Abstract
A common clinical condition, acute kidney injury (AKI) significantly influences morbidity and mortality, particularly in critically ill patients. The pathophysiology of AKI is complex and involves multiple pathways including inflammation, autophagy, cell cycle progression, and oxidative stress. Recent evidence suggests that a single insult to the kidney significantly enhances the propensity to develop chronic kidney disease. Therefore, generation of effective therapies against AKI are timely. In this context, the cytoprotective effects of heme oxygenase 1 (HO-1) in animal models of AKI are well documented. HO-1 modulates oxidative stress, autophagy, and inflammation, and regulates the progression of cell cycle via direct and indirect mechanisms. These beneficial effects of HO-1 induction during AKI are, in part, mediated by the by-products of the HO reaction (iron, carbon monoxide, and bile pigments). This review highlights the recent advances in the molecular mechanisms of HO-1–mediated cytoprotection and discusses the translational potential of HO-1 induction in AKI.
Keywords: acute kidney injury (AKI), heme oxygenase 1 (HO-1), renal failure, translational research, cytoprotection, pathophysiology, oxidative stress, inflammation, cell cycle regulation, autophagic response, biomarker, HMOX1, review
Background
Acute kidney injury (AKI) is a common clinical condition, particularly in critically ill patients, that is associated with increased morbidity, mortality, and hospital cost.1–3 Given the diversity of insults, lack of a reliable early biomarker, and markedly heterogeneous nature of pathways involved in the pathophysiology of AKI, identification and implementation of novel therapeutic options (apart from conservative measures and kidney replacement therapy) has remained elusive. Heme oxygenase 1 (HO-1) is an inducible enzyme with potent anti-oxidant, anti-inflammatory, and anti-apoptotic attributes4–6 that was first recognized as a rapid and protective response in the context of rhabdomyolysis and heme pigment–induced AKI.7 Extensive evidence has demonstrated the beneficial effects of HO-1 to be mediated via the breakdown of heme, a vigorous pro- oxidant molecule, and the generation of protective products–namely, carbon monoxide (CO), biliverdin with the subsequent formation of bilirubin, and ferritin via iron release from the heme moiety.6 These findings have been reported in multiple animal models of AKI, including nephrotoxins, sepsis, kidney transplantation, and ischemia-reperfusion (IR)–mediated AKI among others, and also corroborated in human studies where HO-1 promoter polymorphism (leading to variable levels of HO-1 expression) is associated with several clinical conditions including AKI.8–13 Despite the overwhelming evidence that highlights the promising nature of HO-1 induction as a therapeutic target, the translational aspects of HO-1 application in human AKI settings are yet to be investigated. Such a limitation is based on multifaceted challenges, which will be discussed in this review along with potential solutions and recent advances in the field.
Case Vignette
A 42-year-old man with no major past medical history is brought to the hospital after work equipment fell on his legs at a construction site. Per reports, he was unable to extricate himself and only received medical attention three hours following the accident. On presentation, his vital signs were stable and his physical examination revealed multiple bruises on both lower extremities. He was oliguric, and imaging revealed no evidence of bone fractures. Laboratory evaluation found multiple biochemical abnormalities. Serum creatinine was 4.2 (reference range, 0.4–1.2) mg/dL (corresponding to an estimated glomerular filtration rate (eGFR) of 16 mL/min/1.73 m2 based on the MDRD [Modification of Diet in Renal Disease] Study equation) up from a previous creatinine measurement obtained during an annual evaluation of 1 mg/dL (eGFR, 82 mL/min/1.73 m2). Serum urea nitrogen was 56 (reference range, 5–22) mg/dL; potassium, 6.1 (reference range, 3.1–5.1) mMol/L; phosphorus, 8.3 (reference range, 2.4–5) mg/dL; calcium, 7.1 (reference range, 8.4–10.4) mg/dL; serum creatine kinase, 81,000 (reference range, 25–190) U/L; and troponin, 0.01 (reference range, 0.00–0.039) ng/mL. Urinalysis was notable for dark urine, protein (1+), and blood (3+) with no red blood cells. Further urine analysis revealed the presence of myoglobin. He was diagnosed with rhabdomyolysis-induced AKI, intravenous fluid administration was initiated, and he was admitted to the intensive care unit. Over the course of his hospital stay, his urine output improved, his electrolyte abnormalities resolved, and his serum creatinine decreased to 1.2 mg/dL (eGFR, 66 mL/min/1.73 m2).
Acute kidney injury from rhabdomyolysis was first described by Bywaters and Beall, who noted dark urine, reduction in urinary output, hyperkalemia, and ultimately death in victims of crush injuries at the time of the London blitz in World War II.14 Interestingly, the first evidence for a protective role for HO-1 was demonstrated in rhabdomyolysis-induced AKI by Nath and colleagues some fifty years later.7 The field has since grown exponentially and expanded to multiple other organ systems and disciplines. There are several excellent reviews that discuss the cytoprotective nature of HO-1 in kidney physiology and disease states. 15–19 The elegant review by Courtney and colleagues discussed the role of HO-1 in mediating protection during kidney diseases, with emphasis on transplantation.15 The focus of this current review is to highlight key aspects of the mechanisms by which HO-1 confers protection during AKI, and to discuss the most recent translational advances in this field.
Pathogenesis
Mechanisms of HO-1–Mediated Cytoprotection in AKI
Immunomodulation During AKI
The inflammatory response plays a key role in the pathogenesis and resolution of AKI. Exaggerated inflammation following an acute insult leads to increased severity of AKI, incomplete recovery, and increased propensity to progress to CKD. This unfettered immune response has been well documented in AKI following IR, rhabdomyolysis, and sepsis.20–24 Studies in animal models have identified a prominent role for both innate and adaptive immune mechanisms. Of these, innate immune cells, such as macrophages, dendritic cells (DC), and neutrophils are the primary responders to the acute insult and continue to remain on the forefront of research in the AKI field. Interestingly, studies demonstrate an important immunomodulatory role for HO-1 in these cells.25–28 Global HO-1 knockout mice and HO-1–deficient humans demonstrate increased leukocytosis, erythrophagocytosis, hepatosplenomegaly, and renal tubulointerstitial injury with inflammatory cell infiltration and fibrosis, underscoring the anti-inflammatory actions of HO-1.25, 29–31 Global HO-1–deficient mice also express high levels of circulatory monocyte chemoattractant protein 1 (MCP-1) in the quiescent state and following AKI (Table 1).32 Additionally, macrophages that express HO-1 polarize towards the anti-inflammatory phenotype, secrete anti-inflammatory cytokines (eg. IL-10), and express reparative genes that are critical for tissue recovery after AKI.28, 33 Additionally, the beneficial effects of IL-10 expression are dependent on HO-1 expression and activity.34, 35 Following IR, macrophages that accumulate in the injured kidney of HO-1–deficient mice express significantly higher levels of the cytokine IL-6, while expressing low levels of the anti-inflammatory cytokine IL-10.33, 36 These findings were recapitulated using an animal model of unilateral ureteral obstruction, highlighting the salutary effect of HO-1 in the immune responses during AKI.37, 38
TABLE 1.
The effects of manipulation of HO-1 or its by-products during AKI
| Manipulation | Phenotype | Model | Effect | Ref |
|---|---|---|---|---|
| HO-1 knockout | Global HO-1 deficiency | Mouse kidney IR | Increased MCP-1, IL-6; reduced IL-10 | 32, 33 |
| HO-1 knockout | Global HO-1 deficiency | Mouse unilateral ureteral obstruction | Increased MCP-1, IL-6; reduced IL-10 | 37 |
| Adenovirus-mediated transduction of kidney with HO-1 expression vector | Kidney-specific HO-1 overexpression | Rat kidney IR | Preserved kidney function | 11 |
| HO-1 knockout | Global HO-1 deficiency | Mouse cisplatin nephrotoxicity | Worse kidney structural & functional injury | 9, 72 |
| HO-1 overexpression | Global HO-1 overexpression | Mouse cisplatin nephrotoxicity, rhabdomyolysis | Preserved kidney structure & function | 77 |
| HO-1 knockout in PTCs | PTC-specific HO-1 deficiency | Mouse cisplatin nephrotoxicity | Worse kidney structural & functional injury | 76 |
| HO-1 overexpression in PTCs | PTC-specific HO-1 overexpression | Mouse cisplatin nephrotoxicity | Preserved kidney structure & function | 76 |
| Adenovirus-mediated transduction of BMDM with HO-1 expression vector | BMDM-specific HO-1 overexpression | LPS/IFN injury | Increased IL-10-1; reduced TNF-α, NO | 28 |
| HO-1 knockout in myeloid cells | Myeloid cell–specific HO-1 deficiency | Mouse kidney IR | Egress of kidney dendritic cells to lymph node for antigen presentation | 33 |
| GFP-positive, HO-1 knockout mice | Global HO-1 deficiency and GFP expression | Mouse syngeneic kidney transplant | Egress of donor kidney dendritic cells to lymph node for antigen presentation | 33 |
| HO-1-overexpressing HEK293 | HO-1 overexpression | Human renal epithelial cells | Inhibits ROS generation, autophagy, & apoptosis; promotes cell survival during oxidative stress | 72 |
| Mitochondria-targeted HO-1 expression vector in PTCs from HO-1 knockout mice | Mitochondria-specific overexpression of human HO-1 in murine HO-1-deficient PTCs | Mouse PTCs: hypoxia | Inhibits apoptosis & promotes cell survival during hypoxic stress | 81 |
| Mitochondria-targeted HO-1 expression vector | Mitochondria-specific HO-1 overexpression | Human renal epithelial cells | Inhibits ROS generation & apoptosis following hypoxia & oxidative stress | 81 |
| Gunn rat | Increased bilirubin | Rat hyperoxia | Increased resistance to free radical injury | 89 |
| Acute cholestatic liver disease | Increased bilirubin | Rat rhabdomyolysis | Protects against rhabdomyolysis injury | 88 |
Abbreviations: ischemia reperfusion, IR; monocyte chemoattractant protein 1, MCP-1; interleukin, IL; bone marrow–derived macrophages, BMDM; tumor necrosis factor, TNF; nitric oxide, NO; green fluorescent protein, GFP; reactive oxygen species, ROS; Ref, reference; proximal tubular cell, PTC; HO-1, heme oxygenase 1; IFN, interferon; LPS, lipopolysaccharide
The actions of HO-1 are not confined to the regulation of cellular gene expression and maturation of immune cells (eg. DC), but also extend to their ability to migrate.33, 39–41 Previous studies have demonstrated that following AKI, global HO-1 deficiency leads to increased accumulation of macrophages in the injured kidney.37 A recent study by Hull et al demonstrated that deletion of HO-1 in myeloid cells (macrophages, neutrophils, and dendritic cells) facilitates the egress of these cells from the injured kidney to peripheral lymphoid tissue, presumably for antigen presentation and amplification of the immune response.33 They confirmed these findings using multiple models, including a syngeneic kidney transplantation utilizing fluorescent protein–labeled HO-1–deficient donor kidneys into a wild-type mouse, and bilateral IR in a transgenic mouse with specific deletion of HO-1 in the myeloid cells. This study provided evidence to suggest that manipulation of HO-1 expression in the myeloid cells may be an exciting avenue to minimize the immune response following IR and transplant-associated AKI. Interestingly, CO was also able to exert this inhibitory effect on the migration of DC.41, 42 Additionally, HO-1 expression in antigen-presenting cells such as DC is required for optimal regulatory T cell function, which has been shown to facilitate recovery following AKI.26, 43
It should be noted that while the aforementioned studies utilized genetic manipulation of HO-1, some of these findings were also recapitulated using by-products of the HO reaction. For instance, CO inhibits T cell proliferation via downregulation of IL-2 and caspase activity, thereby dampening inflammation.44 In addition, numerous studies using chemical and pharmacologic modulators of HO-1 expression and/or activity have confirmed these immunoregulatory effects of HO-1 during AKI (Table 2).4, 26, 45–50
TABLE 2.
Agents reported to be protective during AKI through HO-1 or its by-products
| Agent | Property | Model | Effect | Ref |
|---|---|---|---|---|
| Methylene chloride | Induction of CO | Rat allogeneic kidney transplant | Reduced anti-donor immunogenicity | 41, 42 |
| Cobalt protoporphyrin | Induction of HO-1 | Rat allogeneic kidney transplant | Inhibition of DC migration and anti-donor immunogenicity | 41 |
| Methylene chloride | Induction of CO | Rat allogeneic kidney transplant | Decrease in DCs, alloreactive and CD4+ T cells and chronic allograft dysfunction | 42 |
| CO | 250 ppm CO | T Lymphocytes | Inhibits T cell proliferation | 44 |
| Cerivastatin* | Induction of HO-1 | Rat renal IR | Reduced kidney injury and dysfunction | 45 |
| Capsaicin* | Induction of HO-1 | Mouse cisplatin nephrotoxicity | Inhibits oxidative stress, inflammation, and kidney injury | 46 |
| Adiponectin | Induction of HO-1 | Mouse renal IR | Inhibits apoptosis, inflammation and kidney injury | 47 |
| Hepatocyte growth* factor | Induction of HO-1 | Mouse endotoxemia | Prevention of acute kidney failure | 48 |
| Hydrogen gas* | Induction of HO-1 | Mouse Sepsis (CLP) | Promotes survival | 49 |
| Bardoxolone methyl | Induction of HO-1 | Mouse IR | Reduced structural and functional kidney injury | 147 |
| Bardoxolone methyl | Induction of HO-1 | Mouse aristolochic acid nephropathy | Reduced structural and functional kidney injury | 148 |
| Epigallocatechin-3- gallate* | Induction of HO-1 | Rat contrast-induced nephropathy | Inhibits oxidative stress and inflammation | 149 |
| Epigallocatechin-3- gallate | Induction of HO-1 | Mouse unilateral ureteral obstruction | Inhibits oxidative stress and inflammation | 150 |
| IL-10* | Induction of HO-1 | Mouse LPS-induced septic shock | Anti-inflammatory effect | 35 |
| Heme, iron, CO* | Induction of HO-1 | TNF-α/cycloheximide, staurosporine, serum deprivation | Increased sensitivity to apoptosis in the absence of HO-1 and p21 | 58 |
| Myoglobin* | Induction of HO-1, p21 | Human renal epithelial cells | Promotes cell cycle arrest | 60 |
| Hemin* | Induction of HO-1 | Mouse renal epithelial cells | Induces p18 and protects against cisplatin injury | 64 |
| Rapamycin | Induction of HO-1 | Human renal cancer cells | Inhibits autophagy and apoptosis and promotes cell survival | 73 |
| Sorafenib | Induction of HO-1 | Human renal cancer cells | Inhibits autophagy and apoptosis | 73 |
| Hemoglobin | Induction of HO-1 | Rat rhabdomyolysis | Induces HO-1 and protects against rhabdomyolysis | 7 |
| Hemin | Induction of HO-1 | Rat renal IR | Induces HO-1 and protects against IR | 82 |
| Erythropoietin* | Induction of HO-1 | Rat chronic tubulointerstitial injury (salt sensitive Dahl rat model) | Induces HO-1 and protects against oxidative stress | 151 |
| NGAL | Induction of HO-1 | Mouse renal IR | Inhibits azotemia and protects against injury | 152 |
| Bilirubin | Increased bilirubin | Rat kidney IR | Improved vascular resistance, tubular function, mitochondrial integrity | 90 |
| Carbon monoxide | Increased CO | Pig cardiopulmonary bypass-AKI | Reduced kidney injury and dysfunction | 94 |
| CORM-2 | Increased CO | Rat sepsis induced AKI | Inhibits oxidative stress, inflammation and kidney injury | 96 |
| Bilirubin | Increased bilirubin | Rat endotoxin-mediated toxicity | Inhibits NADPH Oxidase and NOS2 expression and prevents mortality | 86 |
Abbreviations: lipopolysaccharide, LPS; dendritic cells, DC; carbon monoxide, CO; ischemia reperfusion, IR; cecal ligation puncture, CLP; carbon monoxide–releasing molecule 2, CORM-2; HO-1, heme oxygenase 1; NADPH, reduced nicotinamide adenine dinucleotide phosphate; Ref, reference
reversal/rescue of effect with inhibitors of heme oxygenase activity or supplementation of heme oxygenase by-products.
The constitutive isoform of the enzyme, HO-2, also plays an important role in immunomodulation. Global HO-2 deficiency leads to a dysregulated inflammatory response and subsequently impairs the reparative response following injury.51, 52 Recent studies have demonstrated that deletion of HO-2 in macrophages significantly impedes their phagocytic ability and promotes expression of inflammatory genes, while downregulating the expression of anti-inflammatory markers leading to diminished wound healing.53 Interestingly, these deleterious effects were reversed by supplementation of biliverdin.53, 54 These results highlight the importance of HO enzyme activity in the modulation of immune responses following injury.
Cell Cycle Regulation
The inherent capacity of the injured kidney to recover from an acute insult is vital for maintaining homeostasis. The key mechanisms that drive the reparative process include upregulation of anti-oxidant defense systems, inhibition of oxidative stress and associated apoptosis, and finally regeneration of the injured tubules. The latter process is finely orchestrated by a group of proteins having roles in cell cycle regulation, namely cyclins and cyclin-dependent kinases (CDKs). The cell cycle is a meticulously controlled process that occurs under both physiologic and pathophysiologic conditions. It consists of a cyclic pathway of specific phases: G1 (gap 1), S (DNA synthesis), G2 (gap 2), and M (mitosis). The progression of each phase into the next is tightly regulated by the expression of specific cyclins and CDKs, ultimately determining cell cycle arrest or proliferation. Interaction of a cyclin with its respective CDK leads to phosphorylation of target proteins, which in turn initiates or restricts progression into the next phase of the cell cycle. This complex system is further controlled by the expression of CDK inhibitors, INK4a/ARF (inhibitor of kinase 4/alternative reading frame), and the Cip/Kip (interacting protein/kinase inhibitory protein) family of proteins.
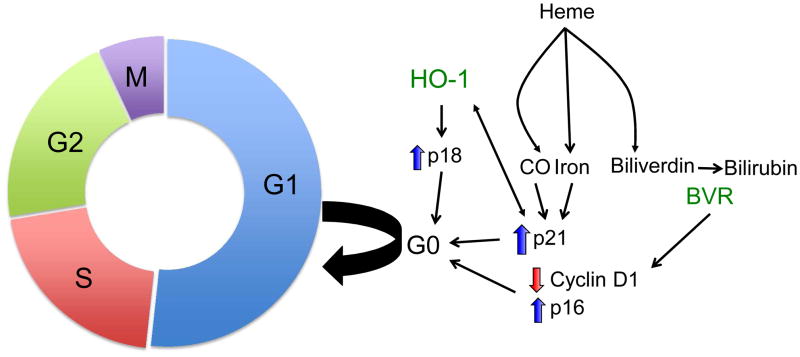
During AKI, seminal studies have delineated a critical role for these proteins in mitigating injury. In this connection, Megyesi et al demonstrated that during cisplatin-induced AKI, expression of p21 (a member of Cip/Kip family) was significantly upregulated in the kidney, associated with an inhibition of cell cycle progression, and subsequently led to marked reduction in injury during ischemia reperfusion and cisplatin nephrotoxicity.55, 56 Following these studies, Nath and colleagues were the first to identify a direct regulatory effect of p21 expression by HO-1 in the kidney .57 They demonstrated a beneficial effect of HO-1 and p21 expression in tubular injury mediated by heme, TNF- α, and serum deprivation.57, 58 Studies utilizing transgenic mice deficient in p21 or HO-1 confirmed a protective role for these proteins in IR- and cisplatin-induced AKI.4, 55, 56, 59 In the quiescent state, tubular cells constitutively express low levels of p21, which is rapidly co-induced with HO-1 upon injury in vitro.57 In cells that constitutively overexpress HO-1, basal p21 expression was reported to be elevated and associated with diminished hyperplastic growth and cell cycle arrest in the G0/G1 phase of the cell cycle. Further work demonstrated that p21 induction is dependent on HO activity, in that inhibition of HO activity was observed to lead to marked reduction in p21 expression and increased apoptosis following injury.57 These findings suggest that the by-products of the HO reaction may contribute to regulation of p21 (Figure 1). In fact, studies demonstrate an inducible effect of iron on p21 expression, which is abolished in the presence of an iron chelator, deferoxamine.58 In addition, CO is also capable of eliciting a similar response.58 Another heme-iron–containing protein, myoglobin, also promotes cell cycle arrest, an effect that reversed in the presence of deferoxamine.60 Recent evidence suggests that HO-1–mediated p21 regulation is not only confined to tubular epithelial cells, but also extends to mesangial cells in the kidney.61
Figure 1.
Heme oxygenase-1 (HO-1) modulates cell cycle progression. HO-1 upregulates the expression of cell cycle regulators p21 and p18, and arrests cell cycle progression from G1 to S phase. By-products of the HO reaction, CO, and iron also mediate arrest via p21. Biliverdin reductase expression leads to cellular senescence via regulation of p16 and cyclin D1.
Interestingly, biliverdin reductase A (BVRA), a key enzyme in the heme oxygenase system, also mediates a dominant role in cell cycle regulation. Kim et al demonstrated that BVRA knockdown leads to marked reduction in the expression of cyclin D1 and phosphorylated pRb, whereas it increases the expression of p16 (member of INK4 family), leading to premature cellular senescence.62 Another inhibitor of the INK4 family of proteins, p18, which arrests the cell cycle at the G1 phase, was shown to be protective during cisplatin nephrotoxicity.63 Recent evidence also suggests that p18 expression is regulated by HO-1 expression, in that HO-1 upregulates p18 expression while inhibition of HO activity was found to ablate such induction during cisplatin injury.64
Taken together, HO-1–mediated cell cycle regulation plays a pivotal role in tubular injury and repair during AKI. A recent study by Kashani and colleagues identified two novel urinary biomarkers (insulin-like growth factor-binding protein 7 [IGFBP7] and tissue inhibitor of metalloproteinases 2 [TIMP2]) for AKI using a multicenter observational study involving large cohort of critically ill patients (about 700).65 Interestingly, both of these markers are potent inducers of G1 cell cycle arrest, underscoring the mechanistic role of cell cycle regulation in the pathogenesis of AKI.65 Additionally, studies have identified a significant role for maladaptive repair in the induction and progression of CKD. Given the increasing evidence for the convergence of AKI to CKD, regulation of cell cycle provides an interesting avenue for the generation of effective therapeutics for AKI and the prevention of CKD as reported from the Bonventre laboratory.66
HO-1 Regulates Autophagic Response During AKI
Autophagy refers to an evolutionarily conserved and physiologically regulated intracellular degradation process by which cytoplasmic components (eg, damaged organelles, protein aggregates, and other macromolecules) are sent to the lysosome for breakdown and ultimate disposal.67, 68 Recent data suggests that autophagy is induced in several animal models of AKI, including IR, nephrotoxin and immunosuppressive agent-mediated injury69, 70. Additionally, the presence of autophagosomes in the transplanted human kidney underscores the relevance of this pathway in injury-mediated responses.71 Increasing evidence suggests that in quiescent cells, constitutively activated autophagy serves as a physiologic homeostatic process. However, under stress conditions such as AKI, autophagy induction may promote survival or converge into apoptotic cell death depending on the extent of injury and activation of pro-survival pathways.69 Interestingly, evidence gathered over the past decade identifies HO-1 as a potent regulator of autophagy.72–74 Proximal tubules deficient in HO-1 display elevated basal autophagy, as evident by increased accumulation of autophagic vesicles and expression of light chain 3 (LC3)-II and beclin.72 This increased activity is presumably to maintain cellular homeostasis and is reflective of an oxidative environment (increased levels of heme, oxidized proteins, and lipid peroxidation) in the absence of HO-1. Following cisplatin administration, absence of HO-1 expression is linked with failed autophagy induction and converges into increased apoptosis, underscoring the effect of cumulative stress in the interplay between autophagy and apoptosis. On the other hand, HO-1 overexpression in kidney epithelial cells during cisplatin nephrotoxicity delays the autophagic response and concomitantly inhibits apoptosis.72 Whether this delayed response is attributed to decreased generation of reactive oxygen species (ROS) and heme content is not known. Another mechanism by which HO-1 regulates autophagy is through inhibition of beclin expression, a key protein responsible for autophagy initiation.73, 74 These studies suggest that the cellular fate, as determined by the competence or perturbation of autophagy, is an HO-1 mediated process, and targeting the HO system may provide effective strategies against AKI.
Oxidative Stress and HO-1
Irrespective of the etiology of injury, oxidative stress is a common denominator in the AKI pathogenesis. Oxidative stress results when the cellular anti-oxidant machinery is exhausted by an overwhelming imbalance in the accumulation of oxidants, such as ROS. These reactive species contain an oxygen atom with an unpaired valence electron, which quickly interacts with various functional groups and propagates a vicious cycle of free radical generation, ultimately culminating in amplified cellular oxidative stress and death. Nath and colleagues provided the first evidence for the cytoprotective role of HO-1 in vivo during AKI.7 This elegant study demonstrated for the first time that following glycerol induced rhabdomyolysis (a model in which oxidative stress is a predominant pathogenic mechanism), HO-1 is quickly induced in the kidney, and prior induction of HO-1 with hemoglobin ameliorates injury. Furthermore, this functional protection is abrogated in the presence of tin protoporphyrin, an HO enzyme activity inhibitor, underscoring the HO-1 mediated beneficial response to oxidant injury.7 This study propelled investigators to determine protective mechanisms modulated by HO-1 during oxidant injury in other organ systems as well. Indeed, it is clear now from multiple studies that oxidative stress induces HO-1, and such induction mediates protection during AKI in animal models.
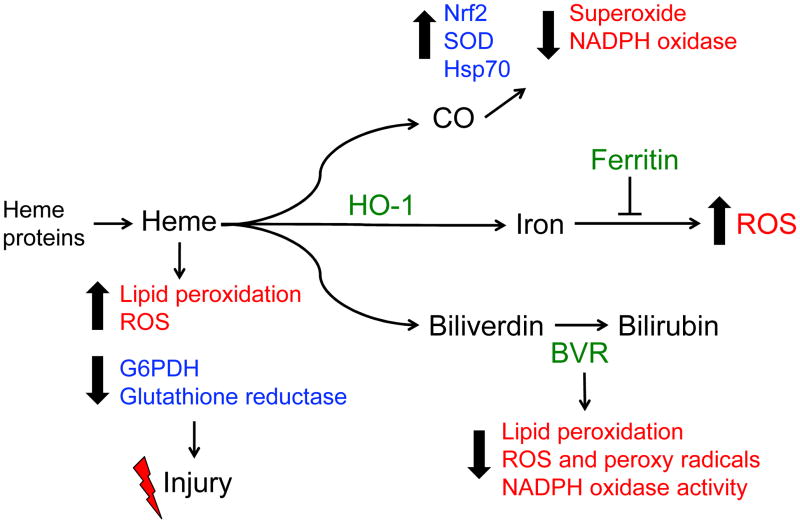
The salutary beneficial effect of HO-1 expression during oxidative stress is twofold. First, HO-1 catalyzes the breakdown of heme, a potent pro-oxidant and a noxious stimulus that amplifies oxidative insult in several models of injury. Heme is an integral functional component of several proteins, intracellular and extracellular, that are involved in cellular homeostasis. Cellular heme levels are maintained at 100 nM through two opposing processes, namely, synthesis and degradation. While heme synthesis is regulated by aminolevulinic acid (ALA) synthase activity, the enzymatic degradation of heme is managed by HO enzymes. During injury (ischemic or nephrotoxic), destabilization of heme proteins, which are ubiquitous in cells, leads to a significant increase in free heme. Heme is lipophilic and freely permeates the lipid membranes (plasma and organellar), causing oxidization of lipids and accentuating oxidative stress (Figure 2). Furthermore, heme not only stimulates the generation of hydrogen peroxide in tubular epithelial cells but also amplifies oxidative stress through interaction with hydrogen and lipid peroxides to form potent pro-oxidant ferryl forms of heme.75 Animal models using global or kidney specific HO-1-deficient mice confirm the damaging role of heme in kidney injury.59, 76, 77 Moreover, patients with rhabdomyolysis and acute intermittent porphyria who use hematin develop severe AKI, supporting the clinical relevance of heme burden and injury.78, 79 Furthermore, genetic HO-1 deficiency in humans results in significantly higher levels of heme in the plasma and renal tubulointerstitial injury.29, 79, 80
Figure 2.
HO-1 inhibits oxidative stress. HO-1 degrades pro-oxidant heme and generates potent anti-oxidant molecules, bile pigments, and carbon monoxide (CO). Heme amplifies oxidative stress by inducing lipid peroxidation and ROS generation, while inhibiting the activity of glutathione reductase and glucose-6-phosphate dehydrogenase (G6PDH). CO and bile pigments decrease oxidative stress via inhibiting NADPH Oxidase and sequestering ROS. CO also upregulates the anti-oxidant machinery, including superoxide dismutase (SOD), heat shock protein 70 (Hsp70), and activation of Nrf2. Iron released from the reaction is safely sequestered by ferritin, and thereby mitigates ROS generation.
While heme is required for the optimal function of metabolic enzymes such as cytochrome c oxidase and NOS, free heme also impairs the activity of several enzymes including glucose-6-phosphate dehydrogenase and glutathione reductase.79 Mitochondrial-targeting of HO-1 in kidney epithelial cells leads to decreased expression of multiple subunits of cytochrome c oxidase without altering basal mitochondrial function.81 Additionally, these cells demonstrate significant protection against hypoxic and oxidative insults, as evident by reduced ROS generation and apoptosis.81 Interestingly, a few studies demonstrate a protective role for low-dose heme infusion during IR-induced injury and attribute these effects to the induction of HO-1.82
The second salutary effect of HO activity is the generation of by-products that possess anti-oxidant and anti-apoptotic properties. The bile pigments, bilirubin and bilverdin, scavenge free radicals such as peroxynitrite, inhibit lipid peroxidation, and, thereby, mitigate oxidative stress.83, 84 Additionally, the cyclic interconversion of biliverdin to bilirubin mitigates oxidative stress through sequestration of hydrogen peroxide during the reaction.84, 85 Bilirubin also suppresses the activity of NADPH (educed nicotinamide adenine dinucleotide phosphate) oxidase, a major source of ROS during oxidative insults.86, 87 Interestingly, ligation of the bile duct during glycerol-induced rhabdomyolysis leads to mitigation of AKI, suggesting a beneficial role for bilirubin during injury.88 Furthermore, neonatal Gunn rats demonstrate increased resistance to free radical injury, an effect that is attributed to increased bilirubin.89 Additional studies have demonstrated that exogenous bilirubin supplementation provides marked protection against oxidative stress during AKI, validating the anti-oxidant effect of this metabolite.90, 91 Another by-product of the HO reaction is CO, a powerful anti-apoptotic and anti-inflammatory molecule (Figure 2). Endogenous CO production is exclusively mediated by the HO enzymes. However, exogenous supplementation is achieved through inhalation of CO (usually at 250 ppm) or by treatment with CO releasing molecules (CORMs). Studies using both these modes of CO supplementation in vivo have demonstrated that CO is capable of mitigating oxidative stress through direct and indirect mechanisms. It has to be noted, however, that supra-therapeutic levels of CO may also amplify ROS generation through its interaction with mitochondrial proteins such as cytochrome c oxidase, which hinders its potential as a therapeutic intervention.
In summary, research over the past few decades has provided indisputable evidence of HO-1 as a beneficial anti-oxidant during AKI. Mechanistically, HO-1 converts a toxic oxidative microenvironment to an anti-oxidant and reparative milieu that facilitates recovery from injury. Therefore, current research efforts must be directed toward translating these pre-clinical studies to generate effective therapies aimed at inducing this cytoprotective enzymatic system in humans with AKI.
Recent Advances
HO-1: The Murine Versus Human Paradox
Introduction of HO-1−/− mice transformed this field of research by providing a valuable tool for investigating various aspects of HO-1 gene regulation and its role in injury settings.30 Several pathologic findings obtained in these transgenic mice have been corroborated in patients with human HO-1 deficiency.101, 102 Despite such remarkable advances in this field, certain limitations to fully explore the potential of HO-1 as a therapeutic target still remain.
First, the global deletion of HO-1 did not allow for meticulous examination of different cell types and dissecting their sequential and temporal effects following injury. To overcome this limitation, and by taking advantage of Cre-Lox site-specific recombinase technology, mouse models have been developed that are deficient or overexpress HO-1 in proximal tubular cells (most susceptible to different forms of insults) or myeloid cells.76, 103 These novel animal models would allow for a more in-depth analysis of the mechanisms that are involved with cellular injury, inflammation, and repair in AKI as it relates to HO-1. Furthermore, they will provide valuable information on the role of cell-specific HO-1 expression in cross-talk of tubular and inflammatory cells, and the contribution of each cell type during injury and repair.
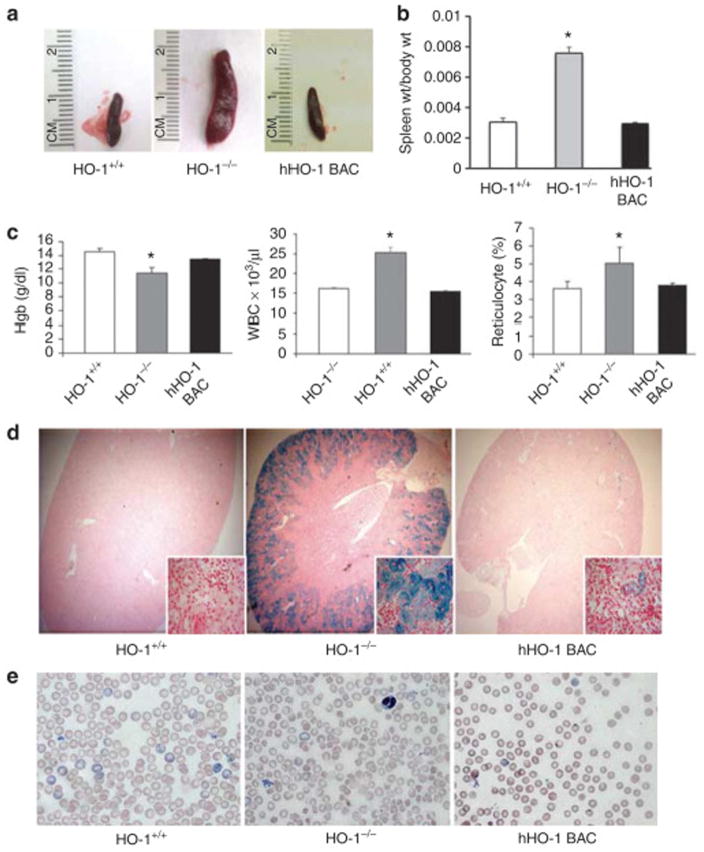
Second, HO-1 gene expression is regulated differently in mice and humans104, 105. These dissimilarities include contrasting responses to various stimuli such as hypoxia, heat shock, hyperosmolarity, and cytokines such as interferon γ . In addition, the molecular mechanisms of human HO-1 gene regulation by an intronic enhancer that facilitates HO-1 gene expression via chromatin looping have not been shown in the rodent HO-1 genes.104, 105 These limitations prompted generation of a novel “humanized” transgenic mouse model, consisting of the human HO-1 gene in addition to its regulatory regions on a HO-1−/− background.77 This study confirmed rescue of the pathologic phenotypes observed in HO-1−/− mice via functional presence of the human HO-1 gene (Figure 3). These mice should serve as an important tool to study the mechanisms of human HO-1 gene regulation in vivo and should enable identification of novel therapeutic agents to target HO-1 expression in AKI.
Figure 3.
Human heme oxygenase-1 (HO-1) in hHO-1 bacterial artificial chromosome (BAC) transgenic mice rescues the pathologic phenotype of HO-1−/− mice. Spleens from 25- to 35-week-old HO-1+/+, HO-1−/−, and hHO-1 BAC transgenic mice were compared for their (a) lengths and (b) weights (wt). Weights of spleen were normalized to body weight of each animal, and average normalized values were plotted for each group (mean±standard error of the mean). *P<0.05 vs. HO-1+/+ and hHO-1 BAC mice. (c) Whole blood from 30- to 40-week-old HO-1+/+, HO-1−/−, and hHO-1 BAC mice were analyzed for hemoglobin (Hgb), leukocyte (white blood cell (WBC)), and reticulocyte counts. *P<0.05 vs. HO-1+/+ and hHO-1 BAC mice. (d) Prussian blue staining was performed on kidneys from 25- to 35-week-old HO-1+/+, HO-1−/−, and hHO-1 BAC mice to detect tissue iron. (e) red blood cell (RBC) morphology was determined by Wright-Giemsa staining on peripheral blood smears from HO-1+/+, HO-1−/−, and hHO-1 BAC mice. Reproduced from Kim et al.77 with permission of the International Society of Nephrology.
Micro-RNA and HO-1
The discovery of micro-RNAs (miRNAs) in the past two decades has led to an exciting new field of investigation.106, 107 These miRNAs are small, non-coding RNA molecules that are involved in gene silencing and post-transcriptional regulation of gene expression.108 They have been shown to have essential roles in many forms of kidney diseases 109, 110. Also, miRNAs may regulate HO-1 gene expression. This notion has been confirmed in different cell lines including podocytes, renal proximal tubular cells, and endothelial cells, among others.111–117 In contrast, there is also evidence that HO-1 expression modulates certain miRNAs.118, 119 Such reciprocal interactions and regulatory involvements between HO-1 and miRNAs provides an innovative platform for further investigation that could lead to identification of novel pathways and potential therapeutic targets in different clinical settings, including AKI and other kidney diseases.
HO-1 Gene Polymorphisms
Translational efforts to study effects of HO-1 expression in human diseases have identified polymorphisms in the 5’ flanking region of the HO-1 gene120. These include a (GT)n dinucleotide length polymorphism and two single-nucleotide polymorphisms (SNPs), G(-1135)A and T(-413)A.120 Among these the (GT)n polymorphism in the promoter region has been extensively studied in different clinical conditions120–123. Shorter (GT)n repeats (n < 27) are associated with greater HO-1 expression and confer protection against many diseases.124–126 Within the context of kidney diseases, HO-1 promoter polymorphism is associated with IgA nephropathy, transplant rejection, patency of arteriovenous fistula, and progression of CKD.127–132 More recently, a study examined such polymorphism and its relationship with development of AKI in patients undergoing cardiac surgery. Importantly, the authors found that longer (GT)n repeat (associated with lower HO-1 expression) in these patients led to increased risk of AKI following cardiac surgery.13 It should be noted that some studies did not find any significant association between the HO-1 promoter polymorphism and disease development or progression.133–135 However, more elaborate recent meta-analysess were performed to address this issue, and after careful review of the literature and accounting for various methods of randomization, the authors have corroborated the protective effects of HO-1 promoter polymorphisms.121, 122 Furthermore, these studies identified potential confounding factors, such as age, race, gender and preexisting conditions/risk factors, that need to be accounted for during design and interpretation of these studies. In this age of precision medicine, taking these factors into consideration would potentially enable a more personalized approach to patients.
Iron and AKI
Iron is an absolute requirement for life, but can also pose deleterious effects via participation in the Fenton reaction and generation of ROS. Recently, the role of the kidney in iron handling and metabolism has gained significant attention.136, 137 Based on experimental and human studies, iron is increasingly recognized to be a major culprit in AKI.138–140 To study iron trafficking and its role in AKI, we recently generated a novel transgenic mouse model with conditional deletion of heavy chain ferritin (FtH) in renal proximal tubules.100 Interestingly, these mice exhibit significantly higher levels of HO-1 expression both under basal and injury conditions. However, despite such significantly higher levels of HO-1, FtH deletion has been associated with worse kidney function and morphologic changes in two different models of AKI.100 These results underscore the crucial significance of FtH co-expression during HO-1 induction to safely sequester the released iron from the breakdown of heme and prevent its participation in the Fenton reaction. Clinical studies have further corroborated these findings. Recently, Leaf et al. conducted a prospective cohort of 250 patients undergoing cardiac surgery and found a direct relationship between plasma catalytic iron levels and higher likelihood of AKI, hospital mortality, and postoperative myocardial injury.139 While results from clinical trials using iron chelators in AKI have not been reported, targeting FtH may represent a novel strategy to sequester iron during AKI.
HO-1 as a Biomarker of AKI
The traditional reliance of clinicians on serum urea nitrogen and/or serum creatinine, as well as urine output, to diagnose AKI has been a major obstacle to the early recognition of this condition as well as potential implementation of therapeutic modalities in a timely manner. Therefore, identification of novel biomarkers in AKI has been a foremost priority in recent years. Several candidates have been identified and many are being evaluated as “point of care” testing in AKI (eg. IGFBP7, TIMP2).141–143 In this regard, given the localization of the HO-1 enzyme to the endoplasmic reticulum and lack of an identified secretory pathway, the probable role of HO-1 as a biomarker has only recently been examined. Zager and colleagues invesigated urinary and plasma levels of HO-1 in four models of AKI that included ischemia/reperfusion, glycerol-induced rhabdomyolysis, cisplatin nephrotoxicity, and bilateral ureteral obstruction.144 Interestingly, following AKI, induction of renal HO-1 was found to be accompanied with elevation of its levels in both serum and urine. Moreover, urinary and plasma levels of HO-1 were seen to be significantly higher in ten patients with AKI when compared to ten critically ill patients without AKI and twenty patients with CKD, including ESRD.144 Such potential to utilize HO-1 as a biomarker in kidney disease is also corroborated by other investigators. For instance, a recent study reported higher levels of urinary HO-1 in patients with type 2 diabetes. Intriguingly, the increment in urinary HO-1 levels in these patients precedes significant proteinuria and also inversely correlates with glomerular filtration rate.145 Another study found increased levels of plasma HO-1 in patients who developed AKI following cardiopulmonary bypass; this is associated with duration of the bypass, hemolysis, and inflammation.146 Given the overwhelming reliance on serum creatinine, urine output, and degree of proteinuria to monitor kidney function, the aforementioned findings of plasma and urinary HO-1 are timely and add to our armamentarium of biomarkers of AKI. However, it must also be noted that utilization of HO-1 as a novel biomarker of AKI requires additional investigation. Two major concerns that require in-depth analysis are related to the following. First, the origin of the observed HO-1 in serum and urine following AKI is interesting, as it is known to be localized to the endoplasmic reticulum; this localization raises the question as to whether the observed HO-1 in serum and urine is a mere reflection of cellular damage and release of intracellular proteins, or if it involves a more active cellular secretion pathway. Second, as shown by Zager and colleagues, the immunoreactive HO-1 in both urine and serum is present as a 16 kDa protein that is likely reflective of a cleavage of the two approximately equal-sized bound helices of HO-1. 144 Further studies are required to validate the functionality of this protein, its precise amino acid sequence, and mechanism(s) leading to such fragmentation.
Summary
The incidence of AKI is growing at an alarming rate and its role in the development of CKD is increasingly recognized, making identification and implementation of novel therapeutics an exigent challenge. The presented case vignette focuses on rhabdomyolysis-induced AKI, and based on seminal findings reported by Nath and colleagues, it is evident that HO-1 is robustly induced in the kidney during rhabdomyolysis.7 The significance of such induction was elegantly underscored by pre-induction of HO-1 being shown to improve kidney function and survival, and conversely chemical inhibition of HO being obeserved to lead to exacerbation of AKI. These pivotal findings have been corroborated in different animal models of AKI, and more recent human studies have validated these findings.13 As discussed in this review, HO-1 mediates cytoprotection during AKI through regulation of several different pathways (Box 1). Future studies should aim to utilize the aforementioned transgenic animals to discover novel therapeutics to induce HO-1 in a timely manner to prevent and/or treat AKI. Recognition of early biomarkers will be a valuable asset to expedite these studies. Furthermore, human studies ought to expand our knowledge regarding HO-1 gene polymorphisms in different AKI settings. Knowledge gained from these studies could be implemented to identify patients at higher risk, paving the way towards a more “personalized medicine” approach. There are a number of ongoing clinical trials targeting the HO-1 pathway in the kidney, heart, and other organ systems, or in various phases of completion (ClinicalTrials.gov study numbers NCT01430156, NCT00483587, NCT02142699, and NCT00531856). A broader understanding of how endogenous adaptive responses such as HO-1 can be exploited as an approach to developing new therapeutic strategies in the setting of AKI would allow us to translate findings in the laboratory to the clinic.
BOX 1. Regulatory actions of HO-1 during AKI.
| Property |
|---|
| Apoptosis |
| Autophagy |
| Inflammation |
| Oxidative stress |
| Regulation of miRNA |
| Biomarker function; Endothelial cell integrity |
| Immune cell trafficking; Regulation of cell cycle |
HO-1, heme oxygenase 1; miRNA, microRNA
Acknowledgments
Support: This work was supported by National Institutes of Health grant R01 DK59600, the core resource of the University of Alabama at Birmingham–University of California San Diego O’Brien Center P30 DK079337, and Department of Veterans Affairs grant IP1-BX001595 (all to Dr Agarwal), as well as K01 DK103931 (to Dr Bolisetty) and an American Society of Nephrology Ben Lipps Fellowship grant (to Dr Zarjou).
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Peer Review: Evaluated by 2 external peer reviewers, Feature Editor Rosner, Education Editor Gilbert, and Editor-in-Chief Levey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zarjou A, Sanders PW, Mehta RL, Agarwal A. Enabling innovative translational research in acute kidney injury. Clin Transl Sci. 2012;5(1):93–101. doi: 10.1111/j.1752-8062.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaushal GP, Shah SV. Challenges and advances in the treatment of AKI. J Am Soc Nephrol. 2014;25(5):877–883. doi: 10.1681/ASN.2013070780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A, Dong Z, Harris R, et al. Cellular and Molecular Mechanisms of AKI. J Am Soc Nephrol. 2016;27(5):1288–1299. doi: 10.1681/ASN.2015070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol. 2000;11(5):965–973. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- 5.Ryter SW, Choi AM. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res. 2016;167(1):7–34. doi: 10.1016/j.trsl.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 7.Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90(1):267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48(4):1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- 9.Shiraishi F, Curtis LM, Truong L, et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000;278(5):F726–736. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- 10.Tracz MJ, Juncos JP, Grande JP, et al. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1−/− mice. Am J Pathol. 2007;170(6):1820–1830. doi: 10.2353/ajpath.2007.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blydt-Hansen TD, Katori M, Lassman C, et al. Gene transfer-induced local heme oxygenase-1 overexpression protects rat kidney transplants from ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14(3):745–754. doi: 10.1097/01.asn.0000050760.87113.25. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu H, Takahashi T, Suzuki T, et al. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med. 2000;28(3):809–817. doi: 10.1097/00003246-200003000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Leaf DE, Body SC, Muehlschlegel JD, et al. Length Polymorphisms in Heme Oxygenase-1 and AKI after Cardiac Surgery. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bywaters EG, Beall D. Crush Injuries with Impairment of Renal Function. Br Med J. 1941;1(4185):427–432. doi: 10.1136/bmj.1.4185.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtney AE, Maxwell AP. Heme oxygenase 1: does it have a role in renal cytoprotection? Am J Kidney Dis. 2008;51(4):678–690. doi: 10.1053/j.ajkd.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23(1):17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70(3):432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 18.Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep. 2009;11(1):56–62. doi: 10.1007/s11906-009-0011-z. [DOI] [PubMed] [Google Scholar]
- 19.Lever JM, Boddu R, George JF, Agarwal A. Heme Oxygenase-1 in Kidney Health and Disease. Antioxid Redox Signal. 2016;25(3):165–183. doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabb H, Griffin MD, McKay DB, et al. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J Am Soc Nephrol. 2016;27(2):371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinsey GR, Sharma R, Okusa MD. Regulatory T cells in AKI. J Am Soc Nephrol. 2013;24(11):1720–1726. doi: 10.1681/ASN.2013050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11(2):88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 23.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109(4):e102–107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood. 2010;116(26):6054–6062. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hull TD, Agarwal A, George JF. The mononuclear phagocyte system in homeostasis and disease: a role for heme oxygenase-1. Antioxid Redox Signal. 2014;20(11):1770–1788. doi: 10.1089/ars.2013.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovtunovych G, Ghosh MC, Ollivierre W, et al. Wild-type macrophages reverse disease in heme oxygenase 1-deficient mice. Blood. 2014;124(9):1522–1530. doi: 10.1182/blood-2014-02-554162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferenbach DA, Ramdas V, Spencer N, et al. Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol Ther. 2010;18(9):1706–1713. doi: 10.1038/mt.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohta K, Yachie A, Fujimoto K, et al. Tubular injury as a cardinal pathologic feature in human heme oxygenase-1 deficiency. Am J Kidney Dis. 2000;35(5):863–870. doi: 10.1016/s0272-6386(00)70256-3. [DOI] [PubMed] [Google Scholar]
- 30.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94(20):10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapturczak MH, Wasserfall C, Brusko T, et al. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165(3):1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pittock ST, Norby SM, Grande JP, et al. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int. 2005;68(2):611–622. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 33.Hull TD, Kamal AI, Boddu R, et al. Heme Oxygenase-1 Regulates Myeloid Cell Trafficking in AKI. J Am Soc Nephrol. 2015;26(9):2139–2151. doi: 10.1681/ASN.2014080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S, Kapturczak MH, Wasserfall C, et al. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci U S A. 2005;102(20):7251–7256. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 36.Tracz MJ, Juncos JP, Croatt AJ, et al. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int. 2007;72(9):1073–1080. doi: 10.1038/sj.ki.5002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolisetty S, Zarjou A, Hull TD, et al. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int. 2015;88(1):95–108. doi: 10.1038/ki.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Wei SY, Li JS, et al. Overexpression of Heme Oxygenase-1 Prevents Renal Interstitial Inflammation and Fibrosis Induced by Unilateral Ureter Obstruction. PLoS One. 2016;11(1):e0147084. doi: 10.1371/journal.pone.0147084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park DJ, Agarwal A, George JF. Heme oxygenase-1 expression in murine dendritic cell subpopulations: effect on CD8+ dendritic cell differentiation in vivo. Am J Pathol. 2010;176(6):2831–2839. doi: 10.2353/ajpath.2010.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George JF, Braun A, Brusko TM, et al. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol. 2008;173(1):154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotsch K, Martins PN, Klemz R, et al. Heme oxygenase-1 ameliorates ischemia/reperfusion injury by targeting dendritic cell maturation and migration. Antioxid Redox Signal. 2007;9(12):2049–2063. doi: 10.1089/ars.2007.1801. [DOI] [PubMed] [Google Scholar]
- 42.Martins PN, Reutzel-Selke A, Jurisch A, et al. Induction of carbon monoxide in donor animals prior to organ procurement reduces graft immunogenicity and inhibits chronic allograft dysfunction. Transplantation. 2006;82(7):938–944. doi: 10.1097/01.tp.0000232716.91887.c5. [DOI] [PubMed] [Google Scholar]
- 43.Brusko TM, Wasserfall CH, Agarwal A, Kapturczak MH, Atkinson MA. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174(9):5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- 44.Song R, Mahidhara RS, Zhou Z, et al. Carbon monoxide inhibits T lymphocyte proliferation via caspase-dependent pathway. J Immunol. 2004;172(2):1220–1226. doi: 10.4049/jimmunol.172.2.1220. [DOI] [PubMed] [Google Scholar]
- 45.Gueler F, Park JK, Rong S, et al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am J Pathol. 2007;170(4):1192–1199. doi: 10.2353/ajpath.2007.060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung SH, Kim HJ, Oh GS, et al. Capsaicin ameliorates cisplatin-induced renal injury through induction of heme oxygenase-1. Mol Cells. 2014;37(3):234–240. doi: 10.14348/molcells.2014.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng CF, Lian WS, Chen SH, et al. Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARalpha-heme oxygenase-1 signaling pathway. J Cell Physiol. 2012;227(1):239–249. doi: 10.1002/jcp.22726. [DOI] [PubMed] [Google Scholar]
- 48.Kamimoto M, Mizuno S, Matsumoto K, Nakamura T. Hepatocyte growth factor prevents multiple organ injuries in endotoxemic mice through a heme oxygenase-1-dependent mechanism. Biochem Biophys Res Commun. 2009;380(2):333–337. doi: 10.1016/j.bbrc.2009.01.080. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Xie K, Chen H, Wang G, Yu Y. Hydrogen gas inhibits high-mobility group box 1 release in septic mice by upregulation of heme oxygenase 1. J Surg Res. 2015;196(1):136–148. doi: 10.1016/j.jss.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 50.Ferenbach DA, Kluth DC, Hughes J. Hemeoxygenase-1 and renal ischaemia-reperfusion injury. Nephron Exp Nephrol. 2010;115(3):e33–37. doi: 10.1159/000313828. [DOI] [PubMed] [Google Scholar]
- 51.Seta F, Bellner L, Rezzani R, et al. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol. 2006;169(5):1612–1623. doi: 10.2353/ajpath.2006.060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellner L, Martinelli L, Halilovic A, et al. Heme oxygenase-2 deletion causes endothelial cell activation marked by oxidative stress, inflammation, and angiogenesis. J Pharmacol Exp Ther. 2009;331(3):925–932. doi: 10.1124/jpet.109.158352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellner L, Marrazzo G, van Rooijen N, Dunn MW, Abraham NG, Schwartzman ML. Heme oxygenase-2 deletion impairs macrophage function: implication in wound healing. FASEB J. 2015;29(1):105–115. doi: 10.1096/fj.14-256503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellner L, Wolstein J, Patil KA, Dunn MW, Laniado-Schwartzman M. Biliverdin Rescues the HO-2 Null Mouse Phenotype of Unresolved Chronic Inflammation Following Corneal Epithelial Injury. Invest Ophthalmol Vis Sci. 2011;52(6):3246–3253. doi: 10.1167/iovs.10-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1998;101(4):777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Megyesi J, Andrade L, Vieira JM, Jr, Safirstein RL, Price PM. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int. 2001;60(6):2164–2172. doi: 10.1046/j.1523-1755.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 57.Inguaggiato P, Gonzalez-Michaca L, Croatt AJ, Haggard JJ, Alam J, Nath KA. Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int. 2001;60(6):2181–2191. doi: 10.1046/j.1523-1755.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez-Michaca L, Farrugia G, Croatt AJ, Alam J, Nath KA. Heme: a determinant of life and death in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286(2):F370–377. doi: 10.1152/ajprenal.00300.2003. [DOI] [PubMed] [Google Scholar]
- 59.Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol. 2000;156(5):1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwata M, Zager RA. Myoglobin inhibits proliferation of cultured human proximal tubular (HK-2) cells. Kidney Int. 1996;50(3):796–804. doi: 10.1038/ki.1996.378. [DOI] [PubMed] [Google Scholar]
- 61.Kumar D, Bhaskaran M, Alagappan L, et al. Heme oxygenase-1 modulates mesangial cell proliferation by p21 Waf1 upregulation. Ren Fail. 2010;32(2):254–258. doi: 10.3109/08860220903491240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim SY, Kang HT, Choi HR, Park SC. Biliverdin reductase A in the prevention of cellular senescence against oxidative stress. Exp Mol Med. 2011;43(1):15–23. doi: 10.3858/emm.2011.43.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Yuan L, Fu L, Liu C, Liu D, Mei C. Overexpression of p18INK(4)C in LLC-PK1 cells increases resistance to cisplatin-induced apoptosis. Pediatr Nephrol. 2011;26(8):1291–1301. doi: 10.1007/s00467-011-1877-y. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Zhang Y, Yuan L, Liu C, Fu L, Mei C. Cyclin-dependent kinase inhibitor p18INK4c is involved in protective roles of heme oxygenase-1 in cisplatin-induced acute kidney injury. Int J Mol Med. 2014;34(3):911–917. doi: 10.3892/ijmm.2014.1828. [DOI] [PubMed] [Google Scholar]
- 65.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543. doi: 10.1038/nm.2144. 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 68.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 69.He L, Livingston MJ, Dong Z. Autophagy in acute kidney injury and repair. Nephron Clin Pract. 2014;127(1–4):56–60. doi: 10.1159/000363677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaushal GP, Shah SV. Autophagy in acute kidney injury. Kidney Int. 2016;89(4):779–791. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki C, Isaka Y, Takabatake Y, et al. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368(1):100–106. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 72.Bolisetty S, Traylor AM, Kim J, et al. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol. 2010;21(10):1702–1712. doi: 10.1681/ASN.2010030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banerjee P, Basu A, Wegiel B, et al. Heme oxygenase-1 promotes survival of renal cancer cells through modulation of apoptosis- and autophagy-regulating molecules. J Biol Chem. 2012;287(38):32113–32123. doi: 10.1074/jbc.M112.393140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim HP, Wang X, Chen ZH, et al. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008;4(7):887–895. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- 75.Nath KA, Balla J, Croatt AJ, Vercellotti GM. Heme protein-mediated renal injury: a protective role for 21-aminosteroids in vitro and in vivo. Kidney Int. 1995;47(2):592–602. doi: 10.1038/ki.1995.75. [DOI] [PubMed] [Google Scholar]
- 76.Bolisetty S, Traylor A, Joseph R, Zarjou A, Agarwal A. Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2016;310(5):F385–394. doi: 10.1152/ajprenal.00335.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J, Zarjou A, Traylor AM, et al. In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int. 2012;82(3):278–291. doi: 10.1038/ki.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dhar GJ, Bossenmaier I, Cardinal R, Petryka ZJ, Watson CJ. Transitory renal failure following rapid administration of a relatively large amount of hematin in a patient with acute intermittent porphyria in clinical remission. Acta Med Scand. 1978;203(5):437–443. doi: 10.1111/j.0954-6820.1978.tb14903.x. [DOI] [PubMed] [Google Scholar]
- 79.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18(2):414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 80.Yachie A, Niida Y, Wada T, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103(1):129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bolisetty S, Traylor A, Zarjou A, et al. Mitochondria-targeted heme oxygenase-1 decreases oxidative stress in renal epithelial cells. Am J Physiol Renal Physiol. 2013;305(3):F255–264. doi: 10.1152/ajprenal.00160.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demirogullari B, Ekingen G, Guz G, et al. A comparative study of the effects of hemin and bilirubin on bilateral renal ischemia reperfusion injury. Nephron Exp Nephrol. 2006;103(1):e1–5. doi: 10.1159/000090113. [DOI] [PubMed] [Google Scholar]
- 83.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6(5):841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 84.Kaur H, Hughes MN, Green CJ, Naughton P, Foresti R, Motterlini R. Interaction of bilirubin and biliverdin with reactive nitrogen species. FEBS Lett. 2003;543(1–3):113–119. doi: 10.1016/s0014-5793(03)00420-4. [DOI] [PubMed] [Google Scholar]
- 85.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99(25):16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lanone S, Bloc S, Foresti R, et al. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005;19(13):1890–1892. doi: 10.1096/fj.04-2368fje. [DOI] [PubMed] [Google Scholar]
- 87.Matsumoto H, Ishikawa K, Itabe H, Maruyama Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol Cell Biochem. 2006;291(1–2):21–28. doi: 10.1007/s11010-006-9190-y. [DOI] [PubMed] [Google Scholar]
- 88.Leung N, Croatt AJ, Haggard JJ, Grande JP, Nath KA. Acute cholestatic liver disease protects against glycerol-induced acute renal failure in the rat. Kidney Int. 2001;60(3):1047–1057. doi: 10.1046/j.1523-1755.2001.0600031047.x. [DOI] [PubMed] [Google Scholar]
- 89.Dennery PA, McDonagh AF, Spitz DR, Rodgers PA, Stevenson DK. Hyperbilirubinemia results in reduced oxidative injury in neonatal Gunn rats exposed to hyperoxia. Free Radic Biol Med. 1995;19(4):395–404. doi: 10.1016/0891-5849(95)00032-s. [DOI] [PubMed] [Google Scholar]
- 90.Adin CA, Croker BP, Agarwal A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol. 2005;288(4):F778–784. doi: 10.1152/ajprenal.00215.2004. [DOI] [PubMed] [Google Scholar]
- 91.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290(3):F563–571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 92.Boczkowski J, Poderoso JJ, Motterlini R. CO-metal interaction: Vital signaling from a lethal gas. Trends Biochem Sci. 2006;31(11):614–621. doi: 10.1016/j.tibs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Sandouka A, Balogun E, Foresti R, et al. Carbon monoxide-releasing molecules (CO-RMs) modulate respiration in isolated mitochondria. Cell Mol Biol (Noisy-le-grand) 2005;51(4):425–432. [PubMed] [Google Scholar]
- 94.Goebel U, Siepe M, Schwer CI, et al. Inhaled carbon monoxide prevents acute kidney injury in pigs after cardiopulmonary bypass by inducing a heat shock response. Anesth Analg. 2010;111(1):29–37. doi: 10.1213/ANE.0b013e3181e0cca4. [DOI] [PubMed] [Google Scholar]
- 95.Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42(9):2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang P, Huang J, Li Y, et al. Exogenous Carbon Monoxide Decreases Sepsis-Induced Acute Kidney Injury and Inhibits NLRP3 Inflammasome Activation in Rats. Int J Mol Sci. 2015;16(9):20595–20608. doi: 10.3390/ijms160920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53(2):394–401. doi: 10.1046/j.1523-1755.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 98.Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988;34(4):474–480. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- 99.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31(4):971–997. doi: 10.1081/dmr-100101947. [DOI] [PubMed] [Google Scholar]
- 100.Zarjou A, Bolisetty S, Joseph R, et al. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest. 2013;123(10):4423–4434. doi: 10.1172/JCI67867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol. 2002;33(1):125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 102.Radhakrishnan N, Yadav SP, Sachdeva A, et al. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol. 2011;33(1):74–78. doi: 10.1097/MPH.0b013e3181fd2aae. [DOI] [PubMed] [Google Scholar]
- 103.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med. 2009;206(5):1167–1179. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lever JM, Boddu R, George JF, Agarwal A. Heme Oxygenase-1 in Kidney Health and Disease. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: Molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol. 2004;286(3):F425–441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- 106.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 107.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 108.Erson AE, Petty EM. MicroRNAs in development and disease. Clin Genet. 2008;74(4):296–306. doi: 10.1111/j.1399-0004.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 109.Gomez IG, Nakagawa N, Duffield JS. MicroRNAs as novel therapeutic targets to treat kidney injury and fibrosis. Am J Physiol Renal Physiol. 2016;310(10):F931–944. doi: 10.1152/ajprenal.00523.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhatt K, Kato M, Natarajan R. Mini-review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. 2016;310(2):F109–118. doi: 10.1152/ajprenal.00387.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang H, Wang Q, Li S. MicroRNA-218 promotes high glucose-induced apoptosis in podocytes by targeting heme oxygenase-1. Biochem Biophys Res Commun. 2016;471(4):582–588. doi: 10.1016/j.bbrc.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 112.Stachurska A, Ciesla M, Kozakowska M, et al. Cross-talk between microRNAs, nuclear factor E2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol Nutr Food Res. 2013;57(3):504–515. doi: 10.1002/mnfr.201200456. [DOI] [PubMed] [Google Scholar]
- 113.Schrottmaier WC, Oskolkova OV, Schabbauer G, Afonyushkin T. MicroRNA miR-320a modulates induction of HO-1, GCLM and OKL38 by oxidized phospholipids in endothelial cells. Atherosclerosis. 2014;235(1):1–8. doi: 10.1016/j.atherosclerosis.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 114.Pulkkinen KH, Yla-Herttuala S, Levonen AL. Heme oxygenase 1 is induced by miR-155 via reduced BACH1 translation in endothelial cells. Free Radic Biol Med. 2011;51(11):2124–2131. doi: 10.1016/j.freeradbiomed.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 115.Beckman JD, Chen C, Nguyen J, et al. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J Biol Chem. 2011;286(5):3194–3202. doi: 10.1074/jbc.M110.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kozakowska M, Szade K, Dulak J, Jozkowicz A. Role of heme oxygenase-1 in postnatal differentiation of stem cells: a possible cross-talk with microRNAs. Antioxid Redox Signal. 2014;20(11):1827–1850. doi: 10.1089/ars.2013.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Go H, La P, Namba F, et al. MiR-196a regulates heme oxygenase-1 by silencing Bach1 in the neonatal mouse lung. Am J Physiol Lung Cell Mol Physiol. 2016 doi: 10.1152/ajplung.00428.2015. ajplung 00428 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin SH, Song W, Cressatti M, Zukor H, Wang E, Schipper HM. Heme oxygenase-1 modulates microRNA expression in cultured astroglia: implications for chronic brain disorders. Glia. 2015;63(7):1270–1284. doi: 10.1002/glia.22823. [DOI] [PubMed] [Google Scholar]
- 119.Kozakowska M, Ciesla M, Stefanska A, et al. Heme oxygenase-1 inhibits myoblast differentiation by targeting myomirs. Antioxid Redox Signal. 2012;16(2):113–127. doi: 10.1089/ars.2011.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37(8):1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 121.Daenen KE, Martens P, Bammens B. Association of HO-1 (GT)n Promoter Polymorphism and Cardiovascular Disease: A Reanalysis of the Literature. Can J Cardiol. 2016;32(2):160–168. doi: 10.1016/j.cjca.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 122.Qiao H, Sai X, Gai L, et al. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: a HuGE review and meta-analysis. Am J Epidemiol. 2014;179(9):1039–1048. doi: 10.1093/aje/kwu024. [DOI] [PubMed] [Google Scholar]
- 123.Bao W, Song F, Li X, et al. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol. 2010;172(6):631–636. doi: 10.1093/aje/kwq162. [DOI] [PubMed] [Google Scholar]
- 124.Hirai H, Kubo H, Yamaya M, et al. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102(5):1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- 125.Taha H, Skrzypek K, Guevara I, et al. Role of heme oxygenase-1 in human endothelial cells: lesson from the promoter allelic variants. Arterioscler Thromb Vasc Biol. 2010;30(8):1634–1641. doi: 10.1161/ATVBAHA.110.207316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen YH, Lin SJ, Lin MW, et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002;111(1):1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 127.Chin HJ, Cho HJ, Lee TW, et al. The heme oxygenase-1 genotype is a risk factor to renal impairment of IgA nephropathy at diagnosis, which is a strong predictor of mortality. J Korean Med Sci. 2009;24(Suppl):S30–37. doi: 10.3346/jkms.2009.24.S1.S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baan C, Peeters A, Lemos F, et al. Fundamental role for HO-1 in the self-protection of renal allografts. Am J Transplant. 2004;4(5):811–818. doi: 10.1111/j.1600-6143.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 129.Exner M, Bohmig GA, Schillinger M, et al. Donor heme oxygenase-1 genotype is associated with renal allograft function. Transplantation. 2004;77(4):538–542. doi: 10.1097/01.tp.0000113467.36269.f8. [DOI] [PubMed] [Google Scholar]
- 130.Lin CC, Yang WC, Lin SJ, et al. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int. 2006;69(1):165–172. doi: 10.1038/sj.ki.5000019. [DOI] [PubMed] [Google Scholar]
- 131.Lin CC, Chung MY, Yang WC, Lin SJ, Lee PC. Length polymorphisms of heme oxygenase-1 determine the effect of far-infrared therapy on the function of arteriovenous fistula in hemodialysis patients: a novel physicogenomic study. Nephrol Dial Transplant. 2013;28(5):1284–1293. doi: 10.1093/ndt/gfs608. [DOI] [PubMed] [Google Scholar]
- 132.Chen YH, Kuo KL, Hung SC, Hsu CC, Chen YH, Tarng DC. Length polymorphism in heme oxygenase-1 and risk of CKD among patients with coronary artery disease. J Am Soc Nephrol. 2014;25(11):2669–2677. doi: 10.1681/ASN.2013111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holweg CT, Balk AH, Uitterlinden AG, et al. Functional heme oxygenase-1 promoter polymorphism in relation to heart failure and cardiac transplantation. J Heart Lung Transplant. 2005;24(4):493–497. doi: 10.1016/j.healun.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 134.Courtney AE, McNamee PT, Heggarty S, Middleton D, Maxwell AP. Association of functional haem oxygenase-1 gene promoter polymorphism with polycystic kidney disease and IgA nephropathy. Nephrol Dial Transplant. 2008;23(2):608–611. doi: 10.1093/ndt/gfm736. [DOI] [PubMed] [Google Scholar]
- 135.Courtney AE, McNamee PT, Middleton D, Heggarty S, Patterson CC, Maxwell AP. Association of functional heme oxygenase-1 gene promoter polymorphism with renal transplantation outcomes. Am J Transplant. 2007;7(4):908–913. doi: 10.1111/j.1600-6143.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- 136.Martines AM, Masereeuw R, Tjalsma H, Hoenderop JG, Wetzels JF, Swinkels DW. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat Rev Nephrol. 2013;9(7):385–398. doi: 10.1038/nrneph.2013.98. [DOI] [PubMed] [Google Scholar]
- 137.Walker VJ, Agarwal A. Targeting Iron Homeostasis in Acute Kidney Injury. Semin Nephrol. 2016;36(1):62–70. doi: 10.1016/j.semnephrol.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Waikar SS. Plasma catalytic iron, AKI, and death among critically ill patients. Clin J Am Soc Nephrol. 2014;9(11):1849–1856. doi: 10.2215/CJN.02840314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leaf DE, Rajapurkar M, Lele SS, et al. Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int. 2015;87(5):1046–1054. doi: 10.1038/ki.2014.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scindia Y, Dey P, Thirunagari A, et al. Hepcidin Mitigates Renal Ischemia-Reperfusion Injury by Modulating Systemic Iron Homeostasis. J Am Soc Nephrol. 2015;26(11):2800–2814. doi: 10.1681/ASN.2014101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gunnerson KJ, Shaw AD, Chawla LS, et al. TIMP2*IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg. 2016;80(2):243–249. doi: 10.1097/TA.0000000000000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gocze I, Koch M, Renner P, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One. 2015;10(3):e0120863. doi: 10.1371/journal.pone.0120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pajenda S, Ilhan-Mutlu A, Preusser M, Roka S, Druml W, Wagner L. NephroCheck data compared to serum creatinine in various clinical settings. BMC Nephrol. 2015;16:206. doi: 10.1186/s12882-015-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zager RA, Johnson AC, Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol. 2012;23(6):1048–1057. doi: 10.1681/ASN.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Z, Xu Y, Liu X, Nie Y, Zhao Z. Urinary Heme Oxygenase-1 as a potential biomarker for early diabetic nephropathy. Nephrology (Carlton) 2016 doi: 10.1111/nep.12719. [DOI] [PubMed] [Google Scholar]
- 146.Billings FTt, Yu C, Byrne JG, Petracek MR, Pretorius M. Heme Oxygenase-1 and Acute Kidney Injury following Cardiac Surgery. Cardiorenal Med. 2014;4(1):12–21. doi: 10.1159/000357871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu QQ, Wang Y, Senitko M, et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARgamma, and HO-1. Am J Physiol Renal Physiol. 2011;300(5):F1180–1192. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu J, Liu X, Fan J, et al. Bardoxolone methyl (BARD) ameliorates aristolochic acid (AA)- induced acute kidney injury through Nrf2 pathway. Toxicology. 2014;318:22–31. doi: 10.1016/j.tox.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 149.Gao Z, Han Y, Hu Y, et al. Targeting HO-1 by Epigallocatechin-3-Gallate Reduces Contrast-Induced Renal Injury via Anti-Oxidative Stress and Anti-Inflammation Pathways. PLoS One. 2016;11(2):e0149032. doi: 10.1371/journal.pone.0149032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y, Wang B, Du F, et al. Epigallocatechin-3-Gallate Attenuates Oxidative Stress and Inflammation in Obstructive Nephropathy via NF-kappaB and Nrf2/HO-1 Signalling Pathway Regulation. Basic Clin Pharmacol Toxicol. 2015;117(3):164–172. doi: 10.1111/bcpt.12383. [DOI] [PubMed] [Google Scholar]
- 151.Katavetin P, Inagi R, Miyata T, et al. Erythropoietin induces heme oxygenase-1 expression and attenuates oxidative stress. Biochem Biophys Res Commun. 2007;359(4):928–934. doi: 10.1016/j.bbrc.2007.05.207. [DOI] [PubMed] [Google Scholar]
- 152.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]