Abstract
Protein Kinase C-epsilon (PKCε) is an isoform of a large PKC family of enzymes that has a variety of functions in different cell types. Here we discuss two major roles of PKCε in cardiac muscle cells; specifically, its role in regulating cardiac muscle contraction via targeting the sarcomeric proteins, as well as modulating cardiac cell energy production and metabolism by targeting cardiac mitochondria. The importance of PKCε action is described within the context of intracellular localization, as substrate selectivity and specificity is achieved through spatiotemporal targeting of PKCε. Accordingly, the role of PKCε in regulating myocardial function in physiological and pathological states has been documented in both cardioprotection and cardiac hypertrophy.
Keywords: myocardial function, phosphorylation, cardiac hypertrophy, cardioprotection, drug target, substrates
1. PRKCE Structure and Activation in Cardiomyocytes
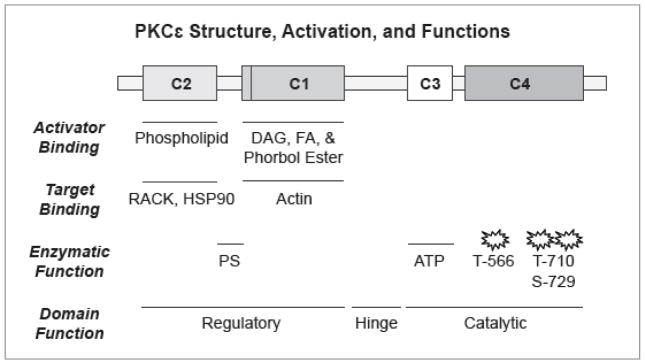
The PRKCE gene (Ensembl ID: ENSG00000171132 (WTSI/EMBL-EBI, 2015)) encodes protein kinase C epsilon (PKCε, Uniprot ID: Q02156 (EMBL-EBI, 2002)). PKC is comprised of a family of serine-threonine kinases that contains thirteen PKC isoforms which differ in primary structure, protein expression, subcellular localization, and modes of activation (Dekker and Parker, 1994). PKCε is a PKC isoform highly expressed in adult cardiomyocytes (Rybin and Steinberg, 1994) (Disatnik et al., 1994) (Bogoyevitch et al., 1993) (Puceat et al., 1994), and it is the most abundantly expressed novel PKC isoform in cardiac muscle, relative to other novel PKCs (δ, θ, and η) (Ping et al., 1997). PKCε shares similar kinase and C-terminal domains with other novel, conventional (α, βI, βII, and γ) and atypical (ζ and ι/λ) PKC isoforms, thus it is no surprise that the requisite phosphorylation at sites Threonine-566, Threonine-710, and Serine-729 for kinase maturation found within this domain are consistent among all PKC isoform classes (Akita, 2002) (Figure 1). PKCε shows distinction in the position of its novel C2, pseudosubstrate and C1 domains. The C2 domain of PKCε and other novel PKCs binds minimally to calcium as in other PKC families; rather, the C2 domain in novel isoforms serves to catalyze important protein-protein interactions (Igumenova, 2015). Moreover, unlike conventional PKCs, in novel PKCs the C1 domain follows the C2 domain, and it has been shown that different second messengers in different combinations acting on the C1 domain can in part direct the translocation of PKCε (Shirai et al., 1998) (Disatnik et al., 1994).
Figure 1. Structural and Functional Regions of PKCε.
PKCε is a novel PKC that houses a C2 domain that is modified to be void of calcium binding, as in conventional PKC isoforms (α, β, and γ), and binds phospholipid activators as well as the RACK scaffolding protein (Z-line targeting) and Hsp90 (mitochondrial inner membrane targeting). Downstream of C2 is the C1 region that is responsible for binding activators diacylglycerol (DAG), fatty acids (FA) and phorbol esters, as well as binding to filamentous (F)-actin. The C-terminal catalytic domain in PKCε is similar to all other PKC isoforms, having an ATP binding domain in C3, and the activating phosphorylation sites in C4. (Recommended Figure Size—1 column width).
Seminal work by Mochley-Rosen et al. identified receptors for activated C-kinase (RACK), which advanced our understanding of how PKCε translocation to discrete subcellular locations is accomplished. Specifically, RACKs are thought to facilitate and to anchor active PKC in close proximity to its phosphorylatable substrate (Mochly-Rosen, 1995). The specific region of PKCε responsible for its translocation and binding to its preferred RACK isoform, RACK2, has been identified within the C2 domain as the first unique region of the protein (amino acids 14–21, also known as εV1–2); peptide inhibitors targeting εV1–2 antagonize the translocation and function of PKCε in cardiomyocytes (Johnson et al., 1996) and peptide agonists enhance translocation (Dorn et al., 1999). The dynamics of the interaction between PKCε and RACK isotypes likely influence cardiac phenotypes, as enhanced localization of PKCε-RACK2 occurs in cardioprotection, whereas enhanced localization of both RACK1 and RACK2 with PKCε occurs during maladaptive cardiac hypertrophy (Pass et al., 2001). Indeed, it was found that muscle ring finger protein-1 (MURF1) is an endogenous inhibitor and direct binding partner of RACK1 that inhibits PKCε translocation to sarcomeric structures at focal adhesions and inhibits hypertrophic cell growth in cardiomyocytes (Arya et al., 2004).
It has classically been understood that PKCs are activated by alpha-adrenergic/Gq- -linked pathways, which activate phospholipase-C and the hydrolysis of phosphatidylinositol-4,5-bisphosphate to create inositol-1,4,5-triphosphate and diacylglycerol (Duquesnes et al., 2011). However, it has also been discovered that phospholipase-D and phospholipase A2-linked signaling resulting in the generation of choline phospholipids may also play a role (Nishizuka, 1995). Moreover, it was recently shown that PKCε can be activated via beta-adrenergic stimulation with isoproterenol (Oestreich et al., 2009). Upon activation, PKCε translocates to multiple intracellular targets, which has been nicely reviewed (Akita, 2002; Newton, 2010). During development and cardiomyocyte differentiation, the time-sensitive expression of PKCε is critical for regulating nkx2.5 and gata4, acting through a phospho-extracellular-regulated kinase (ERK)1/2-mediated pathway (Galli et al., 2013). In adult cardiomyocytes, target organelles include mitochondria (Baines et al., 2003); cross-striated structures or sarcomeres (Disatnik et al., 1994; Huang et al., 1997b; Robia et al., 2001; Huang and Walker, 2004); and perinuclear membranes (Disatnik et al., 1995; Vincent et al., 2006). Localization of PKCε to mitochondria and sarcomere targets in cardiomyocytes is the specific focus of this paper. The translocation of PKCε to mitochondria from the cytosol is a critical component of most cardioprotective regimens, which are discussed in detail below. In addition, PKCε translocates to cardiac sarcomeres; specifically, to Z-line structures upon α-adrenergic and endothelin (ET)A-receptor stimulation by a variety of agonists (Robia et al., 2001) (Disatnik et al., 1994). PKCε was first found to translocate from the cytosolic to particulate fraction following PMA or norepinephrine activation of PKCε (Disatnik et al., 1994). It has since been shown that a myriad of agonists can activate and translocate the PKCε isoform specifically (see Table 1). The activation of PKCε by ET-1 and phenylephrine (PE) signals to phosphorylate and activate p42- and p44-mitogen activated protein kinase (MAPK) (Clerk et al., 1994), which contributes to the PKCε-mediated protection against ischemic injury (Ping et al., 1999). In summary, the importance of PKCε action in cardiomyocytes must be understood within the context of both the stimulus and intracellular localization, as substrate targeting and specificity is achieved through spatiotemporal targeting of PKCε.
Table 1.
Agonists that activate the PKCε isoform and induce its translocation.
| Agonist | References |
|---|---|
| Arachidonic acid | (Huang et al., 1997a) |
| Endothelin-1 | (Clerk et al., 1994) |
| Phenylephrine | (Grimm et al., 2006) |
| Angiotensin-II | (Paul et al., 1997) |
| Myocardial stretch | (Paul et al., 1997; Vincent et al., 2006) |
| Adenosine | (Yang et al., 2012) |
| Hypoxia and Akt-induced stem cell factor | (Huang et al., 2014) |
| ROS from activation of mitoK(ATP) | (Garlid et al., 2013; Li et al., 2014) |
| Apelin | (Perjes et al., 2014) |
| Isoproterenol | (Oestreich et al., 2009) |
2. Sarcomeric Targeting for Modulation of Cardiac Contractile Function and Hypertrophy
Upon activation by various cellular stimuli, one target of PKCε translocation is to cardiac sarcomeres where PKCε plays a role in contractility of the myocardium (see Figure 2). PKCε docks at cardiac Z-lines with an EC50 of 86 nM, likely via RACK2 (Huang and Walker, 2004), and PKCε binds to syndecan-4 (syn-4) and focal adhesion complexes at cardiac costameres (VanWinkle et al., 2002; Heidkamp et al., 2003); in both cases positioning PKCε to phosphorylate sarcomeric targets. Though not yet conclusively demonstrated in cardiomyocytes, it is plausible that PKCε may also localize to sarcomeric actin, as actin was detected in endogenous cardiac PKCε complexes (Ping et al., 2001) and the PKCε isoform specifically has been shown to bind F-actin in neurons to regulate synaptic function and differentiation (Prekeris et al., 1996) (Zeidman et al., 2002). It was first demonstrated by J.F. Kuo’s laboratory that PKCε has a specific affinity for and phosphorylates cardiac troponin I (cTnI) and cardiac troponin T (cTnT) in complex with troponin C (cTnC) (Jideama et al., 1996). Specific phosphorylatable residues on cTnI, including Serine-43, Serine-45, and Threonine-144, showed modulation of contractile function (Noland et al., 1996) (Noland et al., 1995), thus these sites arose as likely sites for endogenous PKCε modulation. Indeed, in vitro phosphorylation of cTnI by PKCε or mutation of Serine-43 and Serine-45 to Glutamate to mimic phosphorylation induced a desensitization of the contractile apparatus to calcium and an overall depression of contractile function (Burkart et al., 2003). Additional studies demonstrated that alteration of the endogenous level of phosphorylation via Serine/Alanine mutation of a putative PKCε site on the N-terminus of cTnI (Serine-5/6) depresses myofilament function (Henze et al., 2013). In vivo studies further supported these findings in showing that mice expressing a mutant cTnI harboring Serine-43/Serine-45 to Alanine mutations showed enhanced contractility (Roman et al., 2004). Studies of PKCε phosphorylation in mouse have been verified in human cardiac fibers, where PKCε has been shown to phosphorylate cTnI, cTnT and myosin binding protein-C (MyBPC), which desensitizes the contractile apparatus to calcium (Kooij et al., 2010).
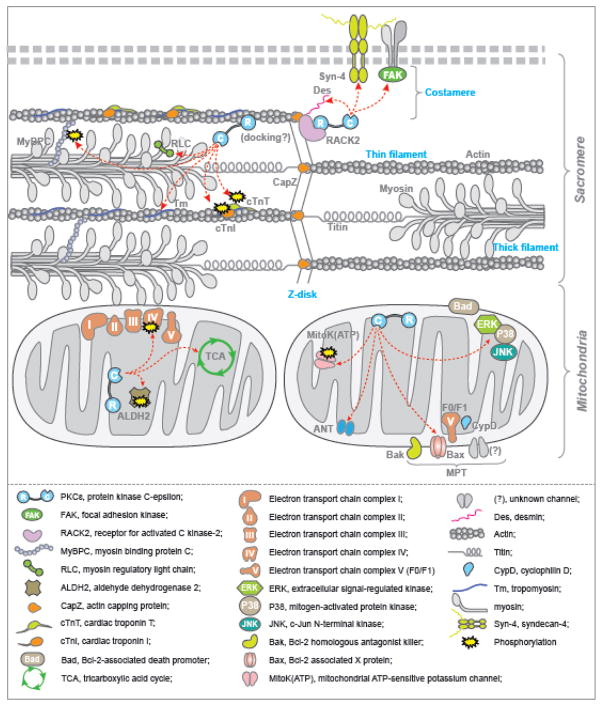
Figure 2. PKCε Interactome and Phospho-proteome in Cardiac Sarcomeres and Mitochondria.
Shown is a schematic of cardiac sarcomeres and mitochondria with actions of PKCε within these organelles. All abbreviations are defined in the inset. Binding partners of PKCε are denoted by arrows, and verified substrates of PKCε phosphorylation are denoted by yellow bursts. PKCε is shown in light blue and illustrated as two domains, regulatory (R) and catalytic (C), connected by a linker region. At sarcomeres, data have shown that PKCε is tethered to Z-lines via receptor for activated C kinase 2 (RACK2). At costameres, PKCε has also been demonstrated to activate focal adhesion kinase (FAK) and bind to the proteoglycan syndecan-4 (Syn-4). On cardiac thin and thick filaments, PKCε binds to tropomyosin (Tm), myosin regulatory light chain (RLC), troponin I (cTnI) and troponin T (cTnT), and evidence shows that PKCε phosphorylates cTnI, cTnT and myosin binding protein-C (MyBPC); in this case, PKCε may dock via its actin-binding region directly on cardiac Actin. At the inner mitochondrial membrane, PKCε has been shown to bind to and phosphorylate certain members of metabolic pathways (left mitochondrion) as well as pathways involving apoptosis and mitochondrial permeability transition (MPT, right mitochondrion). Metabolic pathways targeted by PKCε include the electron transport chain (I–V), tricarboxylic acid (TCA) cycle, and alcohol dehydrogenase 2 (ALDH2). PKCε direct substrates linked to MPT include the mitochondrial ATP-sensitive potassium channel (mitoK(ATP)); adenine nucleotide transporter (ANT); F0/F1 ATP synthase; Bcl-2-associated X protein (Bax); and the c-Jun N-terminal kinase (JNK)/p38 mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) cascade and the downstream effector Bcl-2-associated death promoter (Bad). (Recommended Figure Size—2 column width).
The PKCε isoform has been shown to be specifically induced in response to hypertrophic stimuli in cardiac myocytes; it is the only PKC isoform that translocates following an acute pressure overload stimulus (Li et al., 2015). Hypertrophic stimuli linked to PKCε induction include myotrophin (Sil et al., 1998), mechanical stretch and hypertension (i.e., left ventricular pressure overload) (Inagaki et al., 2002). PKCε inhibition during the transition from compensatory hypertrophy to heart failure has shown to prolong life (Inagaki et al., 2008). However, the story is further complicated by the finding that inhibition of PKCε translocation to the particulate fraction also stimulates a hypertrophic phenotype; specifically, increased cardiomyocyte size and hypertrophic gene expression (Mochly-Rosen et al., 2000). It is known that PKCε elicits effects on hypertrophy in part through regulation of Ras-Raf-mitogen/extracellular signal-regulated kinase (MEK)-extracellular signal-regulated kinase (ERK) signaling, which has been shown to mediate several effects including alterations in cardiomyocyte gene expression (Clerk et al., 1994; Jiang et al., 1996; Heidkamp et al., 2001; Sabri and Steinberg, 2003). In parallel, there is strong evidence suggesting that a site of PKCε action in hypertrophic induction is at cardiac sarcomeric structures involved in strain-sensing and mechanotransduction for controlling optimal sarcomere length. PKCε activates focal adhesion kinase (FAK) at costameres following a hypertrophic stimulus, which is important for sarcomere assembly during cardiac hypertrophy (Heidkamp et al., 2003). Mansour et al. demonstrated that in cardiomyocytes subjected to mechanical strain, PKCε is critical for the recovery of sarcomere length back to normal (Mansour et al., 2004). Additionally, functional PKCε is required for the enhanced actin-capping protein (CapZ) dynamics observed at Z-lines following cyclic strain (Lin et al., 2015). These data indicate that PKCε is a critical modulator of mechanosensory pathways that integrate myocardial strain with sarcomere dynamics, including filament assembly.
Studies investigating the in vivo function using transgenesis have shed additional light on the effects of sustained PKCε activation in cardiac hypertrophy and failure. The first study to generate a mouse harboring cardiac-specific overexpression of constitutively-active PKCε, in which total cellular PKCε protein expression showed a 9-fold increase and PKCε activity in the particulate fraction was elevated 4-fold, demonstrated that these mice develop a concentric hypertrophic phenotype with increased anterior and posterior left ventricular wall thickness albeit normal fractional shortening and end diastolic/end systolic dimensions (Takeishi et al., 2000). A later study serially investigated these mice over a 12-month period and demonstrated that, in agreement with the previous study, the functional parameters and hypertrophic gene expression of PKCε overexpressing mice are normal at 3 months of age (Goldspink et al., 2004). However, alterations in both the sensitivity of sarcomeric contractile apparatus to calcium and the phosphorylation of thin filament proteins cTnI and cTnT were detected at this time point albeit normal hemodynamic function, suggesting that sarcomere proteins represent an early site of cardiac transformation in response to constitutively-active PKCε. At 6 months of age, these mice exhibited progressive decline in both functional and molecular parameters, and an eccentric hypertrophic/dilated cardiomyopathy and failing phenotype was evident by 12 months. To investigate the energetic characteristics of the dilated cardiomyopathy in these mice, Montgomery et al. conducted a detailed functional analysis that showed a preserved Frank-Starling mechanism with an exhausted contractile reserve in that β1-adrenergic stimulation with dobutamine did not increase cardiac output (Montgomery et al., 2005). Moreover, constitutively-active PKCε overexpressing animals crossed with mice expressing cTnI lacking putative PKCε phosphorylation sites (Serine-43/Serine-45 mutated to Alanine) offered critical mechanistic insights (Scruggs et al., 2006). Mutation of these sites to non-phosphorylatable forms attenuated the contractile dysfunction and hypertrophic marker expression observed in the PKCε transgenic mice, thus indicating that Serine-43/Serine-45 on cTnI are relevant endogenous sites of PKCε phosphorylation that are targeted and detrimental during hypertrophic disease progression.
It is noteworthy to mention that PKCε acting at cardiac sarcomeres has also been shown to have cardioprotective effects during ischemia-reperfusion injury. Studies by Ping et al. identified sarcomeric proteins in PKCε signaling complexes, including actin, cTnT, tropomyosin, desmin, and myosin light chain-2 (Ping et al., 2001). All sarcomeric proteins showed greater association with PKCε in mice expressing a constitutively-active PKCε, and the cTnT, tropomyosin, desmin and myosin light chain-2 exhibited changes in post-translational modifications. Evidence has shown that PKCε translocation to sarcomeres and phosphorylation of cTnI and cMyBPC is involved in the κ-opioid- and α-adrenergic-dependent PC that decreases contractile cycling rate, thus protecting myofibrils during injury and better restoring contractile function post-ischemia (Pyle et al., 2000) (Pyle et al., 2003). Phosphorylation of ventricular myosin light chain-2 was also demonstrated following direct activation of PKCε by ΨεRACK prior to ischemia (Budas et al., 2012), however the functional significance of this remains unknown. Additionally, the Z-line resident, actin-capping protein, CapZ, appears to affect the localization of PKCε to sarcomeric Z-lines (Pyle et al., 2002) and plays a role in ischemia-reperfusion injury. Cardioprotection in mice harboring transgenic reduction of CapZ was correlated with a significant increase in the amount of PKCε translocated to myofilaments (Yang and Pyle, 2012). This may indicate that CapZ competes with the binding of RACK2, but further studies are required to sort this out.
3. Mitochondrial Targeting for Modulation of Metabolic Pathways and Mitochondrial Function
The PKC hypothesis of cardioprotection was first introduced by JM Downey in 1994 (Ytrehus et al., 1994), which spawned a series of exciting studies investigating isoform-specific effects of PKC in protection against ischemic injury. PKCε has been strongly implicated in preconditioning (PC), and PKCε acting at mitochondria is the most well-described cardioprotective paradigm. Ping et al. conducted a comprehensive study examining PKC isoform-specific profiles following five different PC regimens in conscious rabbits, and unequivocally determined the translocation of the PKCε isoform from the cytosolic to particulate fraction in all modes of PC tested (Ping et al., 1997) (Bolli et al., 1998). An independent study published shortly after by Gray et al. mutually corroborated and supported this finding in rat cardiomyocytes by determining that an inhibitory peptide specific for the PKCε isoform was sufficient to abolish hypoxic-induced PC (Gray et al., 1997). Liu et al. then confirmed this in adult rabbit ventricular cardiomyocytes using a standard ischemic PC challenge (Liu et al., 1999). PKCε translocation was also observed following ischemic PC in several animal models (Wilson et al., 1996) (Kawamura et al., 1998) (Tong et al., 2000), as well as in human atria (Hassouna et al., 2004), and studies employing transgenesis and selective PKCε activators/inhibitors have further demonstrated the protective role of PKCε translocation against ischemic injury (Gregory et al., 2004).
Despite multiple independent demonstrations of the effectiveness of PKCε to protect injured myocardium, little was known at the time regarding molecular targets of PKCε. Significant advances in our understanding of PKCε targeting of mitochondria came from studies by Baines et al. (Baines et al., 2003) and Ping et. al (Ping et al., 2001), which provided the first documentation of mitochondrial targeting of PKCε. Studies investigating molecular interactions between PKCε and mitochondrial targets commenced to identify signaling molecules involved in the cardioprotective paradigm. Detailed proteomic analysis of PKCε signaling complexes by Ping et al. (Ping et al., 2001) identified 36 proteins that form an endogenous complex with PKCε. This study identified changes in PKCε complex assembly with respect to protein content and post-translational modifications triggered by a constitutively-active PKCε. Mitochondrial proteins were integral components of these complexes, and PKCε was found for the first time at the inner mitochondrial membrane in the form of signaling complexes in healthy hearts (Ping et al., 2001). This group further delineated that PKCε resides in complexes with several key mitochondrial proteins in glycolysis, TCA cycle, beta oxidation, and ion transport signaling pathways, including but not limited to, adenine nucleotide transporter (ANT), ATP synthase, creatine kinase, enolase, GAPDH, succinate dehydrogenase and voltage dependent anion channel (VDAC) (Edmondson et al., 2002).
D Mochley-Rosen’s group was the first to unequivocally identify a target of PKCε involved in cardioprotection (Chen et al., 2008). This study detailed the specific targeting and phosphorylation of alcohol dehydrogenase 2 (ALDH2) by PKCε following protective stimuli, which enhanced the activity of ALDH2 and reduced infarct size (Ping, 2009). Another study by Ogbi and Johnson identified a direct interaction between PKCε and cytochrome c oxidase subunit IV (COIV), and PC stimuli were associated with PKCε phosphorylation of COIV and preservation of COIV protein and activity levels (Ogbi and Johnson, 2006). In addition, Jaburek et al. elucidated an interaction between PKCε and mitoK(ATP) (Jaburek et al., 2006); opening of of mitoK(ATP) locally enhances levels of reactive oxygen species (ROS), specifically attributed to the hydroxyl radical (HO(•)), which appears to both activate mitoK(ATP)-bound PKCε (Garlid et al., 2013) and induce the translocation of PKCε from cytosolic to particulate cell components (Li et al., 2014). PKCε phosphorylation of mitoK(ATP) potentiates channel opening and this interaction has also since been shown to be pivotal in the PC response (Costa and Garlid, 2008). Subunit Kir6.1 of mitoK(ATP) binds connexin-43 and confers cardioprotection through a PKCε-regulated interaction (Waza et al., 2014). It is likely that this is modulated through PKCε phosphorylation of connexin-43 at presumed PKCε sites, Serine-262 and Serine-368, as these have been shown to have a protective effect on mitochondrial function (Srisakuldee et al., 2014; Shan et al., 2015). A phosphoproteomics study by Budas et al. further illuminated putative mitochondrial targets of PKCε phosphorylation upon PKCε-specific activation with ΨεRACK prior to ischemic injury. Enhanced phosphorylation of inner mitochondrial respiratory complexes I, II and III, as well as proteins involved in glycolysis, lipid oxidation, ketone body metabolism and heat shock proteins was reported (Budas et al., 2012). Though these and other targets residing on the inner mitochondrial membrane had been identified, it remained unclear how PKCε translocates from the outer to inner mitochondrial membrane. Another study by Budas et al. unveiled a role for heat shock protein 90 (Hsp90) in this process (Budas et al., 2010) (Yang et al., 2012). They were the first to show that Hsp90 acting in concert with the translocase of the outer mitochondrial membrane-20 (Tom20) is required for PKCε translocation to the inner mitochondrial membrane following a PC stimulus. A seven amino acid peptide homologous to the HSP90 sequence within the novel C2 domain of PKCε (termed TAT-ΨεHSP90) was derived and showed activation of the mitochondrial-targeted translocation of PKCε in conjunction with cardioprotection (Budas et al., 2010). However, it remains to be determined precisely how PKCε docks to the inner mitochondrial membrane. Taken together, these findings support a role for PKCε in the regulation of fatty acid, carbohydrate and protein metabolism, as well as cellular energy balance.
In addition to roles in cellular metabolism, a role for PKCε in the mitochondrial permeability transition (MPT) has been evolving for several decades. Historically, this role has been linked to the association of PKCε with mitochondrial VDAC1, ANT, and hexokinase II (Baines et al., 2003); however, this is currently back on the table of investigation due to genetic studies showing negative findings on roles for VDAC and ANT (Kokoszka et al., 2004; Baines et al., 2007), combined with recent studies showing developments in pore components (Bernardi and Di Lisa, 2015). The finding that proapoptotic Bax and Bak may function as outer membrane components of MPT (Karch et al., 2013), along with studies unveiling a central role for the F1/F0 ATP synthase as the inner membrane component (Bonora et al., 2013; Giorgio et al., 2013; Alavian et al., 2014) has significantly transformed our understanding. The new knowledge brings uncertainty regarding whether the inner and outer mitochondrial membranes physically couple during MPT; new models indicate that these function independently, with the primary site of regulation being on the inner membrane. These developments open up exciting new avenues for understanding molecular targets of PKCε cardioprotective action at mitochondria. It has been previously shown that PKCε, ERK, JNKs and p38 form signaling modules at cardiac mitochondria, and that PKCε activity is required for the phosphorylation of ERK and downstream effector Bad (Baines et al., 2002). This finding was more recently supported in studies showing that inactivation or activation of PKCε in diseased cardiac tissue are associated with enhanced association of Bad or inactivation/phosphorylation of Bad at mitochondria, respectively, with opposite effects on mitochondrial function and apoptosis (Malhotra et al., 2005; Tsai et al., 2014). Though not in cardiomyocytes, a direct interaction between PKCε and Bax has been elucidated in cancer cells (McJilton et al., 2003); and the overexpression of PKCε inhibits Bax dimerization and translocation, while the siRNA knockdown of PKCε has the reverse effect (Lu et al., 2007). Moreover, phosphorylation of mitochondrial F1/F0 ATP synthase subunits has been shown to increase in hearts treated with the PKCε activator ΨεRACK prior to ischemic injury, suggesting that PKCε could potentially be involved in regulation of MPT at this site (Budas et al., 2012). It should also be noted here that ANT, though not an indispensable component of the pore, appears to regulate the pore, as mitochondria from ANT1/2 null mice exhibited dysregulated calcium homeostasis (Kokoszka et al., 2004). This finding combined with earlier studies showing the endogenous binding of PKCε to ANT1 (Baines et al., 2003), suggest that ANT may serve as a site where PKCε can execute cardioprotective effects in mitochondria. Furthermore, Baines et al. conclusively demonstrated that addition of active, recombinant PKCε to cardiomyocytes can inhibit MPT, indexed by calcium-induced mitochondrial swelling (Baines et al., 2003). Taken together, these data suggest that PKCε plays a cardioprotective role acting either directly or indirectly at molecular site(s) involved in MPT; future studies will undoubtedly unfold new PKCε-directed molecular mechanisms in cardiomyocyte MPT, necrosis, and apoptosis.
4. Conclusion
Findings presented here clearly demonstrate a prominent role for the ε isoform of PKC acting on sarcomeres and mitochondria in cardiac health and disease. Translation of these findings into treatment for ischemic injury and heart failure will require the integration of dynamic actions of PKCε at each subcellular location into one comprehensive picture. Innovative research aimed at elucidating the endogenous positioning and presentation of PKCε have come from Daria Mochley-Rosen and colleagues in their successful identification of targeting sequences for docking and translocation of PKC, as well as from multiple laboratories, including our own, which have profiled endogenous PKCε signaling modules and interacting partners. Furthermore, strong supporting evidence for the importance of PKCε in cardioprotection came from the finding that micro-RNAs modulate the cardioprotective effects of PKCε. Both miR-1 and miR-31 are upregulated following ischemia/reperfusion injury, they both specifically target the PRKCE gene, and their inhibition is cardioprotective (Pan et al., 2012) (Wang et al., 2015). Twenty years of investigation on PKCε have presented a beautiful example where our view of single gene-single kinase-single substrate paradigms has been transformed and elevated to a multi-layered understanding of the PRKCE gene and the PKCε protein. Our investigations have illuminated their involvement in temporal regulation as well as specific targeting in distinct subcellular organelles and locations. A systems biology approach has enabled us to decipher the PKCε signaling networks and identify its molecular targets. Future efforts in characterization of PKCε-dependent therapeutic actions will shed additional light on its clinical significance.
Highlights.
PKCε action in cardiomyocytes is spatiotemporally regulated.
PKCε acts on sarcomeric targets and regulates cardiac contraction and hypertrophy.
PKCε targets mitochondria to modulate metabolism and elicit cardioprotection.
Acknowledgments
The authors are supported by the National Institutes of Health (R37 HL063901, R01 HL129723, and U54 GM114833).
This review and the corresponding Gene Wiki article are written as part of the Cardiac Gene Wiki Review series--a series resulting from a collaboration between the journal GENE, the Gene Wiki Initiative, and the NIH BD2K Initiative. The Cardiac Gene Wiki Initiative is supported by National Institutes of Health (R01 GM089820 and U54 GM114833). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The corresponding Gene Wiki entry for this review can be found here: https://en.wikipedia.org/wiki/PRKCE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References Cited
- Akita Y. Protein kinase C-epsilon (PKC-epsilon): its unique structure and function. J Biochem. 2002;132:847–52. doi: 10.1093/oxfordjournals.jbchem.a003296. [DOI] [PubMed] [Google Scholar]
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA. 2014;111:10580–5. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya R, Kedar V, Hwang JR, McDonough H, Li HH, Taylor J, Patterson C. Muscle ring finger protein-1 inhibits PKC{epsilon} activation and prevents cardiomyocyte hypertrophy. J Cell Biol. 2004;167:1147–59. doi: 10.1083/jcb.200402033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–5. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–80. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–7. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–6. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Parker PJ, Sugden PH. Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-epsilon is a major isotype present, and it is activated by phorbol esters, epinephrine, and endothelin. Circ Res. 1993;72:757–67. doi: 10.1161/01.res.72.4.757. [DOI] [PubMed] [Google Scholar]
- Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK, Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol. 1998;93:325–38. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12:674–83. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas G, Costa HM, Jr, Ferreira JC, Teixeira da Silva Ferreira A, Perales J, Krieger JE, Mochly-Rosen D, Schechtman D. Identification of epsilonPKC targets during cardiac ischemic injury. Circ J. 2012;76:1476–85. doi: 10.1253/circj.cj-11-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Disatnik MH, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88:83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. Journal of Biological Chemistry. 2003;278:11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk A, Bogoyevitch MA, Anderson MB, Sugden PH. Differential activation of protein kinase C isoforms by endothelin-1 and phenylephrine and subsequent stimulation of p42 and p44 mitogen-activated protein kinases in ventricular myocytes cultured from neonatal rat hearts. J Biol Chem. 1994;269:32848–57. [PubMed] [Google Scholar]
- Costa AD, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am J Physiol Heart Circ Physiol. 2008;295:H874–82. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker LV, Parker PJ. Protein kinase C--a question of specificity. Trends Biochem Sci. 1994;19:73–7. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210:287–97. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Jones SN, Mochly-Rosen D. Stimulus-dependent subcellular localization of activated protein kinase C; a study with acidic fibroblast growth factor and transforming growth factor-beta 1 in cardiac myocytes. J Mol Cell Cardiol. 1995;27:2473–81. doi: 10.1006/jmcc.1995.0235. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci USA. 1999;96:12798–803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquesnes N, Lezoualc’h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers? J Mol Cell Cardiol. 2011;51:665–73. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Edmondson RD, Vondriska TM, Biederman KJ, Zhang J, Jones RC, Zheng Y, Allen DL, Xiu JX, Cardwell EM, Pisano MR, Ping P. Protein kinase C epsilon signaling complexes include metabolism- and transcription/translation-related proteins: complimentary separation techniques with LC/MS/MS. Mol Cell Proteomics. 2002;1:421–33. doi: 10.1074/mcp.m100036-mcp200. [DOI] [PubMed] [Google Scholar]
- EMBL-EBI, 2002. UniProtKB - Q02156 (KPCE_HUMAN)
- Galli D, Gobbi G, Carrubbi C, Di Marcantonio D, Benedetti L, De Angelis MG, Meschi T, Vaccarezza M, Sampaolesi M, Mirandola P, Vitale M. The role of PKCepsilon-dependent signaling for cardiac differentiation. Histochem Cell Biol. 2013;139:35–46. doi: 10.1007/s00418-012-1022-4. [DOI] [PubMed] [Google Scholar]
- Garlid AO, Jaburek M, Jacobs JP, Garlid KD. Mitochondrial reactive oxygen species: which ROS signals cardioprotection? Am J Physiol Heart Circ Physiol. 2013;305:H960–8. doi: 10.1152/ajpheart.00858.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA. 2013;110:5887–92. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink PH, Montgomery DE, Walker LA, Urboniene D, McKinney RD, Geenen DL, Solaro RJ, Buttrick PM. Protein kinase Cepsilon overexpression alters myofilament properties and composition during the progression of heart failure. Circ Res. 2004;95:424–32 E. doi: 10.1161/01.RES.0000138299.85648.92. pub 2004 Jul 8 Write to the Help Desk NCBI | NLM | NIH Department of Health & Human Services Privacy Statement | Freedom of Information Act | Disclaimer Nov 6 2006 15:24:20. [DOI] [PubMed] [Google Scholar]
- Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945–51. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- Gregory KN, Hahn H, Haghighi K, Marreez Y, Odley A, Dorn GW, 2nd, Kranias EG. Increased particulate partitioning of PKC epsilon reverses susceptibility of phospholamban knockout hearts to ischemic injury. J Mol Cell Cardiol. 2004;36:313–8. doi: 10.1016/j.yjmcc.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Grimm M, Mahnecke N, Soja F, El-Armouche A, Haas P, Treede H, Reichenspurner H, Eschenhagen T. The MLCK-mediated alpha1-adrenergic inotropic effect in atrial myocardium is negatively modulated by PKCepsilon signaling. Br J Pharmacol. 2006;148:991–1000. doi: 10.1038/sj.bjp.0706803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassouna A, Matata BM, Galinanes M. PKC-epsilon is upstream and PKC-alpha is downstream of mitoKATP channels in the signal transduction pathway of ischemic preconditioning of human myocardium. Am J Physiol Cell Physiol. 2004;287:C1418–25. doi: 10.1152/ajpcell.00144.2004. [DOI] [PubMed] [Google Scholar]
- Heidkamp MC, Bayer AL, Martin JL, Samarel AM. Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase C epsilon and delta in neonatal rat ventricular myocytes. Circ Res. 2001;89:882–90. doi: 10.1161/hh2201.099434. [DOI] [PubMed] [Google Scholar]
- Heidkamp MC, Bayer AL, Scully BT, Eble DM, Samarel AM. Activation of focal adhesion kinase by protein kinase C epsilon in neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H1684–96. doi: 10.1152/ajpheart.00016.2003. [DOI] [PubMed] [Google Scholar]
- Henze M, Patrick SE, Hinken A, Scruggs SB, Goldspink P, de Tombe PP, Kobayashi M, Ping P, Kobayashi T, Solaro RJ. New insights into the functional significance of the acidic region of the unique N-terminal extension of cardiac troponin I. Biochim Biophys Acta. 2013;1833:823–32. doi: 10.1016/j.bbamcr.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Guo J, Beigi F, Hodgkinson CP, Facundo HT, Zhang Z, Espinoza-Derout J, Zhou X, Pratt RE, Mirotsou M, Dzau VJ. HASF is a stem cell paracrine factor that activates PKC epsilon mediated cytoprotection. J Mol Cell Cardiol. 2014;66:157–64. doi: 10.1016/j.yjmcc.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Walker JW. Myofilament anchoring of protein kinase C-epsilon in cardiac myocytes. J Cell Sci. 2004;117:1971–8. doi: 10.1242/jcs.01044. [DOI] [PubMed] [Google Scholar]
- Huang XP, Pi Y, Lokuta AJ, Greaser ML, Walker JW. Arachidonic acid stimulates protein kinase C-epsilon redistribution in heart cells. J Cell Sci. 1997a;110:1625–34. doi: 10.1242/jcs.110.14.1625. [DOI] [PubMed] [Google Scholar]
- Huang XP, Pi Y, Lokuta AJ, Greaser ML, Walker JW. Arachidonic acid stimulates protein kinase C-epsilon redistribution in heart cells. J Cell Sci. 1997b;110( Pt 14):1625–34. doi: 10.1242/jcs.110.14.1625. [DOI] [PubMed] [Google Scholar]
- Igumenova TI. Dynamics and Membrane Interactions of Protein Kinase C. Biochemistry. 2015;54:4953–68. doi: 10.1021/acs.biochem.5b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Iwanaga Y, Sarai N, Onozawa Y, Takenaka H, Mochly-Rosen D, Kihara Y. Tissue angiotensin II during progression or ventricular hypertrophy to heart failure in hypertensive rats; differential effects on PKC epsilon and PKC beta. J Mol Cell Cardiol. 2002;34:1377–85. doi: 10.1006/jmcc.2002.2089. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Koyanagi T, Berry NC, Sun L, Mochly-Rosen D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension. 2008;51:1565–9. doi: 10.1161/HYPERTENSIONAHA.107.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaburek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99:878–83. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- Jiang T, Pak E, Zhang HL, Kline RP, Steinberg SF. Endothelin-dependent actions in cultured AT-1 cardiac myocytes. The role of the epsilon isoform of protein kinase C. Circ Res. 1996;78:724–36. doi: 10.1161/01.res.78.4.724. [DOI] [PubMed] [Google Scholar]
- Jideama NM, Noland TA, Jr, Raynor RL, Blobe GC, Fabbro D, Kazanietz MG, Blumberg PM, Hannun YA, Kuo JF. Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties. J Biol Chem. 1996;271:23277–83. doi: 10.1074/jbc.271.38.23277. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271:24962–6. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J, Kinnally KW, Molkentin JD. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife. 2013;2:e00772. doi: 10.7554/eLife.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Yoshida K, Miura T, Mizukami Y, Matsuzaki M. Ischemic preconditioning translocates PKC-delta and -epsilon, which mediate functional protection in isolated rat heart. Am J Physiol. 1998;275:H2266–71. doi: 10.1152/ajpheart.1998.275.6.H2266. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–5. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij V, Boontje N, Zaremba R, Jaquet K, dos Remedios C, Stienen GJ, van der Velden J. Protein kinase C alpha and epsilon phosphorylation of troponin and myosin binding protein C reduce Ca2 sensitivity in human myocardium. Basic Res Cardiol. 2010;105:289–300. doi: 10.1007/s00395-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yang T, Long Z, Cheng J. Effect of mitochondrial ATP-sensitive potassium channel opening on the translocation of protein kinase C epsilon in adult rat ventricular myocytes. Genet Mol Res. 2014;13:4516–22. doi: 10.4238/2014.June.17.3. [DOI] [PubMed] [Google Scholar]
- Li M, Tan J, Miao Y, Lei P, Zhang Q. The dual role of autophagy under hypoxia-involvement of interaction between autophagy and apoptosis. Apoptosis. 2015;20:769–77. doi: 10.1007/s10495-015-1110-8. [DOI] [PubMed] [Google Scholar]
- Lin YH, Swanson ER, Li J, Mkrtschjan MA, Russell B. Cyclic mechanical strain of myocytes modifies CapZbeta1 post translationally via PKCepsilon. J Muscle Res Cell Motil. 2015 doi: 10.1007/s10974-015-9420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein kinase C-epsilon is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol. 1999;31:1937–48. doi: 10.1006/jmcc.1999.1026. [DOI] [PubMed] [Google Scholar]
- Lu D, Sivaprasad U, Huang J, Shankar E, Morrow S, Basu A. Protein kinase C-epsilon protects MCF-7 cells from TNF-mediated cell death by inhibiting Bax translocation. Apoptosis. 2007;12:1893–900. doi: 10.1007/s10495-007-0111-7. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Begley R, Kang BP, Rana I, Liu J, Yang G, Mochly-Rosen D, Meggs LG. PKC-{epsilon}-dependent survival signals in diabetic hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1343–50. doi: 10.1152/ajpheart.01200.2004. [DOI] [PubMed] [Google Scholar]
- Mansour H, de Tombe PP, Samarel AM, Russell B. Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cepsilon and focal adhesion kinase. Circ Res. 2004;94:642–9. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- McJilton MA, Van Sikes C, Wescott GG, Wu D, Foreman TL, Gregory CW, Weidner DA, Harris Ford O, Morgan Lasater A, Mohler JL, Terrian DM. Protein kinase Cepsilon interacts with Bax and promotes survival of human prostate cancer cells. Oncogene. 2003;22:7958–68. doi: 10.1038/sj.onc.1206795. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268:247–51. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Wu G, Hahn H, Osinska H, Liron T, Lorenz JN, Yatani A, Robbins J, Dorn GW., 2nd Cardiotrophic effects of protein kinase C epsilon: analysis by in vivo modulation of PKCepsilon translocation. Circ Res. 2000;86:1173–9. doi: 10.1161/01.res.86.11.1173. [DOI] [PubMed] [Google Scholar]
- Montgomery DE, Rundell VL, Goldspink PH, Urboniene D, Geenen DL, de Tombe PP, Buttrick PM. Protein kinase C epsilon induces systolic cardiac failure marked by exhausted inotropic reserve and intact Frank-Starling mechanism. Am J Physiol Heart Circ Physiol. 2005;289:H1881–8. doi: 10.1152/ajpheart.00454.2005. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–96. [PubMed] [Google Scholar]
- Noland TA, Jr, Guo X, Raynor RL, Jideama NM, Averyhart-Fullard V, Solaro RJ, Kuo JF. Cardiac troponin I mutants. Phosphorylation by protein kinases C and A and regulation of Ca(2)-stimulated MgATPase of reconstituted actomyosin S-1. J Biol Chem. 1995;270:25445–54. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- Noland TA, Jr, Raynor RL, Jideama NM, Guo X, Kazanietz MG, Blumberg PM, Solaro RJ, Kuo JF. Differential regulation of cardiac actomyosin S-1 MgATPase by protein kinase C isozyme-specific phosphorylation of specific sites in cardiac troponin I and its phosphorylation site mutants. Biochemistry. 1996;35:14923–31. doi: 10.1021/bi9616357. [DOI] [PubMed] [Google Scholar]
- Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem. 2009;284:1514–22. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbi M, Johnson JA. Protein kinase Cepsilon interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393:191–9. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Sun X, Ren J, Li X, Gao X, Lu C, Zhang Y, Sun H, Wang Y, Wang H, Wang J, Xie L, Lu Y, Yang B. miR-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS One. 2012;7:e50515. doi: 10.1371/journal.pone.0050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass JM, Zheng Y, Wead WB, Zhang J, Li RC, Bolli R, Ping P. PKCepsilon activation induces dichotomous cardiac phenotypes and modulates PKCepsilon-RACK interactions and RACK expression. Am J Physiol Heart Circ Physiol. 2001;280:H946–55. doi: 10.1152/ajpheart.2001.280.3.H946. [DOI] [PubMed] [Google Scholar]
- Paul K, Ball NA, Dorn GW, 2nd, Walsh RA. Left ventricular stretch stimulates angiotensin II--mediated phosphatidylinositol hydrolysis and protein kinase C epsilon isoform translocation in adult guinea pig hearts. Circ Res. 1997;81:643–50. doi: 10.1161/01.res.81.5.643. [DOI] [PubMed] [Google Scholar]
- Perjes A, Skoumal R, Tenhunen O, Konyi A, Simon M, Horvath IG, Kerkela R, Ruskoaho H, Szokodi I. Apelin increases cardiac contractility via protein kinase Cepsilon- and extracellular signal-regulated kinase-dependent mechanisms. PLoS One. 2014;9:e93473. doi: 10.1371/journal.pone.0093473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping P. Getting to the heart of proteomics. N Engl J Med. 2009;360:532–4. doi: 10.1056/NEJMcibr0808487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping P, Zhang J, Cao X, Li RC, Kong D, Tang XL, Qiu Y, Manchikalapudi S, Auchampach JA, Black RG, Bolli R. PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol. 1999;276:H1468–81. doi: 10.1152/ajpheart.1999.276.5.H1468. [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Pierce WM, Jr, Bolli R. Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–14. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puceat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994;269:16938–44. [PubMed] [Google Scholar]
- Pyle WG, Chen Y, Hofmann PA. Cardioprotection through a PKC-dependent decrease in myofilament ATPase. Am J Physiol Heart Circ Physiol. 2003;285:H1220–8. doi: 10.1152/ajpheart.00076.2003. [DOI] [PubMed] [Google Scholar]
- Pyle WG, Hart MC, Cooper JA, Sumandea MP, De Tombe PP, Solaro RJ. Actin capping protein: an essential element in protein kinase signaling to the myofilaments. Circulation Research. 2002;90:1299–1306. doi: 10.1161/01.res.0000024389.03152.22. [DOI] [PubMed] [Google Scholar]
- Pyle WG, Smith TD, Hofmann PA. Cardioprotection with kappa-opioid receptor stimulation is associated with a slowing of cross-bridge cycling. Am J Physiol Heart Circ Physiol. 2000;279:H1941–8. doi: 10.1152/ajpheart.2000.279.4.H1941. [DOI] [PubMed] [Google Scholar]
- Robia SL, Ghanta J, Robu VG, Walker JW. Localization and kinetics of protein kinase C-epsilon anchoring in cardiac myocytes. Biophys J. 2001;80:2140–51. doi: 10.1016/S0006-3495(01)76187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman BB, Goldspink PH, Spaite E, Urboniene D, McKinney R, Geenen DL, Solaro RJ, Buttrick PM. Inhibition of PKC phosphorylation of cTnI improves cardiac performance in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H2089–95. doi: 10.1152/ajpheart.00582.2003. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994;74:299–309. doi: 10.1161/01.res.74.2.299. [DOI] [PubMed] [Google Scholar]
- Sabri A, Steinberg SF. Protein kinase C isoform-selective signals that lead to cardiac hypertrophy and the progression of heart failure. Mol Cell Biochem. 2003;251:97–101. [PubMed] [Google Scholar]
- Scruggs SB, Walker LA, Lyu T, Geenen DL, Solaro RJ, Buttrick PM, Goldspink PH. Partial replacement of cardiac troponin I with a non-phosphorylatable mutant at serines 43/45 attenuates the contractile dysfunction associated with PKCepsilon phosphorylation. J Mol Cell Cardiol. 2006;40:465–73. doi: 10.1016/j.yjmcc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Shan H, Wei J, Zhang M, Lin L, Yan R, Zhang R, Zhu YH. Suppression of PKCepsilon-mediated mitochondrial connexin 43 phosphorylation at serine 368 is involved in myocardial mitochondrial dysfunction in a rat model of dilated cardiomyopathy. Mol Med Rep. 2015;11:4720–6. doi: 10.3892/mmr.2015.3260. [DOI] [PubMed] [Google Scholar]
- Shirai Y, Kashiwagi K, Yagi K, Sakai N, Saito N. Distinct effects of fatty acids on translocation of gamma- and epsilon-subspecies of protein kinase C. J Cell Biol. 1998;143:511–21. doi: 10.1083/jcb.143.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil P, Kandaswamy V, Sen S. Increased protein kinase C activity in myotrophin-induced myocyte growth. Circ Res. 1998;82:1173–88. doi: 10.1161/01.res.82.11.1173. [DOI] [PubMed] [Google Scholar]
- Srisakuldee W, Makazan Z, Nickel BE, Zhang F, Thliveris JA, Pasumarthi KB, Kardami E. The FGF-2-triggered protection of cardiac subsarcolemmal mitochondria from calcium overload is mitochondrial connexin 43-dependent. Cardiovasc Res. 2014;103:72–80. doi: 10.1093/cvr/cvu066. [DOI] [PubMed] [Google Scholar]
- Takeishi Y, Ping P, Bolli R, Kirkpatrick DL, Hoit BD, Walsh RA. Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy. Circ Res. 2000;86:1218–23. doi: 10.1161/01.res.86.12.1218. [DOI] [PubMed] [Google Scholar]
- Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–15. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- Tsai KL, Liang HJ, Yang ZD, Lue SI, Yang SL, Hsu C. Early inactivation of PKCepsilon associates with late mitochondrial translocation of Bad and apoptosis in ventricle of septic rat. J Surg Res. 2014;186:278–86. doi: 10.1016/j.jss.2013.08.010. [DOI] [PubMed] [Google Scholar]
- VanWinkle WB, Snuggs MB, De Hostos EL, Buja LM, Woods A, Couchman JR. Localization of the transmembrane proteoglycan syndecan-4 and its regulatory kinases in costameres of rat cardiomyocytes: a deconvolution microscopic study. Anat Rec. 2002;268:38–46. doi: 10.1002/ar.10130. [DOI] [PubMed] [Google Scholar]
- Vincent F, Duquesnes N, Christov C, Damy T, Samuel JL, Crozatier B. Dual level of interactions between calcineurin and PKC-epsilon in cardiomyocyte stretch. Cardiovasc Res. 2006;71:97–107. doi: 10.1016/j.cardiores.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Wang Y, Men M, Yang W, Zheng H, Xue S. MiR-31 Downregulation Protects Against Cardiac Ischemia/Reperfusion Injury by Targeting Protein Kinase C Epsilon (PKCepsilon) Directly. Cell Physiol Biochem. 2015;36:179–90. doi: 10.1159/000374062. [DOI] [PubMed] [Google Scholar]
- Waza AA, Andrabi K, Hussain MU. Protein kinase C (PKC) mediated interaction between conexin43 (Cx43) and K()(ATP) channel subunit (Kir6.1) in cardiomyocyte mitochondria: Implications in cytoprotection against hypoxia induced cell apoptosis. Cell Signal. 2014;26:1909–17. doi: 10.1016/j.cellsig.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Wilson S, Song W, Karoly K, Ravingerova T, Vegh A, Papp J, Tomisawa S, Parratt JR, Pyne NJ. Delayed cardioprotection is associated with the sub-cellular relocalisation of ventricular protein kinase C epsilon, but not p42/44MAPK. Mol Cell Biochem. 1996;160–161:225–30. doi: 10.1007/BF00240053. [DOI] [PubMed] [Google Scholar]
- WTSI/EMBL-EBI, 2015. Gene: PRKCE ENSG00000171132
- Yang FH, Pyle WG. Reduced cardiac CapZ protein protects hearts against acute ischemia-reperfusion injury and enhances preconditioning. J Mol Cell Cardiol. 2012;52:761–72. doi: 10.1016/j.yjmcc.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sun W, Hu K. Molecular mechanism underlying adenosine receptor-mediated mitochondrial targeting of protein kinase C. Biochim Biophys Acta. 2012;1823:950–8. doi: 10.1016/j.bbamcr.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol. 1994;266:H1145–52. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- Zeidman R, Troller U, Raghunath A, Pahlman S, Larsson C. Protein kinase Cepsilon actin-binding site is important for neurite outgrowth during neuronal differentiation. Mol Biol Cell. 2002;13:12–24. doi: 10.1091/mbc.01-04-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]