Abstract
Background and purpose
Radiotherapy is an effective treatment for managing breast cancer, but patients may experience shoulder morbidity after completing radiotherapy. There is a knowledge gap regarding how the inclusion of the regional lymphatics in radiation treatment regimens influence the radiation dose delivered to the underlying shoulder musculature.
Material and methods
Five standardized radiation treatment regimens were developed from the computed tomography (CT) scans of 11 patients: tangent fields only (T), high tangent fields (HT), T + supraclavicular fossa and axillary apex with an anterior oblique beam (SCV), T + SCV + axillary nodes with an anterior oblique beam (SCV + AX), and T + SCV + AX with the nodal regions treated with a directly opposed beam configuration (DO). The muscle volumes for nine shoulder muscles anatomically located with the treatment regimens were segmented from the same CT scans. The effect of the nine muscles and five treatment regimens on the percentage of each muscle receiving at least 48 Gy (V48 Gy) was analyzed with two-way and one-way repeated measures ANOVAs.
Results
A statistically significant interaction existed between the nine shoulder muscles and five treatment regimens (p < 0.001) on the V48 Gy dose. Subsequent one-way analyses found statistically significant main effects of treatment plan on the V48 Gy dose for each muscle (p < 0.001). The pectoralis major and minor had the greatest V48 doses across the five treatments regimens. The HT, SCV + AX and DO treatment regimens produced statistically significant increases in the V48 dose of the latissimus dorsi and teres major. The infraspinatus, subscapularis, supraspinatus, teres minor, and trapezius only observed statistically significant V48 doses when treated with a DO plan.
Conclusions
These findings highlight the muscles (pectoralis major, pectoralis minor, latissimus dorsi, and teres major) that may exhibit future morbidity after radiation, and indicate that nodal RT delivered with a DO beam arrangement delivers the highest muscle dose.
Keywords: Breast cancer, Radiotherapy treatment planning, Radiotherapy morbidity, Dose–volume histograms, Axillary radiotherapy, Computed tomography
Nearly 70% of women diagnosed with early-stage breast cancer are treated with breast-conserving surgery and radiotherapy (RT) [1]. RT is effective in managing breast cancer, reducing the recurrence of breast cancer and increasing long-term survival [2]. External beam radiation is the most widely used form of RT, often in the supine position with two coplanar tangential photon beams encompassing the entire breast [3]. The standard tangential beam arrangement can be expanded to include a portion of the axillary nodes within the treatment volume [4], otherwise known as a “high-tangent” field. Alternatively, the treatment volume can be expanded to include the supraclavicular and/or axillary nodes by adding a third beam oriented in an anterior oblique direction [5]. The use of this third beam to treat node-positive breast cancer patients is expected to increase in the coming years. The AMAROS trial recently found in a subset of clinically node negative but pathologically node positive patients that RT to the axillary nodes is as effective at regional control as an axillary lymph node dissection, but with a reduced risk of lymphedema [6].
It is important to address the long-term sequelae of axillary RT treatments as they become more frequent in future years. The increased treatment volume to encompass regional nodes may increase the dose to shoulder muscles and could explain the greater rate of shoulder morbidity in patients treated with nodal RT [7]. In support of the effect of RT on shoulder morbidity, the AMAROS trial found that axillary RT yielded impairment in upper extremity range of motion and quality of life that was equivalent to the axillary dissection arm [6]. Furthermore, axillary RT trended toward greater restriction in arm mobility when compared to axillary dissection. Similarly, RT has been identified as a significant source of arm and shoulder morbidity across a variety of patient-reported outcome surveys and biomechanical assessments [7-12].
The current study addresses how different beam configurations utilized in breast cancer RT influence the radiation dose delivered to individual shoulder muscles. The dose delivered to the shoulder musculature within the treatment volume is not routinely measured in clinical practice and has only been reported for the entire shoulder musculature [13]. The radiation delivered to individual shoulder muscles that are anatomically located within the planned treated fields for breast cancer patients has yet to be measured. Identifying the individual muscles most impacted by various beam configurations is important for elucidating the underlying causes of shoulder morbidity after RT and optimizing future RT protocols. Therefore, the objective of this study was to describe how different RT beam arrangements influence the radiation dose incidentally delivered to a various shoulder muscles that reside within the treatment volume.
Materials and methods
A retrospective review of patient CT treatment simulations was approved by the Institutional Board Review of Northwestern University. Eleven consecutive patients with unilateral breast cancer treated with breast-conserving surgery and sentinel-node biopsy followed by chemotherapy and RT to the breast and axilla were identified. An a priori sample size calculation was performed after the first five patients were analyzed (partial eta squared = 0.861). Using a repeated measures ANOVA, a statistically significant within-subjects interaction (alpha = 0.05 level) could be detected with 80% power with ten patients. The female patients had a mean age of 52 years (range: 36–70 years), 3 patients were treated on the right side and 8/11 were treated on the left side, and breast cancer staging was IIA for 3 patients, IIB for 4 patients, IIIA for 1 patient, and IIIC for 3 patients. CT simulation scans were acquired previously for clinical care (Phillips Healthcare, Andover, MA; Brilliance Big Bore Oncology scanner, 85 cm bore size, 60 cm scan field of view). In all scans, the patient's arms were fully abducted with the patient's elbow flexed and hands placed behind the head. The selection of patients that underwent axillary RT insured that the CT scans included the entire shoulder musculature within the image volume. Treatment regimens were created with the Pinnacle treatment planning system (Phillips Radiation Oncology Systems, Fitchburg, WI). Five treatment regimens were created for each patient (Table 1) to simulate how the variations in treatment regimens affect the radiation dose delivered to individual muscles.
Table 1.
Treatment plans used to simulate radiation dose delivered to individual muscles.
| Abbreviation | Scan information | Total dose (Gy) |
|---|---|---|
| T | Tangent beams inclusive of the whole breast volume | 50 |
| HT | High tangent beams inclusive of the whole breast volume and level I axillary nodes | 50 |
| T + SCV | Tangent beams inclusive of the whole breast volume and anterior oblique beam inclusive of level III axillary nodes | 50 |
| T + SCV + AX | Tangent beams inclusive of the whole breast volume and anterior oblique beam inclusive of level I-III axillary nodes | 50 |
| T + SCV + AX (DO) | Tangent beams inclusive of the whole breast volume and directly opposed anterior-posterior oblique beams inclusive of level I-III axillary nodes | 50 |
A treatment regimen of 50 Gy in 2 Gy fractions was prescribed for all treatment regimens. A field-in-field technique was used in the tangent fields to optimize homogeneity and keep the breast to within ±7% of the prescription dose. In brief, the five beam arrangements were designed as follows:
Tangent (T): tangents fields included the entire breast without intentional coverage of the adjacent lymph nodes.
High Tangent (HT): the standard tangent fields were modified to also include the entirelevel 1 axillary nodes.
Supraclavicular field (SCV): an anterior obliqued nodal field was added to the standard tangent fields using a mono-isocentric technique. This was designed to encompass the supraclavicular fossa and level III of the axilla. The lateral boundary was placed at the medial aspect of the humeral head. The prescription point was selected to limit the hot spot to <110% of the rx dose and to cover the nodal contours with at least 90% of the rx dose.
Supraclavicular + axilla field (SCV + AX): A nodal field was added to the standard tangent fields. This was designed to encompass the supraclavicular fossa and levels I–III of the axilla. The lateral boundary was placed past the lateral aspect of the humeral head. The prescription point was selected to limit the hot spot to <110% of the rx dose and to cover the nodal contours with at least 90% of the rx dose.
Directly opposed fields (DO): The anterior obliqued nodal field used in SCV + AX was directly opposed with an identical field. The prescription point was set at midplane. The beams were weighted in a 60/40 ratio favoring the anterior obliqued field. This represents a historical beam arrangement still used at a handful of centers.
The boundaries for nine shoulder muscles that are anatomically located within the planned treatment fields described above were segmented on each axial slice of the CT simulation. The segmented muscles were the infraspinatus, latissimus dorsi, pectoralis major, pectoralis minor, subscapularis, supraspinatus, teres major, teres minor, and trapezius. The latissimus dorsi was not fully visible within the CT scan's field of view, so segmentation was limited to the portion of the muscle superior of the T7 thoracic vertebrae. The radiodensity window was adjusted to distinguish between the individual muscles and their respective borders with adjacent muscles. Segmentation was initially performed by a specialist in human anatomy and imaging (DBL), and was further reviewed by an attending radiation oncologist (JBS). Radiation dose to the individual muscles was measured within the Pinnacle software for each treatment plan. Representative CT scans are shown in Fig. 1 along with the radiation isodose curves and individual muscle segmentations.
Fig. 1.
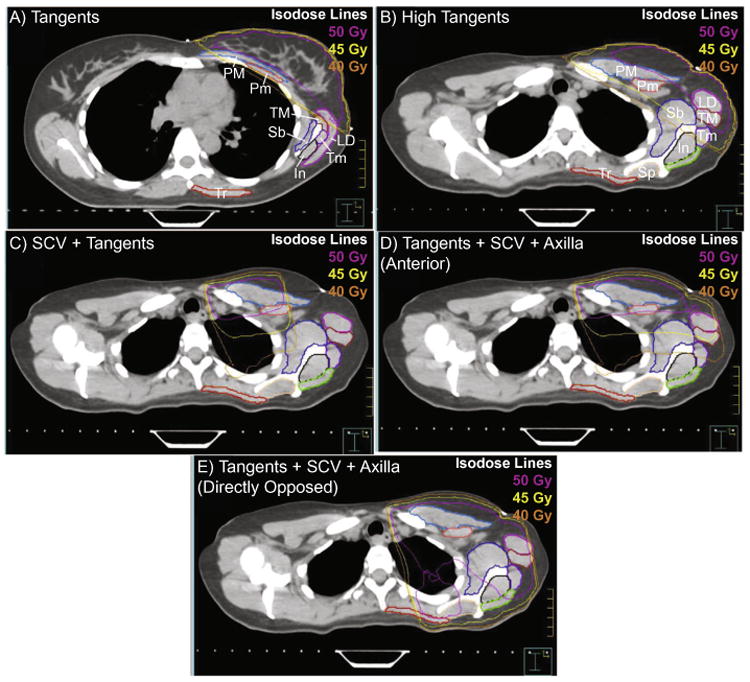
Representative treatment regimens for one individual highlight how the radiation treatments (shown as isodose lines of 40,45, and 50 Gy) alter the dose delivered to the segmented muscles. (A) depicts the tangent field on an inferior slice at the level of the breast, while (B)–(E) show the radiation field on a superior slice at the level of the scapular spine for a (B) high tangents field, (C) supraclavicular field, (D) combined supraclavicular and axillary field, and (E) a combined supraclavicular and axillary field using directly opposed anterior and posterior beams. Nine muscles were segmented on each slice, and are abbreviated on images (A) and (B): infraspinatus (In), latissimus dorsi (LD), pectoralis major (PM), pectoralis minor (Pm), subscapularis (Sb), supraspinatus (Sp), teres major (TM), teres minor (Tm), and trapezius (Tr).
The outcome measurements for this study were the mean radiation dose in Gy delivered to each muscle and the percentage volume of the individual muscles that receive a radiation dose of at least 30 Gy (V30 Gy) or 48 Gy (V48 Gy). The V30 Gy measurement signifies individual muscles that received a moderate dose of radiation with a standardized 50 Gy treatment regimen. The V48 Gy measurement signifies individual muscles that received a near maximal dose for patients treated with a standardized 50 Gy treatment regimen. Statistical analyses were performed in SPSS (v24, IBM Corp., Armonk, NY). A two-way repeated measures ANOVA was used to test the V48 Gy measurement for a significant interaction between the five treatment regimens and nine individual muscles. Both muscle and treatment plan were treated as within-subject factors. One-way repeated measure ANOVAs were individually ran for each shoulder muscle to test for the effect of treatment regimen. Significant was set at p < 0.05, with Greenhouse-Geisser corrections and Bonferroni-corrected post hoc comparisons (relative to the tangent field condition) performed when applicable.
Results
The mean radiation dose delivered across the entire muscle region included in our analysis increased as the treatment volume was expanded to include the regional lymphatics (95% confidence intervals: T: 8.1–9.8 Gy; H: 15.4–17.1 Gy; SCV: 19.0–20.7 Gy; SCV + AX: 24.7–26.4 Gy; DO: 28.8–30.3 Gy). The percentage volume of the entire muscle region that exceeded the V48 Gy dose also increased as the treatment volume was expanded (95% confidence intervals: T: 4.7–7.7%; H: 8.7–12.9%; SCV: 10.3–13.1%; SCV + AX: 13.6–16.9%; DO: 36.8–40.9%). Representative dose–volume histograms from one representative subject across the five treatment regimens are shown for all nine muscles in Fig. 2. These individual results indicate that certain muscle groups like the pectoralis major or pectoralis receive a large radiation dose regardless of treatment regimen, while the dose delivered to other seven muscles are sensitive to the regimen utilized to treat the axillary regions. These results were confirmed by group results for each of the nine muscles examined across all 11 subjects for the mean dose, V30 Gy dose and V48 Gy dose (Table 2).
Fig. 2.

Representative dose–volume histograms for one individual for the nine segmented muscles. Each plot contains five lines indicating how the dose–volume changes for a given muscle across the five treatment regimens.
Table 2.
The mean dose (in Gy) and the percentage volumes (%) of each shoulder muscle that received at least 30 Gy or 48 Gy of radiation during the course of a standard radiation treatment plan for breast cancer (50 Gy fractionized over 25 sessions).
| Radiation treatment regimen | |||||
|---|---|---|---|---|---|
|
| |||||
| T | HT | SCV | SCV + AX | DO | |
| Infraspinatus | |||||
| Mean dose (Gy) | 0.8 ± 0.4 | 2.2 ± 1.7 | 6.0 ± 2.1 | 12.5 ± 3.3 | 17.7 ± 4.8 |
| V30 Gy dose (%) | 0.0 ± 0.0 | 1.2 ± 2.0 | 1.8 ± 2.9 | 15.0 ± 11.7 | 30.6 ± 9.2 |
| V48 Gy dose (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 17.5 ± 6.6*** |
| Latissimus dorsi | |||||
| Mean dose (Gy) | 10.7 ± 3.6 | 16.4 ± 4.1 | 11.5 ± 3.7 | 14.7 ± 3.3 | 15.1 ± 3.2 |
| V30 Gy dose (%) | 17.6 ± 7.9 | 30.0 ± 8.7 | 19.4 ± 8.0 | 25.3 ± 8.8 | 26.1 ± 7.3 |
| V48 Gy dose (%) | 9.4 ± 7.5 | 19.2 ± 10.7*** | 11.9 ± 7.3 | 13.7 ± 7.6* | 17.4 ± 6.7** |
| Pectoralis major | |||||
| Mean dose (Gy) | 19.9 ± 3.9 | 29.3 ± 3.6 | 34.3 ± 1.9 | 38.6 ± 2.7 | 39.3 ± 2.6 |
| V30 Gy dose (%) | 37.1 ± 8.1 | 56.5 ± 7.9 | 65.4 ± 4.4 | 74.6 ± 5.5 | 74.9 ± 5.4 |
| V48 Gy dose (%) | 24.6 ± 12.9 | 33.6 ± 16.1** | 48.0 ± 9.4*** | 57.1 ± 11.3*** | 62.8 ± 9.4*** |
| Pectoralis minor | |||||
| Mean dose (Gy) | 28.0 ± 4.3 | 41.9 ± 2.8 | 44.9 ± 2.1 | 47.9 ± 0.9 | 49.5 ± 0.6 |
| V30 Gy dose (%) | 55.9 ± 8.6 | 87.2 ± 6.4 | 92.2 ± 6.7 | 100.0 ± 0.1 | 100.0 ± 0.1 |
| V48 Gy dose (%) | 16.7 ± 15.2 | 22.6 ± 18.3 | 34.9 ± 15.9** | 49.4 ± 17.9*** | 77.2 ± 12.4*** |
| Subscapularis | |||||
| Mean dose (Gy) | 4.5 ± 2.7 | 13.7 ± 4.7 | 15.9 ± 3.1 | 24.7 ± 4.1 | 30.1 ± 5.1 |
| V30 Gy dose (%) | 4.9 ± 4.8 | 22.8 ± 11.9 | 22.2 ± 13.7 | 42.9 ± 21.1 | 55.3 ± 10.8 |
| V48 Gy dose (%) | 0.3 ± 0.9 | 1.0 ± 1.2 | 1.0 ± 1.3 | 1.8 ± 2.0 | 34.7 ± 12.8*** |
| Supraspinatus | |||||
| Mean dose (Gy) | 0.3 ± 0.1 | 0.7 ± 0.3 | 29.9 ± 2.8 | 31.0 ± 2.4 | 44.0 ± 3.3 |
| V30 Gy dose (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 57.8 ± 38.0 | 60.4 ± 38.3 | 85.7 ± 6.8 |
| V48 Gy dose (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 69.7 ± 12.0*** |
| Teres major | |||||
| Mean dose (Gy) | 12.9 ± 5.9 | 27.5 ± 4.8 | 15.6 ± 6.1 | 24.8 ± 6.0 | 26.4 ± 6.2 |
| V30 Gy dose (%) | 19.5 ± 13.7 | 52.0 ± 13.7 | 25.8 ± 14.1 | 43.5 ± 21.4 | 46.4 ± 15.8 |
| V48 Gy dose (%) | 4.5 ± 7.6 | 19.9 ± 15.0* | 9.2 ± 8.1 | 15.1 ± 9.2* | 27.5 ± 11.9*** |
| Teres minor | |||||
| Mean dose (Gy) | 3.0 ± 2.8 | 14.1 ± 8.1 | 4.9 ± 3.6 | 19.9 ± 4.9 | 25.3 ± 6.1 |
| V30 Gy dose (%) | 1.9 ± 3.1 | 21.2 ± 19.9 | 3.9 ± 6.0 | 33.6 ± 22.7 | 44.2 ± 13.4 |
| V48 Gy dose (%) | 0.1 ± 0.2 | 1.3 ± 2.6 | 0.1 ± 0.3 | 0.1 ± 0.4 | 25.3 ± 11.6*** |
| Trapezius | |||||
| Mean dose (Gy) | 0.2 ± 0.1 | 0.3 ± 0.1 | 15.8 ± 3.2 | 15.8 ± 3.3 | 18.8 ± 3.7 |
| V30 Gy dose (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 25.0 ± 16.8 | 25.0 ± 17.0 | 30.5 ± 9.6 |
| V48 Gy dose (%) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 17.6 ± 6.5*** |
Results reported as mean ± 1 standard deviation.
Abbreviations: Tangent (T), High tangent (HT), Supraclavicular field (SCV), Supraclavicular + axilla field (SCV + AX), Directly opposed fields (DO).
The Bonferroni-corrected significance level for multiple comparisons on V48 Gy doses (compared to T):
p < 0.05.
p < 0.01.
p < 0.001.
Statistical analyses were performed on the V48 Gy dose results to interpret how the five different treatment regimens affect the percentage of each muscle that receive a near maximal dose from a standardized 50 Gy treatment regimen. A statistically significant interaction between the nine muscle and five treatment regimens was found for the percentage volume that received at least 48 Gy (F (32,320) = 41.59; ε = 0.156, p < 0.001). The two-way ANOVA analysis was followed up by one-way ANOVAs for each muscle. There was a statistically significant main effect of treatment plan for all nine muscles analyzed (p < 0.001 for all comparisons). Multiple comparisons were performed to investigate how the inclusion of the axillary fields influences radiation dose for individual muscles when compared to tangent fields only (Table 2). A statistically significant increase in the V48 Gy dose delivered to the pectoralis major and pectoralis minor muscles was found as the treatment volume was expanded to include the axilla. The lone exception that did not produce a statistically significant difference in the V48 Gy dose was for the pectoralis minor between the T and HT plans. The V48 Gy dose delivered to the latissimus dorsi and teres major muscles was sensitive to the simulated treatment plan as statistically significant increases were found for the HT, SCV + AX, and DO plans but not for the SCV plan. The infraspinatus, subscapularis, supraspinatus, teres minor, and trapezius only received a statistically significant increase in the 48 Gy dose when treatments were simulated with the DO condition.
Discussion
Radiotherapy is an effective treatment for eradicating cancer cells and reducing the risk of breast cancer recurrence and death. There is a need to address the potential comorbidities of RT as more and more women become long-term survivors of breast cancer. The current study examined how different treatment regimens that are commonly used in clinical practice may influence the radiation dose received by individual muscles. These findings will allow clinicians and therapists to gain important regional insights into where radiation may produce future morbidity. Treatment of the whole breast deposited high doses of radiation within the pectoralis major and pectoralis minor. Expansion of the treatment volume to include the axillary and supraclavicular nodes increased radiation dose to portions of the latissimus dorsi and teres major. Of the five treatment regimens examined in the study, the DO beam arrangement exposed the shoulder musculature to the greatest radiation dose. The findings of this study provide new insights for radiation oncologists, physiatrists, and physical therapists regarding the specific muscles that should be monitored in breast cancer patients after they complete RT.
The delivery of incidental radiation to the shoulder joint during axillary RT (specifically the humeral head) has been previously identified, with the shoulder joint being identified as an organ at-risk with a 15 Gy dose [12]. However, the thresholds of radiation dose and volume that an individual shoulder muscle can withstand before functional impairment occurs have not been fully elucidated. Clinical guidelines suggest that the maximal safe dosage for muscle tissue treated with fractionized radiation in adults is 60–80 Gy [14]. These guidelines inherently suggest that all muscles examined in this study are safe in terms of the development of fibrosis and atrophy. By contrast, there is evidence to suggest that the threshold for functional impairment in muscle may be considerably lower than 60–80 Gy. Functional changes to muscle, including atrophy and weakness, have been reported in Hodgkin lymphoma patients treated with 40 Gy of radiation using a DO mantle field configuration [15]. Atrophy of the pectoralis major and pectoralis minor has also been documented in breast cancer patients undergoing standardized RT protocols [16]. Similarly, patients that undergo standardized RT protocols of 50 Gy to the breast and axilla exhibit statistically significant reductions in shoulder range of motion and/or strength [7,17,18]. This suggests that underlying changes to the mechanical properties of muscle and/or neuromuscular control of a particular muscle are potentially impacted by RT. Given the lack of clarity, more research is needed to assess how clinically applicable levels of radiation delivered to individual muscle influence functional outcomes. The current study demonstrates a methodology that radiation oncologists can utilize to compare arm and shoulder morbidity to the dose delivered to individual shoulder muscles.
The objective of this study was to highlight the individual muscle or muscles at risk for impairment after RT. The large radiation dose delivered to the pectoralis major and pectoralis minor is not surprising given their anatomical position immediately deep to the breast tissue [19]. The more important findings for clinical practice are that regions within the latissimus dorsi and teres major muscles received near maximal doses as a third anterior beam was used to treat the axillary regions. Future work is needed to monitor impairment in these muscles given the impending increase in the number of patients treated with axillary RT. Recent clinical trials demonstrate a reduction in breast cancer death is associated with axillary RT [20,21] as well as the reduction in lymphedema risk as compared to axillary node dissection [6]. Advances in medical imaging could provide clinicians with improved potential new avenues for assessing clinically relevant changes to the shoulder and its underlying musculature [22,23].
Irradiating muscles and other connective tissues within the shoulder girdle may lead to the development of fibrosis and atrophy [24–26]. These structural changes to these soft tissues could produce functional impairments in breast cancer patients treated with RT. Patients treated with RT after mastectomy show operated-to-opposite side reductions in internal rotation and adductor strength, while patients receiving a mastectomy alone showed no side-to-side weaknesses [18]. Similarly, expanding the treatment volume to include the axillary regions may lead to reductions in range of motion in abduction and internal/external rotation, as well as strength deficient in the adductors and internal rotators of the arm [7]. The functional reductions in shoulder adduction and internal rotation previously observed in patients following RT corresponds with the muscles that the current study identified are most at-risk for receiving a significant dose of radiation as RT fields are expanded to include axilla and supraclavicular nodes. Our study found that axillary radiotherapy delivered statistically significant increases in the V48 Gy dose to individual muscles whose primary actions involved shoulder adduction (pectoralis major, pectoralis minor, latissimus dorsi, and teres major) and internal rotation (latissimus dorsi and teres major). Combined, these results suggest a potential relationship between our muscle radiation dosimetry findings and functional patient outcomes, but more direct measures are necessary.
The treatment regimens simulated in this study were found to deliver varying amounts of radiation to the musculature. The most striking finding in regards to the beam configurations were the large increase in near maximal dosing that the entire shoulder musculature receives in response to the DO configuration. Muscles like the infraspinatus, subscapularis, supraspinatus, teres minor, and trapezius had regions with statistically significant increases in the V48 Gy dose delivered from the DO beam arrangement, but barely achieved any V48 Gy dose in the other axillary RT treatment regimens. While treating the axillary region with a DO beam is used less in clinical practice than an anterior oblique beam, the current study indicates that patients that are treated with DO beams may warrant greater monitoring for shoulder morbidity after completing RT.
The current study serves as a sensitivity analysis of individual muscle doses across varying simulated treatment regimens. One previous study has explored muscle radiation dosimetry [13], but its results only reported the radiation delivered to the entire muscle region. This makes it difficult to compare the dosimetry findings to patient outcomes given the number of muscles anatomically located within the breast cancer treatment volume and the vast three-dimensional mobility of the shoulder. Future prospective studies examining patient functional outcomes correlated to the dose delivered to individual muscles are needed. Despite these limitations, the current study provides robust insights into the radiation dose delivered to muscle tissue within the shoulder girdle. This methodology may provide a new tool for clinicians and scientist to better understand the pathophysiology of shoulder impairment after radiation treatment to optimize RT treatment regimens and develop targeted rehabilitation strategies to improve a breast cancer survivor's quality of life.
Acknowledgments
We acknowledge financial support for this work from NIH T32-HD07418 and Susan G. Komen Postdoctoral Clinical Fellowship PDF15329262. The study sponsors had no involvement in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest statement: The authors have no financial or personal relationships with other people or organizations that could inappropriately influence the submitted manuscript.
References
- 1.Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149:267–74. doi: 10.1001/jamasurg.2013.3049. [DOI] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoskin P. External beam therapy (radiotherapy in practice) 2nd. Oxford University Press; 2012. [Google Scholar]
- 4.Alco G, Igdem SI, Ercan T, Dincer M, Senturk R, Atilla S, et al. Coverage of axillary lymph nodes with high tangential fields in breast radiotherapy. Br J Radiol. 2010;83:1072–6. doi: 10.1259/bjr/25788274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoebers FJ, Borger JH, Hart AA, Peterse JL, Th EJ, Lebesque JV. Primary axillary radiotherapy as axillary treatment in breast-conserving therapy for patients with breast carcinoma and clinically negative axillary lymph nodes. Cancer. 2000;88:1633–42. doi: 10.1002/(sici)1097-0142(20000401)88:7<1633::aid-cncr18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–10. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingvar C, Johansson K, Albertsson M, Ekdahl C. Arm lymphoedema, shoulder mobility and muscle strength after breast cancer treatment – a prospective 2-year study. Adv Physiother. 2001;3:55–66. 2009-07-11. [Google Scholar]
- 8.Hwang JH, Chang HJ, Shim YH, Park WH, Park W, Huh SJ, et al. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008;49:443–50. doi: 10.3349/ymj.2008.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janz NK, Mujahid M, Chung LK, Lantz PM, Hawley ST, Morrow M, et al. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt) 2007;16:1348–61. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 10.Karki A, Simonen R, Malkia E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37:180–8. doi: 10.1080/16501970410024181. [DOI] [PubMed] [Google Scholar]
- 11.Adriaenssens N, Vinh-Hung V, Miedema G, Versmessen H, Lamote J, Vanhoeij M, et al. Early contralateral shoulder-arm morbidity in breast cancer patients enrolled in a randomized trial of post-surgery radiation therapy. Breast Cancer (Auckl) 2012;6:79–93. doi: 10.4137/BCBCR.S9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansen S, Fossa K, Nesvold IL, Malinen E, Fossa SD. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol. 2014;53:521–9. doi: 10.3109/0284186X.2014.880512. [DOI] [PubMed] [Google Scholar]
- 13.Farace P, Deidda MA, Iamundo de Cumis I, Deiana E, Farigu R, Lay G, et al. Bi- tangential hybrid IMRT for sparing the shoulder in whole breast irradiation. Strahlenther Onkol. 2013;189:967–71. doi: 10.1007/s00066-013-0428-9. [DOI] [PubMed] [Google Scholar]
- 14.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 15.van Leeuwen-Segarceanu EM, Dorresteijn LD, Pillen S, Biesma DH, Vogels OJ, van Alfen N. Progressive muscle atrophy and weakness after treatment by mantle field radiotherapy in Hodgkin lymphoma survivors. Int J Radiat Oncol Biol Phys. 2012;82:612–8. doi: 10.1016/j.ijrobp.2010.11.064. [DOI] [PubMed] [Google Scholar]
- 16.Shamley DR, Srinanaganathan R, Weatherall R, Oskrochi R, Watson M, Ostlere S, et al. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat. 2007;106:19–27. doi: 10.1007/s10549-006-9466-7. [DOI] [PubMed] [Google Scholar]
- 17.Nesvold IL, Dahl AA, Lokkevik E, Marit Mengshoel A, Fossa SD. Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol. 2008;47:835–42. doi: 10.1080/02841860801961257. [DOI] [PubMed] [Google Scholar]
- 18.Blomqvist L, Stark B, Engler N, Malm M. Evaluation of arm and shoulder mobility and strength after modified radical mastectomy and radiotherapy. Acta Oncol. 2004;43:280–3. doi: 10.1080/02841860410026170. [DOI] [PubMed] [Google Scholar]
- 19.Contant CM, van Geel AN, van der Holt B, Griep C, Tjong R, Joe Wai, et al. Morbidity of immediate breast reconstruction (IBR) after mastectomy by a subpectorally placed silicone prosthesis: the adverse effect of radiotherapy. Eur J Surg Oncol. 2000;26:344–50. doi: 10.1053/ejso.1999.0896. [DOI] [PubMed] [Google Scholar]
- 20.Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–27. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 21.Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–16. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klatt D, Papazoglou S, Braun J, Sack I. Viscoelasticity-based MR elastography of skeletal muscle. Phys Med Biol. 2010;55:6445–59. doi: 10.1088/0031-9155/55/21/007. [DOI] [PubMed] [Google Scholar]
- 23.Gennisson JL, Deffieux T, Mace E, Montaldo G, Fink M, Tanter M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol. 2010;36:789–801. doi: 10.1016/j.ultrasmedbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13–22. doi: 10.1080/02841860510007440. ed Norway. [DOI] [PubMed] [Google Scholar]
- 25.Willey JS, Bracey DN, Gallagher PE, Tallant EA, Wiggins WF, Callahan MF, et al. Angiotensin-(1–7) attenuates skeletal muscle fibrosis and stiffening in a mouse model of extremity sarcoma radiation therapy. J Bone Joint Surg Am. 2016;98:48–55. doi: 10.2106/JBJS.O.00545. [DOI] [PubMed] [Google Scholar]
- 26.Gillette EL, Mahler PA, Powers BE, Gillette SM, Vujaskovic Z. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys. 1995;31:1309–18. doi: 10.1016/0360-3016(94)00422-H. [DOI] [PubMed] [Google Scholar]


