Abstract
Background: In cross-sectional studies and short-term clinical trials, it has been suggested that there is a positive dose-response relation between alcohol consumption and HDL concentrations. However, prospective data have been limited.
Objective: We sought to determine the association between total alcohol intake, the type of alcohol-containing beverage, and the 6-y (2006–2012) longitudinal change in HDL-cholesterol concentrations in a community-based cohort.
Design: A total of 71,379 Chinese adults (mean age: 50 y) who were free of cardiovascular diseases and cancer and did not use cholesterol-lowering agents during follow-up were included in the study. Alcohol intake was assessed via a questionnaire in 2006 (baseline), and participants were classified into the following categories of alcohol consumption: never, past, light (women: 0–0.4 servings/d; men: 0–0.9 servings/d), moderate (women: 0.5–1.0 servings/d; men: 1–2 servings/d), and heavy (women: >1.0 servings/d; men: >2 servings/d). HDL-cholesterol concentrations were measured in 2006, 2008, 2010, and 2012. We used generalized estimating equation models to examine the associations between baseline alcohol intake and the change in HDL-cholesterol concentrations with adjustment for age, sex, smoking, physical activity, obesity, hypertension, diabetes, liver function, and C-reactive protein concentrations.
Results: An umbrella-shaped association was observed between total alcohol consumption and changes in HDL-cholesterol concentrations. Compared with never drinkers, past, light, moderate, and heavy drinkers experienced slower decreases in HDL cholesterol of 0.012 mmol · L−1 · y−1 (95% CI: 0.008, 0.016 mmol · L−1 · y−1), 0.013 mmol · L−1 · y−1 (95% CI: 0.010, 0.016 mmol · L−1 · y−1), 0.017 mmol · L−1 · y−1 (95% CI: 0.009, 0.025 mmol · L−1 · y−1), and 0.008 mmol · L−1 · y−1 (95% CI: 0.005, 0.011 mmol · L−1 · y−1), respectively (P < 0.0001 for all), after adjustment for potential confounders. Moderate alcohol consumption was associated with the slowest increase in total-cholesterol:HDL-cholesterol and triglyceride:HDL-cholesterol ratios. We observed a similar association between hard-liquor consumption and the HDL-cholesterol change. In contrast, greater beer consumption was associated with slower HDL-cholesterol decreases in a dose-response manner.
Conclusion: Moderate alcohol consumption was associated with slower HDL-cholesterol decreases; however, the type of alcoholic beverage had differential effects on the change in the HDL-cholesterol concentration.
Keywords: alcohol, cardiovascular disease risk, epidemiology, HDL, lipids, prospective cohort, triglyceride
INTRODUCTION
Previous observational studies have consistently reported that individuals with moderate alcohol consumption have a lower risk of cardiovascular disease (CVD)7 compared with that of nondrinkers and heavy drinkers (1, 2). The consumption of 1–2 drinks/d (generally 10–30 g alcohol/d) has been associated with a 20–25% decrease in risk of CVD (3–5); however, greater alcohol consumption increased the risk of CVD and total mortality (4, 5). The beneficial effects of moderate drinking could be attributed to more-favorable inflammation and fibrinolytic status but are primarily a result of an increased HDL concentration (6, 7). It was estimated that a higher HDL concentration could explain ∼50% of the coronary heart disease preventive effect of alcohol consumption (8).
Unlike the U-shaped relation between alcohol consumption and CVD risk, greater alcohol consumption has been associated with a higher HDL concentration in a dose-response manner in previous studies (7, 9, 10). However, most observational studies have been cross-sectional (9, 11, 12), and the long-term impact of alcohol on HDL remains to be elucidated. Experimental studies that have examined the potential effects of higher intake of alcohol have been limited by the sample size (n < 100) and a short follow-up period (<8 wk) (10). Note that long-term high alcohol intake (>2 drinks/d) could result in adverse effects such as inflammation, hypertension, hypertriglyceridemia (11), and liver disease (13); the latter 2 effects each have well-known associations with HDL concentrations and function (13, 14). Regardless, because of the development of pharmaceuticals, which have successfully increased HDL but have not lowered risk (15), alcohol remains an important factor in understanding the relation between HDL and CVD risk.
Alcohol-induced increases in triglyceride could be another factor that mediates the effect of alcohol on CVD risk. The triglyceride:HDL-cholesterol ratio, which is a well-known predictive biomarker of insulin resistance, is associated with CVD risk (16). Alcohol consumption has been reported to be associated with insulin sensitivity (17, 18) and, thus, may affect the triglyceride:HDL-cholesterol ratio. Because there is more cholesterol in the VLDL of patients with elevated triglyceride concentrations, the total-cholesterol:HDL-cholesterol ratio is thought to be preferable to estimate the dyslipidemia status (19). However, to the best of our knowledge, no previous study has examined the longitudinal change of these ratios in relation to alcohol consumption.
In this context, we hypothesized that the association between total alcohol consumption and longitudinal changes in HDL-cholesterol concentrations was not linear and that moderate alcohol consumption was associated with the slowest decline in HDL cholesterol over time. Thus, we tested this hypothesis in a large, community-based cohort including >70,000 participants with repeated HDL-cholesterol measurements. For the secondary analysis, we used changes in other lipid indexes, including the triglyceride:HDL-cholesterol ratio and total-cholesterol:HDL-cholesterol ratio, as outcomes. We also assessed the effect of individual alcoholic beverages on HDL-cholesterol concentrations and the previously mentioned biomarkers.
METHODS
Study population
We used data from the Kailuan Study, which is an ongoing, prospective cohort study being conducted in the Kailuan community in Tangshan City, China. In 2006–2007 (i.e., baseline), 101,050 subjects (81,110 men and 20,400 women; age range: 18–97 y) were recruited. All participants completed a standardized questionnaire and underwent physical examinations and laboratory assessments at recruitment. Participants were followed biennially through 2012 via questionnaires and clinical and laboratory examinations.
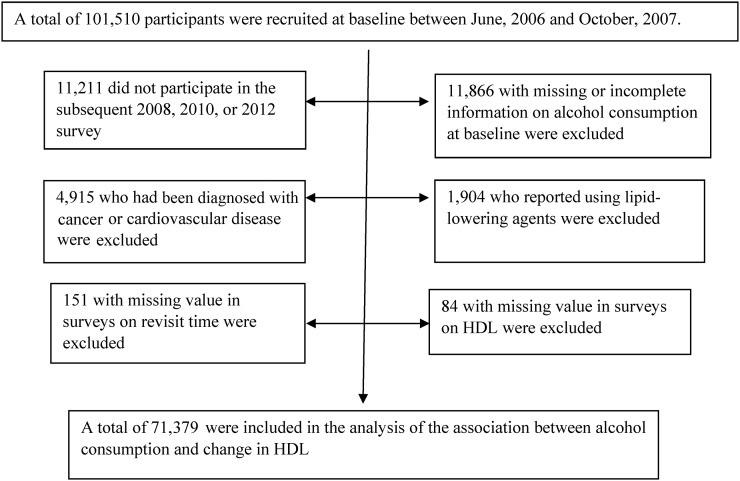
In total, 90,299 subjects (89.4%) participated in ≥1 of the subsequent surveys. In the current study, we further excluded participants 1) if they had diagnosed cancer or CVD (myocardial infarction or stroke) (n = 4915), 2) if they reported the use of lipid-lowering agents at baseline or during follow-up (n = 1904), 3) if they had no information or incomplete information on alcohol consumption at baseline (n = 11,866), or 4) if they had missing values in the surveys for the revisit time (n = 151) or for HDL cholesterol (n = 84). A total of 71,379 individuals were included in the current analysis (Figure 1), and of these participants, 55,371 subjects (77.6%) and 38,089 subjects (53.4%) had 3 and 4 measurements, respectively. Compared with subjects who were included in the analyses, participants who were excluded because of missing information on alcohol intake were older (mean ± SE age: 51.3 ± 0.11 compared with 50.3 ± 0.05 y, respectively), had greater proportions of men (90.7% compared with 76.6%, respectively) and smokers (58.3% compared with 31.6%, respectively), respectively (both P < 0.05), had lower LDL-cholesterol concentrations (2.28 ± 0.008 compared with 2.35 ± 0.003 mmol/L, respectively; P < 0.0001) and slightly lower HDL-cholesterol concentrations (1.54 ± 0.003 compared with 1.55 ± 0.001 mmol/L, respectively; P < 0.05) and BMI (in kg/m2; 24.9 ± 0.03 compared with 25.0 ± 0.01, respectively; P < 0.05) but had similar triglyceride:HDL-cholesterol ratios (1.16 ± 0.02 compared with 1.17 ± 0.008, respectively; P = 0.69), and total-cholesterol:HDL-cholesterol ratios (3.43 ± 0.03 compared with 3.38 ± 0.01, respectively; P = 0.09). This study was approved by the Ethics Committee of the Kailuan General Hospital. All participants provided written informed consent.
FIGURE 1.
Flowchart of the study.
Assessment of alcohol consumption
Information on alcohol use was collected with the use of a questionnaire. Participants were asked to report whether they consumed alcoholic beverages in the past 12 mo and, if so, the beverage type (beer, wine, and hard liquor) and the amount and frequency of intake. Alcohol consumption was calculated in grams per day by multiplying the average frequency (times per day) by the usual consumption amount of each beverage and its average ethanol content (5.0 g for 100 g beer, 12.0 g for wine, and 40.0 g for hard liquor). A standard drink was ∼15 g ethanol. According to the number of standard servings, participants were classified into the following categories of alcohol consumption: never, past, light (women: 0–0.4 servings/d; men: 0–0.9 servings/d), moderate (women: 0.5–1.0 servings/d; men: 1–2 servings/d), and heavy drinkers (women: >1.0 servings/d; men: >2 servings/d) (20). For each category of alcoholic beverage, participants who did not drink or did not drink the indicated alcohol type were grouped as none. Because of the small number of subjects who reported drinking wine (n = 311), participants were classified into 2 groups (yes or no).
Assessment of lipid profiles
Overnight fasting blood samples were drawn from the antecubital vein with the use of vacuum tubes that contained EDTA for storage and were repeatedly collected at baseline and in the subsequent surveys in 2008, 2010, and 2012. Plasma was separated and stored at −80°C for subsequent analyses. Total cholesterol and triglyceride were both measured with the use of an enzymatic colorimetric method (Mind Bioengineering Co. Ltd.) with an upper limit of detection of 20.68 and 11.30 mmol/L; HDL cholesterol and LDL cholesterol were measured via a direct test method (Mind Bioengineering Co. Ltd.) with upper limits of detection of 12.90 and 3.88 mmol/L, respectively. Less than 0.1% of measured values were within 5% of either limit. The interassay CV for each measurement was <10%. All plasma samples were analyzed with the use of an autoanalyzer (Hitachi 747; Hitachi) at the central laboratory of the Kailuan General Hospital.
Assessment of potential covariates
Information on age, sex, socioeconomic status, lifestyle behaviors, and medical history was collected with the use of questionnaires at baseline. Participants were categorized as never, past, current occasional smoker (<1 cigarette/d), and current daily smoker (≥1 cigarette/d) on the basis of self-report smoking status. Physical activity was evaluated from responses to questions regarding the frequency of physical activity (≥20 min/time) during leisure time with possible responses including never, sometimes, and ≥4 times/wk. According to their response, participants were classified as inactive, moderately active, and active. Previous studies have suggested an inverse association between physical activity levels and risk of developing stroke in participants in the Kailuan study (21).
Body weight and height were measured with participants standing without shoes and outer clothing. Body weight was measured to the nearest 0.1 kg with the use of calibrated platform scales, and height was measured to the nearest 0.1 cm with the use of a tape rule. BMI (in kg/m2) was calculated as body weight divided by the square of height. Waist circumference was measured in centimeters at the narrowest point between the lowest rib and the iliac crest. Blood pressure was measured twice at a 5-min interval on the left arm with participants in a seated position after a ≥5-min rest with the use of a mercury sphygmomanometer. The average of the 2 readings was used for analysis. If the 2 measurements differed by >5 mm Hg, a third measurement was taken, and the average of the 3 readings was used for the analysis. Hypertension was defined systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or a self-report history of hypertension. Prehypertension was defined as a systolic blood pressure ranging between 120 and 140 mm Hg or a diastolic blood pressure ranging between 80 and 90 mm Hg.
Fasting blood glucose was measured according to the hexokinase/glucose-6-phosphate dehydrogenase method. Plasma high-sensitivity C-reactive protein (CRP) concentrations were measured with the use of a high-sensitivity, particle-enhanced immunonephelometry assay. Alanine aminotransferase was measured with the use of an enzymatic rate method. All plasma samples were measured with the use of the aforementioned autoanalyzer (Hitachi 747). Abdominal ultrasonography was performed and interpreted by experienced radiologists who were blinded to the clinical presentation and laboratory findings (HD-15; Philips). Fatty liver was diagnosed and graded as mild, moderate, and severe according to ultrasonographic liver features by referring to established criteria (22, 23). Diabetes was defined as a fasting blood glucose concentration >7.0 mmol/L or a self-report history of a diagnosis of diabetes; prediabetes was defined as a fasting blood glucose range of 5.6–6.9 mmol/L.
Statistical analysis
We used generalized estimating equation (GEE) models to estimate the longitudinal association between alcohol consumption and the change rates in HDL-cholesterol and lipid indexes over the 6 y. As previously noted, drinking status was categorized into 5 groups. Never drinkers were taken as the reference group. Three multivariate models were fitted as follows: model 1 was adjusted for age and sex; model 2 was further adjusted for smoking status, physical activity, BMI, presence of hypertension, presence of hyperglycemia, waist circumference, serum concentrations of high-sensitivity CRP, and the presence of fatty liver; and model 3 was further adjusted for triglyceride, total cholesterol, and LDL cholesterol when appropriate. Trends in mean differences in HDL-cholesterol change rates across groups of total alcohol consumption were assessed in GEE models by taking the alcohol-consumption group number as a continuous variable. Associations between alcoholic beverage types and changes in HDL cholesterol were further assessed.
To test the robustness of the main findings, several sensitivity analyses were conducted by excluding smokers, overweight and obese individuals, hypertensive individuals, patients with diabetes, subjects with a high CRP concentration (≥3 mg/L), or subjects who had fatty liver because all of these factors could affect HDL-cholesterol concentrations. We also explored potential interactions between alcohol consumption and age, sex, smoking status, physical activity, and waist circumference in relation to changes in HDL cholesterol by adding multiplicative terms in the GEE models.
All analyses were performed with the use of SAS software (version 9.4; SAS Institute). P < 0.05 was regarded as significant for 2-sided tests.
RESULTS
Of the total number of participants, 30.7% of subject were current drinkers (light drinkers: 16.0%; moderate drinkers: 2.0%; and heavy drinkers: 12.7%). Compared with any drinkers, never drinkers had a lower prevalence of smoking and lower LDL-cholesterol concentrations (P < 0.001) (Table 1). As expected, alcohol intakes were associated with higher baseline HDL-cholesterol concentrations. In subjects who drank alcohol, there was a dose-response relation between alcohol consumption and baseline HDL-cholesterol concentrations (Supplemental Figure 1).
TABLE 1.
Baseline characteristics according to alcohol consumption in 20061
| Alcohol consumption |
||||||
| Never (n = 46,902; 65.7%) | Past (n = 2536; 3.6%) | Light (n = 11,449; 16.0%) | Moderate (n = 1431; 2.0%) | Heavy (n = 9061; 12.7%) | P | |
| Men, % | 65.7 | 97.2 | 95.5 | 99.3 | 99.7 | <0.0001 |
| Age, y | 51.3 ± 0.062 | 53.8 ± 0.23 | 44.5 ± 0.11 | 56.4 ± 0.31 | 50.3 ± 0.12 | <0.0001 |
| BMI, kg/m2 | 25.0 ± 0.02 | 25.2 ± 0.07 | 25.2 ± 0.03 | 24.5 ± 0.09 | 24.8 ± 0.04 | <0.0001 |
| Waist circumstance, cm | 86.7 ± 0.05 | 86.4 ± 0.19 | 86.4 ± 0.09 | 85.2 ± 0.25 | 86.2 ± 0.10 | <0.0001 |
| Physical activity, % | <0.0001 | |||||
| Inactive | 4.9 | 12.9 | 15.4 | 10.6 | 18.6 | |
| Moderately active | 83.6 | 60.7 | 67.3 | 53.5 | 59.8 | |
| Active | 11.5 | 25.7 | 17.1 | 35.8 | 21.3 | |
| Smoking status, % | <0.0001 | |||||
| Never | 85.7 | 22.0 | 27.9 | 21.5 | 13.2 | |
| Past | 1.7 | 34.3 | 8.9 | 10.1 | 5.7 | |
| Occasional | 1.2 | 4.5 | 10.0 | 3.1 | 3.3 | |
| Daily | 11.3 | 39.0 | 53.2 | 65.3 | 77.7 | |
| Diabetes, % | <0.0001 | |||||
| No | 73.0 | 65.9 | 70.3 | 70.7 | 64.9 | |
| Prediabetes | 18.5 | 21.2 | 23.1 | 19.9 | 26.9 | |
| Yes | 8.5 | 12.9 | 6.7 | 9.4 | 8.3 | |
| Hypertension, % | <0.0001 | |||||
| No | 20.8 | 18.0 | 27.5 | 21.1 | 14.2 | |
| Prehypertension | 36.8 | 33.4 | 39.2 | 32.9 | 37.0 | |
| Yes | 41.9 | 48.2 | 33.0 | 45.7 | 48.6 | |
| CRP,3 mg/L | 0.72 ± 1.00 | 0.80 ± 1.03 | 0.79 ± 1.01 | 0.69 ± 1.04 | 0.71 ± 1.02 | <0.0001 |
| FPG, mmol/L | 5.44 ± 0.01 | 5.52 ± 0.03 | 5.42 ± 0.02 | 5.32 ± 0.04 | 5.45 ± 0.02 | <0.0001 |
| Total cholesterol, mmol/L | 4.89 ± 0.005 | 4.90 ± 0.02 | 4.94 ± 0.01 | 5.00 ± 0.03 | 5.17 ± 0.01 | <0.0001 |
| LDL cholesterol, mmol/L | 2.28 ± 0.004 | 2.48 ± 0.02 | 2.49 ± 0.008 | 2.44 ± 0.02 | 2.52 ± 0.01 | <0.0001 |
| HDL cholesterol, mmol/L | 1.55 ± 0.002 | 1.48 ± 0.008 | 1.50 ± 0.004 | 1.54 ± 0.01 | 1.61 ± 0.004 | <0.0001 |
| Triglyceride, mmol/L | 1.65 ± 0.01 | 1.56 ± 0.03 | 1.65 ± 0.01 | 1.51 ± 0.04 | 1.80 ± 0.01 | <0.0001 |
Participants were classified into the following categories of alcohol consumption: never, past, light (women: 0–0.4 servings/d; men: 0–0.9 servings/d), moderate (women: 0.5–1.0 servings/d; men: 1–2 servings/d), and heavy (women: >1.0 serving/d; men: >2 servings/d). CRP, C-reactive protein; FPG, fasting plasma glucose.
Mean ± SE adjusted for age and sex (all such values unless otherwise indicated). Means were compared with the use of a general linear model.
Values are geometric means ± SEs. Means were compared with the use of a general linear model.
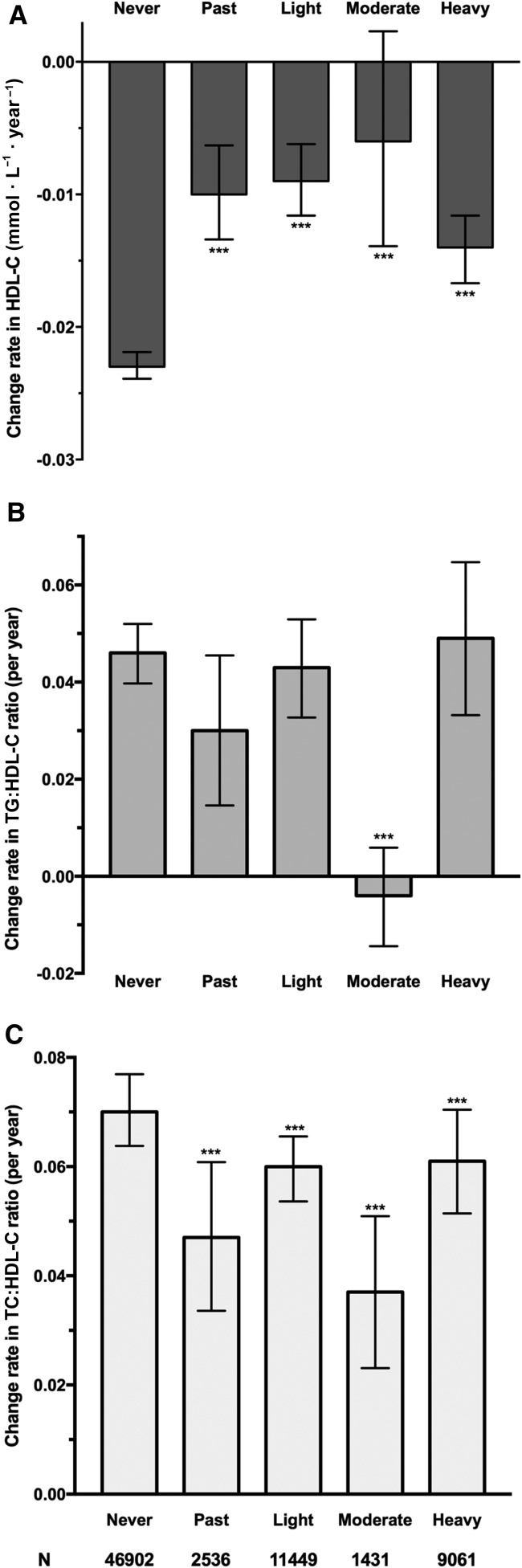
An umbrella-shaped association was observed between total alcohol consumption and changes in HDL-cholesterol concentrations (Supplemental Table 1, Figure 2A), after adjustment for age, sex, and other potential confounders. Consistently, moderate alcohol intake was associated with the slowest increase in the triglyceride:HDL-cholesterol ratio and the total-cholesterol:HDL-cholesterol ratio across all alcohol-drinking groups (Figure 2B, C, Supplemental Table 1). Sensitivity analyses that excluded participants with diabetes, hypertension, obesity, fatty liver, and elevated CRP, respectively, generated similar results (Supplemental Table 2).
FIGURE 2.
Mean (95% CI) time-dependent change rates in HDL-C concentrations (mmol · L−1 · y−1) (A), the TC:HDL-C ratio (B), and the TG:HDL-C ratio (C) according to alcohol consumption. Models were adjusted for age, sex (men or women), physical activity (inactive, moderately active, or active), smoking status (never, past, occasionally, or daily), patients with diabetes (no, prediabetes, or yes), hypertension (no, prehypertension, or yes), BMI (in kg/m2; <24, 24–27.9, 28–29.9, or ≥30), waist circumference (<85 or ≥85 cm for women and <90 or ≥90 cm for men), C-reactive protein (<1, 1–2.9, or ≥3 mg/L), fatty liver (none, mild, or heavy), LDL cholesterol, and triglyceride. Participants were classified into the following categories of alcohol consumption: never, past, light (women: 0–0.4 servings/d; men: 0–0.9 servings/d), moderate (women: 0.5–1.0 servings/d; men: 1–2 servings/d), or heavy (women: >1.0 servings/d; men: >2 servings/d). Generalized estimating equation models were used to model the change rates and to test the differences in change rates compared with never drinkers. ***Compared with never drinkers, P < 0.001. HDL-C, HDL cholesterol; TC, total cholesterol; TG, triglyceride.
The type of alcohol that was consumed modified the change in HDL-cholesterol concentrations. As beer consumption increased, mean differences increased in HDL cholesterol (P-trend < 0.0001) and decreased in the total-cholesterol:HDL-cholesterol ratio (P trend = 0.0007). However, for hard-liquor consumption, an umbrella-shaped trend was observed for the changes in both HDL-cholesterol concentrations and total-cholesterol:HDL-cholesterol ratios (Table 2).
TABLE 2.
Differences in change rates in HDL cholesterol and triglyceride:HDL-cholesterol and total-cholesterol:HDL-cholesterol ratios during 2006–2012 according to the consumption of beer, hard liquor, and wine in 20061
| Alcohol consumption |
|||||
| None | Light | Moderate | Heavy | P-trend | |
| Beer | |||||
| n | 66,806 | 4483 | 55 | 35 | — |
| Change, mmol · L−1 · y−1 | |||||
| HDL cholesterol | Reference | 0.008 (0.005, 0.011)2 | 0.035 (0.012, 0.058) | 0.035 (0.010, 0.060) | <0.0001 |
| Triglyceride:HDL-cholesterol ratio | Reference | 0.008 (−0.008, 0.025) | −0.050 (−0.083, −0.017) | −0.059 (−0.131, 0.014) | 0.40 |
| Total-cholesterol:HDL-cholesterol ratio | Reference | −0.015 (−0.025, −0.005) | −0.068 (−0.124, −0.013) | −0.112 (−0.197, −0.027) | 0.0007 |
| Liquor | |||||
| n | 54,132 | 6794 | 1444 | 9009 | — |
| Change, mmol · L−1 · y−1 | |||||
| HDL cholesterol | Reference | 0.014 (0.010, 0.018) | 0.016 (0.008, 0.024) | 0.007 (0.004, 0.010) | <0.0001 |
| Triglyceride:HDL-cholesterol ratio | Reference | −0.005 (−0.018, 0.008) | −0.049 (−0.061, −0.038) | 0.004 (−0.012, 0.021) | 0.70 |
| Total-cholesterol:HDL-cholesterol ratio | Reference | −0.021 (−0.031, −0.011) | −0.038 (−0.053, −0.023) | −0.016 (−0.028, −0.005) | <0.0001 |
| Wine3 | |||||
| n | 71,064 | — | 311 | — | — |
| Change, mmol · L−1 · y−1 | |||||
| HDL cholesterol | Reference | — | 0.016 (0.005, 0.026) | — | 0.0003 |
| Triglyceride:HDL-cholesterol ratio | Reference | — | −0.097 (−0.242, 0.048) | — | 0.21 |
| Total-cholesterol:HDL-cholesterol ratio | Reference | — | −0.024 (−0.070, 0.022) | — | 0.13 |
All models were adjusted for age, sex, physical activity (inactive, moderately active, or active), smoking status (never, past, occasionally, or daily), patients with diabetes (no, prediabetes, or yes), hypertension (no, prehypertension, or yes), BMI (in kg/m2; <24, 24–27.9, 28–29.9, or ≥30), waist circumference (<85 or ≥85 cm for women and <90 or ≥90 cm for men), C-reactive protein (<1, 1–2.9, or ≥3 mg/L), and fatty liver (none, mild, or severe). Models were further adjusted for triglyceride and LDL cholesterol for the change in HDL cholesterol; for LDL cholesterol for the change in the triglyceride:HDL-cholesterol ratio; or for triglyceride for the change in the total-cholesterol:HDL-cholesterol ratio. Generalized estimating equation models were used to model change rates and to test the differences in change rates compared with in never drinkers.
Mean; 95% CI in parentheses (all such values).
Data for all wine drinkers are presented in the moderate group.
We showed significant interactions between alcohol intake and age, sex, smoking status, and waist circumference. Compared with never drinkers, HDL cholesterol decreased slowest in light drinkers for women but in moderate drinkers for men. The mean difference in the HDL-cholesterol change in moderate drinkers was 0.025 mmol/L in never or past smokers and 0.009 mmol/L in current smokers although alcohol intake was still umbrella-shaped in terms of the change in HDL cholesterol (Supplemental Table 3).
DISCUSSION
In this large, community-based, longitudinal study, we showed an umbrella-shaped association between total alcohol consumption and the longitudinal change in HDL cholesterol. A decreasing rate in the HDL-cholesterol concentration was significantly lower with any amount of alcohol intake compared with in never drinkers but was the lowest in moderate alcohol drinkers. Moderate alcohol consumption was also associated with the smallest rise in the total-cholesterol:HDL-cholesterol ratio. The exclusion of participants with diabetes, hypertension, fatty liver, or high inflammation status did not materially change the results. In this study, the observed nonlinear relation between alcohol intake and HDL-cholesterol change during 6 y of follow-up was consistent with the reported U-shaped association between alcohol consumption and CVD risk.
Although in a cross-sectional analysis we observed a dose-response relation between greater alcohol intakes and higher baseline HDL-cholesterol concentrations in alcohol drinkers, which was consistent with previous studies (9–12), the long-term effect of total alcohol consumption on the change in HDL cholesterol was nonlinear. One possible explanation is that heavy drinkers are more likely to develop severe liver diseases in which case the liver’s production of HDL decreases (13). Although adjustment for fatty liver status and alanine aminotransferase did not change the umbrella-shaped relation, alanine aminotransferase may have a low sensitivity for alcoholic liver injury (24, 25). Furthermore, the impact of alcohol on liver function is generally chronic (24). However, previous observational studies on this topic (9, 11, 12, 26, 27) were predominantly cross-sectional; therefore, whether the long-term impact of alcohol consumption on HDL concentration remains a linear pattern could not be inferred. The only 2 prospective studies on this topic (28, 29) that reported a linear relation were based on only 2 measurements of HDL cholesterol, which made it impossible to describe the time-dependent change. Note that the studies investigated the association between changes in alcohol consumption (instead of in baseline alcohol consumption) and changes in HDL cholesterol, and their analyses should be considered cross-sectional.
Most of the clinical trials regarding alcohol intake and HDL-cholesterol concentrations failed to investigate the dose-response relation and were limited because they were short term and had small sample sizes (n < 100) (10, 30). The trials that examined 2 or 3 doses of alcohol intake often targeted only light-to-moderate doses (31–33). However, 2 meta-analyses (10, 30) that used a meta-regression approach reported a dose-response relation between alcohol consumption and HDL-cholesterol concentrations. The discrepancy between these meta-analyses of trials and our study could have been because the clinical trials did not test the long-term effects of high-dose alcohol intake on the changed in HDL-cholesterol concentration. The dearth of data has limited the ability of meta-analyses to address the association of alcohol consumption with the 6-y change in HDL cholesterol. The dose-response was based on the various doses from different trials, and the estimation of beneficial effects of heavy drinking is questionable. Last, heterogeneity in the study design and study population as well as the small sample size in each study would have led to greater residual confounding when the data were pooled together.
Because an increase in the total cholesterol concentration is an atherogenic marker, whereas lower HDL-cholesterol concentrations are related to risk factors such as metabolic syndrome, the total-cholesterol:HDL-cholesterol ratio is considered to be a sensitive index of CVD risk (34). The triglyceride:HDL-cholesterol ratio reflects the interaction of the overall lipoprotein metabolism and is a useful marker for predicting plasma atherogenicity and insulin resistance (34). The inverse relation of moderate alcohol intake to these lipid indexes confirms previous findings that light-to-moderate alcohol consumption is associated with reduced risk of coronary heart disease.
We observed that different types of alcoholic beverages had different impacts on the change in lipids. Previous studies have generated inconsistent results with some studies having reported that HDL cholesterol increased more after wine consumption (3, 11, 35), and some studies have reported that no difference was observed for the effect on HDL cholesterol or CVD risk for different types of alcoholic beverages (8–10, 36–38). In the current study, because of the small sample size of wine consumers, we dichotomized wine drinking (yes or no) and showed that the consumption of wine could be protective against an HDL-cholesterol decrease over time. However, because of the low rate of wine intake, the results need to be interpreted with caution. We showed that there was a diversity of beer and hard-liquor consumption in relation to the changes in HDL-cholesterol and lipid ratios. Similar to the relation that was observed in total alcohol consumption, a moderate consumption of hard liquor was associated with a greater effect on HDL-cholesterol and lipid ratios, whereas higher intake of beer was associated with better lipid profiles. Because we adjusted for alanine aminotransferase and fatty liver as diagnosed with the use of an ultrasonic wave, it was unlikely that such heterogeneity was totally explained by the confounding that was caused by the difference in liver function in beer drinkers and liquor drinkers. Another possible explanation is that other components in beer (e.g., polyphenols) could have conferred a more-protective effect on the reduction of CVD risk and, probably, on HDL-cholesterol concentrations (39). Note that, in our study, heavy beer drinkers had lower mean alcohol intake compared with that of heavy hard-liquor drinkers (3.2 ± 1.0 compared with 4.1 ± 1.8 servings/d, respectively).
In this study, we showed an average lower decrease in HDL of 0.017 mmol · L−1 · y−1 (95% CI: 0.009, 0.025 mmol · L−1 · y−1), which amounted to 0.102 mmol/L over the 6-y follow-up. On the basis of previous data, this decrease would be associated with a risk reduction in CVD of ∼13.1% (40). The protective effect of alcohol on CVD risk has been recognized mainly through its role in the increase of HDL concentrations (8, 41–43). The underlying mechanisms for this protective effect involve alcohol’s effect on raising concentrations of HDL and its subfractions (10, 43) and on increasing total phospholipid and PUFA contents of HDL (7). By affecting the concentration and composition of HDL and, therefore, the fluidity, alcohol is thought to promote HDL binding to endothelial cells, reverse cholesterol transport, and upregulate secretion of vascular endothlial growth factor, thereby protecting against atherosclerosis (44).
Several limitations should be considered. First, for those carrying some social characteristic, such as lower education level or being in a poor economic situation, alcohol intake could have been underreported, particularly for heavy drinkers, because data on alcohol consumption were collected via self-report. This underreporting might have led to a misclassification and underestimation of the potential effect of heavy drink. However, social drinking is generally not discouraged in Chinese culture and is generally accepted in men (45). Therefore, the underreporting of alcohol consumption would have had modest effects on the results if it existed. Furthermore, in this study, we did not combine past drinkers with never drinkers because the time and reason for quitting drinking were not reported. However, compared with never drinkers, past drinkers experienced a significantly slower decreasing rate in HDL cholesterol over time. Second, although we controlled for most potential confounders, we could not exclude the possibility of residual confounding. For example, we did not collect comprehensive dietary intake information. Because moderate drinkers might follow a healthy diet pattern, which is associated with better lipid profiles, we might have overestimated the association between alcohol intake and the change in HDL-cholesterol concentrations over time. However, we adjusted for factors such as BMI and waist circumference, which reflected dietary intake to some extent. We did not collect information on estrogen therapy until 2010. However, the prevalence of estrogen-therapy use was very low in the Kailuan study; in 2010, only 54 women reported having taken hormone replacement treatment. Although we could not exclude the possibility of underreporting, the impact of estrogen therapy on the observed alcohol-HDL relation could have been small. At baseline, we did not assess prothrombin time or albumin in this cohort. However, we adjusted for fatty liver status and concentrations of amino transferases, and the results did not change materially. Finally, all participants were from the Kailuan community. Therefore, this group could not be viewed as representative of the Chinese population. The results could not be generalized directly to other populations with different cultures and social-economic backgrounds. However, this geographical confinement might have greatly reduced residual confounding that was due to the variance caused by the unmeasured social-economic factors.
In conclusion, our study results suggest an umbrella-shaped relation between total alcohol consumption and the longitudinal change in the blood HDL-cholesterol concentration over a 6-y period, and the decreasing rate is the lowest in individuals who consume light-to-moderate alcohol. The umbrella-shaped association was mainly driven by hard liquor, not by beer, whose association with HDL-cholesterol changes is linear. These findings support the possible beneficial effect of moderate alcohol consumption on cardiovascular health. Additional prospective studies are needed to confirm our findings and to investigate the potential different impacts of different alcoholic beverages on lipid metabolism.
Acknowledgments
The authors’ responsibilities were as follows—SH: wrote the manuscript; SH, JL, and CJ: analyzed the data; JL, XZ, YW, and CJ: conducted the research; GCS and AHL: provided critical study oversight and contributed to the critical revision of the manuscript for important intellectual content; SW and XG: designed the research; XG: had primary responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CRP, C-reactive protein; CVD, cardiovascular disease; GEE, generalized estimating equation.
REFERENCES
- 1.Marmot M, Brunner E. Alcohol and cardiovascular disease: the status of the U shaped curve. BMJ 1991;303:565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maclure M. Demonstration of deductive meta-analysis: ethanol intake and risk of myocardial infarction. Epidemiol Rev 1993;15:328–51. [DOI] [PubMed] [Google Scholar]
- 3.Di Castelnuovo A, Rotondo S, Iacoviello L, Donati MB, De Gaetano G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 2002;105:2836–44. [DOI] [PubMed] [Google Scholar]
- 4.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437–45. [DOI] [PubMed] [Google Scholar]
- 5.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimm EB, Moats C. Alcohol and coronary heart disease: drinking patterns and mediators of effect. Ann Epidemiol 2007;17:S3–7. [Google Scholar]
- 7.Brinton EA. Effects of ethanol intake on lipoproteins. Curr Atheroscler Rep 2012;14:108–14. [DOI] [PubMed] [Google Scholar]
- 8.Gaziano JM, Hennekens CH, Godfried SL, Sesso HD, Glynn RJ, Breslow JL, Buring JE. Type of alcoholic beverage and risk of myocardial infarction. Am J Cardiol 1999;83:52–7. [DOI] [PubMed] [Google Scholar]
- 9.Hansel B, Thomas F, Pannier B, Bean K, Kontush A, Chapman MJ, Guize L, Bruckert E. Relationship between alcohol intake, health and social status and cardiovascular risk factors in the Urban Paris-Ile-de-France Cohort: is the cardioprotective action of alcohol a myth? Eur J Clin Nutr 2010;64:561–8. [DOI] [PubMed] [Google Scholar]
- 10.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foerster M, Marques-Vidal P, Gmel G, Daeppen J-B, Cornuz J, Hayoz D, Pécoud A, Mooser V, Waeber G, Vollenweider P, et al. . Alcohol drinking and cardiovascular risk in a population with high mean alcohol consumption. Am J Cardiol 2009;103:361–8. [DOI] [PubMed] [Google Scholar]
- 12.Marques-Vidal P, Bochud M, Paccaud F, Waterworth D, Bergmann S, Preisig M, Waeber G, Vollenweider P. No interaction between alcohol consumption and HDL-related genes on HDL cholesterol levels. Atherosclerosis 2010;211:551–7. [DOI] [PubMed] [Google Scholar]
- 13.Devenyi P, Robinson GM, Kapur BM, Roncari DA. High-density lipoprotein cholesterol in male alcoholics with and without severe liver disease. Am J Med 1981;71:589–94. [DOI] [PubMed] [Google Scholar]
- 14.Miller M, Langenberg P, Havas S. Impact of lowering triglycerides on raising HDL-C in hypertriglyceridemic and non-hypertriglyceridemic subjects. Int J Cardiol 2007;119:192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet 2014;384:618–25. [DOI] [PubMed] [Google Scholar]
- 16.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clin Biochem 2001;34:583–8. [DOI] [PubMed] [Google Scholar]
- 17.Miyake T, Kumagi T, Hirooka M, Furukawa S, Yoshida O, Koizumi M, Yamamoto S, Watanabe T, Yamamoto Y, Tokumoto Y, et al. . Low alcohol consumption increases the risk of impaired glucose tolerance in patients with non-alcoholic fatty liver disease. J Gastroenterol 2016;51:1090–1100. [DOI] [PubMed] [Google Scholar]
- 18.Marques-Vidal P, Vollenweider P, Waeber G. Alcohol consumption and incidence of type 2 diabetes. Results from the CoLaus study. Nutr Metab Cardiovasc Dis 2015;25:75–84. [DOI] [PubMed] [Google Scholar]
- 19.Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, Dagenais GR, Després J-P. Total holesterol/HDL cholesterol ratio vs. LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med 2001;161:2685–92. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th edition. Washington (DC): US Government Printing Office; 2010. [DOI] [PMC free article] [PubMed]
- 21.Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li N, Bian L, Wu J, Jia Q, et al. . Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke 2013;44:2451–6. [DOI] [PubMed] [Google Scholar]
- 22.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745–50. [DOI] [PubMed] [Google Scholar]
- 23.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough AJ, O’Connor JFB. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol 1998;93:2022–36. [DOI] [PubMed] [Google Scholar]
- 25.O’Shea RS, Dasarathy S, McCullough AJ, Practice Guideline Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010;51:307–28. [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi I. Increased body mass index modifies associations between alcohol intake and blood cholesterol profile. Eur J Clin Invest 2012;42:179–85. [DOI] [PubMed] [Google Scholar]
- 27.Park H, Kim K. Association of alcohol consumption with lipid profile in hypertensive men. Alcohol Alcohol 2012;47:282–7. [DOI] [PubMed] [Google Scholar]
- 28.Anderson KM, Wilson PWF, Garrison RJ, Castelli WP. Longitudinal and secular trends in lipoprotein cholesterol measurements in a general population sample. Atherosclerosis 1987;68:59–66. [DOI] [PubMed] [Google Scholar]
- 29.Koppes LLJ, Twisk JWR, Van Mechelen W, Snel J, Kemper HCG. Cross-sectional and longitudinal relationships between alcohol consumption and lipids, blood pressure and body weight indices. J Stud Alcohol 2005;66:713–21. [DOI] [PubMed] [Google Scholar]
- 30.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ 1999;319:1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pikaar NA, Wedel M, van der Beek EJ, van Dokkum W, Kempen HJ, Kluft C, Ockhuizen T, Hermus RJ. Effects of moderate alcohol consumption on platelet aggregation, fibrinolysis, and blood lipids. Metabolism 1987;36:538–43. [DOI] [PubMed] [Google Scholar]
- 32.Baer DJ, Judd JT, Clevidence BA, Muesing RA, Campbell WS, Brown ED, Taylor PR. Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet. Am J Clin Nutr 2002;75:593–9. [DOI] [PubMed] [Google Scholar]
- 33.Hartung GH, Foreyt JP, Reeves RS, Krock LP, Patsch W, Patsch JR, Gotto AM. Effect of alcohol dose on plasma lipoprotein subfractions and lipolytic enzyme activity in active and inactive men. Metabolism 1990;39:81–6. [DOI] [PubMed] [Google Scholar]
- 34.Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF, Masana L, Mangas A, Hernández-Mijares A, González-Santos P, et al. . Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]
- 35.Marques-Vidal P, Montaye M, Haas B, Bingham A, Evans A, Juhan-Vague I, Ferrieres J, Luc G, Amouyel P, Arveiler D, et al. . Relationships between alcoholic beverages and cardiovascular risk factor levels in middle-aged men, the PRIME Study. Prospective Epidemiological Study of Myocardial Infarction Study. Atherosclerosis 2001;157:431–40. [DOI] [PubMed] [Google Scholar]
- 36.Volcik KA, Ballantyne CM, Fuchs FD, Sharrett AR, Boerwinkle E. Relationship of alcohol consumption and type of alcoholic beverage consumed with plasma lipid levels: differences between whites and African Americans of the ARIC study. Ann Epidemiol 2008;18:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rimm EB, Klatsky A, Grobbee D, Stampfer MJ. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits. BMJ 1996;312:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arranz S, Chiva-Blanch G, Valderas-Martínez P, Medina-Remón A, Lamuela-Raventós RM, Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012;4:759–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, De Gaetano G. Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: a meta-analysis. Eur J Epidemiol 2011;26:833–50. [DOI] [PubMed] [Google Scholar]
- 40.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med 1991;325:373–81. [DOI] [PubMed] [Google Scholar]
- 41.Suh I. Alcohol use and mortality from coronary heart disease: the role of high-density lipoprotein cholesterol. The Multiple Risk Factor Intervention Trial Research Group. Ann Intern Med 1992;116:881–7. [DOI] [PubMed] [Google Scholar]
- 42.Langer RD, Criqui MH, Reed DM. Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation 1992;85:910–5. [DOI] [PubMed] [Google Scholar]
- 43.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med 1993;329:1829–34. [DOI] [PubMed] [Google Scholar]
- 44.Liisanantti MK, Savolainen MJ. Phosphatidylethanol mediates its effects on the vascular endothelial growth factor via HDL receptor in endothelial cells. Alcohol Clin Exp Res 2009;33:283–8. [DOI] [PubMed] [Google Scholar]
- 45.Cochrane J, Chen H, Conigrave KM, Hao W. Alcohol use in China. Alcohol Alcohol 2003;38:537–42. [DOI] [PubMed] [Google Scholar]