Abstract
Introduction
Tobacco advertising can create false beliefs about health harms that are reinforced by product design features. Reduced nicotine content (RNC) cigarettes may reduce harm, but research has not addressed advertising influences. This study examined RNC cigarette advertising effects on false harm beliefs, and how these beliefs – along with initial subjective ratings of RNC cigarettes – affect subsequent smoking behaviors. We further explored whether subjective ratings moderate associations between false beliefs and behavior.
Methods
Seventy-seven daily, non-treatment-seeking smokers (66.2% male) participated in the first 15 days of a randomized, controlled, open-label RNC cigarette trial. Participants viewed an RNC cigarette advertisement at baseline before completing a 5-day period of preferred brand cigarette use, followed by a 10-day period of RNC cigarette use (0.6 mg nicotine yield). Participants provided pre- and post-advertisement beliefs, and subjective ratings and smoking behaviors for cigarettes smoked during laboratory visits.
Results
Viewing the advertisement increased beliefs that RNC cigarettes contain less nicotine and are healthier than regular cigarettes (p’s < 0.001 and 0.011), and decreased the belief that they are less likely to cause cancer (p = 0.046). Neither false beliefs nor subjective ratings directly affected smoking behaviors. Significant interactions of strength and taste ratings with beliefs (p’s < 0.001), however, indicated that among smokers with less negative initial subjective ratings, greater false beliefs were associated with greater RNC cigarette consumption.
Conclusions
Smokers may misconstrue RNC cigarettes as less harmful than regular cigarettes. These beliefs, in conjunction with favorable subjective ratings, may increase product use.
Keywords: low nicotine, reduced nicotine content, false beliefs, subjective ratings, tobacco product advertising, smoking behaviors
1. INTRODUCTION
The U.S. Food and Drug Administration (FDA) has the authority to regulate tobacco products (U.S. Congress, 2009), including the ability to mandate a reduction in cigarette nicotine content. This action is proposed to decrease tobacco-related morbidity and mortality (Benowitz and Henningfield, 1994; Hatsukami et al., 2010b; Henningfield et al., 1998; USDHHS, 2014) and is supported empirically: reduced nicotine content (RNC) cigarette use generally decreases dependence and toxicant exposure without increasing smoking behaviors, and may facilitate cessation (Benowitz et al., 2007, 2012; Benowitz et al., 2006; Donny et al., 2015; Hammond and O’Connor, 2014; Hatsukami et al., 2010a; Hatsukami et al., 2012b). If the FDA implements a reduced nicotine content standard, however, it is unclear how product marketing (e.g., labeling, advertising) may affect RNC cigarette use and acceptance. The tobacco industry falsely marketed “light” cigarettes as reduced harm products to maintain sales, and may promote RNC cigarettes similarly. Studies are thus needed to evaluate the impact of RNC cigarette marketing and determine if additional regulation is warranted.
RNC cigarettes are not equivalent to “light” or “ultra-light” cigarettes. The former contain tobacco genetically modified to have lower nicotine content; the latter manipulate product design features (e.g., filter ventilation) to deliver less nicotine yield yet have nicotine content comparable to regular/“full-flavor” cigarettes (USDHHS, 2001). Smokers can “compensate” for such design features to increase nicotine intake by modifying their smoking behaviors (e.g., increasing daily consumption, blocking filter vents), increasing intake of other, harmful constituents (USDHHS, 2001). In contrast, little to no compensation occurs with long-term RNC cigarette use (Bandiera et al., 2015; Donny et al., 2015; Hatsukami et al., 2015; Mercincavage et al., 2016) because they contain insufficient extractable nicotine and do not reward these behaviors.
Despite these distinctions, a concern with a nicotine reduction approach is that consumers may believe RNC cigarettes to be less harmful, as occurred with “light” cigarettes and other potential reduced exposure products (PREPs) largely due to their marketing (Hamilton et al., 2004; O’Connor et al., 2005; Parascandola et al., 2009; Shadel et al., 2006; Shiffman, 2004; Shiffman et al., 2007; Shiffman et al., 2001). Thus, if RNC cigarettes are marketed similarly, false beliefs about their safety may increase smokers’ use or decrease quitting likelihood. Further, at-risk, non-smoking youth may be more likely to experiment with these products and potentially initiate longer-term use. To our knowledge, however, no studies have experimentally tested how RNC cigarette beliefs affect smoking behavior (i.e., product use) – a critical indicator of both abuse liability and product acceptance. Additionally, data is needed regarding the impact of RNC advertisements and their content (e.g., implicit and explicit claims about product safety) on beliefs about product risks. Explicit content may directly affect specific false beliefs: e.g., “low tar” statement increases belief that product has less tar. Conversely, implicit content can indirectly affect specific beliefs that consequently determine overall product impressions: e.g., lighter colors within advertisements incrementally increase individual beliefs that product has less nicotine and tar, resulting in an overall impression of reduced harm (Bansal-Travers et al., 2011).
Research must also consider RNC cigarette design features, which may produce subjective responses that strengthen false product beliefs. For example, in addition to their misleading marketing, “light” cigarettes contained filter-ventilation that produced sensory perceptions of a “lighter” or “smoother” taste (Kozlowski and O’Connor, 2002; O’Connor et al., 2013), reinforcing smokers’ false beliefs about lower harm (Elton-Marshall et al., 2015; Green et al., 2015; Mutti et al., 2011). While smokers generally provide negative subjective ratings of RNC cigarettes (Benowitz et al., 2007, 2012; Mercincavage et al., 2016; Strasser et al., 2007), implying lower use likelihood, few studies have associated these ratings with subsequent smoking behaviors. The available evidence demonstrates no clear association (Mercincavage et al., 2016). Studies thus must evaluate how subjective ratings of RNC cigarettes affect product use behaviors and beliefs to address whether their design features, like those of “light” cigarettes, reinforce false beliefs about product safety.
Our prior work demonstrated that advertising for a previously commercially-available RNC product that heavily marketed its low nicotine appeal (i.e., Quest®; Vector Tobacco Inc.) affected smokers’ beliefs about the product’s overall health risks (Lochbuehler et al., 2016; Strasser et al., 2008). This work, however, did not consider product use behaviors or subjective ratings. Because no studies have investigated how beliefs about RNC cigarette risks influence actual product use, the present exploratory study examined changes in product risk beliefs after viewing an unaltered advertisement, and how these beliefs and subjective ratings affected subsequent smoking behaviors (i.e., daily cigarette consumption, total puff volume) when provided with the first in a series of “stepdown” RNC cigarette products (i.e., Quest 1® cigarettes). Finally, we investigated possible moderating effects of subjective ratings on associations between false beliefs and use behaviors. This approach, although exploratory, is high novel, as this this study is the first to use experimental data to examine the interplay between RNC cigarette advertising, subjective responses, and smoking behaviors – a critical next step in providing the FDA with comprehensive evidence to evaluate implications of a low nicotine content standard. Specifically, we sought to understand marketing influences on these outcomes when using a novel cigarette product with a reduced nicotine content (not yield) similar to what the FDA could mandate in the future. Findings may inform future decisions regarding regulation of cigarette nicotine content and related marketing.
2. MATERIAL AND METHODS
2.1 Participants and Design
We performed secondary analyses on data from the first 15 days of a 35-day, randomized, controlled, open-label laboratory trial of RNC cigarettes, detailed elsewhere (Mercincavage et al., 2016). Smokers interested in trying a new low nicotine cigarette product were recruited from the Philadelphia area using digital and print advertisements, or were former study participants. A telephone interview determined initial eligibility; eligible participants were ≥ 21 years old, exclusively smoked ≥ 15 non-menthol, filtered cigarettes/day, smoked regularly for ≥ five years, and had no plans to quit smoking in the next two months. Participants were excluded if they drank ≥ 25 alcohol-containing drinks/week; were currently using marijuana or nicotine-containing products; self-reported a history of any psychiatric condition other than depression, a past year myocardial infarction, or a substance use disorder in the past five years; were pregnant/lactating; or provided an initial carbon monoxide (CO) sample < 10 ppm.
Analyses included only participants who completed the first 15 trial days, indicated no prior Quest cigarette use, and were randomized to Quest 1® RNC cigarette (as opposed to Quest 2®, 3®, or preferred brand cigarette) use to control for nicotine content effects. Seventy-seven individuals met these criteria; of these individuals, 55.8% and 44.2% indicated they had heard and not heard of Quest cigarettes, respectively.
2.2 Procedure
Participants completed an initial laboratory visit to provide written informed consent, verify eligibility, and smoke three cigarettes with 45 minutes between each: the first standardized time since last cigarette; the next two were smoked through topography equipment to assess puffing behavior. After each cigarette smoked through topography equipment, participants provided pre- and post-cigarette CO samples, and completed post-cigarette subjective rating forms. After the first cigarette, participants completed demographic, smoking history, and Quest cigarette beliefs (i.e., pre-advertisement beliefs) questionnaires. Following the second cigarette, participants viewed a Quest cigarette advertisement and again completed the beliefs questionnaire (i.e., post-advertisement beliefs). Subsequent visits occurred every five days (i.e., not every day) and were identical in format with the exception of viewing the advertisement. Participants smoked their own preferred brand cigarettes during the initial visit and next five days. At the Day 5 visit, before the third cigarette, participants were provided with Quest 1 RNC cigarettes (0.6 mg FTC-measured nicotine yield) free-of-charge for 10 days. Thus, all Day 0 cigarettes and the first two Day 5 cigarettes were participants’ own brand, while the third Day 5 cigarette and all Day 10 cigarettes were RNC cigarettes; this design allowed for direct comparisons in product use behaviors and subjective responses between RNC and own brand cigarettes under identical conditions, as well as over time within a specific cigarette use period (i.e., initial vs. intermediate vs. final RNC cigarette exposure). Participants were instructed to only use study-supplied cigarettes, were given incentives based on returning used and unused cigarettes equal with amount distributed, and were informed that using non-study-supplied cigarettes would result in their removal; further details on cigarette compliance and incentivization are available elsewhere (Mercincavage et al., 2016). The trial was registered according to International Committee of Medical Journal Editors guidelines (ClinicalTrials.gov #NCT01202942); all procedures were approved by the university Institutional Review Board.
2.2.1. Advertisement
Consistent with previous work (Lochbuehler et al., 2016; Shadel et al., 2006; Strasser et al., 2012; Strasser et al., 2008), participants viewed an unaltered company-created advertisement for 30 seconds (Strasser et al., 2008), deliberately presented five days before randomization to approximate real-world conditions (e.g., viewing novel product advertisement in a magazine/online/etc., then purchasing it the next time at store; i.e., not immediately after viewing advertisement).
2.3 Measures
Demographic and smoking history variables assessed at the initial visit included smokers’ age, gender, race, ethnicity, highest education level, body mass index (BMI), daily cigarette consumption, years smoking regularly, preferred brand cigarette type (light/ultra-light vs. full-flavor/medium), and nicotine dependence (assessed by the Fagerström Test of Nicotine Dependence; Heatherton et al., 1991).
Eight items (Lochbuehler et al., 2016; Shadel et al., 2006; Strasser et al., 2008) rated on a 5-point response scale (1= “definitely untrue”, 5=“definitely true”) assessed participants’ Quest cigarette beliefs only at the initial study visit, before and after viewing the advertisement: “Quest cigarettes:” (a) “are lower in nicotine than regular cigarettes”, (b) “are lower in tar than regular cigarettes”, (c) “are less addictive than regular cigarettes”, (d) “are less likely to cause cancer than regular cigarettes”, (e) “have fewer chemicals than regular cigarettes”, (f) “healthier than regular cigarettes”, (g) “make smoking safer”, (h) “help people quit smoking.” Items b–h were summed to create a cumulative false beliefs variable (Cronbach’s α’s = 0.77 and 0.81 for pre- and post-advertisement variables, respectively); consistent with previous work (Lochbuehler et al., 2016; Shadel et al., 2006), the first item was excluded because it was factually correct.
Subjective ratings of RNC cigarettes were assessed immediately following participants’ initial exposure (i.e., third cigarette on Day 5), using a 14-item 100 mm visual analog scale of cigarette characteristics (e.g., taste) used by the tobacco industry (Philip Morris, 1997) and elsewhere (Strasser et al., 2013, 2007). Anchors were item-specific (e.g., taste: 0=“bad”, 100=“good”); lower scores indicated more negative responses. To reduce the number of rating items in subsequent moderation analyses, we conducted an exploratory principal components factor analysis with varimax rotation. We restricted solutions to contain ≤ three factors, conservatively retaining only items with loadings >0.70. We constructed overall ‘strength’ and ‘taste’ subscales by summing responses to “strength”, “harshness”, “mild taste”, and “too mild” items (Cronbach’s α = 0.81), and “taste” and “after taste” items (Cronbach’s α = 0.70), respectively. The third subscale consisted only of the “draw” item.
The primary and secondary behavioral outcomes were daily cigarette consumption and total puff volume, respectively, during the 10-day RNC cigarette use period. Daily cigarette consumption was assessed via self-report using timeline follow-back methods and verified through spent filter collection (Evans et al., 2015; Strasser et al., 2013) for each day within the 10-day period (r for self-reported consumption and spent filters = 0.97, p < .001, mean difference = .23, 95% CI = .07–.39). Total puff volume, an objective measure of puffing behavior, was collected using Clinical Research Support System equipment (Borgwaldt KC, Richmond, VA) for all RNC cigarettes smoked on Days 5, 10, and 15. Given the stability of each outcome assessed repeatedly over the 10-day use period (intraclass correlation coefficients > 0.70; Mercincavage et al., 2016), composite measures were created by averaging all assessments across the RNC use period.
2.4 Statistical Analysis
Paired t-tests compared pre- and post-advertisement beliefs. Pearson correlations examined associations between post-advertisement individual and cumulative false beliefs, initial subjective ratings, and mean smoking behaviors. Using methods demonstrated by Sell and colleagues (Sell et al., 2016), a series of step-wise regression models examined the direct effects of post-advertisement false beliefs and initial (i.e., only the single assessment from Day 5) subjective ratings on mean smoking behaviors, and the moderating effect of subjective ratings on the association between beliefs and behaviors (Figure 1). In the first step of regression models, each smoking behavior (e.g., daily RNC consumption) was regressed onto covariates identified a priori for their potential effects on study outcomes, including: gender (Perkins, 1996; Perkins et al., 1999; Vogel et al., 2014), BMI (Blendy et al., 2005) nicotine dependence (Bandiera et al., 2015), years smoking (Ayanian and Cleary, 1999), and cigarette type (Kozlowski and Pillitteri, 2001). To account for increased consumption of Quest cigarettes relative to participants’ own preferred brand cigarettes (Mercincavage et al., 2016), daily consumption of own brand during the 5-day baseline period was also included as a covariate. The second model step added false beliefs (Path A) and subjective rating subscales (Path B) to estimate main effects. The third and final model step added the interaction term for false beliefs × subjective ratings (Path C). Separate models were run for each false belief measure (i.e., one cumulative and seven individual items) and each of three subjective rating subscales. Significant interactions were graphed using means within one standard deviation. Tukey’s HSD examined mean differences post-hoc. Analyses were conducted using IBM SPSS Statistics v23.
Figure 1.
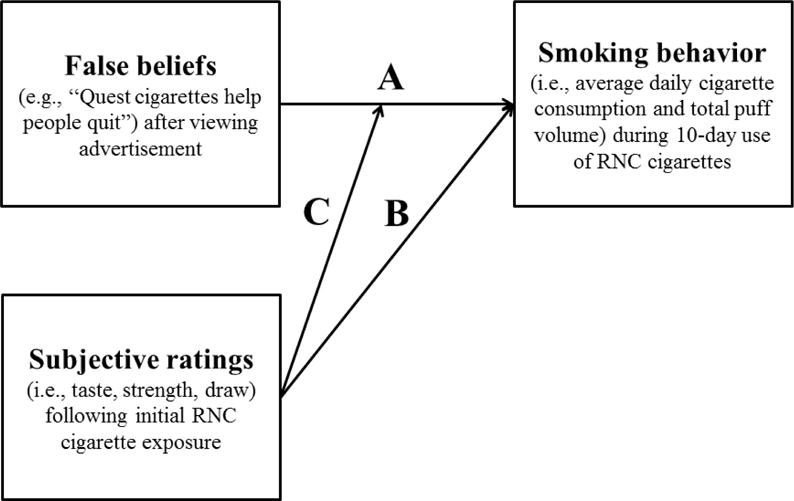
Model conceptualizing independent and interactive effects of false beliefs, subjective ratings, and smoking behavior: Path A represents the main, direct effect of false beliefs on smoking behavior, Path B represents the main, direct effect of subjective ratings on smoking behavior, and Path C represents the moderating effect of subjective ratings on the association between false beliefs and smoking behavior.
3. RESULTS
3.1 Sample characteristics
Of the 83 participants who indicated no prior Quest cigarette use and who were randomized to use the Quest 1 cigarettes, 77 completed the full 10-day use period (i.e., 7.2% attrition). These participants (66.2% male), on average, were 40.49 years old (SD = 13.11), had a BMI of 26.67 (SD = 5.85), reported smoking 21.17 cigarettes/day (SD = 6.00), smoked regularly for 23.57 years (SD = 13.09), and were moderately nicotine dependent (mean FTND score = 5.77, SD = 1.88). The majority was White (94.8%), non-Hispanic (97.4%), never married (55.8%), completed some college/technical training (37.7%), and smoked full-flavor/medium cigarettes (59.7%).
3.2 Advertisement effects on individual and cumulative product beliefs
Viewing the advertisement increased beliefs that Quest cigarettes are lower in nicotine (p < 0.001) and healthier than regular cigarettes (p = 0.011), and decreased the belief that Quest cigarettes are less likely to cause cancer (p = 0.046) (Table 1). There was no change in cumulative false beliefs (p = 0.18).
Table 1.
Mean comparisons of beliefs about Quest cigarettes before and after viewing a Quest cigarette advertisement (n = 77).
| Pre-advertisement rating M (SD) |
Post-advertisement rating M (SD) |
t | |
|---|---|---|---|
| Individual belief items | |||
| (a) Quest cigarettes are lower in nicotine than regular cigarettes | 3.64 (0.79) | 4.19 (0.86) | −4.63*** |
| (b) Quest cigarettes are lower in tar than regular cigarettes | 3.29 (0.65) | 3.31 (0.85) | −0.24 |
| (c) Quest cigarettes are less addictive than regular cigarettes | 2.94 (0.50) | 3.01 (0.98) | −0.76 |
| (d) Quest cigarettes are less likely to cause cancer than regular cigarettes | 2.73 (0.76) | 2.53 (0.87) | 2.03* |
| (e) Quest cigarettes have fewer chemicals than regular cigarettes | 3.04 (0.60) | 3.17 (0.88) | −1.37 |
| (f) Quest cigarettes are healthier than regular cigarettes | 2.73 (0.84) | 2.99 (0.99) | −2.59* |
| (g) Quest cigarettes make smoking safer | 2.57 (0.85) | 2.69 (0.95) | −1.16 |
| (h) Quest cigarettes help people quit smoking | 3.10 (0.60) | 3.21 (1.13) | −0.84 |
| Cumulative false beliefs (items b–h) | 20.39 (3.16) | 20.91 (4.57) | −1.36 |
Note:
indicates significance at the p < 0.05 level;
= p < 0.01,
= p < 0.001
3.3 Effects of individual false beliefs and subjective ratings on smoking behaviors
3.3.1 Correlations
Before conducting regression analyses, we explored independent correlations among false beliefs, subjective ratings, and smoking behaviors (Table 2). Beliefs that Quest cigarettes are less addictive than regular cigarettes and help people quit were positively associated with the taste subscale (p’s = 0.001 and 0.008, respectively) and daily cigarette consumption (p’s = 0.003 and 0.026, respectively). The belief that Quest cigarettes contain fewer chemicals was positively correlated with the taste subscale (p = 0.002) and total puff volume (p = 0.043).
Table 2.
Correlation matrix of individual and cumulative false belief items, subjective ratings during initial exposure to 0.6 mg nicotine yield Quest 1 cigarettes, and smoking behaviors averaged across a 10-day use period.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-advertisement Quest beliefs | ||||||||||||||
| 1. False beliefs | – | |||||||||||||
| 2. Lower in nicotine | .26* | – | ||||||||||||
| 3. Lower in tar | .50*** | .01 | – | |||||||||||
| 4. Less addictive | .71*** | .17 | .19 | – | ||||||||||
| 5. Less likely to cause cancer | .74*** | .20 | .11 | .49*** | – | |||||||||
| 6. Fewer chemicals | .64*** | .01 | .39*** | .27* | .35** | – | ||||||||
| 7. Healthier | .80*** | .28* | .32** | .54*** | .50*** | .48*** | – | |||||||
| 8. Make smoking safer | .76*** | .19 | .27* | .41*** | .65*** | .41*** | .65*** | – | ||||||
| 9. Help people quit smoking | .65*** | .34** | .23* | .44*** | .46*** | .23* | .36** | .28* | – | |||||
| Initial exposure subjective rating subscales | ||||||||||||||
|
| ||||||||||||||
| 10. Strength | .09 | .17 | .03 | .04 | .03 | <.01 | .04 | .17 | .11 | – | ||||
| 11. Taste | .36** | .19 | .21 | .36** | .17 | .34** | .20 | .14 | .30** | .10 | – | |||
| 12. Draw | .06 | .06 | −.01 | .14 | .11 | −.13 | .04 | .11 | .014 | .09 | .17 | – | ||
| RNC cigarette smoking behaviors | ||||||||||||||
|
| ||||||||||||||
| 13. Daily cigarette consumption | .28* | .08 | .08 | .33** | .17 | .18 | .19 | .11 | .25* | .04 | .35** | .19 | – | |
| 14. Total puff volume | .13 | .03 | .04 | .02 | .13 | .23* | .09 | .18 | −.04 | .16 | .08 | −.03 | −.08 | – |
Note:
indicates p < .05,
= p <.01,
= p < .001
3.3.2 Effects on daily RNC cigarette consumption
Models first regressed daily RNC cigarette consumption onto covariates of gender, dependence, years smoking, cigarette type, and own brand daily cigarette consumption. Only own brand daily consumption was significantly associated with RNC cigarette consumption (β = 0.84, SE = 0.08, p < 0.001, 95% CI = 0.68–1.00). After controlling for covariates, neither individual beliefs nor subjective rating subscales had a direct effect on daily RNC cigarette consumption. The strength and taste subscales moderated several associations between daily consumption and individual belief measures. The strength subscale significantly moderated associations of daily RNC cigarette consumption with the beliefs that Quest cigarettes are less likely to cause cancer than regular cigarettes (β = 0.093, SE = 0.028, p = 0.002, 95% CI = 0.002–0.036, R2Δ = 0.038) and make smoking safer (β = 0.08, SE = 0.025, p = 0.001, 95% CI = 0.035–0.14, R2Δ = 0.041). Greater belief in each statement was associated with increased daily RNC consumption among smokers who provided initial above average strength ratings, and decreased daily RNC consumption among smokers who provided below average strength ratings (Figure 2). These beliefs and daily consumption were unassociated among smokers who provided average strength ratings.
Figure 2.
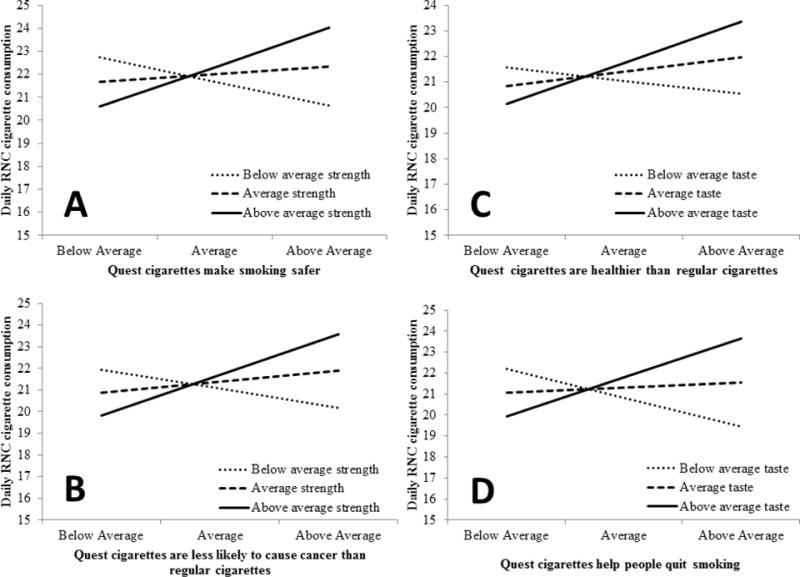
Panels A and B illustrate moderating effects of initial strength ratings on the associations between daily RNC cigarette consumption and the beliefs that (A) “Quest cigarettes make smoking safer” and (B) “Quest cigarettes are less likely to cause cancer than regular cigarettes.” Panels C and D demonstrate moderating effects of initial taste ratings on the associations between daily cigarette consumption and the beliefs that (C) “Quest cigarettes are healthier than regular cigarettes” and (D) “Quest cigarettes help people quit smoking. Greater belief in each statement (i.e., above average ratings) increased RNC cigarette consumption among smokers with above average initial subjective ratings of RNC cigarettes, and decreased cigarette consumption among smokers with below average ratings in panels A, B, and D (C not significant).
The taste subscale significantly moderated the associations of daily RNC cigarette consumption with beliefs that Quest cigarettes were healthier than regular cigarettes (β = 0.06, SE = 0.023, p = .012, 95% CI = 0.014–0.11, R2Δ = 0.024) and help people quit smoking (β = 0.08, SE = 0.02, p < 0.001, 95% CI = 0.041–0.12, R2Δ = 0.054). Among smokers who provided above average initial taste ratings, greater beliefs that Quest cigarettes are healthier and help people quit were associated with greater RNC cigarette consumption. Among smokers who provided below average ratings, greater belief that Quest cigarettes help people quit was associated with lower RNC cigarette consumption. There was no association between these beliefs and daily consumption among smokers who provided average taste ratings, or between the belief that Quest cigarettes are healthier and consumption among smokers who provided below average taste ratings.
3.3.3 Effects on RNC total puff volume
Models regressing RNC cigarette total puff volume onto covariates found significant associations with only own brand total puff volume (β = 0.31, SE = 0.08, p < 0.001, 95% CI = 0.16–0.47) and cigarette type (β = 89.45, SE = 39.68, p = 0.027, 95% CI = 10.32–168.58). Controlling for covariates, individual beliefs and subjective rating subscales had no direct effects on total puff volume (p’s > 0.2). Only the taste subscale moderated the association between total puff volume and the belief that Quest cigarettes help people quit (β = −2.02, SE = 0.87, p = 0.024, 95% CI = −3.75–−0.28, R2Δ = 0.055). Among smokers who provided above average initial taste ratings, greater belief in this statement was associated with decreased total puff volume. This belief was not associated with total puff volume among smokers who provided average or below average taste ratings.
3.4 Effects of cumulative false beliefs and subjective ratings on smoking behaviors
3.4.1 Correlations
Cumulative false beliefs were positively correlated with the taste subscale and daily cigarette consumption (p’s = 0.001 and 0.014, respectively; Table 2).
3.4.2 Effects on daily RNC consumption
After controlling for covariates, models examining cumulative false beliefs as predictors of daily RNC cigarette consumption found that neither cumulative beliefs, nor subjective rating subscales, had a direct effect on consumption (p’s > 0.2). Only the taste subscale significantly moderated the effect of cumulative false beliefs on daily consumption (β = 0.012, SE = 0.005, p = 0.012, 95% CI = 0.003–0.022, R2Δ = 0.024). Among smokers who provided below average initial taste ratings of RNC cigarettes, greater false beliefs were associated with lower daily RNC cigarette consumption. False beliefs and daily cigarette consumption were unassociated among smokers who provided average or above average taste ratings.
3.4.3 Effects on RNC total puff volume
Neither cumulative false beliefs nor subjective rating subscales had a direct effect on RNC cigarette total puff volume after controlling for covariates (p’s > 0.2). Subjective rating subscales did not moderate associations between cumulative false beliefs and total puff volume (p’s > 0.1).
4. DISCUSSION
The findings of this study demonstrate that although neither false beliefs nor subjective ratings directly affected smoking behaviors, taste and strength ratings moderated several associations between beliefs and daily RNC cigarette consumption. These findings are novel because they experimentally demonstrate how sensory perceptions interact with beliefs to influence behavior. Cross-sectional studies (Elton-Marshall et al., 2015; Green et al., 2015; Mutti et al., 2011; O’Connor et al., 2013) indicate that sensory perceptions of a “weaker/lighter” taste reinforce smokers’ beliefs about “light” cigarettes and PREPs; we thus expected false beliefs and consumption to be positively associated among smokers providing lower strength and taste ratings. Instead these associations occurred among smokers who provided greater ratings, and were inverse or nonexistent among smokers providing lower ratings. Because smokers generally rated RNC cigarettes lower than their preferred brand (Mercincavage et al., 2016), findings may still support that weaker/lighter perceptions reinforce false belief effects on behavior. But within RNC cigarette use, false beliefs appear to increase consumption among smokers with greater (i.e., less negative) ratings. Moderation effect sizes appeared small, yet remain significant given that interactions of beliefs from a single, 30-second advertisement exposure with ratings of a single RNC cigarette explained 2–5% of the variance in a behavioral measure of 10-day product use.
Although exploratory, this study improves upon previous cross-sectional research by experimentally modeling how smokers might observe marketing for, and subsequently try, a novel RNC cigarette product. Donny and colleagues (2015) recently demonstrated potential RNC cigarette benefits; unlike the present investigation, however, this and other RNC trials used investigational cigarettes in grey packages without accompanying advertising, and could not evaluate marketing effects. Given that the tobacco industry would likely adjust marketing to promote continued cigarette sales, our study provides important evidence of the independent and interactive effects of marketing-related product beliefs and initial subjective responses on actual use behaviors. Findings suggest that marketing regulations may need to accompany a federal nicotine reduction policy to prevent smokers from developing false beliefs about RNC cigarettes. Future research should further investigate how specific implicit and explicit advertising claims – strategies used by the tobacco industry – affect false beliefs and product use to inform potential regulations on advertising content. The FDA could also consider regulating RNC cigarette design features that increase subjective favorability – also within the FDA’s authority (U.S. Congress, 2009) – to reduce the association between false beliefs and harmful use behaviors.
We also found that RNC cigarette daily consumption and total puff volume were positively related, respectively, to daily consumption and total volume of own brand cigarettes during the baseline period. These findings are not surprising given that smoking behaviors are highly correlated within-subject (Lee et al., 2003; Strasser et al., 2009), and remain so when using a new cigarette product. The finding that medium/full-flavored cigarette smokers had greater total puff volume than light/ultra-light smokers when using RNC cigarettes, is also unsurprising, as the nicotine yield of the study-supplied cigarettes was similar to that of light cigarettes; thus, full-flavor smokers may have engaged in initial compensatory behaviors when using these products. Although studies have shown that RNC cigarettes produce little compensatory smoking behaviors, it is possible that this finding is due to other features of the Quest products (e.g., filter ventilation).
It is interesting to note that exposure to RNC cigarette advertising improved beliefs about the product’s low nicotine content and cancer risk, yet increased the false belief that Quest cigarettes were healthier than regular cigarettes. Despite affecting these individual beliefs, viewing the advertisement did not affect cumulative false product beliefs. Similar to research on “light” cigarettes and PREPs (Hamilton et al., 2004; Shadel et al., 2006; Shiffman, 2004; Shiffman et al., 2007, 2001), these results suggest that without education about RNC cigarette risks, smokers may misconstrue these products as having lower harm in the absence of accurate, explicit contrary claims. Findings illustrate the importance of using both individual and cumulative belief measures to evaluate the complex effects of tobacco product advertising content, as changes may occur in individual belief items due to specific content (e.g., implicit or explicit claims), which are not reflected by changes in a cumulative beliefs measure. These individual and cumulative beliefs may then have distinct, yet important, interactions with product ratings and use.
Several caveats specific to the present study should be considered when interpreting these findings (see Mercincavage et al., 2016 for discussion of general limitations of the full randomized trial). First, analyses tested a large number of effects without accounting for Type 1 error given small anticipated effect sizes. Thus, although findings underscore the importance of examining the interactions of subjective ratings and product risk beliefs, results must be understood as purely exploratory and should be interpreted with caution; larger trials are needed to confirm these findings. Smokers also experienced only a single, 30-second advertisement exposure; true changes in beliefs may require repeated exposures to product advertising. However, smokers were continuously exposed to RNC cigarette marketing through the Quest cigarette packages themselves. These exposures also mimic a real-world marketing campaign in which a smoker might see a product advertisement, form beliefs about the product, purchase it commercially soon after, and make subjective assessments during initial use. Additionally, study cigarettes were the highest nicotine content in a series of novel, marketed-as “step-down” RNC products, and do not necessarily represent marketing effects when using all other RNC levels (investigational or potentially-regulated future commercial products). Because it is unknown at what level, or rate (graduate or immediate), the FDA would mandate a reduction in cigarette nicotine content, future research should evaluate marketing influences on beliefs and use of cigarettes with a variety of nicotine content. Finally, this research is also limited to informing regulatory efforts in countries where tobacco advertising is legal and plain cigarette packaging has not been mandated.
4.1 Conclusions
This study’s exploratory evaluation of RNC cigarette advertising effects on false product beliefs and use, and its examination of initial subjective ratings (i.e., sensory perceptions) as moderators of these associations, are highly novel. Associations between false beliefs and greater smoking behaviors were strongest among smokers who had less negative initial subjective responses to these products. Because the FDA may regulate cigarette nicotine content, design features, and advertising, findings suggest that implementation of a nicotine reduction policy may require additional educational efforts to adequately inform smokers of RNC cigarette harms, and potentially additional regulation on advertising content and design features.
Highlights.
Advertising affected false beliefs about risks of using reduced nicotine content (RNC) cigarettes.
Neither subjective ratings nor false beliefs directly influenced product use.
Subjective ratings and false beliefs interacted to affect RNC cigarette use behaviors.
False beliefs, together with favorable subjective ratings, increased consumption.
Acknowledgments
None.
Role of Funding Sources
Funding for this study was provided by the National Institutes of Health (R01 CA120594 and R01 CA180929 to A.A.S.). M.M.’s training was supported by the National Institutes of Health and FDA Center for Tobacco Products (P50 CA179546). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Neither NIH nor FDA CTP was involved in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
AAS was the principal investigator for the parent RCT; he designed, obtained funding for, and conducted the study. EG and AH assisted with data collection. MM performed the data analyses and wrote the first draft of the manuscript. MLS and DM aided in data interpretation and the development of subsequent drafts. All authors contributed substantially to the writing of this manuscript and approved its submission.
Conflict of Interest
All authors have no conflicts of interest.
References
- Ayanian JZ, Cleary PD. Perceived risks of heart disease and cancer among cigarette smokers. JAMA. 1999;281:1019. doi: 10.1001/jama.281.11.1019. https://doi.org/10.1001/jama.281.11.1019. [DOI] [PubMed] [Google Scholar]
- Bandiera FC, Ross KC, Taghavi S, Delucchi K, Tyndale RF, Benowitz NL. Nicotine dependence, nicotine metabolism, and the extent of compensation in response to reduced nicotine content cigarettes. Nicotine Tob Res. 2015;17:1167–1172. doi: 10.1093/ntr/ntu337. https://doi.org/10.1093/ntr/ntu337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal-Travers M, Hammond D, Smith P, Cummings KM. The impact of cigarette pack design, descriptors, and warning labels on risk perception in the US. Am J Prev Med. 2011;40:674–682. doi: 10.1016/j.amepre.2011.01.021. https://doi.org/10.1016/j.amepre.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21:761–769. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:2479–2485. doi: 10.1158/1055-9965.EPI-07-0393. https://doi.org/10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction: The implications for tobacco regulation. N E J Med. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Herrera B. Nicotine intake and dose response when smoking reduced–nicotine content cigarettes. Clin Pharmacol Ther. 2006;80:703–714. doi: 10.1016/j.clpt.2006.09.007. https://doi.org/10.1016/j.clpt.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, Lerman C. Reduced nicotine reward in obesity: Cross-comparison in human and mouse. Psychopharmacology. 2005;180:306–315. doi: 10.1007/s00213-005-2167-9. https://doi.org/10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK. Randomized trial of reduced-nicotine standards for cigarettes. N E J Med. 2015;373:1340–1349. doi: 10.1056/NEJMsa1502403. https://doi.org/10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton-Marshall T, Fong GT, Yong HH, Borland R, Xu SS, Quah ACK, Feng G, Jiang Y. Smokers’ sensory beliefs mediate the relation between smoking a light/low tar cigarette and perceptions of harm. Tob Control. 2015;24:iv21–iv27. doi: 10.1136/tobaccocontrol-2014-051977. https://doi.org/10.1136/tobaccocontrol-2014-051977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AT, Peters E, Strasser AA, Emery LF, Sheerin KM, Romer D. Graphic warning labels elicit affective and thoughtful responses from smokers: Results of a randomized clinical trial. PLoS One. 2015;10:e0142879. doi: 10.1371/journal.pone.0142879. https://doi.org/10.1371/journal.pone.0142879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AC, Fong GT, Borland R, Quah ACK, Seo HG, Kim Y, Elton-Marshall T. The importance of the belief that “light” cigarettes are smoother in misperceptions of the harmfulness of “light” cigarettes in the Republic of Korea: A nationally representative cohort study. BMC Public Health. 2015;15:1108. doi: 10.1186/s12889-015-2472-0. https://doi.org/10.1186/s12889-015-2472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Norton G diStefano, Ouellette T, Rhodes W, Kling R, Connolly G. Smokers’ responses to advertisements for regular and light cigarettes and potential reduced-exposure tobacco products. Nicotine Tob Res. 2004;6:353–362. doi: 10.1080/14622200412331320752. https://doi.org/10.1080/14622200412331320752. [DOI] [PubMed] [Google Scholar]
- Hammond D, O’Connor RJ. Reduced nicotine cigarettes: Smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol Biomarkers Prev. 2014;23:2032–2040. doi: 10.1158/1055-9965.EPI-13-0957. https://doi.org/10.1158/1055-9965.EPI-13-0957. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. 2015;24:472–476. doi: 10.1158/1055-9965.EPI-14-0739. https://doi.org/10.1158/1055-9965.EPI-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roper-Batker AN, Mackowick KM, Jensen J, Murphy SE, Thomas BF, Donny EC. Dose-response effects of Spectrum research cigarettes. Nicotine Tob Res. 2012b;15:1113–1121. doi: 10.1093/ntr/nts247. https://doi.org/10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS. Reduced nicotine content cigarettes: Effects on toxicant exposure, dependence and cessation. Addiction. 2010a;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, LeSage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger CL, Zeller M. Nicotine reduction revisited: Science and future directions. Tob Control. 2010b;19:e1–e1. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Brit J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Tob Control. 1998;7:281–293. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, O’Connor RJ. Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob Control. 2002;11:i40–i50. doi: 10.1136/tc.11.suppl_1.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Pillitteri JL. Beliefs about “light” and “ultra light” cigarettes and efforts to change those beliefs: An overview of early efforts and published research. Tob Control. 2001;10:i12–i16. doi: 10.1136/tc.10.suppl_1.i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: Reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5:673–679. doi: 10.1080/1462220031000158645. https://doi.org/10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- Lochbuehler K, Tang KZ, Souprountchouk V, Campetti D, Cappella JN, Kozlowski LT, Strasser AA. Using eye-tracking to examine how embedding risk corrective statements improves cigarette risk beliefs: Implications for tobacco regulatory policy. Drug Alcohol Depend. 2016;164:97–105. doi: 10.1016/j.drugalcdep.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercincavage M, Souprountchouk V, Tang KZ, Dumont RL, Wileyto EP, Carmella SG, Hecht SS, Strasser AA. A randomized controlled trial of progressively reduced nicotine content cigarettes on smoking behaviors, biomarkers of exposure, and subjective ratings. Cancer Epidemiol Biomarkers Prev. 2016;25:1125–1133. doi: 10.1158/1055-9965.EPI-15-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti S, Hammond D, Borland R, Cummings MK, O’Connor RJ, Fong GT. Beyond light and mild: Cigarette brand descriptors and perceptions of risk in the International Tobacco Control (ITC) Four Country Survey: Cigarette brands and perceptions of risk. Addiction. 2011;106:1166–1175. doi: 10.1111/j.1360-0443.2011.03402.x. https://doi.org/10.1111/j.1360-0443.2011.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, Caruso RV, Borland R, Cummings KM, Bansal-Travers M, Fix BV, King B, Hammond D, Fong GT. Relationship of cigarette-related perceptions to cigarette design features: Findings from the 2009 ITC U.S. Survey. Nicotine Tob Res. 2013;15:1943–1947. doi: 10.1093/ntr/ntt075. https://doi.org/10.1093/ntr/ntt075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, Hyland A, Giovino GA, Fong GT, Cummings KM. Smoker awareness of and beliefs about supposedly less-harmful tobacco products. Am J Prev Med. 2005;29:85–90. doi: 10.1016/j.amepre.2005.04.013. https://doi.org/10.1016/j.amepre.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Parascandola M, Augustson E, O’Connell ME, Marcus S. Consumer awareness and attitudes related to new potential reduced-exposure tobacco product brands. Nicotine Tob Res. 2009;11:886–895. doi: 10.1093/ntr/ntp082. https://doi.org/10.1093/ntr/ntp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol. 1996;4:166. [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Philip Morris. Sensory evaluation of cigarettes. 1997 Retrieved from http://legacy.library.ucsf.edu/tid/fqu67d00.
- Sell NM, Turrisi R, Scaglione NM, Hultgren BA, Mallett KA. Examining the effects of drinking and interpersonal protective behaviors on unwanted sexual experiences in college women. Addict Behav. 2016;54:40–45. doi: 10.1016/j.addbeh.2015.12.003. https://doi.org/10.1016/j.addbeh.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Lerman C, Cappella J, Strasser AA, Pinto A, Hornik R. Evaluating smokers’ reactions to advertising for new lower nicotine quest cigarettes. Psychol Addict Behav. 2006;20:80–84. doi: 10.1037/0893-164X.20.1.80. https://doi.org/10.1037/0893-164X.20.1.80. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Smoker and ex-smoker reactions to cigarettes claiming reduced risk. Tob Control. 2004;13:78–84. doi: 10.1136/tc.2003.005272. https://doi.org/10.1136/tc.2003.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Jarvis MJ, Pillitteri JL, Di Marino ME, Gitchell JG, Kemper KE. UK smokers’ and ex-smokers’ reactions to cigarettes promising reduced risk. Addiction. 2007;102:156–160. doi: 10.1111/j.1360-0443.2006.01650.x. https://doi.org/10.1111/j.1360-0443.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Pillitteri JL, Burton SL, Rohay JM, Gitchell JG. Smokers’ beliefs about “Light” and “Ultra Light” cigarettes. Tob Control. 2001;10:i17–i23. doi: 10.1136/tc.10.suppl_1.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epidemiol Biomarkers Prev. 2013;22:382–389. doi: 10.1158/1055-9965.EPI-12-1097. https://doi.org/10.1158/1055-9965.EPI-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. https://doi.org/10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Tang KZ, Romer D, Jepson C, Cappella JN. Graphic warning labels in cigarette advertisements. Am J Prev Med. 2012;43:41–47. doi: 10.1016/j.amepre.2012.02.026. https://doi.org/10.1016/j.amepre.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Tang KZ, Sanborn PM, Zhou JY, Kozlowski LT. Behavioral filter vent blocking on the first cigarette of the day predicts which smokers of light cigarettes will increase smoke exposure from blocked vents. Exp Clin Psychopharmacol. 2009;17:405–412. doi: 10.1037/a0017649. https://doi.org/10.1037/a0017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Tang KZ, Tuller MD, Cappella JN. PREP advertisement features affect smokers’ beliefs regarding potential harm. Tob Control. 2008;17:i32–i38. doi: 10.1136/tc.2007.022426. https://doi.org/10.1136/tc.2007.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Congress. Family Smoking Prevention And Tobacco Control Federal Reform. 2009. (Pub. L. No. 111–31). [Google Scholar]
- U.S. Department of Health and Human Services. Risks Associated With Smoking Cigarettes With Low Machine-Measured Yields Of Tar And Nicotine (Smoking and Tobacco Control Monograph No 13) Public Health Service, National Institutes of Health, National Cancer Institute; Bethesda, MD: 2001. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences Of Smoking - 50 Years Of Progress: A Report Of The Surgeon General. Government Printing Office; Atlanta, GA: 2014. [Google Scholar]
- Vogel RI, Hertsgaard LA, Dermody SS, Luo X, Moua L, Allen S, al’Absi M, Hatsukami DK. Sex differences in response to reduced nicotine content cigarettes. Addict Behav. 2014;39:1197–1204. doi: 10.1016/j.addbeh.2014.03.021. https://doi.org/10.1016/j.addbeh.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


