Abstract
Objective
Low mannose-binding lectin (MBL) levels and haplotypes associated with low MBL production have been associated with infection and severe sepsis. We tested the hypothesis that MBL levels would be associated with severe infection in a large cohort of critically ill children.
Design
Prospective cohort study
Setting
Medical and Surgical Pediatric Intensive Care Units (PICUs), Boston Children’s Hospital
Patients
Children < 21 years of age admitted to the intensive care units from November 2009 to November 2010.
Interventions
None
Measurements and Main Results
We measured MBL levels in 479/520 (92%) consecutively admitted children with severe or life-threatening illness. We genotyped 213 Caucasian children for MBL haplotype tagging variants and assigned haplotypes. In the univariate analyses of MBL levels with pre-admission characteristics, levels were higher in patients with pre-existing renal disease. Patients who received >100 ml/kg of fluids in the first 24 hours after admission had markedly lower MBL, as did patients post-spinal fusion surgery. MBL levels had no association with infection status on admission, or with progression from systemic inflammatory response syndrome to sepsis or septic shock. Although MBL haplotypes strongly influenced MBL levels in the predicted relationship, low MBL-producing haplotypes were not associated with increased risk of infection.
Conclusions
Mannose-binding lectin levels are largely genetically determined. This relationship was preserved in children during critical illness, despite the effect of large-volume fluid administration on MBL levels. Previous literature evaluating an association between MBL levels and severe infection is inconsistent; we found no relationship in our PICU cohort. We found that MBL levels were lower after aggressive fluid resuscitation, and suggest that studies of MBL in critically ill patients should assess MBL haplotypes to reflect pre-illness levels.
Keywords: Mannose-Binding Lectin, MBL, complement activation, innate immunity, child, sepsis, intensive care
INTRODUCTION
The innate immune system is the first line of defense against invasive pathologic organisms and its role is essential in controlling infection in the first 24–48 hours before the adaptive immune system is able to mount an adequate response. One of the primary innate immune processes is activation of the complement system for direct pathogen killing and for opsonization, which marks pathogens for destruction by phagocytes. A critical antimicrobial protein in this pathway is mannose-binding lectin (MBL) which recognizes mannose sugars on the periphery of bacteria, viruses, and fungi, and on damaged human cells. (1, 2) Binding of this protein to cell membranes causes conformational changes that activate directed complement deposition for the invading microbe to be opsonized and killed. (2)
In humans, MBL is produced continuously in the liver; serum levels in healthy individuals have been shown to be influenced by host genetic makeup. (3, 4) The MBL protein is coded by the MBL2 gene which is comprised of 4 exons located on the long arm of chromosome 10. (5, 6) Within exon 1, there are three common single nucleotide polymorphisms (SNPs) located at codons 52, 54, and 57 (D, B, C; collectively termed O) and a promoter gene polymorphism at - 221 (termed X/Y). (See Figure 1A) (5–9) Heterozygosity for any one of the high producing variants (A/O) produces MBL levels in the near normal or only mildly reduced range; homozygosity (O/O) or compound heterozygosity with promoter X/Y leads to markedly reduced MBL levels. (8, 10, 11)
Figure 1.
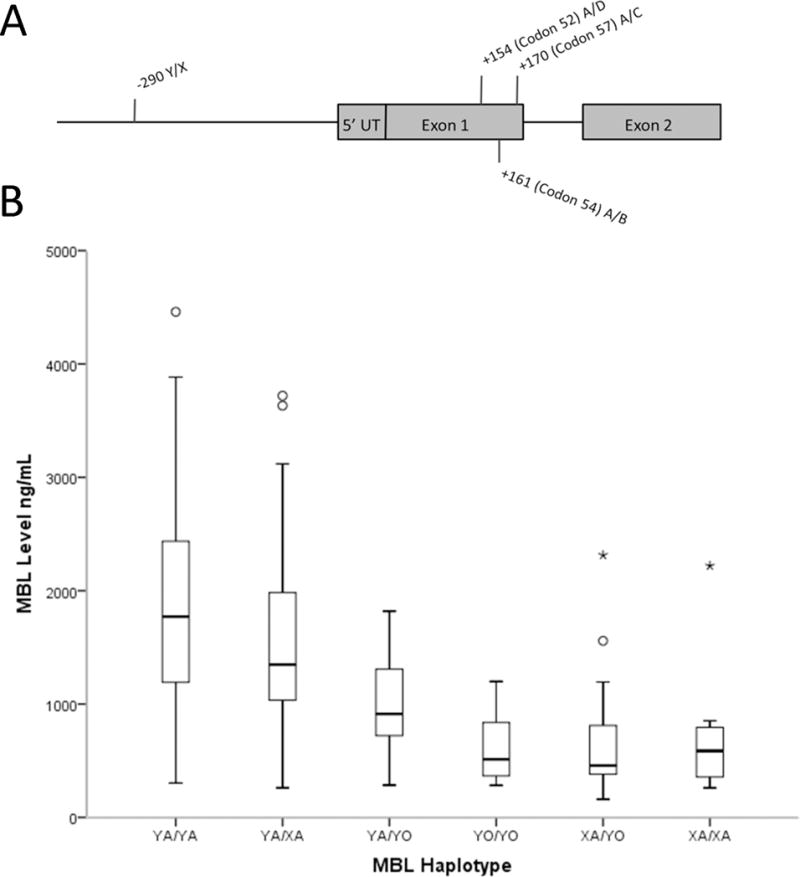
A) Graphical representation of common MBL allelic variants and their location within the 5′ end of the gene. B) Boxplot demonstrating the effect of haplotype on MBL level in a subset of 201 patients that consented for genotyping. Haplotypes are assigned as previously described and ordered by median MBL level in current study.
Low-producing MBL haplotypes remain at a relatively high level in the normal population despite some studies showing that they are associated with worsening severity of illness and increased susceptibility to infection. The level of MBL determining deficiency has not been specifically defined; some use <1000 ng/mL while others hypothesize that clinical consequences begin to occur at levels < 500 ng/mL; homozygous “low-producers” of MBL generally have levels < 50ng/mL. (6, 10, 12–14) It is unclear if there is an advantage to low-producing haplotypes although some have speculated MBL may worsen clinical presentation of infections that cause damage mainly by inflammatory responses.(6, 10, 14–16) Previous studies assessing the relationship between MBL levels and critical illness have varied in cohort size, the population studied, the outcome assessed, and whether/how MBL genotype was evaluated. We speculate that this may have led to the conflicting reports on whether MBL is associated with severe infection.(7, 16–21) In addition, potentially confounding variables such as fluid administration could dilute MBL levels and influence the association.
Because relative deficiency of MBL has been associated with sepsis in some studies, researchers are evaluating recombinant MBL as a therapy for sepsis and other serious infections. (22–24) To better clarify the association between MBL and infection severity in children, we aimed to examine associations between MBL levels, MBL haplotypes and severe infections in children in the pediatric intensive care unit (PICU). We hypothesized that: a) haplotypes would predict serum MBL levels in the previously described manner and b) critically ill children with severe infections (sepsis and septic shock) would have lower serum MBL levels and higher frequencies of low-producing MBL haplotypes in comparison to those without severe infections.
MATERIALS AND METHODS
We included children admitted to the Medical and Surgical Pediatric Intensive Care Units at Boston Children’s Hospital from November 9, 2009 to November 9, 2010. Eligibility criteria were: age < 21 years; estimated PICU stay of ≥ 48 hours (this excluded short-term monitoring patients) or admission due to suspected infection. Patients admitted to the Cardiac ICU were not included in this study due to the alterations in MBL from cardiac bypass as well as the high rate of administration of fresh frozen plasma which contains MBL. Institutional Review Board approval was obtained prior to the beginning of the study. Informed consent was obtained by study coordinators shortly after ICU admission and data were obtained from parent interviews and from the electronic medical record. A second separate consent was obtained for genotyping. Additional details of the study cohort have been published previously. (25)
The Pediatric Risk of Mortality III (PRISM III) score was used to assess illness severity in the first 24 hours. (26) Maximum vasopressor use was scored according to the Sequential Organ Failure Assessment, cardiovascular (CV-SOFA) (27) modified for pediatrics as follows: 0–1: no vasopressors, 2: dopamine < 5 mcg/kg/min, 3: dopamine 5–15 mcg/kg/min or norepinephrine/epinephrine < 0.1 mcg/kg/min, 4: dopamine > 15mcg/kg/min or norepinephrine/epinephrine > 0.1mcg/kg/min.
Suspected infections were those with markers of infection (e.g. cultures sent, antibiotic therapy initiated, chest x-ray findings, etc.) or criteria for community-acquired pneumonia but with negative microbiologic testing. Confirmed infections included 1) bacterial: culture of pathogenic bacteria from blood, CSF, or lung AND treatment with intravenous antibiotics; 2) fungal: positive fungal culture AND antifungal treatment; 3) viral: viral pathogen detected; 4) multiple: more than one of the preceding types. Community-acquired pneumonia was defined as meeting published criteria for pneumonia with bacteria confirmation.(28) Severity of illness categories in the sepsis analysis were as follows: The “SIRS, no infection” category included patients meeting published criteria for systemic inflammatory response syndrome (SIRS) on ICU admission without evidence of infection.(29, 30) Sepsis was defined as suspected or confirmed infection AND SIRS. (29) Severe sepsis was defined as sepsis with an ICU admission day CV-SOFA score ≥ 2, and includes septic shock.
Laboratory Methods and Genotyping
Blood was obtained as close to admission as possible, either from blood drawn at the time of admission for other purposes, or obtained from previous leftover samples stored in the laboratory refrigerator. All plasma was stored refrigerated, frozen at −80°C within 7 days, then shipped frozen for analysis. MBL measurements were done at the Cytokine Reference Lab using a commercial ELISA Kit from R&D Systems (Minneapolis, MN). Samples were diluted 400-fold to achieve levels within the dynamic range of the assay (0.156–10ng/mL).
Genotyping was reserved for patients of Caucasian race to minimize possible confounding by racial stratification. Single nucleotide polymorphisms were determined for rs1800450 (SNP “B”), rs5030737 (SNP “D”), rs1800451 (SNP “C”), and rs7096206 (Promoter “X/Y”) using TaqMan SNP assays (Applied Biosystems) with proprietary primer/probe combinations (See Fig 1A). SNP rs11003125 (Promoter “H/L”) was not included; this SNP had a much weaker effect on MBL levels in preliminary analysis. (31, 32) Haplotypes were determined as previously described, where Y represents the high producing promoter allele and X the low producing allele. The minor pathogenic alleles B, C, and D were collectively grouped as “O”. (6, 10) We compared both the individual haplotypes listed in Figure 1B as well as diplotypes grouped into 3 categories of High (YA/YA, YA/XA), Mid (YA/YO), and Low (XA/YO, YO/YO, and XA/XA) for improved power.
Statistical Methods
Because MBL levels were not normally distributed, we used nonparametric analyses with Spearman correlations for continuous, Mann-Whitney tests for dichotomous, and Kruskal-Wallis tests for categorical variables. Chi-squared tests were used for the association of haplotype with categorical variables and Kruskal-Wallis for association with continuous or ordinal variables. SPSS statistical package was used for computations (version 19.0.0, IBM Corp.).
RESULTS
We screened 2366 consecutive PICU admissions and enrolled 520/818 (62.5%) eligible children in this study. (25) Of 520 enrolled, 41 did not have an MBL levels measured; this yielded 479 patients with MBL levels included in our study. Baseline characteristics for the study population are shown in Table 1. Most children were admitted via the Emergency Department or the Operating Room, so some MBL levels were obtained from samples just prior to ICU admission. Overall, 94% of samples were drawn within 48-hours of PICU admission.
TABLE 1.
Demographic and Other Characteristics of the Subjects Known Previous to PICU Admission and Association with MBL Level
| Characteristic | N | % | MBL, ng/mLa | PValueb | |
|---|---|---|---|---|---|
| Total Sample | 479 | 100.0 | 1197 | (718.9–1878.3) | |
| Gender | 0.84 | ||||
| Male | 241 | 50.3 | 1169 | (767.9–1872.2) | |
| Female | 238 | 49.7 | 1205 | (662.0–1890.5) | |
| Age, y | 0.73c | ||||
| < 1 | 95 | 19.8 | 1309 | (658.6–2052.8) | |
| 1–4 | 141 | 29.4 | 1120 | (720.1–1773.6) | |
| 5–12 | 131 | 27.3 | 1154 | (754.8–1909.5) | |
| >13 | 112 | 23.4 | 1220 | (730.1–1875.4) | |
| Race | 0.01 | ||||
| Caucasian | 353 | 73.7 | 1129 | (694–1786.5) | |
| Black | 42 | 8.8 | 1044 | (415.2–2386.7) | |
| Other | 84 | 17.5 | 1460 | (846.6–2443) | |
| Ethnicity | 0.53 | ||||
| Hispanic | 76 | 15.9 | 1177 | (687.9–1943.3) | |
| Non-Hispanic | 396 | 82.7 | 1185 | (723.1–1854.7) | |
| Unable to Answer | 7 | 1.5 | 1590 | (448.2–2385.6) | |
| Insurance | 0.54 | ||||
| Private | 192 | 40.1 | 1165 | (665.7–1899.2) | |
| Government | 277 | 57.8 | 1202 | (752.7–1838.6) | |
| Underlying Chronic Conditions | 0.56 | ||||
| Any | 403 | 84.1 | 1203 | (734.0–1866.6) | |
| None | 76 | 15.9 | 1118 | (665.7–1896.5) | |
| Underlying Chronic Conditionsd | |||||
| Respiratory | 201 | 42.0 | 1235 | (711.9–1950.1) | 0.95 |
| Asthma | 100 | 20.9 | 1296 | (699.7–1989.7) | 0.86 |
| Neurologic | 191 | 39.9 | 1251 | (820.7–1913.5) | 0.11 |
| Seizure | 103 | 21.5 | 1365 | (876.7–1913.5) | 0.11 |
| Oncologic | 53 | 11.1 | 1202 | (851.4–1942.6) | 0.75 |
| Immunodeficiency | 31 | 6.5 | 1283 | (703.1–1732.0) | 0.98 |
| Renal | 22 | 4.6 | 1619 | (1212.0–2119.9) | 0.03 |
| Liver | 10 | 2.1 | 1385 | (731.9–1853.9) | 0.71 |
| Gastrointestinal | 43 | 9.0 | 1280 | (659.8–1980.3) | 0.94 |
| Nutritional | 49 | 10.2 | 1283 | (694.9–2119.8) | 0.65 |
| Metabolic | 39 | 8.1 | 1280 | (674.5–1753.7) | 0.71 |
Median (Interquartile range)
Testing association with serum MBL level with Mann-Whitney U or Kruskal-Wallis tests
Spearman correlation with age r= 0.016, p=0.73
Compared with patients with other chronic conditions
The univariate analysis of baseline patient characteristics and the effects on MBL levels are shown in Table 1. The median MBL level for the entire sample was 1197 ng/mL (IQR 718–1878 ng/mL; range 110–6154). Of the variables tested, only “Other” race (p = 0.01) and renal disease (p = 0.03) significantly increased MBL levels.
Of the 520 children enrolled in the study; 213 of the 353 parents who self-identified as white race also gave consent for genotype analysis (60%). Of these, 201/213 (94%) also had sufficient samples to obtain MBL level. The proportion carrying the “O/O” diplotype (3.3%) and the distribution of individual haplotypes (YA 0.535, XA 0.249, and YO 0.216) were comparable to previous studies in white populations. (32) MBL level was correlated with underlying haplotype (See Figure 1B, p = < 0.001, using the promoter X/Y allele and the grouped exonic alleles A/O). Our findings were in agreement with previously described MBL linkage disequilibrium, no novel haplotypes were observed. (31) As expected, homozygosity for an exonic allele (“O”), or compound heterozygosity for “O” and “X” produced the lowest levels. Individual allele frequencies were in Hardy-Weinburg equilibrium.
Univariate analyses were performed on the association of MBL levels with non-infection reasons for PICU admission (emergent vs. elective, post-operative versus not, trauma, status asthmaticus, status epilepticus, and admitted for monitoring). Only admission after operative spinal fusion was associated with a lower MBL level (p = 0.04). We did not identify any association between infection-related admission categories with MBL levels (See Table 2). No specific infectious process was associated with lower or higher MBL levels. “MBL deficiency” defined as MBL level < 1000 ng/mL was also not associated with infection categories (p > 0.05 for both suspected and confirmed infections, including all infections, bacterial infections, and other infections separately, DNS).
TABLE 2.
Infectious Reasons for Pediatric Intensive Care Unit Admission and Association with MBL Level
| Characteristic | N | % | MBL, ng/mLa | P Valueb |
|---|---|---|---|---|
| Infection on Intensive Care Unit Admissionc | 0.43 | |||
| No Infection | 238 | 1112 (581.1–1642.6) | ||
| Suspected Infection, not confirmed | 88 | 1389 (490.6–2287.9) | ||
| Bacterial | 90 | 1151 (533.7–1768.3) | ||
| Viral | 45 | 1301 (792.1–1810.7) | ||
| Multiple | 15 | 1280 (607.9–1952.2) | ||
| Lower Respiratory Tract Infection | 0.44 | |||
| No | 434 | 90.6 | 1202 (718.5–1913.7) | |
| Yes | 45 | 9.4 | 1131 (663.3–1784.7) | |
| Community Acquired Pneumonia | 0.24 | |||
| No | 13 | 1278 (844.9–1804.6) | ||
| Yes | 32 | 1122 (565.9–1786.3) | ||
| Sepsisc | 0.6 | |||
| No SIRS | 192 | 40.1 | 1117 (739.2–1975.2) | |
| SIRS, no infectiond | 99 | 20.7 | 1115 (768.1–1681.0) | |
| Sepsise | 112 | 23.4 | 1219 (662.9–2034.4) | |
| Severe Sepsisf | 73 | 15.2 | 1308 (723.8–1851.7) |
Median (Interquartile range)
Testing association with serum MBL level with Mann-Whitney U or Kruskal-Wallis tests
Infection specific data unavailable for 3 patients
No viral, bacterial, or fungal infection
Infection with SIRS
Sepsis with CV-SOFA score ≥ 3; includes septic shock
The univariate analysis of the association between MBL levels and clinical outcomes is shown in Table 3. We found that subjects receiving very large volumes (> 100cc/kg) of fluid prior to or in the first 12 hours after admission had lower MBL levels (p = 0.007 for Spearman’s correlation, r = 0.12; correlation with non-categorized fluid volume). We also found a significant association between MBL level < 1000 ng/mL and fluid administered prior or within the first 12 hours. Patients with MBL level less than 1000ng/mL received 41cc/kg (median, IQR 17–80) vs. 32.7 cc/kg (median, IQR 7.1–64.5) in patients with MBL greater than 1000 ng/mL (p=0.02 Mann-Whitney U)).
TABLE 3.
Treatments and Outcomes of All Patients and Association with MBL Level
| Characteristic | N | % | MBL, ng/mLa | P Valueb | |
|---|---|---|---|---|---|
| Fluid Administration first 24 hours | 0.007c | ||||
| 0–20cc/kg | 173 | 36.1 | 1330 | (796.7–2020.5) | |
| 20–40cc/kg | 91 | 19.0 | 1205 | (661.2–1984.3) | |
| 40–60cc/kg | 72 | 15.0 | 1143 | (767.9–1897.2) | |
| 60–80cc/kg | 48 | 10.0 | 1150 | (657.2–1849.8) | |
| 80–100cc/kg | 31 | 6.5 | 1202 | (697.4–1732.0) | |
| > 100cc/kg | 64 | 13.4 | 899 | (584.1–1542.4) | |
| PRISM III Score | 0.16d | ||||
| 0 | 123 | 25.7 | 1257 | (774.4–1781.5) | |
| 1–3 | 86 | 18.0 | 1148 | (677.8–1952.0) | |
| 4–5 | 85 | 17.7 | 983 | (531.3–1664.2) | |
| 6–9 | 86 | 18.0 | 1314 | (720.6–2074.3) | |
| 10–15 | 69 | 14.4 | 1283 | (818.1–2037.2) | |
| 16+ | 30 | 6.3 | 1149 | (633.6–1565.5) | |
| Vasoactive Infusions (CV-SOFA) | 0.21 | ||||
| 0–1 | 356 | 74.3 | 1186 | (751.7–1934.3) | |
| 2 | 13 | 2.7 | 1320 | (567.4–2567.8) | |
| 3 | 71 | 14.8 | 1235 | (674.2–1819.9) | |
| 4 | 39 | 8.1 | 1167 | (607.0–1810.2) | |
| ARDS/ALI during hospitalization | 0.15 | ||||
| Yes | 13 | 2.7 | 1351 | (918.9–2978.5) | |
| No | 466 | 97.3 | 1185 | (708.8–1869.5) | |
| ECMOe | 0.12 | ||||
| Yes | 7 | 1.5 | 795 | (397.9–1480.9) | |
| No | 471 | 98.3 | 1198 | (721.2–1888.8) | |
| Discharged From Hospital Alivef | 0.51 | ||||
| Yes | 464 | 96.9 | 1169 | (717.8–1865.3) | |
| No | 12 | 2.5 | 1495 | (691.2–1939.0) | |
| FFP Administration | 0.22 | ||||
| Any | 34 | 7.1 | 936 | (638.7–1537.3) | |
| None | 445 | 92.9 | 1202 | (725.0–1911.5) | |
| Respiratory Support | 0.5 | ||||
| None or Oxygen | 166 | 34.7 | 1231 | (725.3–1912.9) | |
| Non-invasive Ventilation | 51 | 10.6 | 1258 | (802.9–1980.5) | |
| Mechanical Ventilation | 243 | 50.7 | 1154 | (718.9–1801.1) | |
| High Frequency Ventilation | 19 | 4.0 | 1038 | (502.0–1814.3) |
Median (Interquartile range)
Testing association with serum MBL level with Mann-Whitney U or Kruskal-Wallis tests except where indicated
Spearman Correlation r = 0.123, p = 0.007; correlation with non-categorized fluid volume
Spearman Correlation
Data unavailable for 1 patient
Data unavailable for 3 patients
We also analyzed the effect of MBL2 genotype on outcomes, comparing diplotypes as High (YA/YA, YA/XA), Mid (YA/YO), and Low (XA/YO, YO/YO, and XA/XA) MBL producers (See Figure 2). As shown in Figure 2, the distribution of diplotypes was similar across categories of no SIRS vs. SIRS vs. sepsis vs. severe septic shock (of 213 patients assigned haplotypes, we were unable to assign an infectious category for 1 patient). The outcome variables listed in Table 3 for serum level analysis were tested for genotype association using Chi-square testing and none were significant (all p>0.05). We then further sub-categorized by age (given previous literature has implied there are some instances where younger children may be more affected for low production of MBL or low-producing diplotypes), however, there was also no statistical difference in distribution of diplotypes across the spectrum of non-infected vs. severe sepsis in the youngest age category (children <1 year old, n=43).
Figure 2.
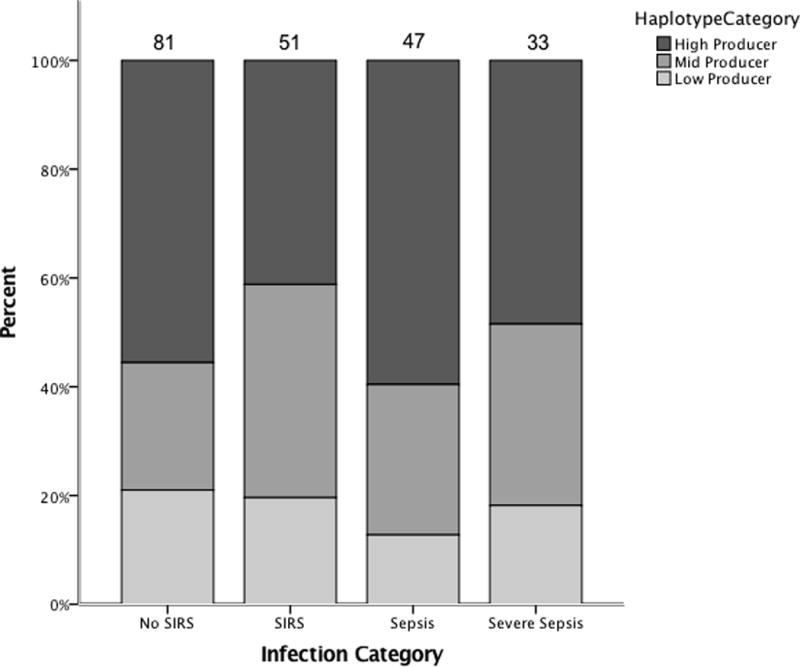
Bar graph showing relative frequencies of High-producing, Mid-producing, and Low-producing MBL diplotypes in patients grouped by infection status.
To determine whether MBL level and MBL2 diplotype may have a stronger influence in patients that do not have other, larger infectious risk factors such as neutropenia (oncologic patients) or chronic respiratory insufficiency, a subpopulation of 107 patients with either no chronic disease or only having asthma was examined. Weak associations between fluid administration (p = 0.04, Spearman’s correlation, r = 0.20) and CV-SOFA score (p = 0.05, Spearman’s correlation, r = −0.19) with lower MBL levels were identified; but no other associations were identified.
DISCUSSION
In contrast to prior reports in adult and pediatric populations, we did not find lower MBL levels in children critically ill from infection compared to those admitted to the PICU for other reasons. (4, 11, 12, 16, 19, 33–38) We did find that MBL levels were diluted in patients that received extremely high volumes of fluid in the first 24 hours; a common occurrence in patients with severe sepsis that could possibly confound the relationship between MBL levels and infection-related critical illness. MBL levels were strongly genetically influenced by the combination of high, intermediate and low producing haplotypes. We found no relationship between carriers of low-producing MBL2 diplotypes and admission for infection or development of sepsis.
In a study of MBL in critically ill children, Fidler et al (4) reported an increasing proportion of low-producing (AO and OO) haplotypes in 50 children with SIRS and sepsis in comparison to children admitted for non-infectious causes. The measured MBL in their population was significantly lower (median MBL level of 100 ng/mL in lowest producing population) than levels in our study. (4) Similarly, Garred et al (37) and Gordon et al (36) reported MBL polymorphisms increasing risk of sepsis in critically ill adult populations. However, other studies in adults have not found MBL to be associated with risk for sepsis (39–42) or have reported a more nuanced picture that includes a mixture of both pro-inflammatory and anti-infection effects which may be beneficial or detrimental in varying disease states. (33, 34, 43) Specifically in pediatrics, six studies have demonstrated an association between lower MBL levels, or low-MBL producing haplotypes, and increased severity of infection-related disease (4, 5, 15, 16, 44–46) while five studies showed similar findings to ours with a lack of association (12, 46–50) and two studies concluded a possible protective role for low-producing MBL genotypes or levels. (12, 51)
There are likely several etiologies behind the lack of association between MBL levels, low-producing MBL haplotypes, and increased susceptibility to severe infection. First, the redundancy in complement activation within the innate immune system may provide relative resilience. (2) Second, our range of MBL levels (IQR 718–1878) are relatively high in comparison to a previously described cut-off of 500 for clinically important MBL deficiency. (4, 7, 12) Severe MBL deficiency has been described by some as <50 ng/ml, a level not identified in our patients; it has been postulated that patients are relatively protected above this low level due to pathway redundancy. (52, 53) Finally, in pro-inflammatory states which are often seen in critical illness, low-producing MBL may be advantageous if higher MBL production is associated with more severe inflammatory damage via a different mechanism. (42, 54–56) Grouping heterogeneous disease-states together under an umbrella of infection-related critical illness could combine cohorts in which MBL is detrimental due to inflammation with those in which it is beneficial.
Strengths of our study included a relatively large cohort of 520 children compared to prior studies and a study design which allowed for comparison of multiple different subsets of critically ill patients while maintaining an appropriate control group of similar patients. We were able to replicate the previously observed relationship between MBL genotype and MBL level (See Figure 1B). Additionally, we were able to evaluate the association between MBL levels and haplotypes in 284 children with SIRS, sepsis, or severe sepsis.
This study had several limitations. Because we a priori limited our population for genetic analysis to whites to prevent racial stratification (6, 32), and because not all patients consented for genotyping, our statistical power for subset group analysis was limited as was our ability to generalize these results to all populations. With limited numbers, we were unable to assess the association of specific types of infections with MBL haplotypes and our findings are limited to the broader category of critically ill patients with infections. Additionally, although patient age may influence the importance of MBL in immune protection (i.e. younger patients lacking humoral immunity may be more dependent on innate immunity), we had relatively few neonates as this study assessed a pediatric ICU population.
CONCLUSIONS
We did not find an association between infection-related causes of illness and MBL levels or MBL2 haplotypes in children admitted to the PICU. As such, our results do not support testing for MBL deficiency or use of recombinant MBL as a potential immunomodulatory therapy for children with severe and life-threatening infections. Because large-volume fluid resuscitation was often a marker of a higher illness severity, we were not able to determine if fluid dilution of plasma proteins such as MBL may predispose patients to worse outcomes. Our study supports that variability in MBL levels is genetically determined. To control for the risk of MBL serum level dilution with large volumes of fluid, we suggest that future studies in critically ill patients use MBL2 haplotype carriage to reflect pre-illness MBL levels.
Acknowledgments
We thank Ellen Smith, BA for her assistance with patient recruitment. We thank Shannon Keisling, BA and Stephanie Cobb, BA for their input on laboratory issues. We thank Hardeep Ranu, PhD from the Genotyping Core Facility, and Partner’s Health Care Center for Personalized Genetic Medicine (PCPGM) for genotyping support.
Financial Support: This work was supported by a grant from the Clinical Research Program (CRP) at Children’s Hospital Boston. EM was supported by the Fred Lovejoy Housestaff Research Fund. KM participated with support from Harvard Catalyst (NIH Award #UL1 RR 025758). Dr. Randolph was supported by the National Institutes of Health (AI084011).
Footnotes
Reprint Request Address: Reprints are not available.
References
- 1.Kilpatrick DC. Mannan-binding lectin and its role in innate immunity. Transfus Med. 2002;12(6):335–52. doi: 10.1046/j.1365-3148.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway–its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 3.Auriti C, Prencipe G, Inglese R, et al. Role of mannose-binding lectin in nosocomial sepsis in critically ill neonates. Hum Immunol. 2010;71(11):1084–8. doi: 10.1016/j.humimm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Fidler KJ, Wilson P, Davies JC, et al. Increased incidence and severity of the systemic inflammatory response syndrome in patients deficient in mannose-binding lectin. Intensive Care Med. 2004;30(7):1438–45. doi: 10.1007/s00134-004-2303-8. [DOI] [PubMed] [Google Scholar]
- 5.Darton TC, Jack DL, Johnson M, et al. MBL2 deficiency is associated with higher genomic bacterial loads during meningococcemia in young children. Clin Microbiol Infect. 2014;20(12):1337–42. doi: 10.1111/1469-0691.12745. [DOI] [PubMed] [Google Scholar]
- 6.De Pascale G, Cutuli SL, Pennisi MA, et al. The role of mannose-binding lectin in severe sepsis and septic shock. Mediators Inflamm. 2013;2013:625803. doi: 10.1155/2013/625803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisen DP, Dean MM, Thomas P, et al. Low mannose-binding lectin function is associated with sepsis in adult patients. FEMS Immunol Med Microbiol. 2006;48(2):274–82. doi: 10.1111/j.1574-695X.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 8.Minchinton RM, Dean MM, Clark TR, et al. Analysis of the relationship between mannose-binding lectin (MBL) genotype, MBL levels and function in an Australian blood donor population. Scand J Immunol. 2002;56(6):630–41. doi: 10.1046/j.1365-3083.2002.01167.x. [DOI] [PubMed] [Google Scholar]
- 9.Bronkhorst MW, Lomax MA, Vossen RH, et al. Risk of infection and sepsis in severely injured patients related to single nucleotide polymorphisms in the lectin pathway. Br J Surg. 2013;100(13):1818–26. doi: 10.1002/bjs.9319. [DOI] [PubMed] [Google Scholar]
- 10.Eisen DP, Dean MM, Boermeester MA, et al. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin Infect Dis. 2008;47(4):510–6. doi: 10.1086/590006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frakking FN, Brouwer N, van Eijkelenburg NK, et al. Low mannose-binding lectin (MBL) levels in neonates with pneumonia and sepsis. Clin Exp Immunol. 2007;150(2):255–62. doi: 10.1111/j.1365-2249.2007.03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frakking FN, van de Wetering MD, Brouwer N, et al. The role of mannose-binding lectin (MBL) in paediatric oncology patients with febrile neutropenia. Eur J Cancer. 2006;42(7):909–16. doi: 10.1016/j.ejca.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 13.van Till JW, Modderman PW, de Boer M, et al. Mannose-binding lectin deficiency facilitates abdominal Candida infections in patients with secondary peritonitis. Clin Vaccine Immunol. 2008;15(1):65–70. doi: 10.1128/CVI.00297-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Benedetti F, Auriti C, D’Urbano LE, et al. Low serum levels of mannose binding lectin are a risk factor for neonatal sepsis. Pediatr Res. 2007;61(3):325–8. doi: 10.1203/pdr.0b013e318030d12f. [DOI] [PubMed] [Google Scholar]
- 15.Broeders EN, Wissing KM, Hazzan M, et al. Evolution of immunoglobulin and mannose binding protein levels after renal transplantation: association with infectious complications. Transpl Int. 2008;21(1):57–64. doi: 10.1111/j.1432-2277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Shang S, Zhang C, et al. Lower mannose-binding lectin contributes to deleterious H1N1 2009 infection in children. Apmis. 2014;122(2):136–9. doi: 10.1111/apm.12111. [DOI] [PubMed] [Google Scholar]
- 17.Koenig KF, Potlukova E, Mueller B, et al. MBL serum levels in patients with sepsis correlate with thyroid function but not with outcome. Clin Immunol. 2012;144(1):80–2. doi: 10.1016/j.clim.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Liman P, Babel N, Schachtner T, et al. Mannose-binding lectin deficiency is not associated with increased risk for polyomavirus nephropathy. Transpl Immunol. 2012;26(2–3):123–7. doi: 10.1016/j.trim.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Peterslund NA, Koch C, Jensenius JC, et al. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358(9282):637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhang AQ, Yue CL, Pan W, et al. Mannose-binding lectin polymorphisms and the risk of sepsis: evidence from a meta-analysis. Epidemiol Infect. 2014;142(10):2195–206. doi: 10.1017/S0950268813003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao DN, Zhang Y, Ren YB, et al. Relationship of serum mannose-binding lectin levels with the development of sepsis: a meta-analysis. Inflammation. 2015;38(1):338–47. doi: 10.1007/s10753-014-0037-5. [DOI] [PubMed] [Google Scholar]
- 22.Frakking FN, Brouwer N, van de Wetering MD, et al. Safety and pharmacokinetics of plasma-derived mannose-binding lectin (MBL) substitution in children with chemotherapy-induced neutropaenia. Eur J Cancer. 2009;45(4):505–12. doi: 10.1016/j.ejca.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Valdimarsson H. Infusion of plasma-derived mannan-binding lectin (MBL) into MBL-deficient humans. Biochem Soc Trans. 2003;31(Pt 4):768–9. doi: 10.1042/bst0310768. [DOI] [PubMed] [Google Scholar]
- 24.Valdimarsson H, Vikingsdottir T, Bang P, et al. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand J Immunol. 2004;59(1):97–102. doi: 10.1111/j.0300-9475.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 25.Madden K, Feldman HA, Smith EM, et al. Vitamin D deficiency in critically ill children. Pediatrics. 2012;130(3):421–8. doi: 10.1542/peds.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care. 2008;12(6):R161. doi: 10.1186/cc7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 30.Williams DJ, Edwards KM, Self WH, et al. Antibiotic Choice for Children Hospitalized With Pneumonia and Adherence to National Guidelines. Pediatrics. 2015;136(1):44–52. doi: 10.1542/peds.2014-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naito H, Ikeda A, Hasegawa K, et al. Characterization of human serum mannan-binding protein promoter. J Biochem. 1999;126(6):1004–12. doi: 10.1093/oxfordjournals.jbchem.a022543. [DOI] [PubMed] [Google Scholar]
- 32.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155(6):3013–20. [PubMed] [Google Scholar]
- 33.Liu L, Ning B. The role of MBL2 gene polymorphism in sepsis incidence. Int J Clin Exp Pathol. 2015;8(11):15123–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Huh JW, Song K, Yum JS, et al. Association of mannose-binding lectin-2 genotype and serum levels with prognosis of sepsis. Crit Care. 2009;13(6):R176. doi: 10.1186/cc8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Laorden MI, Sole-Violan J, Rodriguez de Castro F, et al. Mannose-binding lectin and mannose-binding lectin-associated serine protease 2 in susceptibility, severity, and outcome of pneumonia in adults. J Allergy Clin Immunol. 2008;122(2):368–74. 74.e1–2. doi: 10.1016/j.jaci.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 36.Gordon AC, Waheed U, Hansen TK, et al. Mannose-binding lectin polymorphisms in severe sepsis: relationship to levels, incidence, and outcome. Shock. 2006;25(1):88–93. doi: 10.1097/01.shk.0000186928.57109.8d. [DOI] [PubMed] [Google Scholar]
- 37.Garred P, J JS, Quist L, et al. Association of mannose-binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J Infect Dis. 2003;188(9):1394–403. doi: 10.1086/379044. [DOI] [PubMed] [Google Scholar]
- 38.Frakking FN, Israels J, Kremer LC, et al. Mannose-binding lectin (MBL) and the risk for febrile neutropenia and infection in pediatric oncology patients with chemotherapy. Pediatr Blood Cancer. 2011;57(1):89–96. doi: 10.1002/pbc.22901. [DOI] [PubMed] [Google Scholar]
- 39.Mills TC, Chapman S, Hutton P, et al. Variants in the Mannose-binding Lectin Gene MBL2 do not Associate With Sepsis Susceptibility or Survival in a Large European Cohort. Clin Infect Dis. 2015;61(5):695–703. doi: 10.1093/cid/civ378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osthoff M, Au Yong HM, Dean MM, et al. Significance of mannose-binding lectin deficiency and nucleotide-binding oligomerization domain 2 polymorphisms in Staphylococcus aureus bloodstream infections: a case-control study. PLoS One. 2013;8(9):e76218. doi: 10.1371/journal.pone.0076218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrera-Ramos E, Lopez-Rodriguez M, Ruiz-Hernandez JJ, et al. Surfactant protein A genetic variants associate with severe respiratory insufficiency in pandemic influenza A virus infection. Crit Care. 2014;18(3):R127. doi: 10.1186/cc13934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellemann D, Larsson A, Madsen HO, et al. Heterozygosity of mannose-binding lectin (MBL2) genotypes predicts advantage (heterosis) in relation to fatal outcome in intensive care patients. Hum Mol Genet. 2007;16(24):3071–80. doi: 10.1093/hmg/ddm265. [DOI] [PubMed] [Google Scholar]
- 43.Smithson A, Perello R, Aibar J, et al. Genotypes coding for low serum levels of mannose-binding lectin are underrepresented among individuals suffering from noninfectious systemic inflammatory response syndrome. Clin Vaccine Immunol. 2010;17(3):447–53. doi: 10.1128/CVI.00375-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agbeko RS, Fidler KJ, Allen ML, et al. Genetic variability in complement activation modulates the systemic inflammatory response syndrome in children. Pediatr Crit Care Med. 2010;11(5):561–7. doi: 10.1097/PCC.0b013e3181d900ba. [DOI] [PubMed] [Google Scholar]
- 45.Frakking FN, Brouwer N, Dolman KM, et al. Mannose-binding lectin (MBL) as prognostic factor in paediatric oncology patients. Clin Exp Immunol. 2011;165(1):51–9. doi: 10.1111/j.1365-2249.2011.04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingels C, Vanhorebeek I, Steffensen R, et al. Lectin pathway of complement activation and relation with clinical complications in critically ill children. Pediatr Res. 2014;75(1–1):99–108. doi: 10.1038/pr.2013.180. [DOI] [PubMed] [Google Scholar]
- 47.Ammann RA, Bodmer N, Simon A, et al. Serum Concentrations of Mannan-Binding Lectin (MBL) and MBL-Associated Serine Protease-2 and the Risk of Adverse Events in Pediatric Patients With Cancer and Fever in Neutropenia. J Pediatric Infect Dis Soc. 2013;2(2):155–61. doi: 10.1093/jpids/pit005. [DOI] [PubMed] [Google Scholar]
- 48.Lehrnbecher T, Venzon D, de Haas M, et al. Assessment of measuring circulating levels of interleukin-6, interleukin-8, C-reactive protein, soluble Fc gamma receptor type III, and mannose-binding protein in febrile children with cancer and neutropenia. Clin Infect Dis. 1999;29(2):414–9. doi: 10.1086/520224. [DOI] [PubMed] [Google Scholar]
- 49.Lian YC, Della-Negra M, Rutz R, et al. Immunological analysis in paediatric HIV patients at different stages of the disease. Scand J Immunol. 2004;60(6):615–24. doi: 10.1111/j.0300-9475.2004.01492.x. [DOI] [PubMed] [Google Scholar]
- 50.Lausen B, Schmiegelow K, Andreassen B, et al. Infections during induction therapy of childhood acute lymphoblastic leukemia–no association to mannose-binding lectin deficiency. Eur J Haematol. 2006;76(6):481–7. doi: 10.1111/j.1600-0609.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 51.Sprong T, Mollnes TE, Neeleman C, et al. Mannose-binding lectin is a critical factor in systemic complement activation during meningococcal septic shock. Clin Infect Dis. 2009;49(9):1380–6. doi: 10.1086/606054. [DOI] [PubMed] [Google Scholar]
- 52.Brouwer N, Dolman KM, van Zwieten R, et al. Mannan-binding lectin (MBL)-mediated opsonization is enhanced by the alternative pathway amplification loop. Mol Immunol. 2006;43(13):2051–60. doi: 10.1016/j.molimm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37(11):1496–505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 54.Carroll KE, Dean MM, Heatley SL, et al. High levels of mannose-binding lectin are associated with poor outcomes after lung transplantation. Transplantation. 2011;91(9):1044–9. doi: 10.1097/TP.0b013e318212c7d6. [DOI] [PubMed] [Google Scholar]
- 55.Ling MT, Tu W, Han Y, et al. Mannose-binding lectin contributes to deleterious inflammatory response in pandemic H1N1 and avian H9N2 infection. J Infect Dis. 2012;205(1):44–53. doi: 10.1093/infdis/jir691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilgin YM, Brand A, Berger SP, et al. Mannose-binding lectin is involved in multiple organ dysfunction syndrome after cardiac surgery: effects of blood transfusions. Transfusion. 2008;48(4):601–8. doi: 10.1111/j.1537-2995.2007.01585.x. [DOI] [PubMed] [Google Scholar]


