Abstract
Recent work shows that models based on functional connectivity in large-scale brain networks can predict individuals’ attentional abilities. Some of the first generalizable neuromarkers of cognitive function, these models also inform our basic understanding of attention, providing empirical evidence that (1) attention is a network property of brain computation, (2) the functional architecture that underlies attention can be measured while people are not engaged in any explicit task, and (3) this architecture supports a general attentional ability common to several lab-based tasks and impaired in attention deficit hyperactivity disorder. Looking ahead, connectivity-based predictive models of attention and other cognitive abilities and behaviors may potentially improve the assessment, diagnosis, and treatment of clinical dysfunction.
Keywords: attention, sustained attention, functional magnetic resonance imaging, functional connectivity, connectome, predictive models
What is attention, and how do we measure it?
Perhaps no cognitive capacity is more crucial to navigating daily life than the ability to pay attention. Although we all know what it feels like to pay attention, the concept is notoriously difficult to define. More than a century ago in what has perhaps become one of the most oft-quoted lines in psychology, William James explained attention as “the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought” [1]. Seventy years later Anne Treisman questioned the utility of such folk-psychological definitions, arguing that conceptualizations of attention as “‘the focalization of consciousness’ or ‘the increased clearness of a particular idea’.... [had] proved sterile for empirical research and ended in a series of inconclusive controversies.” She instead suggested that studying attention as information processing could “open the way to a more exact linking of behavioural concepts with underlying physiological mechanisms” [2].
Treisman’s words proved prescient: Psychological research on attention, guided by this approach, has boomed in the last half-century. However, despite the resulting advances in our understanding of attentional processes and neural mechanisms, we still don’t have a clear understanding of what kind of process attention is in the mind and brain, or whether it is one single process at all [3]. One reason for this lack of clarity, Chun and colleagues recently observed, is that “Attention has become a catch-all term for how the brain controls its own information processing” [3]. To advance understanding, they argue, researchers should work to understand the common and distinct mechanisms that support different forms of attention [3].
While the broad scope of what researchers mean when they say “attention” has made the topic unwieldy to study [3], the absence of a standardized way to measure attention may have further hindered basic research and translational applications. Unlike for other abilities, such as memory and intelligence, researchers and clinicians lack a straightforward way to summarize a person’s overall attentional function. Although complex processes often can’t be reduced to a single number, summary indices like capacity for working memory and gF for fluid intelligence are useful for quantifying individual differences and changes in abilities over time. A comparable measure of attention — an objective, standardized summary score — would benefit both research and clinical practice by facilitating comparisons across and within individuals, evaluations of treatments and interventions, and predictions of real-world behavior and clinical symptoms.
Here we propose that attention can be understood as an emergent property of large-scale brain networks, based on a novel framework for measuring attentional abilities with functional magnetic resonance imaging (fMRI). We review empirical work showing that, although the functional organization of the brain is generally consistent across individuals, every person has a unique pattern of functional connectivity (see Glossary) that lies atop a common blueprint and distinguishes them from the group. These distinct connectivity patterns can be used to predict how well individual people pay attention. Predictions can be made from connectivity patterns observed as people perform attention tasks, but also from patterns observed as they are not engaged in any explicit task at all. In other words, we can measure attention using resting-state fMRI data alone, meaning that the neural architecture that supports attention function is reflected in the brain’s intrinsic functional organization. Viewing attention as a network property of brain activity, not unlike how one might characterize the efficiency of a computer or air traffic network, reveals insights about the nature and underlying structure of attention. Looking beyond attention, models that make individualized predictions from brain networks may have clinical benefits in translational settings and offer a new kind of solution to challenges in cognitive, clinical, and developmental neuroscience.
From brain areas to brain networks: What have we learned about attention from cognitive neuroscience?
It’s hard to imagine meeting the demands of daily life without the ability to focus. In fact, impairments in attention, which are common to clinical populations as diverse as attention deficit hyperactivity disorder [4], depression [5], schizophrenia [6], bipolar disorder [7], post-traumatic stress disorder [8], and traumatic brain injury [9], predict a wide range of negative outcomes, from poorer educational achievement to worse employment and job performance, peer acceptance, and family relationships [10].
Although the ability to attend varies widely even in the healthy population [11], until recently cognitive neuroscience studies of attention devoted little focus to individual differences. That is, since the early 1990s, fMRI studies of human attention have focused on identifying regions of the brain where activity and/or functional connectivity is, on average, modulated by specific attentional demands. This work has been vital in identifying the basic neural architecture of attention, and, from a cognitive psychological standpoint, clarifying subcomponents of attention by demonstrating that distinct processes are related to distinct patterns of brain activity. Some findings support distinctions posited by cognitive psychology, such as that between goal-directed and stimulus-driven attention [12], whereas others highlight the importance of dimensions that had been, by comparison, relatively unexplored, such as internal versus external attentional focus [13]. Despite the success of cognitive neuroscience in describing the brain bases of different forms of attention, the focus on group-level rather than single-subject level analyses has resulted in neuroanatomical models that, on the whole, do not account for the individual differences in attention that permeate our everyday experience.
One of the earliest and most influential models of attention divided attention into three subsystems based on behavioral and neural evidence: (1) alerting, or preparing and maintaining alertness and vigilance; (2) orienting, or directing overt or covert attention to a stimulus; and (3) target detection/executive control, or noticing and selecting stimuli for conscious processing [14,15]. One line of behavioral evidence that alerting, orienting, and executive control are independent components of attention comes from the Attention Network Task (ANT), which shows that these abilities are largely independent within subjects ([16,17]; but see [18]). At the group level, these components are related to activity in distinct groups of brain regions [14,15,17]. At the individual subject level, they are related to integrity in distinct white matter tracts [19,20], cortical thickness [21], white matter asymmetry [22], and functional connectivity in the dorsal attention and default mode networks [23,24]. However, precluding the use of these markers as generalizable measures of attention function, so far these studies have been correlational rather than predictive in nature.
A similarly influential model of attention, the dual network approach, describes two neural systems for orienting attention in a goal-directed (top-down) or stimulus-driven (bottom-up) fashion [12]. Functional connectivity studies show that even while people are resting in the fMRI scanner — that is, when they are not performing an explicit task — these two systems are reflected in the functional organization of the brain [25]. In concert with a wide body of behavioral evidence (e.g., [26–28]) this result suggests that a fundamental organizing principle of what we call “attention” is whether it is “pushed” towards a goal-relevant stimulus or “pulled” towards a stimulus with low-level salience. Studies have related attentional orienting to cerebellar volume in children with autism [29], and performance on a perceptual task requiring orienting to functional connectivity between visual and prefrontal areas [30], but so far these relationships have not been leveraged to predict behavior in novel individuals.
Recently attention researchers have described another pair of large-scale brain networks that reveal an important dimension along which attention can vary: the so-called “task-negative” and “task-positive” networks [31]. The default mode, or task-negative, network refers to a set of regions more active during rest than task performance [32,33], and is thought to be related to internally focused attentional processes [13,34] such as mind wandering, off-task performance [35,36], and self-referential thought [13]. “Task-negative” may be a misleading moniker, however, as the network also plays a role in external environment monitoring [13,37,38] and successful sustained attention [39]. Activity in these networks is anticorrelated during task engagement and rest [31], and the degree of this anticorrelation during task is related to individual differences in performance variability [40]. Thus, attention can be subdivided into separate but complementary processes for internal and external focus.
Compared with other attention networks, individual differences in task-negative and task-positive network activity and connectivity have been relatively well explored. For example, many studies have observed aberrant default mode network activity and functional connectivity in psychiatric disorders such as Alzheimer’s disease [13], schizophrenia [41], depression [42], autism [43], and ADHD [44,45] (for reviews see [46,47]). Correlational studies of individual differences in the healthy population report less default network suppression in people who mind-wander [35] and engage in divergent thinking [48]; a pattern of either stronger or weaker default network connectivity in individuals who mind wander [34,49]; and stronger default network connectivity in individuals with high trait mindfulness [50], neuroticism [51], and openness to experience [51,52]. The diversity of these findings and lack of generalizable models, however, so far precludes the use of the task-positive and task-negative networks as generalizable neuromarkers of the ability to focus.
Overall, existing models of attention successfully describe brain activity and functional organization at the group level, making important contributions to knowledge about distinct attentional processes in the mind. They do not, however, predict an individual person’s ability to pay attention, and are limited by their reliance on task-based fMRI data and a circumscribed set of regions or functional connections. Models that account for individual differences using resting-state data offer additional practical and clinical benefits and reveal complementary insights about the nature of attention.
New insights from network neuroscience and predictive modeling
A central question of cognitive neuroscience is how the brain gives rise to the mind and behavior. Some of the earliest evidence in humans came from neuropsychological studies of patients with brain damage, which found that certain lesions were associated with stereotyped deficits, such as spatial neglect [53]. Neuroimaging and electrophysiological studies have also identified process-specific brain regions such as the frontal eye fields, which are responsible for covert and overt shifts of attention [54,55]. Researchers recognize, however, that many processes cannot be localized to a single brain region, and that most of our sophisticated cognitive abilities, such as attention, working memory, and decision-making, rely on the orchestrated activity of a distributed array of structures, as suggested by several meta-analyses [56,57]. Accordingly, the best characterization of individual cognitive ability may lie not in the magnitude of activity in single regions, but rather the degree to which activity is coordinated across large-scale networks [12,14,17,30,31,58,59]. In other words, as the emerging field of network neuroscience suggests, processes like attention and cognition may emerge from dynamic interactions between diverse sets of brain areas [60–63].
That attention is a network property is not a new idea. As early as 1906 psychologists thought that the focus of attention was determined, in part, by, “the play of excitement among the organised systems of neural elements of which the higher levels of the brain are composed” [64], and existing models of attention emphasize the importance of large-scale networks such as the dorsal and ventral attention and default mode networks in attentional performance. However, only recently have methods emerged for predicting individual differences in attention from features of complex brain networks. These methods complement previous work to provide novel evidence that attentional processes arise from interactions between distinct anatomical regions.
Predictive network models
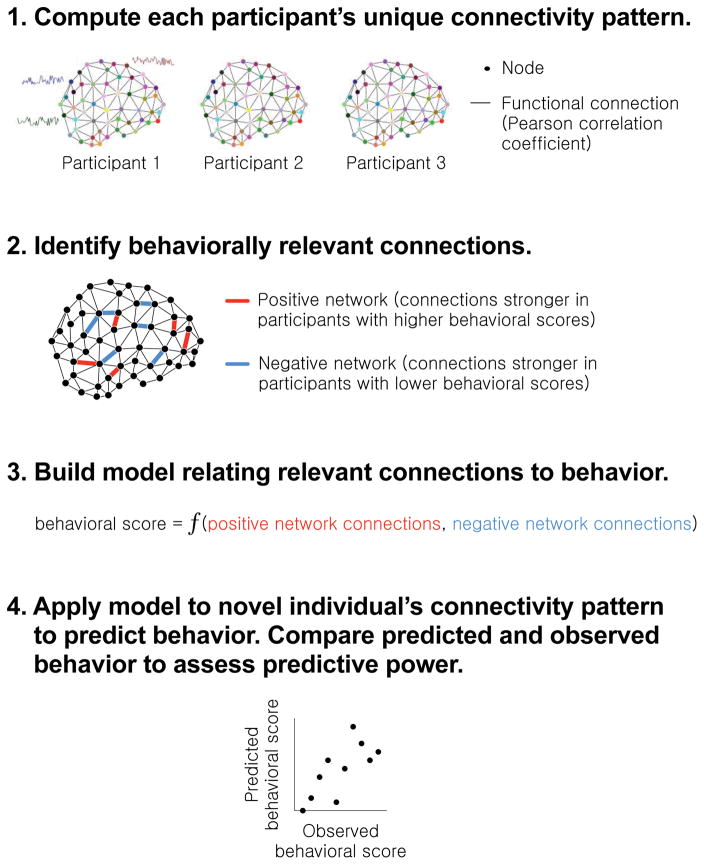
So far, the most well-validated connectivity model of individual differences in attention is the sustained attention connectome-based predictive model (CPM) [65]. The sustained attention CPM was defined using a novel technique, connectome-based predictive modeling [66,67], to predict how well individuals perform on the gradual-onset continuous performance task (gradCPT), a challenging test of attention [39,68]. Briefly, the CPM method identifies functional connections from the set of all possible connections in the brain related to a behavioral measure of interest, and uses the strength of those connections to predict behavior in novel individuals (Figure 1). CPM does not generate cognitive models of representation or computation, but is an approach to develop statistical models of functional connectivity with impressive predictive power for behavior and traits.
Figure 1. Connectome-based predictive modeling.
The connectome-based predictive modeling (CPM) approach identifies functional connectivity networks that are related to behavior, and measures strength in these networks within previously unseen individuals to make predictions about their behavior [65–67]. First, every participant’s whole-brain connectivity pattern is calculated by correlating the fMRI activity timecourses of every pair of regions, or nodes, in a brain atlas. Next, behaviorally relevant connections are identified by correlating every connection in the brain with behavior across subjects. Connections that are most strongly related to behavior in the positive and negative directions are retained for model building. A linear model relates each individual’s positive network strength (i.e., the sum of the connections in their positive network) and negative network strength (i.e., the sum of the connections in their negative network) to their behavioral score. The model is then applied to a novel individual’s connectivity data to generate a behavioral prediction. Predictive power is assessed by correlating predicted and observed behavioral scores across the group.
To define the sustained attention CPM, we first collected fMRI data from a group of healthy participants as they performed the gradCPT and rested. GradCPT performance, assessed with sensitivity (d′), served as a proxy for a person’s overall ability to sustain attention. For each participant, task-based and resting-state connectivity matrices were computed using data from the gradCPT and resting-state runs, respectively. That is, a task-based connectivity matrix was computed by correlating the BOLD signal timecourses of every pair of regions of a 268-node brain atlas [69], and a resting-state connectivity matrix was computed analogously using data collected during rest. In a leave-one-subject-out cross-validation procedure, connections whose strength during gradCPT performance correlated with d′ were identified. Connections that were stronger in participants with higher d′ scores formed the high-attention network, whereas connections that were stronger in participants with low d′ scores formed the low-attention network. Next, models were built relating connectivity in the high- and low-attention networks to d′ across subjects. Finally, the left-out subject’s connectivity strength was input into the model to generate a personalized d′ prediction. Demonstrating that models generalized to previously unseen individuals, predicted and observed d′ values were significantly correlated when models were tested using connection strength calculated during either task or rest [65]. In other words, based on the strength of hundreds of connections across an individual’s brain measured even while they were simply resting, models could predict how well they performed an attention task.
This result was internally valid for the initial group of participants. To be theoretically and practically useful as a measure of attention, however, a predictive model should be externally valid — that is, it should predict attention in completely independent groups of individuals. To test whether the sustained attention CPM generalized to predict a real-world measure of attention, the model was applied, without alteration, to resting-state connectivity data from children and adolescents, some of whom had diagnoses of attention deficit hyperactivity disorder (ADHD) [65]. These data, part of a publicly available repository, had been collected at Peking University in Beijing and included, for both patients and typically developing controls, a clinician-rated measure of ADHD symptom severity [70]. As expected, predictions of the sustained attention CPM were inversely correlated with ADHD symptom scores. In other words, the model predicted that children with few ADHD symptoms would have high d′ scores if they were to perform the gradCPT, and that children with frequent and/or severe ADHD symptoms would have low d′ scores if they were to perform the task [65]. Thus, the sustained attention CPM generalizes across participant population (healthy adults vs. children and adolescents with and without ADHD diagnoses), scan site (Yale University vs. Peking University), imaging parameters, and measures of sustained attention (d′ vs. ADHD symptom scores). Informing models of attention dysfunction, this result also shows that the same functional networks that predict individual differences in the healthy range are compromised in ADHD.
The sustained attention CPM also generalizes to predict pharmacologically induced changes in attention function [71]. Specifically, high- and low-attention network strength were examined in a new group of healthy adults who had been administered a single dose of methylphenidate (trade name: Ritalin), a drug used to treat ADHD symptoms [72], and a group of unmedicated control participants (dataset from [73,74]). As predicted a priori, individuals given methylphenidate showed connectivity signatures of strong attentional abilities: they had stronger high-attention networks and weaker low-attention networks than unmedicated controls as they rested and performed a stop-signal task [71]. In addition, the sustained attention CPM predicted go response rate on the stop-signal task from both task-based and resting-state data [71]. These results demonstrate that the sustained attention CPM predicts attention dynamics, and provide further evidence that the model is a robust and generalizable neuromarker of sustained attention.
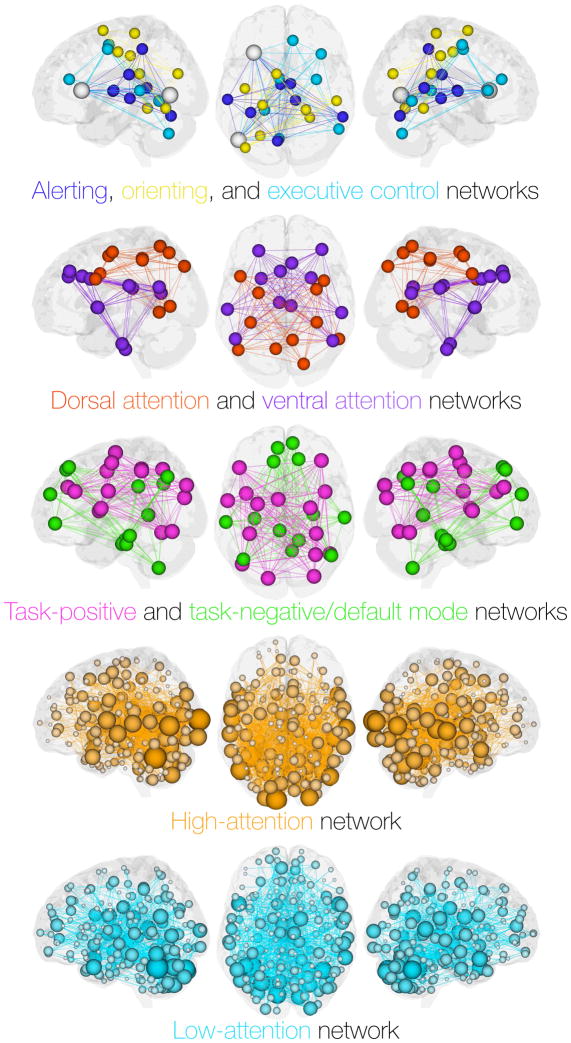
Interestingly, the sustained attention CPM does not rely on canonical brain networks to predict attention function. Rather, the high- and low-attention networks comprise hundreds of connections between cortical, subcortical, and cerebellar regions, and their functional anatomy is rich and extensive in a way that encompasses other attention networks described in the literature (Figure 2). Broadly speaking, connections between motor cortex, occipital lobes, and the cerebellum predict better sustained attention, whereas connections between temporal and parietal regions, as well as connections within the temporal lobe and within the cerebellum, predict worse sustained attention. Computationally “lesioning” the high- and low-attention networks by selectively removing connections from canonical brain networks revealed non-significant trends suggesting that the frontoparietal network and the default network are more relevant than other networks. Overall, however, the sustained attention CPM remained robust and resilient to such subnetwork lesions [65], suggesting that the functional anatomy of networks supporting attention is broadly distributed.
Figure 2. Canonical attention networks and networks used to build the sustained attention CPM.
Nodes of canonical networks were defined using MNI coordinates or Talairach coordinates converted to MNI space from representative articles. For each x, y, z coordinate, the closest node of the Shen et al. (2013) atlas [69] was identified using the knnsearch function in Matlab. Nodes and fully connected networks were then visualized using BioImage Suite [105]. As such, these figures are meant to be illustrative summaries rather than quantitative analyses of well-studied canonical networks. Nodes of the alerting, orienting, and executive control network were defined using Tables 3–5 in Ref. [17]. Six left-hemisphere nodes of the dorsal and ventral attention networks [12] were defined using Table 5 in Ref. [106]; symmetrical right-hemisphere nodes were also included for visualization. Nodes of the task-positive and task-negative networks were defined using Table 1 in Ref. [31]. The high-attention network (orange) and low-attention network (turquoise) were defined in Ref. [65]. Nodes of these networks are sized according to their total number of connections.
That the sustained attention CPM does not neatly overlap with existing neuroanatomical models of attention does not appear to be a fluke of the CPM method, or of the particular attention task used to define the model. One potential explanation for its distributed anatomy is that the high- and low-attention networks involve brain areas with related levels of activity in certain neurotransmitter systems. Preliminary evidence for this hypothesis is found in the significant overlap between the high-attention network and the network stronger in individuals given methylphenidate (known to improve attention function), and the low-attention network shows significant overlap with a network of connections stronger in controls not given methylphenidate. More broadly, the high-attention and methylphenidate networks, and the low-attention and unmedicated control networks, show remarkably similar patterns of connectivity [71]. The sustained attention CPM, therefore, may be related to the expression of dopamine and norepinephrine, neurotransmitters whose extracellular concentration is modulated by methylphenidate [75–77].
In considering the anatomy of the sustained attention CPM, it is worth noting that it does not necessarily represent a dual-systems model of attention. Rather, because the CPM approach retains connections that are both positively and negatively correlated with behavior for model building purposes, the two-network model was inevitable. Predictive power of models based on high-attention and low-attention network strength alone tends to be highly similar, suggesting that there is some degree of redundant information in the two networks. Furthermore, models that make predictions based on strength in both networks generally do not outperform the single-network models [65]. Although a large literature suggests that attention is controlled by two anti-correlated brain networks with distinct functions (i.e., the task-positive and task-negative networks) [31], the predictive power of the sustained attention CPM is not explained by connectivity in these networks alone, and does not, in and of itself, provide evidence for a dual-systems model of attention.
Furthermore, the broadly distributed nature of the sustained attention CPM does not imply that canonical brain networks cannot also predict individual differences in attention function. One study, for example, found that resting-state connectivity between the dorsal and ventral attention and default networks predicted the ability to suppress irrelevant visual distractors measured several months later [78]. Greater connectivity between default mode regions predicted better distractor suppression, supporting evidence of compromised default network function in a variety of clinical disorders [47,78]. Greater connectivity between the default mode and the dorsal and ventral attention networks, on the other hand, predicted worse distractor suppression, reinforcing previous findings that diminished anticorrelation between the task-positive and task-negative networks is related to impaired attention [40,78]. These support vector regression models were internally validated with a leave-one-subject-out approach; however, they were not tested for external validity beyond the study sample.
Another recent study used growth charts based on connectivity networks to predict attention function in children [79]. The authors first computed each child’s full resting-state connectivity matrix by correlating activity timecourses between 1068 regions in the brain, and performed dimensionality reduction using independent components analysis (ICA). A normative growth chart was defined for each component by plotting component expression against biological age. For every individual, a maturation score was calculated by measuring the distance between their actual component expression and the normative component expression for their age. Models based on maturation scores, trained and tested with a split-half cross-validation approach, predicted performance on a sustained attention task and ADHD diagnoses. Although models relied on ICA components defined using whole-brain connectivity data, trends were observed in the relationship between canonical attention networks and age. Similar to the distractibility findings [78], within-default connectivity increased and connectivity between the default and dorsal and ventral attention networks decreased with age [79]. Although it is yet untested whether growth curve models based on connectivity in these networks alone would generate similarly accurate attention predictions, future work should explore the relationship between canonical attention networks and individual differences in attention.
Implications for cognitive psychology and clinical practice
By directly linking brain variables to behavioral ones, models that make individualized attention predictions inform our basic understanding of how the brain gives rise to attention and provide empirical evidence that attention is a network property of the brain. In addition, they make significant contributions to our understanding of the nature of attention, and are potentially applicable in clinical or translational settings.
First, predicting attention from intrinsic brain connectivity represents a new way of measuring not only attention, but potentially any cognitive ability or mental processes. (See Outstanding Questions.) Consider how we currently measure cognitive functions. When we want to know how a person’s memory capacity, we ask them to remember stimuli and count how many they can recall. When we want to know a person’s capacity for self-control, we ask them to override some prepotent behavioral response. When we want to know how well a person can pay attention, however, we do not need to have them perform any attention task at all. Instead, we can apply existing models to fMRI data collected as they simply rested in the scanner, meaning that predictive network models are a way to measure attention without having people actively pay attention. Practically, this means models can be applied retroactively to preexisting fMRI data, and that model predictions are immune to confounds such as motor control that may influence attention task performance in some individuals. In addition, it means that an infinite number of predictive models can be applied to the same resting-state functional connectivity profile to predict a suite of cognitive abilities and behaviors. For example, distinct connectivity-based models already exist to predict fluid intelligence [67] and response to math tutoring [80] (although these have not yet been tested for external validity), and future work could pursue neuromarkers of abilities such as memory, spatial cognition, theory of mind, and emotional regulation; characteristics such as personality or cognitive style; and clinical disorders such as depression, anxiety, autism, and schizophrenia (Figure 3). Theoretically, the success of predictive models shows, for the first time, that the functional infrastructure underlying a person’s ability to attend is reflected in the brain even when they are not engaged in an attention-demanding task.
Outstanding questions.
What attention factors comprise a person’s overall attention profile? What tasks or assessments best capture these factors? Can we develop new behavioral tasks that efficiently test the overall attention profile?
Are connectivity signatures of attention malleable within subjects? In addition to pharmacological intervention, does behavioral training or conscious control affect network connectivity? Can real-time neurofeedback based on attention network connectivity be used to effect lasting improvements in sustained attention?
What else can connectivity-based predictive modeling predict? How effectively can this approach predict clinical symptoms or developmental trajectories? Does predictive power extend to real-world behaviors, such as education success? How are predictions affected by variability due to an fMRI participant’s sleepiness or caffeine intake, for example?
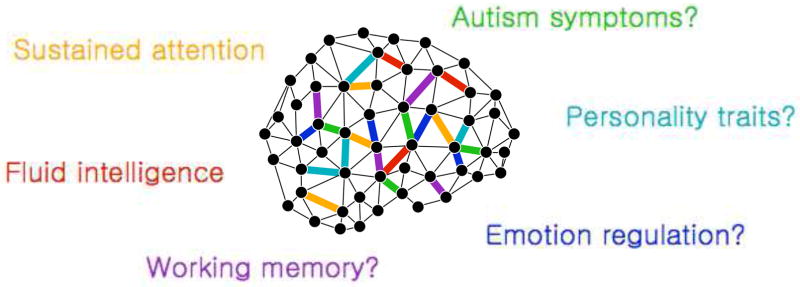
Figure 3. A suite of predictive models to predict behavior.
One benefit of using fMRI data to predict an individual’s behavior and clinical symptoms is that multiple predictive models can be applied to a single fMRI dataset. For example, the sustained attention CPM can be applied to predict sustained attention [65]. At the same time, separate models may be applied to predict fluid intelligence [67], and, hypothetically, a number of behaviors, traits, and symptoms, such as working memory, emotion regulation, personality traits, and symptoms of autism.
Second, predictive models help elucidate the unique and common processes that underlie a person’s ability to pay attention in a variety of contexts. The sustained attention CPM, for example, predicts adults’ performance on two separate tests of attention (the gradCPT and stop-signal task) and children’s ADHD symptom scores, and is flexible in response to an attention-enhancing drug [65,71]. In concert with findings of behavioral individual differences studies [81], these results also suggest a common “attention factor”, analogous to g in intelligence research, and underscore the utility of attention as a psychological construct. Notably, this “attention factor” is distinct from g: The sustained attention CPM predicts ADHD symptoms when controlling for children’s age and IQ, but does not predict IQ when controlling for ADHD symptoms and age [65]. Looking ahead, defining a suite of models that predict different proposed types of attention (such as alerting, orienting, and executive control [14,15]) and evaluating how well they predict attention on variety of tasks can help identify both general and specialized factors of attention (Box 1).
Box 1. A profile of attention function.
Predictive network models show promise for quantifying attentional abilities, particularly sustained attention [65,71,79]. A comprehensive measure of attention function, however, should reflect the fact that there are multiple dimensions along which people’s abilities may vary. For example, one person may struggle to pay attention for long periods of time but have no difficulty switching between tasks, whereas another may have no trouble maintaining uninterrupted focus but lack the ability to multitask.
Although an extensive task battery could provide a behavioral measure of overall attention function, administering a multitude of tests is impractical given that there are dozens of candidates. Instead, a profile of attention could feature a suite of models that predict multiple attention factors from one fMRI scan, analogous to how several blood tests can be performed on a single sample. Although building such a suite would require fMRI and behavioral data from many individuals, the endeavor could be facilitated via large-scale collaborations [88,89]. Once validated, the final set of models could be applied to predict an individual’s “attention profile”. This profile, or set of predictions, would facilitate objective comparisons across individuals, improve longitudinal tracking, and help predict other behaviors or clinical symptoms.
What components of attention constitute the hypothetical attention profile? (See Outstanding Questions.) Behavioral evidence suggests that there are both general and highly specific factors: When individuals were tested on up to seventeen attention tasks, cross-correlations suggested that performance is explained by a general factor that explains performance in variety of tasks such as search, tracking, and visual short-term memory, as well as several specific factors related to spatial orienting, attentional capture, and inhibition of return [81].
To build models that capture general and specific attention factors, a large sample of participants should be administered an assay of behavioral tasks that test the components described above, either during fMRI scanning or after a resting-state scan. The task battery may also include the attention network task (ANT), which measures alerting, exogenous orienting, and executive control [16,17]. Aspects of executive control could be tested with change detection [90–92] or n-back tasks [93,94]. Divided attention to multiple stimuli could be measured with multiple object tracking or dual-tasks [95–97].
Using brain connectivity and behavioral data, separate predictive models can be developed to predict performance on each test. To test the relationship between various tasks, each model can be applied to predict other behavioral measures; patterns of specificity will help identify separable factors. For example, work in our lab has found that the sustained attention model does not predict fluid intelligence, and the fluid intelligence model does not predict sustained attention, suggesting independence between the two models and, therefore, constructs. Thus, the pattern of cross-correlations across tasks form a prediction matrix, from which factor analysis can extract both general factors of attention and specialized factors of attention that will ultimately constitute an overall profile of attention function.
Finally, because network models offer objective measures of attention, they may be useful in clinical contexts [82,83]. They could, for example, be used to identify children at risk of developing attention problems for early intervention, match individuals to the most appropriate attention training or treatment, and track changes in attentional state or attention-related symptoms over time (Box 2). Functional networks that predict attention are also potentially effective targets for pharmacological or behavioral interventions to improve focus. For example, with real-time neurofeedback [84,85], it may be possible to train individuals to express patterns of connectivity associated with strong attentional abilities to improve performance (see Outstanding Questions).
Box 2. Intraindividual attention fluctuations.
The ability to pay attention not only differs across individuals, but also changes over time within a single person. Attention can vary on the order of seconds, fluctuating from one trial to the next of a cognitive task [68]; minutes, declining over the course of a taxing or monotonous task [98]; and hours, varying throughout the day with the circadian rhythm and drugs like caffeine. Attention function also changes over the lifespan, improving with development and declining with healthy aging [11], injury, and disease.
Brain activity in canonical networks is related to fluctuations that occur on relatively short time scales. Default mode network activity, for example, distinguishes periods of mind wandering from periods of focus within-subjects [35,36], characterizes attentional lapses [99], and, along with activity in the dorsal attention network, predicts periods of better and worse sustained attention [39,100–102]. Activity and connectivity in a these networks also differs between populations whose attentional abilities differ, such as ADHD patients and healthy controls [103].
Recent work using dynamic connectivity approaches has examined how changes in functional connectivity over time relate to fluctuations in attention. Although some work suggests that patterns of brain connectivity do fluctuate with attention within a single individual [104], and that networks that predict attention function are perturbed by pharmacological attention intervention [71], the extent of change within individuals and over time is still unknown. Thus, it will be useful to apply predictive models to connectivity data collected at multiple time points (for example, during several blocks of a task in a single fMRI session, longitudinally over the course of days or years, or pre- and post-attention training) and compare predictions to repeated measures of attention Complementary work can address whether the strength of attention-related networks varies with changes in attention related to development, aging, and treatment or disease progression, and whether network models predict future improvements or declines in attentional abilities.
Thus, although attention waxes and wanes within individuals, mean attention function — that is, a person’s overall attentional abilities — appears stable enough from day to day to reveal substantial differences across individuals. One advantage of predictive modeling for measuring attention longitudinally over short and long time intervals is to avoid practice effects that come with repeatedly performing the same task.
Concluding remarks
Models that predict attention from patterns of functional brain connectivity, some of the first generalizable neuromarkers of cognitive function, represent a significant contribution to the fast-growing field of individual differences in fMRI [86,87]. In doing so, they provide empirical support for a central prediction of network neuroscience: that attention and cognition arise from the dynamic interactions of many distinct regions of the brain [60–62]. Further, successful predictions from resting-state data reveal that the infrastructure supporting attentional abilities is reflected in the brain’s intrinsic functional organization. In the future, models that predict individual differences in other abilities and behaviors may help improve clinical diagnosis and treatment and provide a new kind of insight about the nature of other processes in the mind.
Trends.
Valuable research has described the attention system of the human brain using mostly group-level analyses of neuroimaging data.
FMRI research is moving towards single-subject level analyses, which afford significant scientific and practical benefits such as personalized assessment, diagnosis, or prediction.
Recent work shows that models based on functional brain networks can predict how well individual people pay attention.
Predictive models provide empirical evidence that attention is a network property of the brain, and that the functional architecture that underlies attention can be measured while people are not engaged in any explicit task.
Looking ahead, connectivity-based predictive models of attention and other cognitive abilities may improve the assessment, diagnosis, and treatment of clinical dysfunction.
Glossary
- Correlational vs. predictive studies
FMRI studies of individual differences often claim that a brain-based measure “predicts” a behavioral measure when the two are simply correlated across individuals. Following Gabrieli et al., we reserve the term “prediction” for cross-validated models, that is, models that generalize to novel individuals [86]. Although beyond the scope of this article, another sense in which models can be predictive is that they use baseline data from an individual to predict his or her future behavior [86], for example, using functional connectivity to predict performance on perceptual tasks [30].
- External validity
A model is externally valid when it generalizes to novel datasets, that is, when predictions are robust to the specific group of participants or data collection site. For models of traits, behavior, or symptoms to be clinically useful, they must demonstrate external validity.
- Functional brain connectivity
Functional connectivity is measured by correlating the blood oxygenation level–dependent (BOLD) signal timecourse, measured with fMRI, in two spatially distinct regions of the brain. Activity in regions that are strongly functionally connected fluctuates in synchrony, whereas activity in regions that are weakly functionally connected changes out of sync. Functional connectivity does not necessarily imply structural connectivity; rather, functional connections are thought to reflect regions engaged in common or related processing during task performance or rest.
- Functional connectivity matrix
An m x m matrix, where m is the number of nodes (brain regions) in the network. Cells represent functional connections. Cell (i,j) of the matrix represents the temporal correlation between the activity in brain region i and the activity in brain region j. Non-directional connectivity matrices are symmetrical about the diagonal. The diagonal, the correlation of a region with itself, is equal to 1.
- Internal validity
A model is internally valid when it generalizes to novel individuals within a single dataset. Although leave-one-subject-out cross-validation (i.e., k-fold cross-validation with k = number of subjects) tests internal validity, the strictest and least biased method is split-half validation (k = 2).
- Resting-state fMRI
Resting-state fMRI data are collected as participants simply lie in an MRI scanner without engaging in an explicit task. Participants in resting-state scans may be asked to keep their eyes closed or open and focused on a central fixation point. Although functional connectivity can be acquired from resting-state data, overall activity cannot because there is no measure of absolute activity during rest. Compared with task-based data, resting-state data is often easier to collect and share across sites.
References
- 1.James W. The principles of psychology (Vols. 1 & 2) New York Holt. 1890;118:688. [Google Scholar]
- 2.Treisman AM. Selective attention in man. Br Med Bull. 1964;20:12–6. doi: 10.1093/oxfordjournals.bmb.a070274. [DOI] [PubMed] [Google Scholar]
- 3.Chun MM, et al. A Taxonomy of External and Internal Attention. Annu Rev Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- 4.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Hammar Å, Årdal G. Cognitive Functioning in Major Depression–A Summary. Front Hum Neurosci. 2009;3:26. doi: 10.3389/neuro.09.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Arán A, et al. Cognitive Function Across Manic or Hypomanic, Depressed, and Euthymic States in Bipolar Disorder. Am J Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 8.Uddo M, et al. Memory and attention in combat-related post-traumatic stress disorder (PTSD) J Psychopathol Behav Assess. 1993;15:43–52. [Google Scholar]
- 9.Robertson IH, et al. “Oops!”: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 10.Nigg J. Attention-deficit/hyperactivity disorder and adverse health outcomes. Clin Psychol Rev. 2013;33:215–228. doi: 10.1016/j.cpr.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortenbaugh FC, et al. Sustained Attention Across the Life Span in a Sample of 10,000 Dissociating Ability and Strategy. Psychol Sci. 2015 doi: 10.1177/0956797615594896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 13.Buckner RL, et al. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 14.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 15.Petersen SE, Posner MI. The Attention System of the Human Brain: 20 Years After. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan J, et al. Testing the Efficiency and Independence of Attentional Networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 17.Fan J, et al. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.MacLeod JW, et al. Neuropsychology. Vol. 24. American Psychological Association; 2010. Appraising the ANT: Psychometric and theoretical considerations of the Attention Network Test; pp. 637–651. [DOI] [PubMed] [Google Scholar]
- 19.Yin X, et al. Anatomical Substrates of the Alerting, Orienting and Executive Control Components of Attention: Focus on the Posterior Parietal Lobe. PLoS One. 2012;7:e50590. doi: 10.1371/journal.pone.0050590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niogi SN, et al. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Frontiers in Neuroanatomy. 2010;4 doi: 10.3389/neuro.05.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westlye LT, et al. Associations between Regional Cortical Thickness and Attentional Networks as Measured by the Attention Network Test. Cereb Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- 22.Yin X, et al. Inferior frontal white matter asymmetry correlates with executive control of attention. Hum Brain Mapp. 2013;34:796–813. doi: 10.1002/hbm.21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visintin E, et al. Parsing the intrinsic networks underlying attention: A resting state study. Behav Brain Res. 2015;278:315–322. doi: 10.1016/j.bbr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Madhyastha TM, et al. Dynamic connectivity at rest predicts attention task performance. Brain Connect. 2015;5:45–59. doi: 10.1089/brain.2014.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox MD, et al. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonides J. Voluntary versus automatic control over the mind’s eye’s movement. Psychonomic Bulletin & Review. 1998:187–203. [Google Scholar]
- 27.Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- 28.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 29.Harris NS, et al. Neuroanatomic contributions to slowed orienting of attention in children with autism. Cogn Brain Res. 1999;8:61–71. doi: 10.1016/s0926-6410(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 30.Baldassarre A, et al. From the Cover: Individual variability in functional connectivity predicts performance of a perceptual task. Proceedings of the National Academy of Sciences. 2012;109:3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raichle ME, et al. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulman GL, et al. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 34.Andrews-Hanna JR, et al. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104:322–35. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason MF, et al. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science (80- ) 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christoff K, et al. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106:8719–24. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn B, et al. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex. 2007;17:1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert SJ, et al. Comment on "Wandering minds: the default network and stimulus-independent thought". Science (80- ) 2007;317:43. doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- 39.Esterman M, et al. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb Cortex. 2013;23:2712–2723. doi: 10.1093/cercor/bhs261. [DOI] [PubMed] [Google Scholar]
- 40.Clare Kelly AM, et al. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Whitfield-Gabrieli S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheline YI, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Martino a, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014;19:659–67. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fair DA, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:1084–91. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castellanos FX, Aoki Y. Intrinsic Functional Connectivity in Attention-Deficit/Hyperactivity Disorder: A Science in Development. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:253–261. doi: 10.1016/j.bpsc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broyd SJ, et al. Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience and Biobehavioral Reviews. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi H, et al. Failing to deactivate: The association between brain activity during a working memory task and creativity. Neuroimage. 2011;55:681–687. doi: 10.1016/j.neuroimage.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 49.O’Callaghan C, et al. Shaped by our thoughts - A new task to assess spontaneous cognition and its associated neural correlates in the default network. Brain Cogn. 2015;93:1–10. doi: 10.1016/j.bandc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Shaurya Prakash R, et al. Mindfulness disposition and default-mode network connectivity in older adults. Soc Cogn Affect Neurosci. 2013;8:112–117. doi: 10.1093/scan/nss115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adelstein JS, et al. Personality is reflected in the brain’s intrinsic functional architecture. PLoS One. 2011:6. doi: 10.1371/journal.pone.0027633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beaty RE, et al. Personality and complex brain networks: The role of openness to experience in default network efficiency. Hum Brain Mapp. 2016;37:773–779. doi: 10.1002/hbm.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parton A, et al. Hemispatial neglect. J Neurol Neurosurg Psychiatry. 2004;75:13–21. [PMC free article] [PubMed] [Google Scholar]
- 54.Corbetta M, et al. A Common Network of Functional Areas for Attention and Eye Movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 55.Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 56.Yarkoni T, et al. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laird AR, et al. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics. 2005;3:65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- 58.Smith S, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18:1565–1567. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SM, et al. Functional connectomics from resting-state fMRI. Trends in Cognitive Sciences. 2013;17:666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park H-J, Friston K. Structural and Functional Brain Networks: From Connections to Cognition. Science (80- ) 2013:342. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- 61.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nat Neurosci. 2014;17:652–660. doi: 10.1038/nn.3690. [DOI] [PubMed] [Google Scholar]
- 63.Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- 64.McDougall W. III—Physiological factors of the attention-process (IV.) Mind. 1906;15:329–359. [Google Scholar]
- 65.Rosenberg MD, et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19:165–71. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen X, et al. Connectome-based predictive modeling: A framework to develop and test predictive models relating individual behavior to brain connectivity [Google Scholar]
- 67.Finn ES, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18:1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenberg M, et al. Sustaining visual attention in the face of distraction: a novel gradual-onset continuous performance task. Atten Percept Psychophys. 2013;75:426–439. doi: 10.3758/s13414-012-0413-x. [DOI] [PubMed] [Google Scholar]
- 69.Shen X, et al. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403–415. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Consortium T.A.-200. The ADHD-200 Consortium: A Model to Advance the Translational Potential of Neuroimaging in Clinical Neuroscience. Front Syst Neurosci. 2012;6:62. doi: 10.3389/fnsys.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberg MD, et al. Methylphenidate modulates functional network connectivity to enhance attention. J Neurosci. 2016 doi: 10.1523/JNEUROSCI.1746-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldman LS, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- 73.Farr OM, et al. The effects of methylphenidate on cerebral activations to salient stimuli in healthy adults. Exp Clin Psychopharmacol. 2014;22:154–65. doi: 10.1037/a0034465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farr OM, et al. The effects of methylphenidate on resting-state striatal, thalamic and global functional connectivity in healthy adults. Int J Neuropsychopharmacol. 2014;17:1177–1191. doi: 10.1017/S1461145714000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volkow ND, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spencer RC, et al. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biological Psychiatry. 2015;77:940–950. doi: 10.1016/j.biopsych.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berridge CW, et al. Methylphenidate Preferentially Increases Catecholamine Neurotransmission within the Prefrontal Cortex at Low Doses that Enhance Cognitive Function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 78.Poole VN, et al. Intrinsic functional connectivity predicts individual differences in distractibility. Neuropsychologia. 2016 doi: 10.1016/j.neuropsychologia.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 79.Kessler D, et al. Growth charting of brain connectivity networks and the identification of attention impairment in youth. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.0088. at < http://dx.doi.org/10.1001/jamapsychiatry.2016.0088>. [DOI] [PMC free article] [PubMed]
- 80.Supekar K, et al. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc Natl Acad Sci U S A. 2013;110:8230–8235. doi: 10.1073/pnas.1222154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang L, et al. Measuring the interrelations among multiple paradigms of visual attention: An individual differences approach. Journal of Experimental Psychology: Human Perception and Performance. 2012;38:414–428. doi: 10.1037/a0026314. [DOI] [PubMed] [Google Scholar]
- 82.Siegel JS, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci. 2016;113:E4367–E4376. doi: 10.1073/pnas.1521083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baldassarre A, et al. Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain. 2014;137:3267–3283. doi: 10.1093/brain/awu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stoeckel LE, et al. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage: Clinical. 2014;5:245–255. doi: 10.1016/j.nicl.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.deBettencourt MT, et al. Closed-loop training of attention with real-time brain imaging. Nat Neurosci. 2015;18:470–475. doi: 10.1038/nn.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gabrieli JDE, et al. Prediction as a Humanitarian and Pragmatic Contribution from Human Cognitive Neuroscience. Neuron. 2015;85:11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dubois J, Adolphs R. Building a Science of Individual Differences from fMRI. Trends Cogn Sci. 2016;xx:1–19. doi: 10.1016/j.tics.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poldrack RA, Gorgolewski KJ. Making big data open: data sharing in neuroimaging. Nat Neurosci. 2014;17:1510–1517. doi: 10.1038/nn.3818. [DOI] [PubMed] [Google Scholar]
- 89.Milham MP. Open Neuroscience Solutions for the Connectome-wide Association Era. Neuron. 2012;73:214–218. doi: 10.1016/j.neuron.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 91.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- 92.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 93.Jaeggi SM, et al. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- 94.Kane MJ, et al. Working memory, attention control, and the N-back task: a question of construct validity. J Exp Psychol Learn Mem Cogn. 2007;33:615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- 95.Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spat Vis. 1988;3:179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- 96.Scholl BJ, Pylyshyn ZW. Tracking multiple items through occlusion: clues to visual objecthood. Cogn Psychol. 1999;38:259–290. doi: 10.1006/cogp.1998.0698. [DOI] [PubMed] [Google Scholar]
- 97.Lavie N. Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Parasuraman R. Vigilance, monitoring, and search. Handbook of perception and human performance, Vol. 2: Cognitive processes and performance. 1986:1–39. [Google Scholar]
- 99.Weissman DH, et al. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 100.Esterman M, et al. Intrinsic Fluctuations in Sustained Attention and Distractor Processing. J Neurosci. 2014;34:1724–1730. doi: 10.1523/JNEUROSCI.2658-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosenberg MD, et al. Predicting moment-to-moment attentional state. Neuroimage. 2015;114:249–256. doi: 10.1016/j.neuroimage.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 102.Kucyi A, et al. Spontaneous default network activity reflects behavioral variability independent of mind-wandering. Proc Natl Acad Sci. 2016;113:13899–13904. doi: 10.1073/pnas.1611743113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Castellanos FX, Proal E. Large-scale brain systems in ADHD: Beyond the prefrontal-striatal model. Trends in Cognitive Sciences. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shine JM, et al. Temporal metastates are associated with differential patterns of time-resolved connectivity, network topology, and attention. Proc Natl Acad Sci U S A. 2016;113:9888–9891. doi: 10.1073/pnas.1604898113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joshi A, et al. Unified Framework for Development, Deployment and Robust Testing of Neuroimaging Algorithms. Neuroinformatics. 2011;9:69–84. doi: 10.1007/s12021-010-9092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]