-transformed OR divided by the mean allelic effect size. In the Fx-GRS, we used 4 and 2 genotyped and 11 and 13 imputed SNPs in the GARNET and WHIMS+, respectively. We used a typed proxy SNP rs7898709 (r2 = 1) for rs1373004, because the latter SNP was not available in genotype data (http://analysistools.nci.nih.gov/LDlink/) (22).
We constructed another GRS that reflected genetic susceptibility to low BMD (BMD-GRS) with 50 SNPs from 63 established BMD-associated variants from the GEFOS meta-analysis with the use of the method described above. Weights for BMD-GRS were calculated as a β coefficient for femoral neck (FN) BMD obtained from the meta-analysis of both discovery and replication sets in women only, divided by the mean value of the effect size. In the score, we used 13 and 11 typed, 36 and 38 imputed, and 1 and 1 typed proxy SNPs (rs7898709 for rs1373004) in GARNET and WHIMS+, respectively. We excluded 13 of 63 established BMD SNPs for the following reasons: failed quality control (rs12821008, rs1566045, and rs17040773), secondary signals after conditional analysis (rs10226308, rs13245690, rs1564981, rs17482952, rs4792909, rs736825, rs7521902, and rs7751941), or no association with FN BMD in women (β-coefficient for FN BMD = 0) (rs1878526 and rs7071206).
Ascertainment of fracture
The phenotype of interest in the current study was first event of any type of fracture (total fracture) occurring during the WHI CaD CT from enrollment into the CaD trial in 1995 until study close-out in 2005 in the subgroup of CaD CT participants from European ancestry. Total fractures were defined as all reported and adjudicated fractures except for fractures at the skull or face, fingers, toes, ribs, or sternum, and those at cervical vertebrae (11). Participants reported their fracture incidence via semiannual questionnaires, and all reported fractures were adjudicated by centrally trained adjudicators in a blind manner via review of radiologic, operative, or MRI reports (11). For exploratory fracture subtype analyses, type of fracture was stratified into 3 groups based on their anatomic location: central body (hip, spine, and pelvis), lower limb (ankle, patella, shaft of femur, tarsal or metatarsal, tibia or fibula, and tibial plateau) and upper limb (carpal, elbow, metacarpal, radius or ulna, upper radius and ulna, lower humerus, humerus, and upper humerus) (23) (Supplemental Table 1).
Statistical analyses
Baseline participant characteristics between randomization arms were compared with the use of chi-square tests and t tests as appropriate. We tested the interactions between CaD and GRS on the risk of fracture with the use of a time-to-event analysis with Cox proportional hazard regression models adjusted for age and WHI GWAS (WHIMS+ or GARNET). We did not consider any other covariates in the model, because randomization balanced any potential confounders between groups. The time to event was defined as the number of days since CaD CT randomization to the first adjudicated fracture. Both continuous scales and categorized GRS groups were used in the current analyses. For the categorical analyses, we categorized participants into 3 groups based on GRS quartiles by merging the middle 2 groups into 1; quartile 1, quartiles 2–3, and quartile 4 (Supplemental Table 2). We evaluated the proportional hazards assumptions by examining Schoenfeld residuals plots, and no substantial violation was found.
We tested for the presence of both additive and multiplicative interactions between GRS and CaD on fracture risk. The presence of multiplicative interaction was examined by testing the statistical significance of the product term of continuous scale GRS and CaD in a Cox regression model. We also used a case-only approach with 854 total fracture cases to test a multiplicative interaction in a logistic regression model. The independence assumption for a case-only analysis was tested in 4969 noncases (4968 for BMD-GRS analyses), and no violation was observed (Supplemental Table 3).
Additive interaction between genetic susceptibility (quartiles 2–3, quartile 4) and placebo randomization was examined by calculating the relative excess risk due to interaction (RERI) for fracture with CaD active supplementation-assigned women with lowest genetic risk (quartile 1) as the reference. A RERI >0 would imply that there is positive additive interaction, in which the combined effect of 2 factors is greater than the sum of the individual effects of each factor. If there is negative additive interaction (RERI <0), the joint effect is less than the sum of the individual effects. To test and assess RERI, we used the methods described by Li and Chambless (24). We used the δ method from Hosmer and Lemeshow (25) to calculate variances of the RERI estimates.
We further explored the effect modification by genetic risk on the association between CaD and fracture risk by calculating GRS stratum-specific HR for fracture in women assigned to CaD supplementation compared with women assigned to placebo. We tested for heterogeneity of the CaD supplementation effect on fracture across different GRS strata with the use of Cochran’s Q test for meta-analysis.
We conducted sensitivity analyses to assess the impact of nonadherence by censoring participants 6 mo after they became nonadherent. Because participants were advised to reduce the frequency of intake of the study medication in the event of gastrointestinal side effects (11), we considered 2 nonadherence thresholds: taking <50% of or stopped the study medication, and taking <80% of or stopped the study medication.
To examine whether the joint effect of genetic risk and CaD supplementation on fracture differed by a participant’s risk level at baseline, a second sensitivity analysis was conducted by grouping participants based on age (50–59 y, 60–69 y, and ≥70 y) and baseline total daily personal calcium intake level (<800 mg and ≥800 mg but <1200 and ≥1200 mg). Daily total calcium intake at baseline was computed by summing the amount of dietary (assessed with a modification of the Block food-frequency questionnaire) and supplemental calcium intake. We calculated GRS stratum-specific HRs and 95% CIs for fracture with CaD supplementation in each baseline risk factor category, then compared the pattern of heterogeneity across the risk factor categories.
As exploratory analyses, we also evaluated the effect modification between genetic risk and CaD supplementation for the risk of fracture at different anatomic sites (central body, lower limb, and upper limb) by computing GRS stratum-specific HRs for each fracture subgroup in participants assigned to CaD supplementation compared with those assigned to placebo. A separate exploratory analysis was conducted to assess whether CaD-GRS joint effect on fracture differed by HT randomization in a way similar to that described previously.
All statistical analyses were conducted with the use of SAS 9.4. All P values reported were 2-sided with a significance level defined as P < 0.05.
RESULTS
Baseline characteristics
The comparisons of baseline characteristics between the 2 treatment arms of the CaD trial are presented in Table 1. The 2 randomization arms were balanced according to baseline demographic characteristics, including age, BMI, general health status, number of falls during the previous 12 mo, HT arm assignment, WHI GWAS, daily total personal calcium intake, and daily total personal vitamin D intake. Participants had a mean ± SD age of 67.7 ± 6.5 y at CaD randomization. Approximately 8.2% of participants were current smokers, and 4.4% of women experienced ≥3 falls during the previous year. Mean ± SD BMI (in kg/m2) at WHI enrollment was 29.1 ± 5.8, and nearly 40% of the women had a BMI >30. The mean ± SD daily total personal calcium intake reported at baseline was 1132.8 ± 681.8 mg, and the mean ± SD daily total personal vitamin D intake at baseline was 366.1 ± 263.2 IU. The mean ± SD GRSs of the CaD intervention and placebo groups were 14.7 ± 2.5 and 14.7 ± 2.5, respectively, for Fx-GRS and 50.7 ± 4.8 and 50.8 ± 4.7, respectively, for BMD-GRS; these GRS values did not differ according to randomization arm.
TABLE 1.
Baseline characteristics of participants by CaD CT random assignment1
| Characteristics | CaD intervention (n = 2882) | Placebo (n = 2941) | P2 |
| Age at CaD CT random assignment, y | 0.22 | ||
| 50–59 | 428 (14.9) | 420 (14.3) | |
| 60–69 | 1390 (48.2) | 1370 (46.6) | |
| 70–79 | 1064 (36.9) | 1151 (39.1) | |
| Mean | 67.6 ± 6.5 | 67.9 ± 6.4 | 0.07 |
| BMI, kg/m2 | 0.09 | ||
| <20 | 65 (2.3) | 44 (1.5) | |
| 20–24.9 | 692 (24.1) | 753 (25.7) | |
| 25–29.9 | 993 (34.6) | 983 (33.6) | |
| ≥30 | 1117 (39.0) | 1147 (39.2) | |
| Mean | 29.1 ± 5.9 | 29.0 ± 5.7 | 0.50 |
| Self-reported general health | 0.65 | ||
| Excellent, very good, or good | 2690 (94.0) | 2739 (93.7) | |
| Fair or poor | 172 (6.0) | 184 (6.3) | |
| Personal HT use reported at WHI enrollment | 0.79 | ||
| Never used | 1909 (66.3) | 1972 (67.1) | |
| Past user | 741 (25.7) | 743 (25.3) | |
| Recent user | 230 (8.0) | 225 (7.7) | |
| Smoking status | 0.21 | ||
| Never smoked | 1465 (51.4) | 1557 (53.6) | |
| Past smoker | 1140 (40.0) | 1121 (38.6) | |
| Current smoker | 245 (8.6) | 227 (7.8) | |
| Falls in previous 12 mo | 0.97 | ||
| None | 1803 (65.9) | 1817 (65.4) | |
| 1 | 560 (20.5) | 584 (21.0) | |
| 2 | 252 (9.2) | 254 (9.1) | |
| ≥3 | 121 (4.4) | 124 (4.5) | |
| Fracture at age >55 y | 0.19 | ||
| No | 1876 (74.3) | 1880 (73.5) | |
| Yes | 470 (18.6) | 519 (20.3) | |
| Not applicable, age ≤55 y | 178 (7.1) | 159 (6.2) | |
| Alcohol, servings/wk | 0.14 | ||
| Current nondrinker | 1244 (43.3) | 1307 (44.6) | |
| <1 drink | 540 (18.8) | 585 (20.0) | |
| ≥1 drink | 1090 (37.9) | 1040 (35.5) | |
| HT CT arm, E or E+P intervention | 1445 (50.1) | 1486 (50.5) | 0.77 |
| DM CT arm | 0.06 | ||
| Not randomly assigned DM | 2082 (72.2) | 2102 (71.5) | |
| Intervention | 290 (10.1) | 350 (11.9) | |
| Control | 510 (17.7) | 489 (16.6) | |
| WHI GWAS, WHIMS+ | 1657 (57.5) | 1708 (58.1) | 0.65 |
| Daily total calcium intake, mg | 1119.9 ± 668.6 | 1145.5 ± 694.3 | 0.15 |
| Daily total vitamin D intake, IU | 359.4 ± 260.9 | 372.5 ± 265.2 | 0.06 |
| Age at menopause, y | 48.5 ± 6.5 | 48.2 ± 6.4 | 0.12 |
| Total MET-h/wk | 11.2 ± 12.9 | 10.8 ± 12.5 | 0.23 |
| Fx-GRS | 14.7 ± 2.5 | 14.7 ± 2.5 | 0.25 |
| BMD-GRS | 50.7 ± 4.8 | 50.8 ± 4.7 | 0.63 |
Values are means ± SDs or n (%). BMD-GRS, bone mineral density genetic risk score; CaD, calcium with vitamin D; CT, clinical trial; DM, dietary modification; E, conjugated equine estrogen; E+P, conjugated equine estrogen plus medroxyprogesterone acetate; Fx-GRS, fracture genetic risk score; GWAS, genome-wide association study; HT, hormone therapy; MET-h, metabolic equivalent task hours; WHI, Women’s Health Initiative; WHIMS+, Women’s Health Initiative Memory Study +.
Chi-square test for categorical variables or t test for continuous variables.
Fracture incidence rate
During a median follow-up of 6.5 y, 400 participants in the CaD intervention group and 454 women in the placebo group experienced a fracture. The annualized fracture incidence rate was 22 cases/1000 person-years in the CaD intervention group and 25 cases/1000 person-years in the placebo group. There was no statistically significant difference in the risk of total fracture between women assigned to the CaD intervention and those assigned to placebo (HR: 0.89; 95% CI: 0.78, 1.02).
Interaction of genetic susceptibility and CaD supplementation on total fracture risk
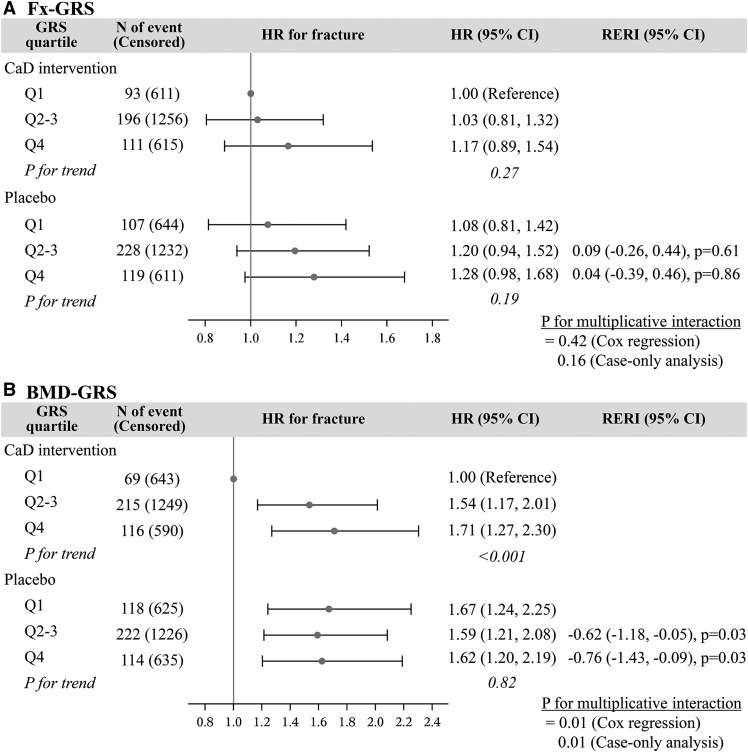
The analysis results of the joint effects of CaD supplementation and GRS on total fracture risk are summarized in Figure 1. The analysis results of the unadjusted and adjusted models are not different. In the analysis of Fx-GRS, we observed a pattern of higher fracture risk with higher GRSs for both the intervention and placebo groups. However, the linear relation was not statistically significant (CaD intervention, P-trend = 0.27; placebo, P-trend = 0.19). We did not find any evidence for multiplicative or additive interaction between Fx-GRS and CaD supplementation regardless of scale examined (Cox regression, P-multiplicative interaction = 0.42; case-only analysis, P-multiplicative interaction = 0.16), and RERI P values were >0.60.
FIGURE 1.
Joint effects of CaD supplementation and genetic risk score on total fracture risk, median follow-up 6.5 y, for Fx-GRS (A) and BMD-GRS (B). Fx-GRS, Q1: <13.0, Q2–3: 13.0 to <16.4, and Q4: ≥16.4. BMD-GRS, Q1: <47.5, Q2–3: 47.5 to <53.9, and Q4: ≥53.9. Cox regression models were adjusted for age (years) and Women’s Health Initiative genome-wide association study (GARNET or WHIMS+). Case-only analysis models were adjusted for age (years) and Women’s Health Initiative genome-wide association study (GARNET or WHIMS+). BMD-GRS, bone mineral density genetic risk score; CaD, calcium with vitamin D; Fx-GRS, fracture genetic risk score; GARNET, Genomics and Randomized Trials Network; GRS, genetic risk score; Q, quartile; RERI, relative excess risk due to interaction; WHIMS+, Women’s Health Initiative Memory Study +.
In the analysis of BMD-GRS, we observed a significant linear trend across GRS categories in relation to total fracture risk only in women assigned to the intervention (P-trend < 0.001). In women assigned to the CaD intervention, those in quartiles 2–3 had 1.54 times (95% CI: 1.17, 2.01 times) and women in quartile 4 had 1.71 times (95% CI: 1.27, 2.30 times) the increased risk of total fracture that of participants at lowest BMD genetic risk. However, the risk of total fracture was similarly increased in the placebo group regardless of genetic susceptibility compared with those with lowest genetic risk of low BMD who were on CaD (P-trend = 0.82) (Figure 1B).
We observed a significant multiplicative interaction between BMD-GRS and CaD supplementation on fracture risk with the use of both methods for detection of interaction (Cox regression, P-interaction = 0.01; case-only P-interaction = 0.01). Moreover, we observed a significant negative additive interaction between placebo and higher genetic predisposition to low BMD. RERI (95% CI) was −0.62 (−1.18, −0.05; P = 0.03) and −0.76 (−1.43, −0.09; P = 0.03) for quartiles 2–3 and quartile 4, respectively. This means that the joint effect of placebo and higher genetic risk of low BMD on total fracture risk was lower than expected from the sum of the individual effects.
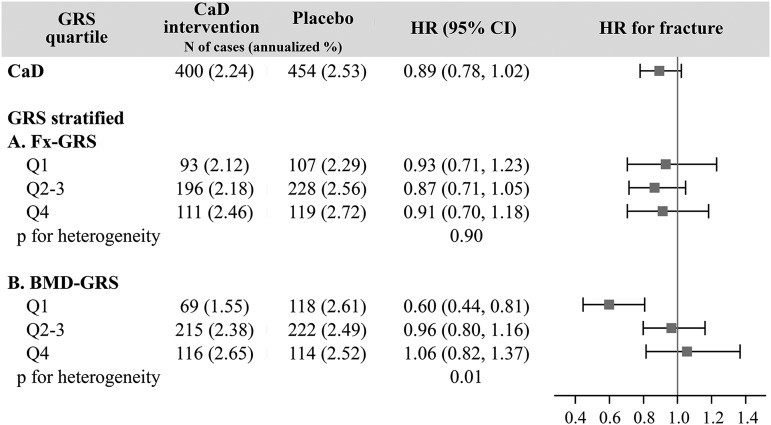
We further evaluated the effect modification of the association between CaD and fracture risk by genetic susceptibility; here, we estimated the CaD effect on total fracture risk while stratifying by categories of GRS (Figure 2). The results of the unadjusted and multivariate adjusted models were not different. For the Fx-GRS strata, we did not observe any difference in the CaD effect on fracture risk (P-heterogeneity = 0.90). However, we observed that genetic predisposition to low BMD significantly modified the association between CaD and total fracture risk (P-heterogeneity = 0.01); we observed reduced total fracture risk with CaD supplementation in women with the lowest genetic risk (BMD-GRS quartile 1) (HR: 0.60; 95% CI: 0.44, 0.81).
FIGURE 2.
The association between calcium with vitamin D supplementation and fracture risk stratified by genetic susceptibility category, median follow-up 6.5 y. Fx-GRS, Q1: <13.0, Q2–3: 13.0 to <16.4, and Q4: ≥16.4. BMD-GRS, Q1: <47.5, Q2–3: 47.5 to <53.9, and Q4: ≥53.9. Cox regression models were adjusted for age (years) and Women’s Health Initiative genome-wide association study (Genomics and Randomized Trials Network or Women’s Health Initiative Memory Study +). BMD-GRS, bone mineral density genetic risk score; CaD, calcium with vitamin D; Fx-GRS, fracture genetic risk score; GRS, genetic risk score; Q, quartile.
There were no significant CaD intervention effects on fracture risk for women with a higher BMD-GRS [HR (95% CI): BMD-GRS quartiles 2–3, 0.96 (0.80, 1.16); quartile 4, 1.06 (0.82, 1.37)].
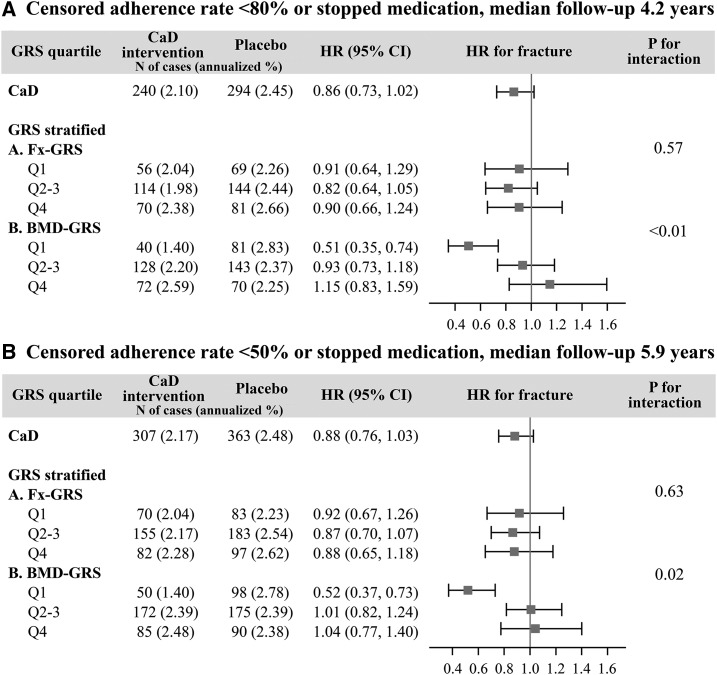
Sensitivity analysis: adherence
We conducted a sensitivity analysis to assess the impact of nonadherence with 2 adherence thresholds levels, 80% and 50% (Figure 3). The median follow-up time was 4.2 and 5.9 y in the 80% and 50% adherence groups, respectively. The adherence sensitivity analyses yielded results that were similar to the main study findings, in which the protective effect of CaD on total fracture risk was evident only in the participants with the lowest genetic predisposition to low BMD (BMD-GRS quartile 1) [HR (95% CI): 80% adherence threshold, 0.51 (0.35, 0.74); 50% adherence threshold, 0.52 (0.37, 0.73)].
FIGURE 3.
Sensitivity analysis: Effect modification of genetic susceptibility on the association between calcium with vitamin D supplementation and fracture risk after censoring data from nonadherent participants. Censored adherence rate <80% (A) and <50% (B). Fx-GRS, Q1: <13.0, Q2–3: 13.0 to <16.4, and Q4: ≥16.4. BMD-GRS, Q1: <47.5, Q2–3: 47.5 to <53.9, and Q4: ≥53.9. Cox regression models were adjusted for age (years) and Women’s Health Initiative genome-wide association study (Genomics and Randomized Trials Network or Women’s Health Initiative Memory Study +). BMD-GRS, bone mineral density genetic risk score; CaD, calcium with vitamin D; Fx-GRS, fracture genetic risk score; GRS, genetic risk score; Q, quartile.
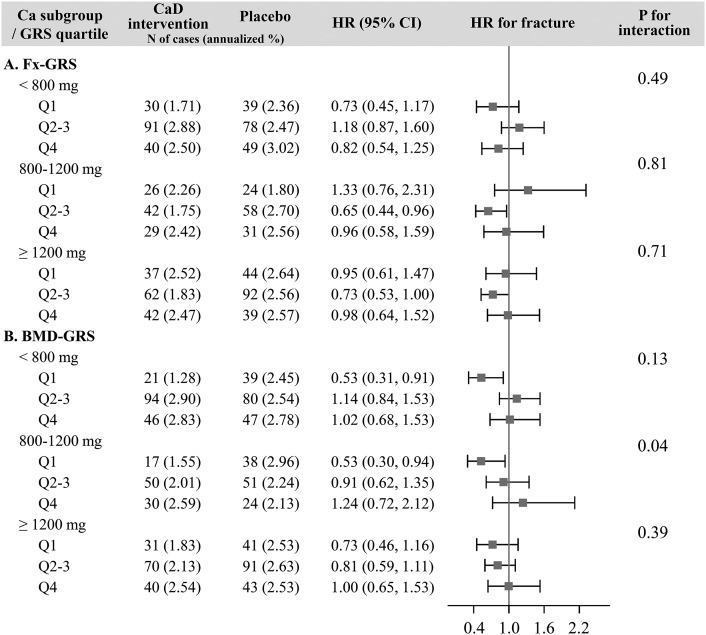
Sensitivity analysis: total personal calcium intake
In the calcium intake subgroup analyses, total fracture risk was significantly reduced by 47% in women in the lowest BMD-GRS quartile whose baseline personal calcium intake was <800 mg/d (HR: 0.53; 95% CI: 0.31, 0.91), whereas no such risk reduction was observed in women with a higher genetic risk of low BMD. A similar result was observed in women whose baseline total personal calcium intake was between 800 and 1200 mg/d, in which fracture risk reduction was observed only in individuals in BMD-GRS quartile 1 (HR: 0.53; 95% CI: 0.30, 0.94) (Figure 4).
FIGURE 4.
Sensitivity analysis: Heterogeneity of the interactions between genetic susceptibility and calcium with vitamin D on fracture across total personal calcium intake subgroups (<800, 800–1200, ≥1200 mg daily) for Fx-GRS (A) and BMD-GRS (B). Fx-GRS, Q1: <13.0, Q2–3: 13.0 to <16.4, and Q4: ≥16.4. BMD-GRS, Q1: <47.5, Q2–3: 47.5 to <53.9, and Q4: ≥53.9. Cox regression models were adjusted for age (years) and Women’s Health Initiative genome-wide association study (Genomics and Randomized Trials Network or Women’s Health Initiative Memory Study +). BMD-GRS, bone mineral density genetic risk score; CaD, calcium with vitamin D; Fx-GRS, fracture genetic risk score; GRS, genetic risk score; Q, quartile.
Exploratory analysis: fracture subgroup
We further explored the potential differential gene-CaD interaction on fractures at different skeletal sites by dividing fracture into 3 groups (central body, lower limb, and upper limb) (Supplemental Figure 2 and Supplemental Tables 3–5). Similar to the results from the total fracture analysis, we observed heterogeneity of the CaD effect for central body (P-heterogeneity = 0.07) and upper limb fracture (P-heterogeneity = 0.02) in the analyses of BMD-GRS (Supplemental Figure 2). Risk reduction with CaD supplementation was observed only for women in the lowest BMD-GRS quartile, with an HR (95% CI) of 0.41 (0.22, 0.78) for central body fracture and 0.53 (0.34, 0.83) for upper limb fracture.
Interaction between CaD supplementation and GWAS SNPs on total fracture risk
To further evaluate the effect of individual SNP on the association between CaD supplementation and fracture risk, we tested the interaction for all 50 BMD GWAS SNPs (Supplemental Table 6). Of 50 SNPs, 4 SNPs (rs479336, rs2062377, rs2887571, and rs1286083) showed significant interaction with CaD supplementation at P < 0.05, with the same direction of interaction as the BMD-GRS. However, after Bonferroni correction for multiple testing (P < 0.001), only 1 variant {rs479336 [dynamin 3 (DNM3)]} remained statistically significant (P-interaction = 0.0007), and another variant, rs2062377 (TNFRSF11B), showed a suggestive interaction with CaD (P-interaction = 0.0083).
DISCUSSION
In our study of 5823 Caucasian postmenopausal women, we observed a significant GRS-CaD interaction in the association with fracture risk. Specifically, we observed a significant risk reduction with CaD randomization for women in the lowest BMD-GRS category only. Our findings were not consistent with our a priori hypothesis. Analyses of subgroups revealed that CaD reduced the risk of fracture in women with the lowest genetic risk of low BMD whose baseline calcium intake was <1200 mg. We did not observe significant interactions between Fx-GRS and CaD on fracture.
One possible explanation for the antifracture effect of CaD supplementation found only in women who have a low genetic predisposition to low BMD is that genes exclusively included in the BMD-GRS contribute to calcium metabolism, so mutations in these genes may have led to an attenuated response to calcium intake. This may have resulted in the selectively observed responsiveness to CaD in women with the lowest genetic predisposition to low BMD in our study, because the proportion of women with mutations in those genes is likely lower in those with a low total genetic risk of low BMD (BMD-GRS quartile 1) than in those in higher BMD-GRS categories.
In an analysis of 987 Koreans, Park et al. (26) found that rs1366594 in the myocyte enhancer factor 2C (MEF2C) gene was associated with serum calcium concentrations in men. Xiao et al. (27) examined the interaction of GEFOS meta-GWAS–identified SNPs with factors associated with calcium and phosphate metabolism on BMD in Chinese individuals. Although no significant interaction was reported for serum calcium concentration, the authors reported a significant interaction between rs11623869 in microtubule affinity–regulating kinase 3 (MARK3) and the concentration of serum alkaline phosphatase, a biomarker for osteoblast activity. Although these findings may provide some insights into the possible role of SNPs in calcium metabolism, results from these studies are difficult to generalize because of small sample sizes and the limited diversity of the study population.
Another possible explanation for the observed selective antifracture effect with CaD supplementation in women with a low genetic risk is that SNPs may not influence actual serum calcium concentrations, but, rather, may have strong calcium-independent effects on BMD, so that the effects of calcium supplementation are unable to overwhelm the genetic effects in women with higher genetic risk. To test this hypothesis, an understanding of the functional significance of the genetic variants and their mechanisms of action on BMD is needed.
Calcium is a nutrient that exerts its effect on bone mass up to certain intake levels, with no further effect above those thresholds (28). Our results show that regardless of the amount of personal calcium intake, women in the lowest BMD-GRS had the greatest fracture risk reduction for groups with a personal intake of <800 and 800–1200 mg Ca. In women in the highest intake category (≥1200 mg), we observed a similar but not significant protection for women at lowest genetic risk. The lack of significance in the highest intake category may be due to low power.
Of the 50 SNPs in the BMD-GRS, rs479336 (DNM3) showed a significant interaction and rs2062377 (TNFRSF11B) showed a suggestive interaction with total fracture risk after Bonferroni correction. DNM3 is speculated to have a role in endocytosis and megakaryocyte development (29, 30). Other variants near DNM3 were reported in previous GWASs for their relation to height (31–33). However, the exact biological function of DNM3 on BMD is as yet unknown. TNFRSF11B, also known as osteoprotegerin (OPG) is a decoy receptor for receptor activator of nuclear factor κ-B ligand, and it prevents excessive bone resorption by blocking receptor activator of nuclear factor κ-B ligand and inhibiting osteoclast differentiation and activation (34, 35). It appears that calcium promotes the expression of OPG in vitro (36). To further illustrate the function of these genes and their interaction with calcium and vitamin D on bone, a functional evaluation of the GWAS hits are required.
We did not observe any significant reduction in fracture risk with CaD, regardless of participants’ Fx-GRS levels. Previous studies have shown the predictive ability of the GRS based on 16 BMD- and fracture-associated SNPs (Fx-GRS) (9, 10). The observed results might be due to the insignificant effect of CaD on fracture. The original WHI CaD trial did not show a significant reduction in total fracture incidence with CaD intervention, and the results did not change after exclusion of nonadherent women (11). Women in the WHI CaD trial were allowed to take their personal calcium in amounts ≤1000 mg/d and vitamin D in amounts ≤600 IU/d (later increased to 1000 IU/d) (11). Furthermore, only a small proportion of our study participants (8.7%) were taking <400 mg Ca daily at study enrollment. These may have limited our ability to observe the effect of the intervention medication. Although our results may indicate the presence of a true association, considering the nonsignificant CaD effect on fracture, the observed interaction between CaD and BMD-GRS could also be a chance finding.
Our study used the powerful design advantage of CTs while using multiple genetic susceptibility variants from a meta-GWAS. Standardized outcome ascertainment through adjudication and comprehensive and uniform data collection on participant characteristics, along with a well-defined cohort, are also notable strengths of our study.
The current study also has some limitations. Because the original trial was designed to assess the combined effect of calcium and vitamin D, we were unable to test the interaction of GRS with calcium and vitamin D separately. Because the study participants were allowed to take their own calcium and vitamin D supplementation and most of the women were not CaD-deficient, we may have a limited ability to examine the impact of CaD supplementation on fracture and the interaction with genetic risk. Our GRSs were calculated from common variants identified from a meta-GWAS, which included 17 GWASs focused primarily on BMD. Therefore, it is likely that some of important fracture-associated genetic variants and rare variants with presumably larger effects sizes are not included in the GRS. Because we included a subgroup of 5823 women who enrolled in both WHI HT and CaD trials, the characteristics of the women who participated in our study may differ from the entire postmenopausal population. Therefore, the external validity of our study may be limited. Furthermore, because we focused on participants with European ancestry only, findings from our study may not be generalized to other race and ethnic groups. Finally, our sample size may have limited statistical power to investigate gene-CaD interaction.
In conclusion, we found a significant interaction between BMD-GRS and CaD supplementation on fracture risk in which fracture risk was significantly reduced with CaD supplementation in women with the lowest genetic risk. These types of pharmacogenetic analyses are needed as we attempt to move toward the goal of precision medicine and understand which individuals can benefit the most from particular therapies. In addition, as we more fully understand the more subtle influence of rare variants on bone phenotypes and a deeper understanding of the function of particular risk variants, we will be able to improve on risk classification beyond the GRS used herein. Further large-scale studies with varied doses of calcium and vitamin D and information from functional analysis of GWAS SNPs are warranted to demonstrate the utility of genetic factors in assessing potential risks and benefits of CaD use on bone health, and which individuals will potentially benefit from CaD supplementation.
Acknowledgments
The datasets used for the analyses described in this manuscript were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap through dbGaP accession phs000675.v2.p3 (WHIMS+) and phs000315.v6.p3 (GARNET). A full listing of Women’s Health Initiative investigators can be found at: http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
The authors’ responsibilities were as follows— JW-W and HMO-B: obtained funding; YW, JW-W, and HMO-B: conceived of and designed the study; JW-W, LP, JN, and HMO-B: collected and assembled the data; YW, LES-C, and HMO-B: analyzed the data; YW, JW-W, LES-C, and HMO-B: interpreted the data; YW and HMO-B: drafted the manuscript; and all authors: critically revised the manuscript for content and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BMD, bone mineral density; BMD-GRS; bone mineral density genetic risk score; CaD, calcium with vitamin D; CT, randomized clinical trial; DNM3, dynamin 3; FN, femoral neck; Fx-GRS, fracture genetic risk score; GARNET, Genomics and Randomized Trials Network; GEFOS, GEnetic Factors for OSteoporosis consortium; GRS, genetic risk score; GWAS, genome-wide association study; HT, hormone therapy; MARK3, microtubule affinity–regulating kinase 3; MEF2C, myocyte enhancer factor 2C; OPG, osteoprotegerin; RERI, relative excess risk due to interaction; SNP, single-nucleotide polymorphism; WHI, Women’s Health Initiative; WHIMS, Women's Health Initiative Memory Study; WHIMS+, Women’s Health Initiative Memory Study +.
REFERENCES
- 1.Office of the Surgeon General. Reports of the Surgeon General. Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD): Office of the Surgeon General (US); 2004. [PubMed]
- 2.Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol 2011;347:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Driel M, van Leeuwen JP. Vitamin D endocrine system and osteoblasts. Bonekey Rep 2014;3:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pike JW, Lee SM, Meyer MB. Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. Bonekey Rep 2014;3:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrew T, Antioniades L, Scurrah KJ, Macgregor AJ, Spector TD. Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J Bone Miner Res 2005;20:67–74. [DOI] [PubMed] [Google Scholar]
- 6.Deng HW, Chen WM, Recker S, Stegman MR, Li JL, Davies KM, Zhou Y, Deng H, Heaney R, Recker RR. Genetic determination of Colles’ fracture and differential bone mass in women with and without Colles’ fracture. J Bone Miner Res 2000;15:1243–52. [DOI] [PubMed] [Google Scholar]
- 7.Michaëlsson K, Melhus H, Ferm H, Ahlbom A, Pedersen NL. Genetic liability to fractures in the elderly. Arch Intern Med 2005;165:1825–30. [DOI] [PubMed] [Google Scholar]
- 8.Ralston SH, Uitterlinden AG. Genetics of osteoporosis. Endocr Rev 2010;31:629–62. [DOI] [PubMed] [Google Scholar]
- 9.Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, et al. . Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 2012;44:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson J, Evans DS, Nielson CM, Shen J, Srikanth P, Hochberg M, McWeeney S, Cawthon PM, Wilmot B, Zmuda J, et al. . Limited clinical utility of a genetic risk score for the prediction of fracture risk in elderly subjects. J Bone Miner Res 2015;30:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, et al. . Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669–83. [DOI] [PubMed] [Google Scholar]
- 12.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, et al. . Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701–12. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 15.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(9 Suppl):S98–106. [DOI] [PubMed] [Google Scholar]
- 16.Craig MC, Maki PM, Murphy DG. The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 2005;4:190–4. [DOI] [PubMed] [Google Scholar]
- 17. NCBI Database of Genotypes and Phenotypes (dbGaP). WHIMS+GWAS. GWAS on selected Women's Health Initiative (WHI) hormone trial European Americans (dbGaP accession e.g. phs000200.v10.p3). [Google Scholar]
- 18.NCBI Database of Genotypes and Phenotypes (dbGaP) [Internet]. [cited 2016 Jan 1]. Available from: http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000746.v1.p3. [DOI] [PMC free article] [PubMed]
- 19.Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet 2009;84:210–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurie CC, Doheny KF, Mirel DB, Pugh EW, Bierut LJ, Bhangale T, Boehm F, Caporaso NE, Cornelis MC, Edenberg HJ, et al. . Quality control and quality assurance in genotypic data for genome-wide association studies. Genet Epidemiol 2010;34:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornelis MC, Agrawal A, Cole JW, Hansel NN, Barnes KC, Beaty TH, Bennett SN, Bierut LJ, Boerwinkle E, Doheny KF, et al. . The Gene, Environment Association Studies consortium (GENEVA): maximizing the knowledge obtained from GWAS by collaboration across studies of multiple conditions. Genet Epidemiol 2010;34:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015;31:3555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crandall CJ, Yildiz VO, Wactawski-Wende J, Johnson KC, Chen Z, Going SB, Wright NC, Cauley JA. Postmenopausal weight change and incidence of fracture: post hoc findings from Women’s Health Initiative Observational Study and Clinical Trials. BMJ 2015;350:h25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol 2007;17:227–36. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology 1992;3:452–6. [DOI] [PubMed] [Google Scholar]
- 26.Park SE, Oh KW, Lee WY, Baek KH, Yoon KH, Son HY, Lee WC, Kang MI. Association of osteoporosis susceptibility genes with bone mineral density and bone metabolism related markers in Koreans: the Chungju Metabolic Disease Cohort (CMC) study. Endocr J 2014;61:1069–78. [DOI] [PubMed] [Google Scholar]
- 27.Xiao SM, Kung AW, Sham PC, Tan KC. Genetic analysis of recently identified osteoporosis susceptibility genes in southern Chinese. J Clin Endocrinol Metab 2013;98:E1827–34. [DOI] [PubMed] [Google Scholar]
- 28.Recker RR. Prevention of osteoporosis: calcium nutrition. Osteoporos Int 1993;3 Suppl 1:163–5. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 2012;13:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Gilligan DM, Sun S, Wu X, Reems J-A. Distinct functional effects for dynamin 3 during megakaryocytopoiesis. Stem Cells Dev 2011;20:2139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berndt SI, Gustafsson S, Magi R, Ganna A, Wheeler E, Feitosa MF, Justice AE, Monda KL, Croteau-Chonka DC, Day FR, et al. . Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet 2013;45:501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudbjartsson DF, Walters GB, Thorleifsson G, Stefansson H, Halldorsson BV, Zusmanovich P, Sulem P, Thorlacius S, Gylfason A, Steinberg S, et al. . Many sequence variants affecting diversity of adult human height. Nat Genet 2008;40:609–15. [DOI] [PubMed] [Google Scholar]
- 33.Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, Willer CJ, Jackson AU, Vedantam S, Raychaudhuri S, et al. . Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 2010;467:832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001;142:5050–5. [DOI] [PubMed] [Google Scholar]
- 35.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008;473:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welldon KJ, Findlay DM, Evdokiou A, Ormsby RT, Atkins GJ. Calcium induces pro-anabolic effects on human primary osteoblasts associated with acquisition of mature osteocyte markers. Mol Cell Endocrinol 2013;376:85–92. [DOI] [PubMed] [Google Scholar]