Abstract
As the population ages, neurodegenerative diseases, such as Alzheimer’s disease (AD), are becoming a significant burden on patients, their families, and health care systems. Neurodegenerative processes may start up to fifteen years before outward signs and symptoms of AD, as evidenced by data from AD patients and mouse models. A major genetic risk factor for late-onset (AD) is the ε4 isoform of apolipoprotein E (ApoE4), which is present in almost 20% of the population. In this review, we discuss the contribution of ApoE receptor signaling to the function of each component of the tripartite synapse - the axon terminal, the post-synaptic dendritic spine, and the astrocyte - and examine how these systems fail in the context of ApoE4 and AD.
Keywords: Endosome, Synaptic Dysfunction, LRP, Calcium Homeostasis, NMDA receptor, Dendrite, Reelin
ApoE and the LDL Receptor Family
The low-density lipoprotein (LDL) receptor family is an evolutionarily ancient and highly conserved receptor family initially identified for its role in carrying lipoprotein particles. The LDL receptor family comprises seven core members: low-density lipoprotein receptor (LDLR), LDLR-related receptor 1 (LRP1), very-low-density lipoprotein receptor (VLDLR), megalin (LRP2), apolipoprotein E receptor 2 (Apoer2 or LRP8), LRP4, and LRP1b [1]. They share a conserved structure: a short intercellular domain containing 1–3 NPXY motifs that mediates signal transduction and trafficking, a transmembrane domain, and a large extracellular domain with a varying number of epidermal growth factor (EGF) precursor homology domains and complement-type repeats, which are responsible for ligand binding and pH-dependent ligand release [1]. All LDL receptor family members share the structural properties that allow them to interact with ApoE, hence the terms ‘LDL receptor family’ and ‘ApoE receptors’ will be used synonymously throughout this review.
The first identified member of the LDL receptor family, LDLR, is expressed on the cell surface of hepatocytes and binds LDL particles through Apolipoprotein B (ApoB); similarly, LDLR and LRP1 bind ApoE on chylomicron remnants and VLDL particles [2]. This process mediates the uptake of lipid and cholesterol-laden particles into the cell. As key components of cholesterol homeostasis, deficiency of either ApoE or LDLR in humans leads to severe hypercholesterolemia and premature atherosclerosis [3].
In the central nervous system (CNS), cholesterol and phospholipids produced by glia are vital for the formation and maintenance of healthy synapses [4]. Most cholesterol is released by astrocytes in the form of ApoE-containing “high-density lipoprotein (HDL) -like” particles [5]. Complete genetic deficiency of ApoE, or deficiency limited to the CNS, results in a reduction of synapse number that is at least partially due to loss of astrocyte-derived particles [6]. In the brain, ApoE binds numerous receptors, including Ldlr, Lrp1, Apoer2, and Vldlr, and these receptors play a variety of roles outside of lipid trafficking and metabolism, including synaptic transmission, modulation of spine structure, and astrocyte function [1].
ApoE, ApoE Receptors, and Alzheimer’s Disease
Alzheimer’s disease (AD) affects over 30 million people worldwide, and one in nine people over 65 years of age [7]. AD is characterized clinically by brain shrinkage accompanied by progressive memory loss and cognitive decline, as well as personality changes later in the disease course. Pathologically, AD is characterized by the progressive buildup of neuritic plaques of amyloid-beta (Aβ), followed by neurofibrillary tangles of hyperphosphorylated tau. Overt clinical symptoms typically do not appear until the underlying pathology is well-developed [8]; however, functional imaging studies suggest that changes in synaptic function occur several years before outward signs of the disease are apparent [9]. Moreover, rising Aβ levels may in part be responsible for the subtle, but also progressive, reduction in cognitive ability that occurs during normal aging, and patients with “subjective cognitive decline” (i.e., patients who perform normally on standardized memory tests, but nevertheless report subjective memory impairment) have generally higher levels of Aβ deposition on positron emission tomography (PET) scanning [10, 11].
Over two decades ago, the ε4 isoform of ApoE (ApoE4) was identified as a major genetic risk factor for late-onset AD (i.e., after 60 years of age) [12]. Possession of one copy of ApoE4 triples the risk of developing AD, while individuals with two copies have a 90% lifetime risk of developing the disease. The allele frequency of ApoE4 is 15–20%, with some variation in incidence between populations [13]. Conversely, ApoE2 is considered to be protective against AD, while ApoE3 is considered risk-neutral, because it is by far the most common of the three isoforms and thus is considered the standard for the general population [13].
Since its identification as an important risk factor, great strides have been produced in understanding the role ApoE4 plays in synapse function and AD. One important role of ApoE is the clearance of Aβ, with ApoE4 hindering Aβ clearance significantly over ApoE3 and ApoE2 [14] and thus directly increasing amyloid pathology. Additional roles for ApoE4 have been indicated by noninvasive imaging studies, which have shown that older individuals who are ApoE4 carriers have structural and functional alterations in AD affected areas in the absence of cognitive dysfunction [15]. Moreover, some of these changes are present much earlier in life [16], indicating a role for ApoE in neuronal function prior to amyloid deposition. There is an enormous amount of literature that explores the interaction between ApoE4 and Aβ, which has been reviewed in depth recently in [1, 17, 18]; therefore, this review will focus on the roles of ApoE and its receptors at the synapse.
We divide our discussion based on the three components of the tripartite synapse – the post-synaptic spine, the axon terminal, and the astrocyte – and delineate the newly discovered roles of ApoE receptor signaling at each of these compartments (Figure 1).
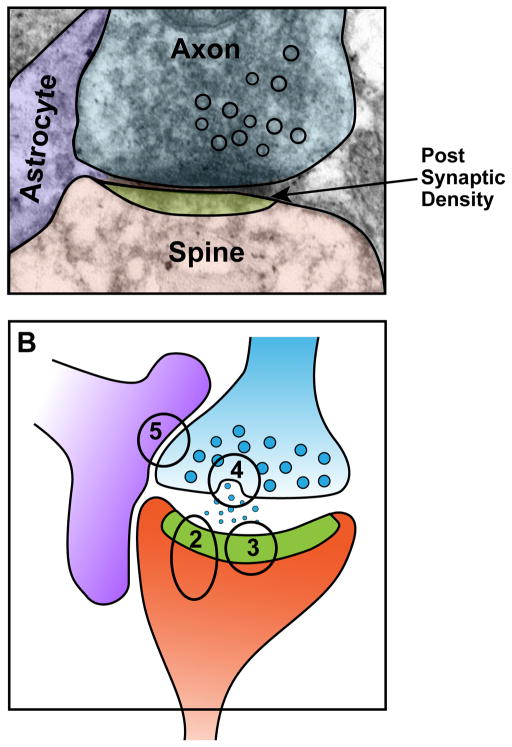
Figure 1. The tripartite synapse and ApoE receptor signaling.
The classic model of a synapse – with the axon terminal of one neuron synapsing onto the dendritic spine of another neuron – has been expanded to include support and signaling from the perisynaptic astrocyte, which has cell processes in close proximity with the synaptic cleft. ApoE receptor signaling affects all three components of the synapse. Panel A, electron microscopy image of a mouse hippocampal synapse courtesy of Bret Evers. Panel B, schematic rendition of the synapse in A. Numbers indicate parts of the synapse shown in greater detail in Figures 2–5.
Reelin, ApoE receptors, and Glutamate Signaling
Several ApoE receptors have been identified in the postsynaptic density, most notably Lrp1, Apoer2, and Vldlr, where they interact with key synaptic components. For example, Lrp1 interacts with the N-methyl-D-aspartate receptor (NMDAR), promoting the endocytosis of NMDAR from the cell surface [19]. Additionally, loss of Lrp1 hinders some elements of NMDAR signaling, such as internalization of GluA1 and degradation of PSD-95 [20].
Similar to Lrp1, the ApoE receptors Apoer2/Lrp8 and Vldlr form a complex with scaffolding proteins, such as PSD-95, and the glutamate receptors [21]. The primary ligand for these receptors in the CNS is not ApoE, but rather the glycoprotein Reelin [22]. Reelin is a large, secreted, extracellular protein with numerous roles in the CNS. During development, Reelin is secreted by Cajal-Retzius cells of the marginal zone and dentate gyrus, as well as cerebellar granule cells, where it is required for appropriate migration of newly-generated neurons and neuronal layering. Mice that are deficient in Reelin (reeler) mice, have abnormal neuronal layering, severe ataxia and learning impairment, and typically die at an early age [23]. In the adult brain, Reelin secretion by Cajal-Retzius neurons declines, as they are replaced by a subset of GABAergic interneurons that populate the cortex by tangential migration emanating from the medial ganglionic eminence, and by glutamatergic neurons within layer II of the entorhinal cortex, a region that is affected early in AD [23, 24].
When Apoer2/Lrp8 and Vldlr are bound by Reelin, they cluster and induce phosphorylation of the adaptor protein Disabled-1 (Dab1), which has several important consequences [25, 26]. First, Dab1-mediated activation of Src and Fyn leads to the tyrosine phosphorylation of the NR2 subunits of NMDARs, which causes increased Ca2+ influx when the receptors are activated and also reduces NMDAR endocytosis [27, 28], thereby producing a large net influx of Ca2+ into the dendrite. Accordingly, when Reelin is applied to hippocampal slices, greater Ca2+ influx through opened NMDARs leads to increased long-term potentiation [29]. This effect is mirrored in vivo by the finding that intraventricular injection of Reelin in mice improves learning and memory [30]. Second, Dab1 activates phosphoinositol-3 (PI3) kinase and subsequently Akt, which phosphorylates glycogen synthase kinase 3β (GSK3β) at its inhibitory Ser-9 phosphorylation site. This reduces the phosphorylation of numerous targets of GSK3β, the most relevant of which in the context of AD is the microtubule-associated protein τ [25].
In opposition to Reelin, Aβ oligomers have antagonistic effects at the post-synaptic spine. Aβ binds to α7 nicotinic acetylcholine receptors (α7 nAChRs) and to metabotropic glutamate receptors, in particular mGluR5 [31–33]. A common consequence of these interactions is the activation of calcineurin (PP2B) [28]. PP2B regulates levels and activity of striatal-enriched protein tyrosine phosphatase (STEP) [34]. Some of the targets of STEP are the same tyrosine residues on NMDAR that are phosphorylated by Reelin-Apoer2 signaling [35]. Thus, Aβ-mediated activation of STEP leads to excessive internalization of NMDARs [28, 35]. This effect is specific to oligomeric, not monomeric, Aβ. STEP additionally dephosphorylates and deactivates Fyn, enhancing the overall effect of STEP on NMDAR endocytosis [36].
Similarly, Aβ activates GSK3β through a signaling cascade involving caspase-3 and Akt [37]. The receptor for this pathway remains unidentified, though there are numerous potential receptors for Aβ (PrPc, α7 nAChRs, PirB, among others [33, 38]). GSK3β activation reduces synaptic long-term potentiation (LTP) and provides a potential biochemical mechanism by which Aβ can induce τ hyperphosphorylation [39]. As a result of these two mechanisms, activated GSK3β and increased calcineurin and STEP activation, Aβ oligomers reduce LTP [40]. Importantly, Reelin application to hippocampal slices prevents the reduction in LTP induced by Aβ [41].
Alterations in Reelin signaling in the adult brain have been implicated in numerous neurodevelopmental disorders including schizophrenia, autism, and mood disorders [43]. With age, Reelin levels are reduced in the brain and in AD, Reelin levels are particularly reduced in the entorhinal cortex [44]. Some SNPs in the Reelin gene have been identified as being protective against AD and are hypothesized to increase Reelin levels in the presence of AD pathology [45]; however, definitive studies showing a protective effect of Reelin in humans are largely lacking. Intriguingly, though Reelin is vital for brain development, another ApoE receptor ligand, ApoE, is not, as patients who are completely ApoE-deficient have broadly normal cognition [3]. This is partially due to the fact that ApoE expression, in both mice and humans, begins relatively late in development [46, 47]. Additionally, adult loss of Reelin and ApoE receptor signaling in mice is well-tolerated in the absence of amyloid pathology, likely due to homeostatic regulation and compensation by other neuromodulatory mechanisms [42].
Taken together, these findings provide a rational concept of Reelin and ApoE receptor signaling whereby Reelin protects the synapse against the deleterious effects of Aβ through a complex signaling cascade to maintain the balance of kinase and phosphatase activity (Figure 2). Previously, it was difficult to examine this protective role of Reelin in vivo, due to the necessity of Reelin in brain development; however, a conditional knockout mouse was recently developed to overcome these challenges. It was found that while adult loss of Reelin does not cause significant cognitive impairment - suggesting an ability of a healthy CNS to homeostatically compensate for Reelin loss - overexpression of Aβ in Reelin-deficient mice leaves them heavily impaired in the Morris Water maze task of learning and memory [42]. These findings highlight the important role for Reelin and ApoE receptor signaling in protecting the synapse from Aβ-induced suppression.
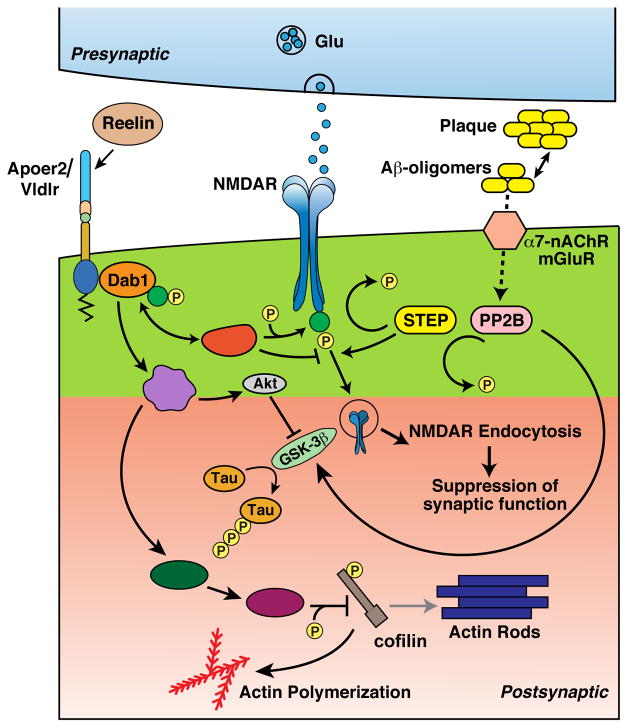
Figure 2. ApoE receptors, Reelin, and Aβ at the post-synaptic neuron.
Reelin binding to Apoer2 and Vldlr clusters the receptors and initiates a downstream signaling cascade via Dab1 that counteracts Aβ signaling at several sites, including NMDAR phosphorylation and endocytosis, tau phosphorylation, and cofilin-mediated actin depolymerization and spine remodeling.
Calcium Dysregulation and ApoE receptors
One intensely discussed mechanism for how Aβ causes synaptic dysfunction early in disease is dysregulation of Ca2+ homeostasis. Ca2+ flux forms the basis of the regulation of synaptic strength: changes in Ca2+ levels in the right context trigger LTP and long-term depression (LTD), respectively. Briefly, large Ca2+ influx through NMDARs stimulates Ca2+/calmodulin dependent protein kinase II (CaMKII) activity and G proteins, ultimately resulting in the insertion of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and growth of the spine [48, 49]. This process underlies part of the molecular basis leading to LTP, in which the synapse responds more robustly to incoming synaptic stimulation. Conversely, lower levels of Ca2+ influx preferentially activate the phosphatase calcineurin, which in turn activates protein phosphatase 1 (PP1) and STEP, resulting in the dephosphorylation of AMPARs and their removal from and long-term depression of the synapse (referred to as LTD) [50].
In AD, Ca2+ homeostasis inclines toward a higher level of resting Ca2+ [51]. This has been shown in AD mouse models, particularly in the area surrounding amyloid plaques, and application of Aβ to hippocampal slices elevates resting Ca2+ levels [52, 53]. Several mechanisms have been proposed for the effect of Aβ on resting Ca2+ levels. First, Aβ may directly enhance Ca2+ entry through receptors. This may involve extrasynaptic NMDARs, which do not participate directly in synaptic transmission, but rather contribute background noise to the otherwise ordered network function coordinated by synaptic transmission [54–56]. Alternatively, Aβ oligomers have been proposed to themselves act as calcium leak channels [57].
Intracellular calcium stores play a vital role in calcium homeostasis and are disrupted in AD. In this concept, presenilin 1 (PS1) – which is more commonly known in AD for regulating amyloid precursor protein (APP) cleavage to Aβ-plays a role in the ER as a regulator of Ca2+ release through interaction with inositol triphosphate receptors (InsP3Rs, or ryanodine receptors) and as a direct mediator of Ca2+ release as a Ca2+ leak channel. In general terms, there is an important role for presenilins in intracellular Ca2+ stores, which have been discussed in depth in other reviews [58].
Finally, tight control of Ca2+ homeostasis is vital for ensuring efficient synaptic transmission and maintaining balance between storage and elimination of a memory trace (i.e., LTP versus LTD). To maintain tight control, there are Ca2+ buffering and Ca2+ sensing systems present in the cytoplasm, such as calbindin D-28k (CB) and calmodulin. CB contains four Ca2+ binding sites, is present in high concentrations in neurons, and acts as a Ca2+ buffer. Levels of CB are reduced with age and in AD. Conversely, expression of CB prevents some of the elevation in resting Ca2+ levels induced by Aβ [59]. Additionally, there are other Ca2+ sensing molecules, like calmodulin, which regulates the activity of both Ca2+ sensitive kinases (CaMKII) as well as phosphatases (calcineurin), and whose levels are also disrupted in AD [51]. Impairments of this buffering system together with enhanced Ca2+ leakage from the intracellular and extracellular pools, leads to further disruption of Ca2+ homeostasis and synaptic transmission.
The main effect of higher resting levels of Ca2+ appears to be to tip the balance of synaptic plasticity towards LTD. The net result of this would be memory trace erasure and loss of synapses. Importantly, the ApoE receptors activated by Reelin are essential in maintaining the signal strength over the noise of rising Ca2+ levels induced by AD-promoting mechanisms. This mechanism affords protection to the synapse by maintaining synaptic strength during the early stages of AD until Aβ and τ accumulate to such levels that the system breaks down and synaptic loss ensues.
Post-synaptic actin polymerization
Maintaining Ca2+ homeostasis is essential for regulating post-synaptic signaling, which is important for the growth and maintenance, and alternatively the shrinkage, of dendritic spines. Dendritic spines are the central sites of post-synaptic transmission, and the formation and elimination of spines is a dynamic process that continues throughout life and that is dependent on many extraneuronal and intraneuronal signals [60]. Here, Reelin and ApoE receptors play a role in directing dendritic complexity through the control of actin polymerization. Briefly, Reelin signaling activates PI3kinase, as described above [61]. In turn, PI3kinase initiates a signaling pathway that induces phosphorylation of LIM kinase-1 (LIMK-1), which in turn phosphorylates cofilin at an inhibitory site, blocking the actin-depolymerizing activity of cofilin [62, 63]. As a result, actin polymerization and dendritic spine growth exhibit a net increase in the presence of Reelin, and mice that overexpress Reelin have higher spine density and increased spine complexity [64, 65].
Conversely, Aβ has the opposite effect on actin microfilament dynamics. Aβ oligomers reduce activity of LIMK, which in turns leads to dephosphorylated, active cofilin, depolymerization of actin filaments and the generation of actin rods [66]. While direct opposition of Reelin and Aβ at the level of actin polymerization, or an effect of ApoE on this process, has yet to be definitively shown, it is important to point out that ApoE4 targeted replacement mice have a reduction in dendritic spine complexity, a finding that is mirrored in human ApoE4 carriers [67]. Overall, however, it is clear that the ApoE receptors play an important role at the post-synaptic neuron separate from modulation of synaptic plasticity by regulating structural changes to the dendritic spine itself.
ApoE and Endocytic Trafficking
We have so far reviewed the role of ApoE receptors and Reelin in the maintenance of synaptic plasticity and actin polymerization. As ligands for ApoE receptors, ApoE isoforms have differential effects on these processes. ApoE binds the receptors at a different site from Reelin, and thus does not directly affect receptor signaling by hindering Reelin engagement; however, the ApoE isoforms do affect receptor trafficking.
ApoE exists in the human population in three isoforms, which differ at only two residues, 112 and 158. ApoE2 has a cysteine at both positions, ApoE3 has a cysteine at position 112 and an arginine at position 158, and ApoE4 has arginines at both positions [68]. ApoE has two structural domains, an N-terminal domain, which is responsible for interaction with ApoE receptors, and a C-terminal domain, which is responsible for lipoprotein binding [68]. The presence of an arginine at position 112 promotes the formation of a salt bridge between Arg-61 and Glu-255, leading to a “domain interaction” between the N- and C-terminals. As a result, ApoE4 has a greater tendency to form molten globules over the other ApoE isoforms, as well as a greater propensity to aggregate at 37°C [69, 70].
The different structural properties of ApoE4 have an important effect on its role in ligand delivery and receptor trafficking. Under normal conditions, ApoE-containing particles are internalized by the ApoE receptors, the lipoprotein particles then begin to disengage from their receptors in the early endosome, and the receptors are finally recycled through recycling endosomes back to the cell surface. However, when ApoE4 is present, the final recycling step is delayed and the process is stalled in the endosome, presumably due to the propensity of ApoE4 to form molten globules at the lower pH of the endosome [69, 71]. Thus, in the presence of ApoE4, the recycling of the Reelin receptors and the glutamate receptors trapped in the same vesicle back to the synapse is stalled, Reelin signaling is blunted and glutamate receptor homeostasis is impaired [72] (Figure 3). As a result, Reelin is compromised in its ability to effectively balance the inhibitory effect of Aβ on LTP (see above) when ApoE4 is present [72]. In this way, ApoE4 induces a state of “Reelin resistance” that likely affects more than synaptic plasticity (e.g., Ca2+ homeostasis, spine remodeling, and the pre-synaptic and astrocytic roles described below).
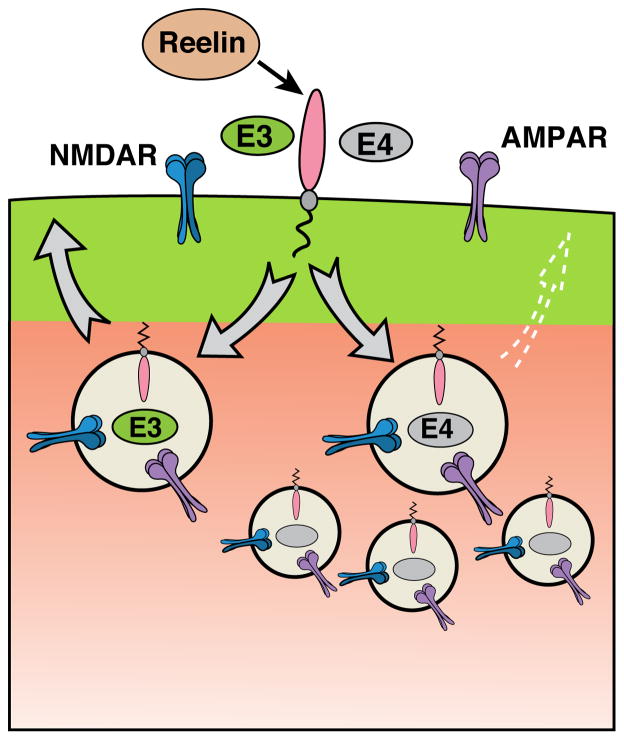
Figure 3. ApoE4 impairs endocytic vesicle recycling.
ApoE receptors are constitutively recycled to and from the surface. ApoE4 is predisposed to form molten globules as the pH drops in the early endosome, which leads to impaired vesicle recycling and reduction of surface levels of ApoE receptors and glutamate receptors.
Presynaptic Roles of ApoE Receptors
Most work on synaptic ApoE receptors has focused on their postsynaptic roles. However, emerging data indicate that ApoE receptors have a similarly important role in regulating presynaptic vesicle release [73]. It was previously thought that LDL receptor family members were mainly expressed in the post-synaptic density; however, recent studies have shown that the ApoE receptors Apoer2 and Vldlr are also expressed at the presynaptic membrane. Briefly, Reelin signaling through Apoer2 and Vldlr receptors on the presynaptic membrane leads to transient elevations of intracellular Ca2+ [73]. This Ca2+ elevation specifically increases the fusion of vesicles containing vesicle-associated membrane protein 7 (VAMP7), an alternative ‘soluble N-ethylmaleimide sensitive factor attachment protein receptor’ (SNARE) protein that participates in spontaneous vesicle release [74]. Reelin does not affect other SNARE protein-mediated signaling [73] (Figure 4). An effect of ApoE isoform on these presynaptic changes has yet to be determined, though given what we know about the postsynaptic mechanisms by which ApoE4 impairs ApoE receptor function, this is likely going to occur at the presynaptic side of the synapse as well.
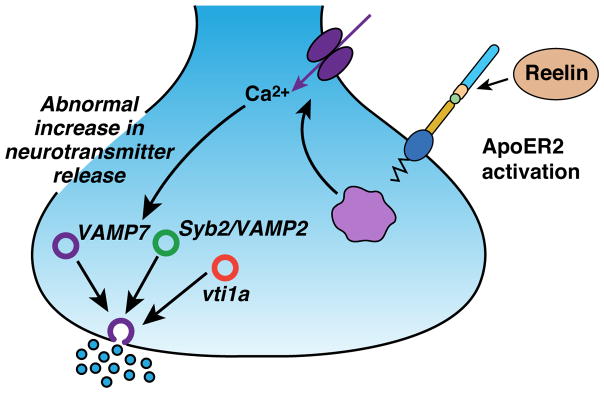
Figure 4. Reelin signaling modulates spontaneous synaptic vesicle release.
Reelin signaling through Apoer2/Vldlr on the pre-synaptic neuron stimulates influx of Ca2+, which specifically increases spontaneous release of VAMP7-containing vesicles.
Another presynaptic role for ApoE receptors may involve the production of glutamate. Studies in ApoE4 targeted replacement (TR) mice have shown decreased levels of glutamate, increased levels of glutamine (the precursor of glutamate), and a late increase in vesicular glutamate transporter 1 (vglut1), suggesting a reduced ability of presynaptic ApoE4 neurons to convert glutamine to glutamate and a compensatory increase in vesicular glutamate loading [75]. Follow-up studies are required to determine the impact this altered glutamate production has in vivo, and what effect, if any, it may have on AD.
Astrocytes
Most ApoE in the brain is expressed by astrocytes. Astrocyte-derived ApoE is important for cholesterol transport through ApoE-containing “HDL-like” particles, which play an important role in synaptic development and maintenance [1, 4, 5]. Interestingly, recent data supports the hypothesis that ApoE and the ApoE receptors mediate processes in the astrocyte outside of lipid trafficking.
One role of ApoE receptor signaling in the astrocytes may be modulation of synaptic pruning. Astrocytes actively partake in synaptic pruning by phagocytosing synaptosomes, and ApoE isoforms differentially affect this process, with ApoE4 limiting the ability of astrocytes to prune synapses [76, 77]. Lrp1 is highly expressed in astrocytes, where it is responsible for phagocytosis of degraded myelin [78, 79]. It is likely, though it remains to be demonstrated, that Lrp1 mediates at least in part the effect of ApoE isoforms on synaptic pruning.
In addition to their role in synaptic pruning, the importance of astrocytes for effective synaptic transmission is rapidly becoming appreciated. Astrocytes are capable of detecting synaptic activity through glutamatergic receptors [80]. Ion flow through these receptors causes alterations in intracellular Ca2+ stores that trigger the release of various substances from the astrocyte, including glutamate, D-serine, and ATP, which can then affect synaptic transmission [81]. This process is termed “gliotransmission,” which has complex effects (thoroughly reviewed in [82]) and this is a rapidly growing field within neuroscience.
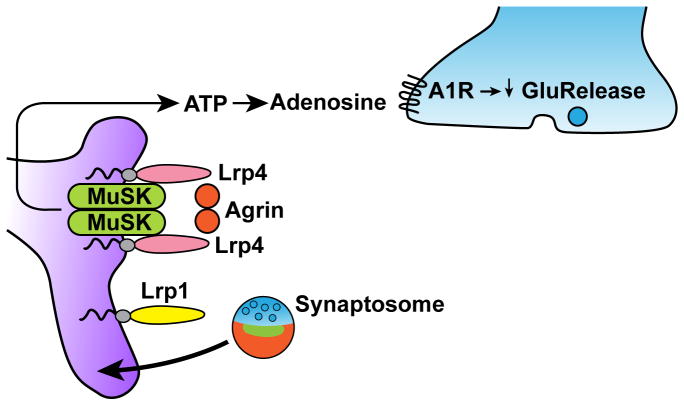
A role for Lrp4 in gliotransmission was recently described. Lrp4 is more commonly known as the gene defective in Cenani-Lenz syndrome [83–86], and for its role in neuromuscular junction (NMJ) development and in the maintenance of the adult NMJ, which is highlighted clinically by the role of anti-Lrp4 and anti-MuSK antibodies in myasthenia gravis [87]. Briefly, at the NMJ, Lrp4, APP and muscle-specific kinase (MuSK) form a complex to prepattern the muscle [88–90]. Agrin is released from the motor neuron and signals through APP, MuSK and Lrp4 to recruit nicotinic acetylcholine receptors (AChRs). Importantly, the absolute requirement of Lrp4 for NMJ development in mice, but intriguingly not in humans [86] and cattle [91], means that mice that are completely deficient in Lrp4 die perinatally, which has precluded effective study of the role of Lrp4 in the CNS until recently [92]. A recent study used a mouse model in which Lrp4 is expressed in the muscle on an Lrp4 knockout background, permitting survival into adulthood. Importantly, these mice have a reduction of synaptic transmission and long-term potentiation [93], and this finding is mimicked in mice carrying a hypomorphic allele for Lrp4 [94]. A further study refined the role of Lrp4, inasmuch as it now appears that it is not neuronally expressed Lrp4, but rather astrocytic Lrp4 that mediates this process. In the astrocyte, Agrin signaling through Lrp4 leads to the increased release of ATP, which is subsequently broken down into adenosine in the extracellular milieu [95]. Adenosine signaling through adenosine A1 (A1A) receptors at the presynaptic membrane leads to a reduced release probability of glutamate-containing vesicles and thus reduced glutamatergic transmission [96] (Figure 5). The mechanism by which Agrin-Lrp4 signaling regulates ATP release from astrocytes remains unclear. It is additionally unknown if other LDL receptor family members take part in gliotransmission, or if ApoE isoforms affect this gliotransmission.
Figure 5. ApoE receptor signaling at the astrocyte.
Agrin stimulates Lrp4/MuSK complexes on astrocytes to induce release of ATP. In the extracellular space, ATP is metabolized to adenosine, which then acts on pre-synaptic A1 receptors to decrease glutamatergic vesicle release. Lrp1 on astrocytes mediates phagocytic uptake of particles, potentially including synaptosomes. ApoE isoforms differentially affect synaptosome uptake.
When discussing effects of ApoE receptor signaling on the astrocyte, it is important to point out that glial activation is affected by ApoE isoform, with ApoE4 causing the greatest activation [97]. Though beyond the scope of this review, glial activation is an indication of pro-inflammatory changes that is commonly found in AD brains, which have been thoroughly discussed by other groups [98].
Overall, the astrocyte has been a long-neglected part of the synapse. As the field becomes more aware of the role of the astrocyte at the tripartite synapse, and the fact that ApoE receptors are actively involved in shaping its functions as well, more studies will emerge that evaluate these new roles in neurodegeneration.
Concluding Remarks and future perspectives
ApoE receptors have a central role as regulators of the synapse, both at pre- and post-synaptic sites, as well as at the peri-synaptic astrocyte. These functions, which are essential for maintaining proper synaptic strength, are differentially affected by ApoE isoforms, with ApoE4 most severely disrupting the neuromodulatory roles of ApoE receptors. Thus, ApoE4 promotes neuronal dysfunction at the earliest stages of the pathology, leading up to the clinical manifestation of AD by two distinct mechanisms: impairing the turnover of Aβ, thereby accelerating amyloid deposition, and weakening the ability of Reelin and ApoE receptor signaling to protect against the deleterious effects of Aβ on the synapse. Many facets of ApoE receptor signaling remain unknown and are outlined in the “Outstanding Questions” box, from mechanistic explanations of Reelin signaling effects on presynaptic transmission to defining the role of ApoE in astrocytic function. Novel pharmacological interventions that target the effect of ApoE4 on endosomal trafficking would be of potential clinical impact by reducing the risk the ApoE4 allele poses for late-onset Alzheimer’s disease.
Outstanding Questions Box.
What are the mechanisms by which Reelin signaling changes postsynaptic protein translation to alter the glutamate receptor composition of the synapse?
How do healthy neurons compensate for loss of Reelin signaling?
Does ApoE4 represent a gain or loss of function?
Do ApoE isoforms affect pre-synaptic function, in particular VAMP7-vesicle mobilization?
Through what mechanism does ApoE affect synaptic pruning by astrocytes?
How does Lrp4 increase ATP release from astrocytes?
What molecular mechanisms could be targeted to ameliorate the effects of ApoE4 on endocytic recycling?
Would increasing or decreasing ApoE levels be a good therapeutic target?
Trends Box.
Reelin signaling through ApoE receptors activates a signaling cascade that protects against Aβ at the level of NMDAR endocytosis, actin polymerization, and tau phosphorylation
ApoE4 induces neuronal resistance to Reelin by impairing recycling of vesicles containing ApoE receptors, which results in reduced surface expression of the receptors
ApoE receptors on the presynaptic neuron affect spontaneous vesicle release by increasing mobilization of VAMP7-containing vesicles
Astrocytes express ApoE receptors, which may play a role in gliotransmission and synaptic pruning
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lane-Donovan C, et al. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron. 2014;83(4):771–87. doi: 10.1016/j.neuron.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161(1):161–72. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mak AC, et al. Effects of the absence of apolipoprotein e on lipoproteins, neurocognitive function, and retinal function. JAMA Neurol. 2014;71(10):1228–36. doi: 10.1001/jamaneurol.2014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Rev. 2010;63(1–2):39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Mahley RW. Central Nervous System Lipoproteins: ApoE and Regulation of Cholesterol Metabolism. Arterioscler Thromb Vasc Biol. 2016;36(7):1305–15. doi: 10.1161/ATVBAHA.116.307023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane-Donovan C, et al. Genetic Restoration of Plasma ApoE Improves Cognition and Partially Restores Synaptic Defects in ApoE-Deficient Mice. J Neurosci. 2016;36(39):10141–50. doi: 10.1523/JNEUROSCI.1054-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Association A.s. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiman EM, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11(12):1048–56. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lista S, et al. Evolving Evidence for the Value of Neuroimaging Methods and Biological Markers in Subjects Categorized with Subjective Cognitive Decline. J Alzheimers Dis. 2015;48(Suppl 1):S171–91. doi: 10.3233/JAD-150202. [DOI] [PubMed] [Google Scholar]
- 11.Zwan MD, et al. Subjective Memory Complaints in APOEvarepsilon4 Carriers are Associated with High Amyloid-beta Burden. J Alzheimers Dis. 2015;49(4):1115–22. doi: 10.3233/JAD-150446. [DOI] [PubMed] [Google Scholar]
- 12.Lutz MW, et al. A Genetics-based Biomarker Risk Algorithm for Predicting Risk of Alzheimer’s Disease. Alzheimers Dement (N Y) 2016;2(1):30–44. doi: 10.1016/j.trci.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CC, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano JM, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, et al. Disrupted functional and structural networks in cognitively normal elderly subjects with the APOE varepsilon4 allele. Neuropsychopharmacology. 2015;40(5):1181–91. doi: 10.1038/npp.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean DC, 3rd, et al. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 2014;71(1):11–22. doi: 10.1001/jamaneurol.2013.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtzman DM, et al. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanekiyo T, et al. ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81(4):740–54. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier W, et al. LRP1 is critical for the surface distribution and internalization of the NR2B NMDA receptor subtype. Mol Neurodegener. 2013;8:25. doi: 10.1186/1750-1326-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima C, et al. Low density lipoprotein receptor-related protein 1 (LRP1) modulates N-methyl-D-aspartate (NMDA) receptor-dependent intracellular signaling and NMDA-induced regulation of postsynaptic protein complexes. J Biol Chem. 2013;288(30):21909–23. doi: 10.1074/jbc.M112.444364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beffert U, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47(4):567–79. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Lee GH, D’Arcangelo G. New Insights into Reelin-Mediated Signaling Pathways. Front Cell Neurosci. 2016;10:122. doi: 10.3389/fncel.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Arcangelo G. Reelin mouse mutants as models of cortical development disorders. Epilepsy Behav. 2006;8(1):81–90. doi: 10.1016/j.yebeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Kobro-Flatmoen A, et al. Reelin-immunoreactive neurons in entorhinal cortex layer II selectively express intracellular amyloid in early Alzheimer’s disease. Neurobiol Dis. 2016;93:172–83. doi: 10.1016/j.nbd.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7(11):850–9. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 26.Strasser V, et al. Receptor clustering is involved in Reelin signaling. Mol Cell Biol. 2004;24(3):1378–86. doi: 10.1128/MCB.24.3.1378-1386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25(36):8209–16. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–8. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 29.Weeber EJ, et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277(42):39944–52. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 30.Rogers JT, et al. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem. 2011;18(9):558–64. doi: 10.1101/lm.2153511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dineley KT, et al. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci. 2001;21(12):4125–33. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renner M, et al. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66(5):739–54. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauren J, et al. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457(7233):1128–32. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102(2):491–6. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelkey KA, et al. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34(1):127–38. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen TH, et al. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277(27):24274–9. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- 37.Jo J, et al. Abeta(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat Neurosci. 2011;14(5):545–7. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 38.Kim T, et al. Human LilrB2 is a beta-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer’s model. Science. 2013;341(6152):1399–404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu LQ, et al. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27(45):12211–20. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 41.Durakoglugil MS, et al. Reelin signaling antagonizes beta-amyloid at the synapse. Proc Natl Acad Sci U S A. 2009;106(37):15938–43. doi: 10.1073/pnas.0908176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lane-Donovan C, et al. Reelin protects against amyloid beta toxicity in vivo. Sci Signal. 2015;8(384):ra67. doi: 10.1126/scisignal.aaa6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folsom TD, Fatemi SH. The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology. 2013;68:122–35. doi: 10.1016/j.neuropharm.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chin J, et al. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci. 2007;27(11):2727–33. doi: 10.1523/JNEUROSCI.3758-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kramer PL, et al. Alzheimer disease pathology in cognitively healthy elderly: a genome-wide study. Neurobiol Aging. 2011;32(12):2113–22. doi: 10.1016/j.neurobiolaging.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JA, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508(7495):199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 48.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herring BE, Nicoll RA. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol. 2016;78:351–65. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- 50.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80(3):704–17. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berridge MJ. Calcium signalling and Alzheimer’s disease. Neurochem Res. 2011;36(7):1149–56. doi: 10.1007/s11064-010-0371-4. [DOI] [PubMed] [Google Scholar]
- 52.Lopez JR, et al. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J Neurochem. 2008;105(1):262–71. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 53.Kuchibhotla KV, et al. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59(2):214–25. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferreira IL, et al. Amyloid beta peptide 1-42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium. 2012;51(2):95–106. doi: 10.1016/j.ceca.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Talantova M, et al. Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82(2):279–93. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 57.Shirwany NA, et al. The amyloid beta ion channel hypothesis of Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3(5):597–612. [PMC free article] [PubMed] [Google Scholar]
- 58.Supnet C, Bezprozvanny I. Presenilins function in ER calcium leak and Alzheimer’s disease pathogenesis. Cell Calcium. 2011;50(3):303–9. doi: 10.1016/j.ceca.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31(9):454–63. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14(8):536–50. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bock HH, et al. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem. 2003;278(40):38772–9. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- 62.Caroni P, et al. Synapse rearrangements upon learning: from divergent-sparse connectivity to dedicated sub-circuits. Trends Neurosci. 2014;37(10):604–14. doi: 10.1016/j.tins.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013;25(2):457–69. doi: 10.1016/j.cellsig.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Chai X, et al. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci. 2009;29(1):288–99. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bosch C, et al. Reelin Regulates the Maturation of Dendritic Spines, Synaptogenesis and Glial Ensheathment of Newborn Granule Cells. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao L, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9(2):234–42. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 67.Ji Y, et al. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122(2):305–15. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Zhong N, Weisgraber KH. Understanding the association of apolipoprotein E4 with Alzheimer disease: clues from its structure. J Biol Chem. 2009;284(10):6027–31. doi: 10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrow JA, et al. Apolipoprotein E4 forms a molten globule. A potential basis for its association with disease. J Biol Chem. 2002;277(52):50380–5. doi: 10.1074/jbc.M204898200. [DOI] [PubMed] [Google Scholar]
- 70.Hatters DM, et al. Amino-terminal domain stability mediates apolipoprotein E aggregation into neurotoxic fibrils. J Mol Biol. 2006;361(5):932–44. doi: 10.1016/j.jmb.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 71.Heeren J, et al. Impaired recycling of apolipoprotein E4 is associated with intracellular cholesterol accumulation. J Biol Chem. 2004;279(53):55483–92. doi: 10.1074/jbc.M409324200. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y, et al. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A. 2010;107(26):12011–6. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bal M, et al. Reelin Mobilizes a VAMP7-Dependent Synaptic Vesicle Pool and Selectively Augments Spontaneous Neurotransmission. Neuron. 2013;80(4):934–46. doi: 10.1016/j.neuron.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hua Z, et al. v-SNARE composition distinguishes synaptic vesicle pools. Neuron. 2011;71(3):474–87. doi: 10.1016/j.neuron.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dumanis SB, et al. APOE genotype affects the pre-synaptic compartment of glutamatergic nerve terminals. J Neurochem. 2013;124(1):4–14. doi: 10.1111/j.1471-4159.2012.07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chung WS, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504(7480):394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung WS, et al. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci U S A. 2016;113(36):10186–91. doi: 10.1073/pnas.1609896113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaultier A, et al. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J Cell Sci. 2009;122(Pt 8):1155–62. doi: 10.1242/jcs.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang X, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9(6):816–23. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 81.Bezzi P, Volterra A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol. 2001;11(3):387–94. doi: 10.1016/s0959-4388(00)00223-3. [DOI] [PubMed] [Google Scholar]
- 82.Araque A, et al. Gliotransmitters travel in time and space. Neuron. 2014;81(4):728–39. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karner CM, et al. Lrp4 regulates initiation of ureteric budding and is crucial for kidney formation--a mouse model for Cenani-Lenz syndrome. PLoS One. 2010;5(4):e10418. doi: 10.1371/journal.pone.0010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson EB, et al. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum Mol Genet. 2005;14(22):3523–38. doi: 10.1093/hmg/ddi381. [DOI] [PubMed] [Google Scholar]
- 85.Johnson EB, et al. Defective splicing of Megf7/Lrp4, a regulator of distal limb development, in autosomal recessive mulefoot disease. Genomics. 2006;88(5):600–9. doi: 10.1016/j.ygeno.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, et al. LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani-Lenz syndrome. Am J Hum Genet. 2010;86(5):696–706. doi: 10.1016/j.ajhg.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Selcen D, et al. Impaired Synaptic Development, Maintenance, and Neuromuscular Transmission in LRP4-Related Myasthenia. JAMA Neurol. 2015;72(8):889–96. doi: 10.1001/jamaneurol.2015.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi HY, et al. APP interacts with LRP4 and agrin to coordinate the development of the neuromuscular junction in mice. Elife. 2013;2:e00220. doi: 10.7554/eLife.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim N, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135(2):334–42. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang B, et al. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60(2):285–97. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Drogemuller C, et al. Congenital syndactyly in cattle: four novel mutations in the low density lipoprotein receptor-related protein 4 gene (LRP4) BMC Genet. 2007;8:5. doi: 10.1186/1471-2156-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weatherbee SD, et al. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133(24):4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- 93.Gomez AM, et al. Synaptic plasticity and cognitive function are disrupted in the absence of Lrp4. Elife. 2014;3:e04287. doi: 10.7554/eLife.04287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pohlkamp T, et al. Lrp4 domains differentially regulate limb/brain development and synaptic plasticity. PLoS One. 2015;10(2):e0116701. doi: 10.1371/journal.pone.0116701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun XD, et al. Lrp4 in astrocytes modulates glutamatergic transmission. Nat Neurosci. 2016;19(8):1010–8. doi: 10.1038/nn.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Serrano A, et al. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26(20):5370–82. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu Y, et al. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia. 2012;60(4):559–69. doi: 10.1002/glia.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heppner FL, et al. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–72. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]