Abstract
Physical frailty is an important prognostic indicator in heart failure (HF); however, few studies have examined the relationship between physical frailty and invasive hemodynamics among adults with HF. The purpose of this study was to characterize physical frailty in HF in relation to invasive hemodynamics. We enrolled 49 patients with New York Heart Association (NYHA) Class II-IV HF when participants were scheduled for a right heart catheterization (RHC) procedure. Physical frailty was measured according to the Frailty Phenotype: shrinking, weakness, slowness, physical exhaustion, and low physical activity. Markers of invasive hemodynamics were derived from a formal review of RHC tracings, and projected survival was calculated using the Seattle HF Model (SHFM). The mean age of the sample (n = 49) was 57.4±9.7 years, 67% were male, 92% had NYHA Class III/IV HF, and 67% had non-ischemic HF. Physical frailty was identified in 24 participants (49%) and was associated with worse SHFM one-year projected survival (p = 0.007). After adjusting for projected survival, physically frail participants had lower cardiac index (by both thermodilution and the Fick equation) and higher heart rates compared with those not physically frail (all p < 0.05). In conclusion, physical frailty is highly prevalent in patients with HF and is associated with low-output HF.
Keywords: Heart Failure, Hemodynamics, Physical Frailty
Heart failure (HF) is an increasingly common condition with approximately 915,000 new cases diagnosed every year.1 The rising numbers of adults with HF coupled with the complexity of clinical management2 highlights the need to pursue new lines of inquiry in HF. Frailty is a highly prevalent condition generally among older adults3 and specifically among those with cardiovascular disease.4,5 A number of studies have demonstrated high prevalence rates of frailty in HF and worse associated clinical outcomes among frail adults with HF.6-10 Following recommendations by several HF groups to include a frailty assessment in HF,11,12 there is a critical need to study all aspects of physical frailty13 in HF, including the relationship between physical frailty and other commonly used markers in HF such as invasive hemodynamics. The purpose of this study was to characterize physical frailty in HF by quantifying differences in invasive hemodynamics between physically frail and non-physically frail patients with HF.
Methods
This article addresses a primary aim of a U.S. National Institutes of Health-funded cross-sectional study that involved comprehensive measurements of physical frailty and invasive hemodynamics in HF. The study was conducted between July 2015 and March 2016. After initial screening and approval by the HF cardiologists, potential participants who met the inclusion criteria were approached when scheduled for a clinically-indicated right heart catheterization (RHC) procedure (Figure 1). Physical frailty criteria were assessed usually on the same day as, or within 7 days of, the RHC procedure.
Figure 1. Enrollment Flow Diagram for Symptom Biology and Accelerated Aging in Heart Failure Study.
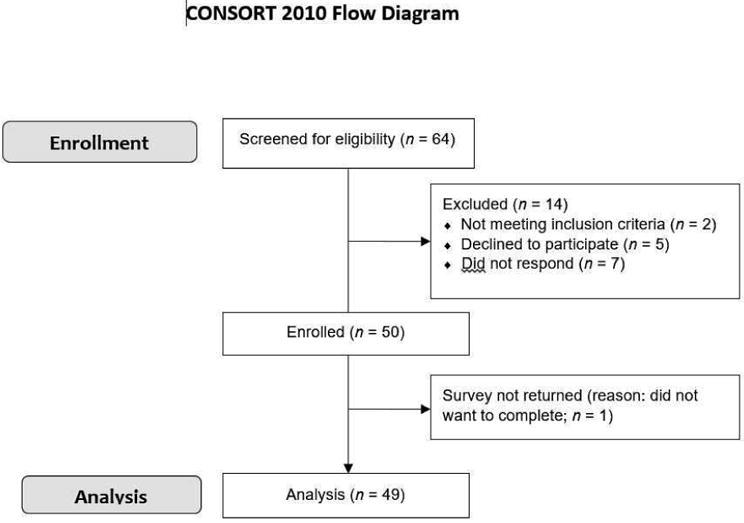
We screened 64 adults for our study, 50 adults were enrolled, and 49 adults were analyzed.
The sampling frame for this study was adult women and men with HF who receive care from a HF practice (outpatient clinic and/or inpatient facilities) at an academic medical center in the Pacific Northwest and required a RHC procedure during the study period. Formal inclusion criteria included age ≥ 21 years, ability to read and comprehend 5th grade English, New York Heart Association (NYHA) functional class II-IV (i.e. current HF symptoms), and undergoing RHC for clinical purposes. Participants were excluded if they had previously had a heart transplant or ventricular assist device, had major and uncorrected hearing dysfunction, or were otherwise unable to complete the requirements of the study (e.g. life-threatening illness). This study was approved by the university Institutional Review Board, and written informed consent was obtained from all participants.
Data on age, gender, and race were obtained using a socio-demographic questionnaire. Functional status (i.e. NYHA) was assessed and documented by an attending HF cardiologist. Data on history, duration, etiology, and treatment of HF along with clinical characteristics were collected through an in-depth review of the electronic medical record. Comorbid conditions were summarized using the Charlson Comorbidity Index.14
All RHC procedures were performed without the use of sedation by either advanced HF cardiologists or interventional cardiologists. Following completion of the RHC procedure, we reviewed the RHC tracings and reports. We collected data and calculated pressures based on waveforms, including right atrial pressure, pulmonary artery pressures, pulmonary capillary wedge pressure, and arterial blood pressure, along with the reported heart rate. We collected data on flow based on cardiac output and cardiac index, both as measured by thermodilution and as calculated by the Fick equation (using assumed VO2). We also calculated the pulmonary artery pulsatility index (pulmonary artery systolic pressure – pulmonary artery diastolic pressure)/right atrial pressure, and the right ventricular systolic work index (pulmonary artery mean pressure – right atrial pressure)*(cardiac index/heart rate).15 Finally, we collected data on oxygen extraction, as measured by mixed venous oxygen saturation.
We collected data from the most recent transthoracic echocardiogram, including left ventricular end-diastolic diameter and visually estimated left ventricular ejection fraction. We also collected data from recent cardiopulmonary exercise testing, including peak oxygen consumption (peak VO2,), respiratory quotient, ventilatory equivalent of carbon dioxide slope coefficient, and oxygen consumption at anaerobic threshold. The Seattle HF Model (SHFM) 1-year projected survival was calculated based on the model developed by Levy and colleagues (2006)16 and available online (https://depts.washington.edu/shfm/); this model uses objective clinical variables and HF treatments to generate estimated projected survival.
We assessed cognitive function in-person using the Montreal Cognitive Assessment (MoCA).17 The MoCA is a cognitive screening tool, designed for first-line clinicians with an adjusted algorithm for persons with chronic cardiovascular disease (score < 24/30) that is 100% sensitive to detect amnestic mild cognitive dysfunction in this population.18 A MoCA score of 24 was used as the cut point for mild cognitive dysfunction in this study.
Based on the Frailty Phenotype,3 a well-validated measure in older adults, we measured 5 physical frailty criteria: shrinking, weakness, physical exhaustion, slowness and low physical activity (Figure 2). We measured shrinking by a self-report of unintentional weight loss of > 10 pounds over the last year. We measured weakness of the upper extremities using a hand-held Smedley III Digital Grip Strength Tester (Takei Scientific Instruments, Japan). Participants were asked to perform standing maximal isometric contraction with their dominant hand 3 consecutive times with a 5-second rest period between each contraction. Weakness was determined using gender and body mass index cut points based on the Frailty Phenotype.3 We also measured weakness of the lower extremities using 5-repeat chair stands. Participants were assessed and timed on their ability to rise out of a chair 5 times without using their arms; a cut point of > 12 seconds or inability to rise 5 times indicated weakness.19 We measured slowness by clocking the time (in seconds) it took a participant to walk 4 meters. Participants were asked to walk at their usual speed, starting at 1 meter before the start line and walking to 1 meter past the finish line. They were permitted to use walking aides (e.g. canes or walkers). Based on a review of cut points for slow gait speed,20,21 we used a cut point of < 0.9 meters per second to indicate slowness. We measured physical exhaustion using the 13-item Functional Assessment of Chronic Illness Therapy Fatigue Scale (FACIT-F; v.4).22 The FACIT-F captures self-reported tiredness, weakness, and inability to perform activities of daily living as a result of fatigue. The 13 items are rated from 0 (not at all) to 4 (very much); cumulative scores range from 0 to 52 with lower scores indicating more fatigue. Cronbach's α of the FACIT-F in this sample was 0.92. Based on the application of the FACIT-F in the general population,23 we used a cut point of < 17 to identify those with severe physical exhaustion. We measured level of physical activity with the question “During the past week, how much total time did you spend exercising?” Those who reported less than 1 hour per week (to approximate expending ∼300 kcal/week in physical activity) were classified as having low physical activity. We compared responses to this question with the 12-item Duke Activity Status Index (DASI), an instrument of functional capacity that assesses activities related to major aspects of physical function24 and has demonstrated good reliability and validity in HF.25 Cronbach's α of the DASI in this sample was 0.83.
Figure 2. Measures to Assess Physical Frailty in Heart Failure Based on the Frailty Phenotype Criteria.
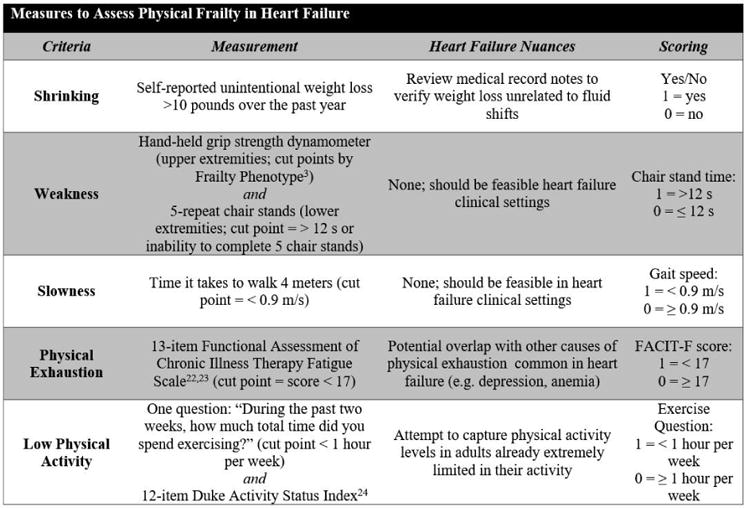
Based on the Frailty Phenotype, we assessed and scored each of the 5 criteria: shrinking, weakness, slowness, physical exhaustion, and low physical activity. Each criteria was reviewed and adapted for nuances specific to the heart failure population.
After completing the measures for each of the 5 criteria, the scores were totaled (range 0 to 5; Figure 2). Each participant was then classified as either “non-frail” (0/5 criteria met), “pre-frail” (1-2 criteria met), or “physically frail” (≥3 criteria met). Because of the small numbers in the non-frail group (n = 1), we combined this group with the pre-frail group (n = 24) (i.e. “not physically frail”), as compared with the “physically frail” group.
Characteristics of the sample are presented using standard descriptive statistics, including measures of central tendency and dispersion. Comparative statistics (Student's t-, Mann-Whitney U, Fisher exact tests, or the Pearson χ2) were used to determine significant differences in demographic and clinical characteristics, invasive hemodynamics, and individual physical frailty criteria measures between the 2 groups. Reported effect sizes for individual physical frailty criteria were calculated using, or converted to, Cohen's d. Multivariate linear regression was used to compare invasive hemodynamics between groups, adjusting for SHFM 1-year projected survival. Significance was set at α < 0.05. All analyses were performed using Stata/MP version 13MP (StataCorp, College Station, TX).
Results
Characteristics of the sample are presented in Table 1. The age of the sample ranged from 27 to 75 years. We identified physical frailty in 24 (49%) participants and pre-frailty in 24 (49%) participants; 1 participant was non-frail. Ten (20%) participants met 1/5 criteria, 14 (29%) met 2/5 criteria, 11 (22%) met 3/5 criteria, 9 (18%) met 4/5 criteria, and 4 (8%) met 5/5 criteria. Seven participants were unable to complete 5 chair stands, and 2 participants were unable to complete the gait speed assessment due to profound weakness. Unintentional weight loss and weakness by grip strength were not significantly different between the groups (Table 2). Gait speed was the most significant discriminatory criteria.
Table 1. Characteristics of the sample and by level of physical frailty*.
| Total (n = 49) | Not Physically Frail (n = 25)† | Physically Frail (n = 24) | p value‡ | |
|---|---|---|---|---|
|
|
||||
| Age (years) | 57.4±9.7 | 54.8±11.7 | 60.1±6.4 | 0.06 |
| Male | 33 (67%) | 19 (76%) | 14 (58%) | 0.19 |
| Non-Hispanic Caucasian | 40 (82%) | 22 (88%) | 18 (75%) | 0.29 |
| Body mass index (kg/m2) | 30.3±7.6 | 31.0±8.4 | 29.5±6.9 | 0.52 |
| Charlson Comorbidity Index (weighted) | 2.3±1.2 | 2.2±1.2 | 2.4±1.2 | 0.53 |
| Atrial fibrillation | 26 (53%) | 15 (60%) | 11 (46%) | 0.32 |
| Stage 3 chronic kidney disease | 9 (18%) | 3 (12%) | 6 (25%) | 0.29 |
| Out-patient (versus in-patient) at enrollment | 34 (69%) | 20 (80%) | 14 (58%) | 0.13 |
| Time with Heart failure (years) | 8.4 [2.4-14.8] | 8.4 [4.8-15.0] | 8.0 [1.0-13.5] | 0.21 |
| New York Heart Association Functional Class | <0.01 | |||
| Class II | 4 (8%) | 4 (16%) | 0 (0%) | |
| Class III | 34 (69%) | 19 (76%) | 15 (63%) | |
| Class IV | 11 (23%) | 2 (8%) | 9 (38%) | |
| Non-ischemic etiology | 33 (67%) | 19 (76%) | 14 (58%) | 0.19 |
| Prescribed a β-blocker | 35 (71%) | 20 (80%) | 15 (63%) | 0.22 |
| Prescribed an angiotensin-converting enzyme-inhibitor or angiotensin II receptor blocker | 39 (80%) | 21 (84%) | 18 (75%) | 0.50 |
| Prescribed an aldosterone antagonist | 25 (51%) | 14 (56%) | 11 (46%) | 0.48 |
| Prescribed digoxin | 11 (23%) | 8 (32%) | 3 (13%) | 0.17 |
| Prescribed a vasodilator (nitrate or hydralazine) | 10 (20%) | 5 (20%) | 5 (21%) | 1.00 |
| ICD or Biventricular ICD | 39 (80%) | 23 (92%) | 16 (67%) | <0.04 |
| Serum sodium (mEq/L) | 136.8±3.9 | 138.1±2.8 | 135.4±4.4 | <0.02 |
| Serum hemoglobin (g/dL) | 13.3±1.7 | 13.4±1.7 | 13.2±1.6 | 0.67 |
| Serum creatinine (mg/dL) | 1.2±0.5 | 1.1±0.3 | 1.3±0.6 | 0.46 |
| Serum B-type natriuretic peptide (pg/mL) | 478 [267-1103] | 349 [111-807] | 699 [347-1323] | 0.08 |
| Serum N-terminal pro-B-type natriuretic peptide (pg/mL) | 1234 [622-2686] | 714 [519-1158] | 1774 [985-2958] | 0.17 |
| Left ventricular end-diastolic diameter (cm) | 6.6±1.0 | 6.7±1.0 | 6.4±1.0 | 0.34 |
| Left ventricular ejection fraction (%) | 24.3±8.9 | 25.2±6.8 | 23.3±10.7 | 0.47 |
| Peak VO2 (mL/kg/min) | 15.4±3.6 | 16.2±3.7 | 13.6±2.8 | <0.05 |
| VO2 at aerobic threshold (mL/kg/min) | 12.0±3.5 | 12.8±3.6 | 10.2±2.6 | 0.07 |
| Respiratory quotient | 1.1±0.2 | 1.1±0.2 | 1.2±0.1 | 0.55 |
| VE/VCO2 slope coefficient | 32.8±5.3 | 32.0±4.9 | 34.5±6.1 | 0.33 |
| Seattle Heart Failure Model projected 1 year survival (%) | 93.0 [81.0-96.0] | 95.0 [92.0-97.0] | 89.0 [70.0-95.0] | <0.01 |
| Mild cognitive dysfunction | 16 (33%) | 2 (8%) | 14 (58%) | <0.001 |
| Right atrial pressure (mmHg) | 8.3±4.2 | 8.0±4.0 | 8.5±4.5 | 0.68 |
| Pulmonary artery systolic pressure (mmHg) | 41.3±15.1 | 38.2±14.0 | 44.6±15.7 | 0.14 |
| Pulmonary artery diastolic pressure (mmHg) | 19.1±7.8 | 16.8±7.2 | 21.4±7.9 | <0.04 |
| Pulmonary capillary wedge pressure (mmHg) | 18.8±8.1 | 17.2±7.1 | 20.4±9.0 | 0.18 |
| Mixed venous oxygen saturation (%) | 61.8±7.3 | 64.2±6.6 | 59.2±7.1 | <0.02 |
| Cardiac output (L/min by thermodilution) | 4.7±1.5 | 5.3±1.6 | 4.2±1.1 | <0.01 |
| Cardiac index (L/min/m2 by thermodilution) | 2.3±0.6 | 2.5±0.6 | 2.1±0.5 | <0.03 |
| Cardiac output (L/min by Fick equation) | 4.0±1.1 | 4.3±1.1 | 3.7±1.0 | 0.05 |
| Cardiac index (L/min/m2 by Fick equation) | 2.0±0.5 | 2.1±0.4 | 1.9±0.5 | 0.18 |
| Arterial systolic blood pressure (mmHg) | 110.7±18.2 | 115.4± 15.8 | 105.8±19.6 | 0.08 |
| Arterial diastolic blood pressure (mmHg) | 69.7±8.6 | 69.1±7.4 | 70.4±9.9 | 0.64 |
| Heart rate (beats per min) | 79.0±16.2 | 73.4±12.7 | 84.8±17.6 | <0.02 |
| Pulmonary artery pulsatility index | 2.7 [2.1-3.4] | 2.8 [2.0-3.4] | 2.7 [2.1-3.6] | 0.99 |
| Right ventricular stroke work index | 0.57±0.28 | 0.59±0.27 | 0.55±0.28 | 0.59 |
Continuous data are presented as mean ± SD or median [interquartile range]; categorical data as number of patients (percentage of sample)
Not physically frail includes both non-frail (n = 1) and pre-frail (n = 24)
p values comparing physically frail versus not physically frail
Abbreviations: ICD, implantable cardioverter defibrillator; VE/VCO2, ventilatory equivalent of carbon dioxide slope coefficient; VO2, peak oxygen consumption.
Table 2. Physical frailty characteristics of the sample and by level of physical frailty*.
| Total (n = 49) | Not Physically Frail (n = 25)† | Physically Frail (n = 24) | p value‡ | Cohen's d Effect Size [95%CI] | |
|---|---|---|---|---|---|
|
|
|||||
| Physical Frailty Measures: | |||||
| Unintentional weight loss | 17 (35%) | 6 (24%) | 11 (46%) | 0.11 | 0.54 [-0.13, 1.22] |
| Weakness by handgrip strength (kg) | |||||
| Women | 24.1±9.3 | 30.6±10.5 | 20.1±6.0 | 0.06 | 1.32 [0.18, 2.42] |
| Men | 41.1±7.8 | 42.0±8.6 | 39.8±6.8 | 0.43 | 0.27 [-0.42, 0.96] |
| Weakness by chair stands (s)§ | 17.6±8.0 | 14.4±7.1 | 21.3±7.6 | <0.005 | 0.95 [0.31, 1.57] |
| Slowness (m/s)§ | 0.9±0.2 | 1.1±0.2 | 0.7±0.2 | <0.001 | 2.15 [1.42, 2.87] |
| Physical exhaustion (FACIT-F; 0-52) | 24.0±10.6 | 28.4±9.6 | 19.5±9.8 | <0.005 | 0.92 [0.33, 1.51] |
| Low physical activity | 33 (67%) | 12 (48.0) | 21 (88%) | <0.01 | 1.12 [0.25, 2.13] |
| Duke Activity Status Index (METS; 0-58.2) | 5.9±1.6 | 6.8±1.4 | 5.1±1.3 | <0.001 | 1.23 [0.62, 1.84] |
Continuous data are presented as mean ± SD or median [interquartile range]; categorical data as number of patients (percentage of sample)
Not physically frail includes both non-frail (n = 1) and pre-frail (n = 24)
p values comparing physical frailty versus not physically frail
data only includes those who could successfully complete these assessments (i.e. several could not complete 5-repeat chair stands or gait speed)
Abbreviations: CI, confidence interval; FACIT-F, Functional Assessment of Chronic Illness Therapy Fatigue Scale; METS, metabolic equivalents.
Compared with those who were not physically frail, physically frail participants had significantly higher proportions of NYHA Class IV functional classification, lower serum sodium levels, and lower peak VO2 (Table 1). Physically frail participants also had significantly higher proportions of mild cognitive dysfunction and worse 1-year projected survival than those who were not physically frail. There were no significant differences between many other demographic and clinical characteristics.
Multiple measures of invasive hemodynamics were significantly different comparing physically frail participants versus those not who were not physically frail (Table 1). Physically frail participants had significantly higher pulmonary artery diastolic pressures, lower mixed venous oxygen saturations, lower cardiac outputs and cardiac indexes (by thermodilution), and higher heart rates than those who were not physically frail in unadjusted models (Table 3). After adjusting for SHFM 1-year projected survival, cardiac output (by both thermodilution and Fick equation) and heart rate remained significantly different (Table 3).
Table 3. Unadjusted and adjusted differences in invasive hemodynamic characteristics between levels of physical frailty.
| Unadjusted* | Adjusted*† | |||
|---|---|---|---|---|
|
| ||||
| β±SE | p value | β±SE | p value | |
|
|
||||
| Right atrial pressure (mmHg) | 0.5±1.2 | 0.68 | -0.8±1.2 | 0.49 |
| Pulmonary artery systolic pressure (mmHg) | 6.5±4.2 | 0.13 | 3.7±4.5 | 0.42 |
| Pulmonary artery diastolic pressure (mmHg) | 4.6±2.2 | <0.04 | 3.2±2.3 | 0.17 |
| Pulmonary capillary wedge pressure (mmHg) | 3.1±2.3 | 0.18 | 0.8±2.4 | 0.74 |
| Mixed venous oxygen saturation (%) | -5.0±2.0 | <0.02 | -3.0±2.0 | 0.14 |
| Cardiac output (L/min by thermodilution) | -1.1±0.4 | <0.01 | -0.9±0.4 | <0.05 |
| Cardiac index (L/min/m2 by thermodilution) | -0.4±0.2 | <0.03 | -0.2±0.2 | 0.16 |
| Cardiac output (L/min by Fick equation) | -0.6±0.3 | 0.05 | -0.8±0.3 | <0.03 |
| Cardiac index (L/min/m2 by Fick equation) | -0.2±0.1 | 0.18 | -0.3±0.1 | 0.09 |
| Heart rate (beats per minute) | 11.3±4.4 | <0.02 | 9.8±4.6 | <0.05 |
| Pulmonary artery pulsatility index | -0.1±0.6 | 0.87 | 0.2±0.7 | 0.78 |
| Right ventricular stroke work index | -0.04±0.08 | 0.59 | -0.02±0.09 | 0.81 |
Slope coefficient for physically frail participants (versus not physically frail)
Adjusting for Seattle Heart Failure Model 1-year survival score (a composite of clinical variables and heart failure treatments)
Abbreviations: SE, standard error
Discussion
The results from this study have generated several novel findings. First, using clinically applicable measures, we have shown that physical frailty is highly prevalent among patients with HF. Second, the characterization of physical frailty in HF in this sample demonstrates that physical frailty is associated with more advanced stages of HF. Finally, we are the first study to comprehensively show multiple invasive hemodynamic measures are significantly worse in physically frail patients with HF compared with those who are not physically frail.
In comparing our findings with other HF studies using the Frailty Phenotype, we observed slightly higher prevalence rates of physical frailty and pre-frailty, although differences in sample characteristics must be considered.7-10,26 Our rates of physical frailty could be higher compared with other HF settings because our academic medical center is a referral site for advanced HF, and all participants required a clinically-indicated RHC procedure (e.g. to stage for advanced therapies). Further, we enrolled from both hospital and clinic settings in order to capture a wide cross-section of patients with moderate to advanced HF.
The findings of this study illustrate physically frail patients with HF have worse functional status, serum sodium levels, cognitive function, and projected survival. It is not surprising that all physically frail patients were either Class III or IV functional class. The inability to rise from a chair and walk down the hall, much less complete any form of physical activity, is characteristic of impaired function in HF. Together with other studies in HF,6,7 we also show evidence that cognitive function is significantly worse among physically frail patients with HF. Finally, the significant difference in 1-year projected survival is in line with other studies that have demonstrated worse clinical outcomes in frail patients with HF.6,7
This was the first study to comprehensively examine the relationship between physical frailty and invasive hemodynamics among patients with HF. After adjusting for SHFM 1-year projected survival, we found physically frail patients with HF had significantly lower cardiac outputs and higher heart rates than those who were not physically frail. Differences in echocardiographic structural and functional parameters between frail and non-frail older adult patients have recently been noted;27 as such, studying differences in other invasive and non-invasive functional markers would help us better understand the biology of physical frailty in HF. In our study, the low mixed venous oxygen saturation and low flow at rest coupled with low peak VO2 and elevated VE/CO2 during exercise provides evidence that physical frailty is, in part, a manifestation of low-output HF. It also aligns with the cycle of physical frailty, which is conceptualized as decreased physiological reserves resulting from the cumulative decline across physiologic systems.3,28 Physical frailty is evidence that the body is slowing down; in HF, the body is slowing down because HF is an inability of the heart to adequately perfuse and deliver oxygen to the tissues. Thus, the findings from this study provide preliminary evidence of some of the similarities between the pathophysiology of HF and the presentation of physical frailty. In future research, as described by Flint and colleagues (2012),29 studying physical frailty in patients receiving mechanical circulatory support or other advanced therapies presents a unique opportunity to dissect the similarities and differences between physical frailty and HF.
The design of this study demonstrates the feasibility of assessing physical frailty in HF based on the Frailty Phenotype.3 Physical frailty was easily assessed in about 5-7 minutes in both outpatient and inpatient settings. Notably, we assessed weakness in both the upper and lower extremities, and we found the lower extremity assessment was more useful. This is the first study to incorporate 5-repeat chair stands as part of an assessment of physical frailty in HF. In the future, we would recommend using 5-repeat chair stands as they were more informative than grip strength, better captured a function most patients with HF encounter every day (e.g. rising from a chair or toilet), and have been shown to predict falls.19
A number of clinical implications can be drawn from these results. First, our assessment of physical frailty was feasible and can easily be adapted for both outpatient and inpatient clinical settings. The 5 criteria together are informative in a comprehensive and additive manner, and we would recommend using all 5 criteria when assessing physical frailty. Second, the presence of physical frailty in a patient with HF could be a useful clinical indicator of low-output HF without having to perform a RHC procedure. Finally, based on the collective significant differences between the groups, we have demonstrated physical frailty is revealing more advanced stages of HF functionally-, cognitively-, and hemodynamically-speaking. An assessment of physical frailty provides the incremental benefit of identifying those advanced HF patients at higher risk for poor clinical- and patient-oriented outcomes. Moreover, given the association with low-output HF, a physical frailty assessment provides a tool by which to gauge changes following advanced therapies such as mechanical circulatory support.
This study has a few limitations. First, beyond the limitations inherent in cross-sectional studies, we had a limited sample size, and thus, we may have been underpowered to detect some differences. Second, our sample was comprised of mostly young, Non-Hispanic Caucasian patients with moderate to advanced HF. All of the participants required a RHC procedure for clinical purposes, indicating that they were relatively sick, and referral bias for advanced HF management must be taken into consideration. Hence, our findings in a sample of relatively young, sick, mostly advanced HF patients are not generalizable to all patients with HF. Finally, only one participant was non-frail, and thus, we were limited to a comparison of physical frailty with pre-frailty; however, the significant differences between these two groups would likely translate to larger differences between physically frail and non-frail patients with HF.
Acknowledgments
Funding: Pre-doctoral funding for Quin Denfeld provided by the National Institutes of Health/National Institute of Nursing Research (NIH/NINR) Ruth L. Kirschstein National Research Service Award (F31NR015936; Denfeld) and the National Hartford Centers of Gerontological Nursing Excellence (NHCGNE) Patricia G. Archbold Scholar Program (Denfeld). Post-doctoral funding for Quin Denfeld provided by NIH/National Institute of Heart, Lung, and Blood (NIH/NHLBI) at Oregon Health & Science University Knight Cardiovascular Institute (T32HL094294; Thornburg). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NINR, NIH/NHLBI, or the NHCGNE.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Afilalo J, Mottillo S, Eisenberg MJ, Alexander KP, Noiseux N, Perrault LP, Morin JF, Langlois Y, Ohayon SM, Monette J, Boivin JF, Shahian DM, Bergman H. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–228. doi: 10.1161/CIRCOUTCOMES.111.963157. [DOI] [PubMed] [Google Scholar]
- 5.Gharacholou SM, Roger VL, Lennon RJ, Rihal CS, Sloan JA, Spertus JA, Singh M. Comparison of frail patients versus nonfrail patients <65 years of age undergoing percutaneous coronary intervention. Am J Cardiol. 2012;109:1569–1575. doi: 10.1016/j.amjcard.2012.01.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 7.Jha SR, Hannu MK, Chang S, Montgomery E, Harkess M, Wilhelm K, Hayward CS, Jabbour A, Spratt PM, Newton P, Davidson PM, MacDonald PS. The prevalence and prognostic significance of frailty in patients with advanced heart failure referred for heart transplantation. Transplantation. 2016;100:429–436. doi: 10.1097/TP.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 8.McNallan SM, Singh M, Chamberlain AM, Kane RL, Dunlay SM, Redfield MM, Weston SA, Roger VL. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1:135–141. doi: 10.1016/j.jchf.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16:208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez-Rodriguez A, Abreu-Gonzalez P, Jimenez-Sosa A, Gonzalez J, Caballero-Estevez N, Martín-Casañas FV, Lara-Padron A, Aranda JM., Jr The impact of frailty in older patients with non-ischaemic cardiomyopathy after implantation of cardiac resynchronization therapy defibrillator. Europace. 2015;17:598–602. doi: 10.1093/europace/euu333. [DOI] [PubMed] [Google Scholar]
- 11.Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin-Adams M, Dickinson MG, Levy P, Stough WG, Sweitzer NK, Teerlink JR, Whellan DJ, Albert NM, Krishnamani R, Rich MW, Walsh MN, Bonnell MR, Carson PE, Chan MC, Dries DL, Hernandez AF, Hershberger RE, Katz SD, Moore S, Rodgers JE, Rogers JG, Vest AR, Givertz MM. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21:519–534. doi: 10.1016/j.cardfail.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail. 2016;22:110–116. doi: 10.1016/j.cardfail.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.McLennan SN, Mathias JL, Brennan LC, Stewart S. Validity of the Montreal Cognitive Assessment (MoCA) as a screening test for Mild Cognitive Impairment (MCI) in a cardiovascular population. J Geriatr Psychiatry Neurol. 2011;24:33–38. doi: 10.1177/0891988710390813. [DOI] [PubMed] [Google Scholar]
- 19.Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37:430–435. doi: 10.1093/ageing/afn100. [DOI] [PubMed] [Google Scholar]
- 20.Bennett JA, Winters-Stone KM, Dobek J, Nail LM. Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol Nurs Forum. 2013;40:E126–E134. doi: 10.1188/13.ONF.E126-E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulignano G, Del Sindaco D, Di Lenarda A, Alunni G, Senni M, Tarantini L, Cioffi G, Tinti MD, Barbati G, Minardi G, Uguccioni M. Incremental value of gait speed in predicting prognosis of older adults with heart failure: insights From the IMAGE-HF study. JACC Heart Fail. 2016;4:289–298. doi: 10.1016/j.jchf.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 24.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 25.Fan X, Lee KS, Frazier SK, Lennie TA, Moser DK. Psychometric testing of the Duke Activity Status Index in patients with heart failure. Eur J Cardiovasc Nurs. 2015;14:214–221. doi: 10.1177/1474515114523354. [DOI] [PubMed] [Google Scholar]
- 26.Reeves GR, Whellan DJ, Patel MJ, O'Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva AM, Kitzman DW. Comparison of frequency of frailty and severely impaired physical function in patients >/=60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol. 2016;117:1953–1958. doi: 10.1016/j.amjcard.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gharacholou SM, Tashiro T, Cha SS, Scott CG, Takahashi PY, Pellikka PA. Echocardiographic indices associated with frailty in adults >/=65 years. Am J Cardiol. 2015;116:1591–1595. doi: 10.1016/j.amjcard.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint KM, Matlock DD, Lindenfeld J, Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circulation. 2012;5:286–293. doi: 10.1161/CIRCHEARTFAILURE.111.963215. [DOI] [PMC free article] [PubMed] [Google Scholar]


