Abstract
Organ transplantation in older people is increasing, but how aging impacts B cell responses to organ transplantation is unknown. Here, we show that the depletion of B cells with anti-CD20 antibodies has disparate effects depending on recipient age. In young murine recipients, anti-CD20 treatment impaired the ability of immune modulation to extend skin allograft survival. In contrast, anti-CD20 treatment extended allograft survival in aged recipients treated with immune modulation. Although regulatory B cell function and the numbers of marginal and follicular B cells were similar between age groups, a subpopulation of B cells, termed age-associated B cells (ABCs), accumulated upon aging. ABCs isolated from aged mice exhibited upregulation of CD73, CD80, CD106, and TLR2 and an increased capacity to augment T cell alloimmunity compared to ABCs from young mice. Importantly, ABCs from aged, but not young, mice impaired the ability of immune modulation to enhance allograft survival after adoptive transfer into young transplant recipients. Our study indicates that ABCs impair the immune regulation of allografts. Thus, recipient age needs to be considered when proposing B cell-depleting immune therapy.
Keywords: Aging, B cells, immune regulation, transplantation
Introduction
B cells produce anti-donor antibodies against transplant antigens that enhance acute and chronic rejection [1–3]. B cells also act as antigen-presenting cells (APC) to enhance cellular immunity towards an allograft [4, 5]. In addition to these pro-immune functions of B cells, a subpopulation of B cells, termed regulatory B cells (B regs), has been identified that promote immune regulation by producing IL-10 [2, 4, 6–9]. Hence, B cells play important and diverse roles that can either enhance or suppress immunity towards organ allografts.
The most rapid subpopulation of patients seeking organ transplants, including kidney and heart transplants, are over 65 years of age [10]. Despite the increasing numbers of older patients that receive organ allografts, our understanding of how aging impacts the immune response to organ transplantation remains unclear. Studies have found that aging impacts T cell and dendritic cell (DC) responses after organ transplantation [11–15]. Yet, the role of aging on B cells following organ transplantation is unexplored.
In this study, we reveal that recipient age plays a critical role in the outcome of B cell depletion following organ transplantation. We found that, in young (2–4 months of age) murine skin allograft recipients, B cell depletion via anti-CD20 treatment impairs the ability of anti-CD45RB and anti-CD154 treatment to maximally extend graft survival. This observation is consistent with previous studies in humans that show that anti-CD20 precipitates acute renal allograft rejection [16]. In sharp contrast, we uncovered that allografts survived longer in aged recipients (16–18 months of age) receiving anti-CD45RB and anti-CD154 when the recipients were also treated with anti-CD20. Upon aging, a subpopulation of non-follicular, non-marginal B cells, termed age-associated B cells (ABCs: CD19+, CD21−, CD23−, CD43−, CD93−), accumulate and exhibit enhanced activation and increased T cell alloimmune priming capabilities. Importantly, adoptive transfer of ABCs isolated from aged mice into young skin allograft recipients impairs the ability of anti-CD45RB and anti-CD154 to extend allograft survival. Our findings indicate that, upon aging, heightened T cell priming by the B cell pool may impair the immune regulation of allografts, which suggests that host age impacts the response to B cell immune depletion.
Results and Discussion
To determine how aging impacts B cell responses to organ transplantation, we evaluated how B cell depletion influenced the immune regulation of skin allografts in young and aged murine recipients. We found that B cell depletion via anti-CD20 antibodies has disparate effects depending on recipient age, findings that should be taken into consideration when treating aged organ recipients and recipients with other inflammatory conditions.
Recipient age critically impacts the outcome of anti-CD20 on the immune regulation of allografts
Anti-CD20 monoclonal antibodies (mAb) have been used to deplete B cells and reduce alloantibody levels prior to transplantation [17]. We found that administering 100 µg mAb against CD20 every 10 days for a total of four serial doses effectively depleted the splenic B cell pool (CD19+ cells) in both young (2–4 months of age) and aged (16–18 months of age) C57BL/6 mice compared to isotype controls (Supplemental Figure 1A). Within the B cell pool, anti-CD20 treatment ablated the follicular (CD19+, CD23hi, CD21mid), the marginal zone B cell pool (CD19+, CD23mid, CD21hi) and the CD19+, CD23−, CD21− population in the spleens of both young and aged mice (Supplemental Figure 1B). Thus, anti-CD20 effectively ablates the B cell pool in both young and aged mice.
We then examined the impact of B cells on the efficacy of anti-CD45RB, an agent that blocks signal one between APC and T cells [18], and anti-CD154, a signal two blocker [19], to maximally enhance BALB/c skin graft survival in young and aged C57BL/6 mice. Anti-CD45RB and anti-CD154 have been shown to extend graft survival in murine transplant recipients [18], although aged recipients do not exhibit the maximal extension of graft survival observed in young recipients [20]. Treatment with anti-CD45RB and anti-CD154 reduced the total B cell pool by 50% seven days after skin allograft transplantation in both young and aged recipients (Supplemental Figure 1C). Additionally, compared to isotype controls, the B cell pool remained ablated at three weeks post transplantation in young and aged mice that received perioperative anti-CD45RB, anti-CD154, and a skin allograft with anti-CD20 mAb (Supplemental Figure 1D). These data show that anti-CD20 mAb is highly effective at ablating B cells in murine recipients treated with anti-CD45RB and anti-CD154.
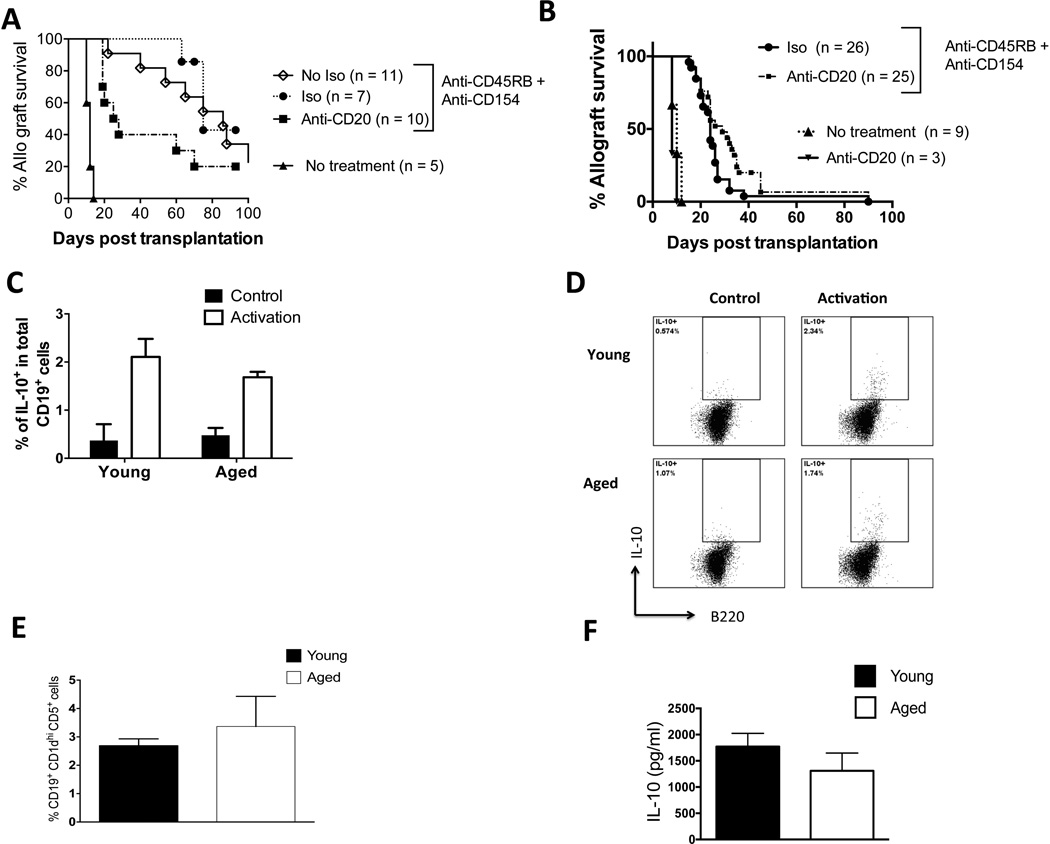
In young skin allograft recipients, we found that B cell depletion with anti-CD20 mAb impaired the ability of anti-CD45RB and anti-CD154 to maximally extend allograft survival compared to an isotype control of anti-CD20 (Figure 1A). (The isotype control did not impact the efficacy of anti-CD45RB and anti-CD154 to extend allograft survival; there was no significant difference in graft survival times between young mice that received anti-CD45RB and anti-CD154 with the isotype control compared to young mice that received anti-CD45RB and anti-CD154 without the isotype control (Figure 1A). In contrast, in aged recipients, B cell depletion led to a small but significantly enhanced ability of anti-CD45RB and anti-CD154 to extend allograft survival compared to aged recipients that received anti-CD45RB, anti-CD154, and an isotype control of anti-CD20 (Figure 1B). B cell depletion alone in aged recipients did not impact the tempo of skin allograft rejection (Figure 1B), an expected finding given the immunogenicity of full major histocompatibility complex skin graft rejection, which is a T cell-dependent process [21]. Thus, these results indicate that recipient age critically impacts the outcome of B cell depletion on the immune regulation of allografts. Specifically, with an immunoregulatory regimen that exhibits an age-dependent effect on graft survival [20], B cell depletion enhances immune regulation in aged recipients but impairs it in young recipients.
Figure 1. Recipient age alters the impact of B cell depletion on the immune regulation of skin allografts.
A: Young C57BL/6 mice received BALB/c skin transplants with either no further treatment or treatment with anti-CD45RB, anti-CD154, and anti-CD20 mAb or an isotype control to anti-CD20 mAb. Allograft survival was assessed. P = 0.006: isotype, median survival time (MST) 75 days vs. anti-CD20, MST = 25 days (Logrank). P <0.0001: anti-CD45RB, anti-CD154 vs. no treatment (MST = 12 days). P = 0.5: isotype control + anti-CD45RB, anti-CD154 vs. anti-CD45RB, anti-CD154 + no isotype control (MST = 86 days).
B: Aged C57BL/6 mice received BALB/c skin transplants with either no further treatment, treatment with anti-CD20 only, or treatment with anti-CD45Rb, anti-CD154, and anti-CD20 mAb or an isotype control to anti-CD20 mAb. Allograft survival was assessed. P = 0.05, isotype (MST = 26 days) vs. anti-CD20 (MST = 30 days) (Logrank). P <0.0001 anti-CD45RB, anti-CD154 vs. no treatment (MST = 10 days). Anti-CD20 treatment alone, MST = 8 days
C–D: Aged and young splenic B cells were enriched by magnetic negative separation and stimulated with LPS, PMA, and ionomycin at the indicated doses (see methods). C: The proportion of IL-10-producing cells was assessed after intracellular cytokine staining (pooled data, n = 12 biological replicates/group). D: Representative flow cytometric plots gated on B220+ cells are shown.
E: Spleens of aged and young mice were stained with fluorescent antibodies, and data were acquired via flow cytometry. The percentage of CD19+, CD1dhi, and CD5+ within total splenic B cell pool was measured (n = 6 mice/group).
F: Enriched B cells from the spleens of young and aged mice one week after skin allograft transplantation and treatment with CD45RB and anti-CD154. The cells were stimulated with LPS, and IL-10 was measured by ELISA (n = 4 biological replicates/group). Non-activated cells did not produce IL-10 (not shown). Representative of one experiment repeated independently with consistent results.
Aging does not alter B reg function
In islet and cardiac allograft models, CD45RB and anti-CD154 are known to act via B regs to enhance graft survival [6, 22]. Thus, an increased potency of B reg function in young recipients than in aged recipients could explain the different effects of B cell depletion observed between young and aged transplant recipients. Production of the immune suppressive cytokine, IL-10, is a consistent hallmark of B reg function [23]. However, we found no difference in the proportion of IL-10-producing B cells upon ex vivo stimulation with lipopolysaccharide (LPS) in the spleens of young and aged mice prior to transplantation (Figure 1C–D). We also found no difference in the proportion of CD19+, CD1dhi, CD5+, cells that contain putative B reg populations [7], between the spleens young and aged mice (Figure 1E). After mice were transplanted and treated with anti-CD45RB and anti-CD154, we found no difference in IL-10 production in the spleens of young and aged mice (Figure 1F). Thus, we did not find that B reg function or numbers are altered upon aging.
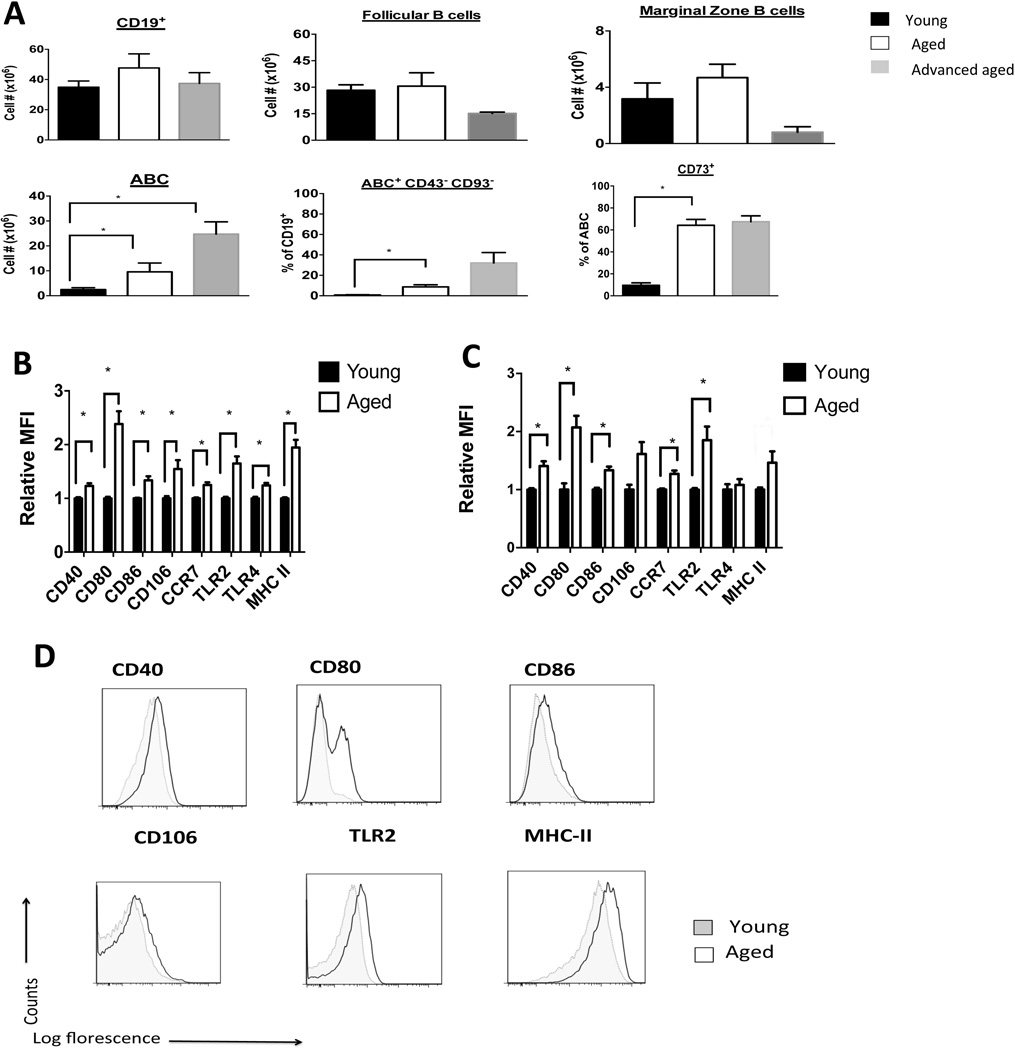
Aging increases the accumulation of ABCs that exhibit immune memory and activation phenotypes
As we did not identify an alteration in B reg number or function between young and aged mice, we next examined other populations of B cells, including follicular and marginal zone B cells. We did not find increases in the total CD19+ B cell pool or in the subpopulations of follicular or marginal zone B cells between the spleens of young, aged, and advanced aged (24 months of age) mice (Figure 2A). However, we found an increase in a CD19+, CD23−, CD21−, CD43−, CD93− population of B cells known as age-associated B cells (ABCs) [24, 25] (Figure 2 and Supplemental Figure 2A). We found low proportions (< 1% BrdU+ cells) of proliferating cells within the ABC population of both young and aged mice. We also found similar proportions of apoptotic (Annexin V+) cells in splenic B cell subpopulations in young and aged mice (Supplemental Figure 2B). These results suggest that neither differences in cellular proliferation nor the degree of apoptosis explain the increased numbers of ABCs in the spleens of aged mice.
Figure 2. Aging increases the accumulation of ABCs that exhibit memory and activation phenotypes.
A: The spleens of young (2–4 months of age), aged (16–18 months of age), and advanced aged mice (22–24 months of age) were harvested and stained with appropriate fluorescent antibodies, and data were acquired via flow cytometry. Absolute cell number or proportions are shown. *, P < 0.01, n = 4–6 mice/group.
B: Enriched splenic B cells from non-transplanted and non-treated young and aged mice were stained with the indicated fluorescent antibody and gated on the ABC subpopulation. Relative median fluorescence intensity (MFI) is normalized to average MFI of young mice. *P<0.01,(t-test) between young and aged groups, n = 16 mice/group.
C: Enriched splenic B cells were obtained 12 days after skin allotransplantation and treatment with anti-CD45RB and anti-CD154 in young and aged recipient mice. Staining is per B. *, P < 0.01, (t-test) n = 6 mice / group.
D: Representative flow cytometry plots gated on ABC subpopulation from non – transplanted mice from B are shown (Gating is shown in Supplemental Figure 2A).
We found that ABCs isolated from aged mice exhibited a three-fold higher proportion of CD73 positive cells, a marker of memory B cells [26, 27], as compared to ABCs from young mice (Figure 2A). Additionally, ABCs isolated from aged mice exhibited a two-fold increase in MHC class II, CD80 (a memory B cell marker [27] and co-stimulatory molecule), a 30% increase in TLR2 and a 10% increase in TLR4 compared to ABCs isolated from young mice, respectively (Figure 2B–C). Additionally, ABCs from aged mice also exhibited a two-fold upregulation in the adhesion molecule, VCAM-1 (CD106), and a 10% increase in the chemokine receptor, CCR7 (Figure 2B and D). In contrast, within the follicular and marginal zone B cell populations, only MHC Class II expression was higher in the aged B cell subpopulations compared to the young ones (Supplemental Figure 2C–D). Besides TLR4, these changes in ABCs persisted after aged and young mice were transplanted and treated with anti-CD45RB and anti-CD154 (Figure 2C). These data indicate that ABCs exhibit an increase in activation and an immune memory phenotype upon aging.
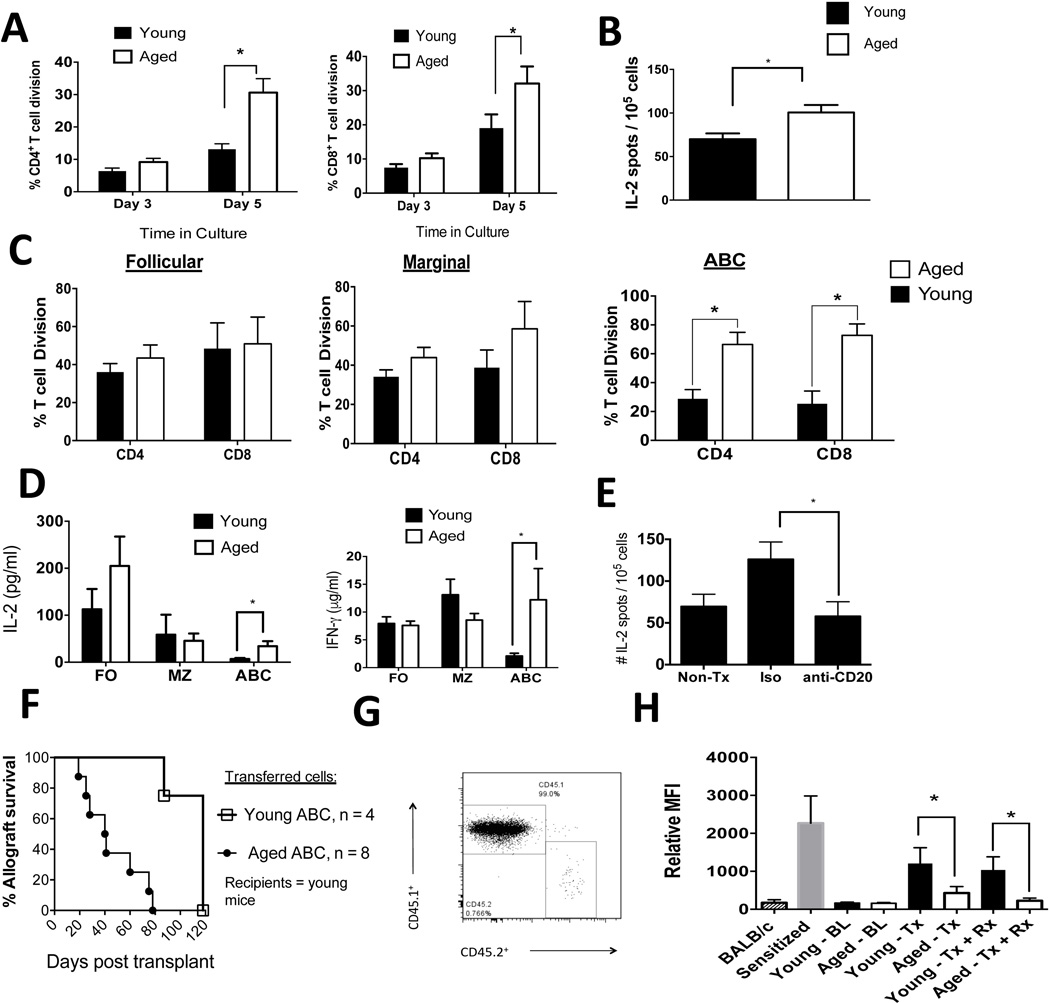
Aging enhances priming of allogeneic T cells in vitro
As ABCs from aged mice exhibited increases in CD80 and MHC class II, which can enhance priming of T cell function [28], we next assessed the alloimmune T cell priming function of aged and young B cells. Enriched splenic B cells (total CD19+ population) from aged C57BL/6 mice exhibited an enhanced ability to induce both BALB/c CD4+ and CD8+ T cell proliferation in vitro compared to B cells purified from young C57BL/6 mice (Figure 3A). Furthermore, splenic B cells that were enriched from aged transplant recipients treated with anti-CD45RB and anti-CD154 exhibited an enhanced ability to activate allogeneic T cells ex vivo compared to splenic B cells isolated from similarly treated young mice (Figure 3B).
Figure 3. Aged B cells enhance anti-donor T cell immunity and impair the immune regulation of allografts.
A: The total B cell pool (CD19+ cells) was enriched by negative magnetic separation from young and aged mice. Cells were irradiated and cultured with allogeneic, (BALB/c), enriched T cells that were stained with violet proliferation dye. The percentage of dividing cells from a representative experiment, repeated twice independently, is shown (n = 3 biological replicates/age group/experiment). *, P < 0.01 (t-test).
B: The total B cell pool (CD19+ cells) was enriched by negative magnetic separation from young and aged mice 12 days post skin allotransplantation and treatment with anti-CD45RB and anti-CD154. The B cells were irradiated and cultured with allogeneic T cells ex vivo for 12h. IL-2 production by T cells was measured via ELISPOT. *, P: 0.01 (t-test). N = 7 biological replicates / group. T cells that were not stimulated ex vivo produced < 10 spots /105 cells.
C: B cells were FACS sorted into follicular cells, marginal cells, or ABCs (see Supplemental Figure 2E for representative FACS plots), irradiated, and cultured with allogeneic T cells as per A. A representative experiment is shown (n = 3 biological replicates/age group/experiment). *, P < 0.01 (t-test). Consistent results were observed in four independent experiments.
D: MLC culture supernatants from C above were harvested, and IL-2 and IFN-γ production was measured by ELISA. Error bars = SEM. *, P < 0.02 (Mann-Whitney). The data shown are pooled from three independent experiments with three biological replicates/experiment.
E: Aged C57LB/6 mice were transplanted with BALB/c skin allografts, treated with CD45RB, anti-CD154, and anti-CD20 mAb or an isotype control. At three weeks post transplantation, spleens were obtained, and T cells were enriched by magnetic negative selection and cultured with irradiated donor spleen cells. The number of IL-2-producing T cells was measured via ELISPOT (n = 6 mice/group). *, P = 0.03 (t-test).
F: Young CD45.1+ mice received 1 × 106 splenic FACS-purified ABCs via tail vein injections from young or aged CD45.2+ mice on day −2 relative to skin allograft transplantation. Mice received anti-CD45RB and anti-CD154 as described in the methods section. The difference between the experimental groups was statistically significant, P = 0.0006 (Logrank).
G: ABCs were isolated from aged CD45.2+ mice and transferred into young CD45.1+ mice that were transplanted with a BALB/c skin graft and then treated with anti-CD45RB and anti-CD154. One hundred days after transfer, the spleens were obtained and the proportion of CD45.2+ cells was determined after appropriate staining and data acquisition via flow cytometry.
H: Serum was obtained from mice, diluted, and cultured with allogeneic donor T cells. After the cells were washed, they were stained with an anti-IgG fluorescent antibody, and the data were acquired via flow cytometry. BL, baseline; Tx, transplantation; Rx, anti-CD45RB + anti-CD154. *, P < 0.01. The data shown are pooled from two independent experiments.
We then further purified aged and young splenic B cells into follicular, marginal, and ABC subtypes via fluorescence-activated cell sorting (FACS; Supplemental Figure 2E) and cultured the purified subpopulations with allogeneic (BALB/c) CD4+ and CD8+ T cells. ABCs, but not marginal or follicular B cells, purified from the spleens of aged C57BL/6 mice exhibited an age-dependent enhancement in the ability to induce T cell alloimmune proliferation (Figure 3C). ABCs purified from aged mice also increased IL-2 and IFN-γ production by allogeneic T cells compared to ABCs purified from young mice, whereas follicular and marginal zone B cells did not exhibit age-dependent increases in IL-2 and IFN-γ production (Figure 3D). IL-17 was detected at low levels in this assay with no differences noted between the cell populations upon aging (Supplemental Figure 2F). Together, these data show that ABC exhibit enhanced in vitro T cell alloimmunity upon aging.
Aged B cells exhibit enhanced IL-2 T cell alloimmune responses in vivo
As anti-CD20 mAb ablates all B cell subpopulations examined (Supplemental Figure 1B), we tested the requirement for B cells in anti-donor T cell priming in vivo. We transplanted aged C57BL/6 mice with a BALB/c skin graft and administered anti-CD45RB and anti-CD154 along with the anti-CD20 mAb or an isotype control of anti-CD20 mAb. We found that, three weeks after transplantation, anti-CD20 treatment reduced IL-2 anti-donor T cell responses in aged recipients but not in isotype control-treated aged recipients (Figure 3F). In similarly treated young transplant recipients, anti-CD20 treatment did not alter the anti-donor IL-2 response (Supplemental Figure 2G). These data reveal that B cell depletion reduces IL-2 anti-donor T cell responses in aged skin allograft recipients who receive anti-CD45RB and anti-CD154.
ABCs isolated from aged mice impair the ability of anti-CD45RB and anti-CD154 to extend allograft survival after adoptive transfers into young transplant recipients
To determine if ABCs from aged, non-transplanted, non-treated mice were sufficient to impair the immune regulation of allografts, we adoptively transferred FACS-sorted ABCs from either young mice or aged CD45.2+ mice into young CD45.1+ mice. Two days after adoptive transfer, mice were transplanted with a skin allograft and treated with anti-CD45RB and anti-CD154. On the day of transplantation, we noted that there were between 0.6–0.8% of CD45.2+ cells in the peripheral blood of recipients. Furthermore, in a separate cohort of young recipient CD45.1+ mice, the engraftment of adoptively transferred ABCs within the spleen was similar between transferred ABCs purified from young or aged mice (0.4–0.6% of splenic ABCs). We found that the transfer of aged, but not young, ABCs impaired the ability of anti-CD45RB and anti-CD154 to extend allograft survival in young recipients (Figure 3G). At 100 days post transplantation, aged ABCs remained detectable (0.76% of spleen cells) within the spleen of the recipients (Figure 3H). These data provide direct in vivo evidence that ABCs from aged mice impair the immune regulation of allografts.
Aged transplant recipients exhibit lower alloantibody levels than young transplant recipients
B cells respond to transplant antigens by producing donor-specific antibodies (DSAs). Prior to transplantation, we found low and similar plasma IgG DSA levels between young and aged C57BL/6 mice. Two weeks after BALB/c skin transplantation, both aged and young mice exhibited increases in the serum levels of anti-donor IgG alloantibodies; however, the aged recipients exhibited significantly lower levels than their younger counterparts (Figure 3H), which is consistent with previous vaccine studies that show that aging impairs antibody production [29]. Administration of anti-CD45RB and anti-CD154 did not impact alloantibody levels, and the reduced anti-donor IgG levels in the aged recipients persisted (Figure 3H). These data indicate that aging impairs the production of DSAs; thus, increased DSA production is unlikely to be a mechanism by which aging influences B cells to enhance anti-donor responses.
Concluding Remarks
In summary, our study indicates that the accumulation of ABCs, likely due to a series of environmental exposures, may override the immunoregulatory functions of B cells in aged transplant recipients. In addition to these quantitative changes, our work indicates that upon aging, ABCs also exhibit qualitative changes, such as an upregulation of costimulatory molecules, which enhances the priming of allogeneic T cells. However, it is possible that ABCs directly interact with immunosuppressive cells, such as B regs, to impair immune regulation; this possibility will require future investigation. The age of the patients is not always stated in clinical studies, such as one reporting that B cell depletion precipitated acute allograft rejection [16]. Our study indicates that recipient age should be considered when investigating the immune response to B cell depletion following organ transplantation and possibly in the treatment of other inflammatory conditions.
Materials and Methods
Mice, transplantation, and reagents administered in vivo
Young (2–4 months) C57BL/6 (H2b), aged (16–18 months), and advanced aged (22–24 months) C57BL/6 mice were obtained from the National Institutes of Aging rodent colony. Young donor BALB/c mice (H2d) and C57BL/6 CD45.1+ were purchased from the Jackson Laboratory (Bar Harbor, ME, USA).
Full-thickness BALB/c skin was transplanted to the dorsum of C57BL/6 recipients as described previously [30]. Recipients were treated with anti-CD45RB (clone HB220, 100 µg intraperitoneally on days −1, 0, 1, 2, 5, and 8 relative to transplantation) and anti-CD154 (clone MR1, 250 µg intraperitoneally on days 0, 2, 6, and 8 relative to transplantation). Both antibodies were purchased from BioXcell (Lebanon, NH, USA). Complete loss of the skin graft area was considered as rejection. B cell depletion was accomplished using an anti-CD20 monoclonal antibody (four doses of 100 µg/dose administered on days −5, +5, +15, and +25 relative to transplantation). The anti-CD20 antibody (clone 5D2) was obtained from Genentech (San Francisco, CA, USA).
The Yale University Institutional Animal Care and Use Committee approved the use of animals in this study.
Cell sorting, flow cytometry, adoptive transfer, and ELISA
The following monoclonal, fluorescently tagged antibodies were obtained from Biolegend (CD19, TCRβ, CD5, CD21, CD23, CD43, CD93, CD73, CD40, CD80, CD86, MHC-II, TLR2, TLR4, B220, CD1b, CD5, TIM-1, and IL-10). Data were acquired on a BD LSRII flow cytometer and analyzed using FlowJo Software (TreeStar). For intracellular cytokine staining, 1 × 106 spleen cells were activated using phorbol 12-myristate 13 acetate (PMA) (50 ng/ml), ionomycin (500 ng/ml), and LPS (10 µg/ml) (Sigma) for 4 h as previously described [7]. Golgi-stop was added to the cultures 1 h before staining with the indicated antibodies. For all flow cytometric analysis, cells were gated on a propidium iodide-negative population. Magnetic negative cell enrichment (> 95% purity, as assessed by flow cytometric staining) of splenic B cells (CD19+) and T cells was performed using kits from Stemcell Technologies (Vancouver, BC, Canada). In specific cases, B cell subpopulations (Supplemental Figure 2E) were further sorted by FACS on a BD FACS Aria II cell sorter. FACS-purified splenic ABCs were transferred at a dose of 1 × 106 cells/mouse on day −2 relative to transplantation. Enriched B cells (1 × 106 cells/well) were stimulated with 10 µg/ml LPS for 2 d, and IL-10 (eBioscience, San Diego, CA, USA) concentrations were measured in the culture supernatants via an enzyme-linked immunosorbent assay (ELISA). IL-2 (eBioscience, San Diego, CA, USA) concentrations were measured in mixed lymphocyte culture (MLC) culture supernatants via ELISA.
MLC
As described above, B cells were enriched from the spleens of young or aged C57BL/6 mice and irradiated (1000 RAD). These cells (2 × 105 cells/well) were then cultured in complete RPMI media at a ratio of 1:2: B cells:CD4+ or CD8+ T cells that were enriched from BALB/c mice. T cells were stained with violet proliferation dye (Thermofisher, Waltham, MA USA) and assessed for proliferation at day 3 of 5 of culture. Percent proliferation was calculated after comparing the values to non-activated control T cells.
ELISPOT
Recipient T cells (1 × 105 cells/well) and irradiated donor splenic cells (5 × 105 cells/well) were cultured in 96-well ELISPOT plates coated with the antibody of interest. After 18 h of culture, the plates were treated with the reagents according to manufacturer’s instructions (BD Biosciences, San Diego, CA, USA). Plates were read on an AID ELISPOT reader and analyzed using AID ELISPOT software (AID, Strassberg, Germany).
Measurement of alloantibodies
Serum was obtained from mice and diluted 1:100 in PBS. The serum was incubated with enriched allogeneic T cells (target cells; 2 × 105 cells/well) in 96-well plates. After incubating for 1 h at 4µC, the cells were washed twice with PBS, re-suspended, and cultured with anti-mouse IgG FITC (Jackson Immuno Research West Grove, PA, USA) on ice for 30 min. The cells were washed twice and re-suspended in PBS, and then data were acquired on a flow cytometer. The degree of fluorescence was reported as median fluorescence intensity (MFI) as a measure of anti-donor antibody attachment to target cells.
Statistics
Means between indicated experimental groups were statistically analyzed by parametric tests (two-way Student t-test) or non-parametric tests (Mann-Whitney), and survival analyses were performed via a log rank test using Graphpad Prism Software. A p-value < 0.05 was considered significant. Error bars shown in figures represent the standard error of the mean (SEM).
Supplementary Material
A: Aged and young mice were either treated with 100 µg anti-CD20 mAb every 10 days in four serial doses or with one dose of 100 µg anti-CD20 mAb. Ten days after the last dose, spleens were obtained, and the proportion of CD19+ cells was assessed via flow cytometry (n = 4 mice/group). The representative experiment shown was repeated once with similar results. Four serial doses led to >97% depletion of B cells in both age groups.
B: B cell subpopulations of the spleens of young or aged mice that were treated as per A. Ctrl = isotype control antibody for anti-CD20 mAb. The percent of total B cells is shown within each B cell subpopulation.
C: Young and aged C57BL/6 mice either received BALB/c skin transplants (Tx) or Tx and treatments (Rx) with anti-CD45RB and anti-CD154, and the number of CD19+ cells were enumerated following flow cytometric staining.
D: Young and aged C57BL/6 mice received BALB/c skin transplants, anti-CD45RB, anti-CD154, and anti-CD20 mAb (four serial doses as described in A) or an isotype control to anti-CD20. Three weeks post transplantation, spleens were obtained, and the number of B cells was enumerated following flow cytometric staining (n = 4–6 mice/group). − = non-transplanted, non-treated baseline, control.
A: Spleens were obtained from young, aged, and advanced aged C57BL/6 mice and stained with the relevant fluorescently tagged monoclonal antibodies. Representative flow cytometry plot gated on CD19+ cells. The percentage of cells with ABC, follicular (FO), or marginal zone (MZ) B cells are shown.
B: Within each of the B cell subpopulations shown in A, cells were assessed for Annexin V expression (an apoptosis marker). The proportion of apoptotic cells for each subpopulation is plotted (n = 3/group). Error bars = SEM.
C–D: Enriched splenic B cells were stained with the indicated fluorescent antibodies and gated on either the follicular cell subpopulation (C) or the marginal zone subpopulation (D). Samples were analyzed at baseline. Relative median fluorescence intensity (MFI) is normalized to average MFI of young mice. (n = 3–5 mice/group). *, P < 0.01 (t-test). Similar results were noted with a repeat experiment.
E: Representative flow plots show the purity of sorting into follicular cells, marginal zone cells, and ABCs.
F: MLC culture supernatants from were harvested from cultures of T cells cultured with the indicated B cell subpopulation, and IL-17 production was measured by ELISA. Error bars = SEM. Data are pooled from two independent experiments with three biological replicates / experiment.
G: Young C57LB/6 mice were transplanted with BALB/c skin allografts, treated with CD45RB, anti-CD154, and anti-CD20 mAb or an isotype control. At three weeks post transplantation, spleens were obtained, and T cells were enriched by magnetic negative selection and cultured with irradiated donor spleen cells. The number of IL-2-producing T cells was measured via ELISPOT (n = 6 mice/group).
Acknowledgments
This study was supported by the NIH grant AG028082 to DRG.
Abbreviations
- ABC
age-associated B cell
- APC
antigen-presenting cell
- B reg
regulatory B cell
- DSA
donor-specific antibodies
- mAb
monoclonal antibody
- MFI
median fluorescence intensity
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Pankewycz O, Soliman K, Laftavi MR. The increasing clinical importance of alloantibodies in kidney transplantation. Immunol Invest. 2014;43:775–789. doi: 10.3109/08820139.2014.910016. [DOI] [PubMed] [Google Scholar]
- 2.Dalloul AH. B-cell-mediated strategies to fight chronic allograft rejection. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tse GH, Johnston CJC, Kluth D, Gray M, Gray D, Hughes J, Marson LP. Intrarenal B Cell Cytokines Promote Transplant Fibrosis and Tubular Atrophy. Am J Transplant. 2015;15:3067–3080. doi: 10.1111/ajt.13393. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Q, Ng Y-H, Singh T, Jiang K, Sheriff KA, Ippolito R, Zahalka S, Li Q, Randhawa P, Hoffman RA, Ramaswami B, Lund FE, Chalasani G. B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest. 2014;124:1052–1056. doi: 10.1172/JCI70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jane-Wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, Abrahimi P, Devalliere J, Moeckel G, Kulkarni S, Tellides G, Pober JS. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-kappaB signaling in endothelial cells. Circulation. 2013;128:2504–2516. doi: 10.1161/CIRCULATIONAHA.113.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JI, Rothstein DM, Markmann JF. Role of B cells in tolerance induction. Curr Opin Organ Transplant. 2015;20:369–375. doi: 10.1097/MOT.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KM, Kim JI, Stott R, Soohoo J, O’Connor MR, Yeh H, Zhao G, Eliades P, Fox C, Cheng N, Deng S, Markmann JF. Anti-CD45RB/Anti-TIM-1-Induced Tolerance Requires Regulatory B Cells. Am J Transplant. 2012;12:2072–2078. doi: 10.1111/j.1600-6143.2012.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KM, Stott RT, Zhao G, SooHoo J, Xiong W, Lian MM, Fitzgerald L, Shi S, Akrawi E, Lei J, Deng S, Yeh H, Markmann JF, Kim JI. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. E J Immunol. 2014;44:1728–1736. doi: 10.1002/eji.201344062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins PNA, Pratschke J, Pascher A, Fritsche L, Frei U, Neuhaus P, Tullius SG. Age and Immune Response in Organ Transplantation. Transplantation. 2005;79:127–132. doi: 10.1097/01.tp.0000146258.79425.04. [DOI] [PubMed] [Google Scholar]
- 11.Tesar BM, Du W, Shirali AC, Walker WE, Shen H, DR G. Aging Augments IL-17 T-Cell Alloimmune Responses. Am J Transplant. 2009;9:54–63. doi: 10.1111/j.1600-6143.2008.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen H, Tesar BM, Du W, Goldstein DR. Aging Impairs Recipient T Cell Intrinsic and Extrinsic Factors in Response to Transplantation. PLoS ONE. 2009;4:e4097. doi: 10.1371/journal.pone.0004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascher A, Reutzel-Selke A, Jurisch A, Bachmann U, Heidenhain C, Nickel P, Reinke P, Brandt C, Pratschke J, Frei U, Neuhaus P, Volk HD, Tullius SG. Alterations of the immune response with increasing recipient age are associated with reduced long-term organ graft function of rat kidney allografts. Transplantation. 2003;11:1560–1568. doi: 10.1097/01.TP.0000090161.79609.D3. [DOI] [PubMed] [Google Scholar]
- 14.Bedi DS, Krenzien F, Quante M, Uehara H, Edtinger K, Liu G, Denecke C, Jurisch A, Kim I, Li H, Yuan X, Ge X, ElKhal A, Tullius SG. Defective CD8 Signaling Pathways Delay Rejection in Older Recipients. Transplantation. 2016;100:69–79. doi: 10.1097/TP.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 15.Oberhuber R, Heinbokel T, Cetina Biefer HR, Boenisch O, Hock K, Bronson RT, Wilhelm MJ, Iwakura Y, Edtinger K, Uehara H, Quante M, Voskuil F, Krenzien F, Slegtenhorst B, Abdi R, Pratschke J, Elkhal A, Tullius SG. CD11c+ Dendritic Cells Accelerate the Rejection of Older Cardiac Transplants via Interleukin-17A. Circulation. 2015;132:122–131. doi: 10.1161/CIRCULATIONAHA.114.014917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, Smith KG. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360:2683–2685. doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnett AN, Hadjianastassiou VG, Mamode N. Rituximab in renal transplantation. Transpl Int. 2013;26:563–575. doi: 10.1111/tri.12072. [DOI] [PubMed] [Google Scholar]
- 18.Rothstein DM, Livak MFA, Kishimoto K, Ariyan C, Qian H-Y, Fecteau S, Sho M, Deng S, Zheng XX, Sayegh MH, Basadonna GP. Targeting Signal 1 Through CD45RB Synergizes with CD40 Ligand Blockade and Promotes Long Term Engraftment and Tolerance in Stringent Transplant Models. J Immunol. 2001;166:322–329. doi: 10.4049/jimmunol.166.1.322. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Ahmed E, Wang T, Wang Y, Ochando J, Chong AS, Alegre M-L. TLR Signals Promote IL-6/IL-17-Dependent Transplant Rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du W, Shen H, Galan A, Goldstein DR. An Age-Specific CD8+ T Cell Pathway That Impairs the Effectiveness of Strategies To Prolong Allograft Survival. J Immunol. 2011;187:3631–3640. doi: 10.4049/jimmunol.1100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger NR, Yin DP, Fathman CG. CD4+ but not CD8+ cells are essential for allorejection. J. Exp Med. 1996;184:2013–2018. doi: 10.1084/jem.184.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lal G, Nakayama Y, Sethi A, Singh AK, Burrell BE, Kulkarni N, Brinkman CC, Iwami D, Zhang T, Bromberg JS. Interleukin-10 From Marginal Zone Precursor B-Cell Subset Is Required for Costimulatory Blockade-Induced Transplantation Tolerance. Transplantation. 2015;99:1817–1828. doi: 10.1097/TP.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolp J, Turka LA, Wood KJ. B cells with immune-regulating function in transplantation. Nat Rev Nephrol. 2014;10:389–397. doi: 10.1038/nrneph.2014.80. [DOI] [PubMed] [Google Scholar]
- 24.Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)–driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS. Cutting Edge: CTLA-4Ig Inhibits Memory B Cell Responses and Promotes Allograft Survival in Sensitized Recipients. J Immunol. 2015;195:4069–4073. doi: 10.4049/jimmunol.1500940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting Edge: Hierarchy of Maturity of Murine Memory B Cell Subsets. J Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharpe AH. Mechanisms of costimulation. Immunological Reviews. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng J, Stout-Delgado HW, Kavita U, Jacobs A, Tang J, Du W, Tussey L, Goldstein DR. Efficacy of a vaccine that links viral epitopes to flagellin in protecting aged mice from influenza viral infection. Vaccine. 2011;29:8147–8155. doi: 10.1016/j.vaccine.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen H, Goldstein DR. IL-6 and TNF-α Synergistically Inhibit Allograft Acceptance. J Am Soc Nephrol. 2009;20:1032–1040. doi: 10.1681/ASN.2008070778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Aged and young mice were either treated with 100 µg anti-CD20 mAb every 10 days in four serial doses or with one dose of 100 µg anti-CD20 mAb. Ten days after the last dose, spleens were obtained, and the proportion of CD19+ cells was assessed via flow cytometry (n = 4 mice/group). The representative experiment shown was repeated once with similar results. Four serial doses led to >97% depletion of B cells in both age groups.
B: B cell subpopulations of the spleens of young or aged mice that were treated as per A. Ctrl = isotype control antibody for anti-CD20 mAb. The percent of total B cells is shown within each B cell subpopulation.
C: Young and aged C57BL/6 mice either received BALB/c skin transplants (Tx) or Tx and treatments (Rx) with anti-CD45RB and anti-CD154, and the number of CD19+ cells were enumerated following flow cytometric staining.
D: Young and aged C57BL/6 mice received BALB/c skin transplants, anti-CD45RB, anti-CD154, and anti-CD20 mAb (four serial doses as described in A) or an isotype control to anti-CD20. Three weeks post transplantation, spleens were obtained, and the number of B cells was enumerated following flow cytometric staining (n = 4–6 mice/group). − = non-transplanted, non-treated baseline, control.
A: Spleens were obtained from young, aged, and advanced aged C57BL/6 mice and stained with the relevant fluorescently tagged monoclonal antibodies. Representative flow cytometry plot gated on CD19+ cells. The percentage of cells with ABC, follicular (FO), or marginal zone (MZ) B cells are shown.
B: Within each of the B cell subpopulations shown in A, cells were assessed for Annexin V expression (an apoptosis marker). The proportion of apoptotic cells for each subpopulation is plotted (n = 3/group). Error bars = SEM.
C–D: Enriched splenic B cells were stained with the indicated fluorescent antibodies and gated on either the follicular cell subpopulation (C) or the marginal zone subpopulation (D). Samples were analyzed at baseline. Relative median fluorescence intensity (MFI) is normalized to average MFI of young mice. (n = 3–5 mice/group). *, P < 0.01 (t-test). Similar results were noted with a repeat experiment.
E: Representative flow plots show the purity of sorting into follicular cells, marginal zone cells, and ABCs.
F: MLC culture supernatants from were harvested from cultures of T cells cultured with the indicated B cell subpopulation, and IL-17 production was measured by ELISA. Error bars = SEM. Data are pooled from two independent experiments with three biological replicates / experiment.
G: Young C57LB/6 mice were transplanted with BALB/c skin allografts, treated with CD45RB, anti-CD154, and anti-CD20 mAb or an isotype control. At three weeks post transplantation, spleens were obtained, and T cells were enriched by magnetic negative selection and cultured with irradiated donor spleen cells. The number of IL-2-producing T cells was measured via ELISPOT (n = 6 mice/group).