Abstract
It is well known that cardiac dysfunction develops during sepsis in both humans and in rodents (rats, mice). These defects appear to be reversible, since after “recovery” from sepsis, cardiac dysfunction disappears and the heart returns to its function that was present before the onset of sepsis. Our studies, using in vivo and in vitro models, have demonstrated that C5a and its receptors (C5aR1 and C5aR2) play key roles in cardiac dysfunction developing during sepsis. Use of a neutralizing antibody to C5a largely attenuates cardiac dysfunction and other adverse events developing during sepsis. The molecular basis for cardiac dysfunctions is linked to generation of C5a and its interaction with C5a receptors present on surfaces of cardiomyocytes (CMs). It is established that C5a interactions with C5a receptors leads to significant reductions involving faulty contractility and relaxation in CMs. In addition, C5a interactions with C5a receptors or CMs results in reductions in Na+/K+-ATPase in CMs. This ATPase is essential for intact action potentials in CMs. The enzymatic activity and protein for this ATPase were strikingly reduced in CMs during sepsis by unknown mechanisms. In addition, C5a interactions with C5aRs also caused reductions in CM homeostatic proteins that regulate cytosolic [Ca2+]i in CMs: sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2) and Na+/Ca2+ exchanger (NCX). In the absence of C5a receptors, defects in SERCA2 and NCX in CMs after sepsis are strikingly attenuated. These observations suggest new strategies to protect the heart from dysfunction developing during sepsis.
Keywords: Complement C5a, C5aR1, C5aR2 (GPR77), Na+/K+-ATPase, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2), Na+/Ca2+ exchanger (NCX)
1. Introduction
It has long been known in humans with sepsis that, as the situation progresses to septic shock, this may result in high lethality (≥70%) (Russell, 2006). Rather surprisingly, if patients survive septic shock, the post-sepsis situation usually involves hearts that resume their function that existed before the onset of sepsis (Kan and Finkel, 2003; Parker et al., 1984). In other words, the events that lead to septic shock and heart dysfunction may be completely reversible. Many decades ago, it was postulated that “warm shock” in sepsis progressed to “cold shock” linked to low cardiac output (MacKenzie, 2001; Rabuel and Mebazaa, 2006). It was postulated that early in shock there was a “hyperdynamic phase” followed by a “hypodynamic phase”. However, recent studies suggest that aggressive fluid resuscitation of septic patients results in abolition of the early “hyperdynamic” phase. The hypodynamic phase was thought to be associated with reduced cardiac output and elevated systemic vascular resistance (Clowes et al., 1966; MacKenzie, 2001).
However, over the past several decades, the use of sophisticated physiological monitoring of septic humans has described normal or elevated cardiac output and decreased systemic vascular resistance in septic patients aggressively treated with fluid resuscitation (Gunnar et al., 1973; Marik and Varon, 1998; Packman and Rackow, 1983; Sharma and Dellinger, 2006; Winslow et al., 1973). It is important to note that direct measurements of coronary artery blood flow during sepsis have revealed increased blood flow. Furthermore, myocardial necrosis or apoptosis had not generally been found in hearts from patients who have died during sepsis (Hotchkiss et al., 1999; Lanone et al., 2000; Rabuel and Mebazaa, 2006). Together, it appears that global ischemia of the heart during sepsis does not occur during sepsis.
There has been evidence that septic shock was associated with suppressive factors in plasma negatively affecting cardiac function (Alhamdi et al., 2015; Kalbitz et al., 2015; Parrillo et al., 1985). Circulating cardio-suppressive factors altered [Ca2+]i flux in cardiomyocytes (CMs). These factors have been identified with TNF, IL-1 and C5a (Atefi et al., 2011; Finkel et al., 1992; Kumar et al., 1996; Niederbichler et al., 2006; Parrillo et al., 1985). While these cytokines impair heart function ex vivo, there is no consensus that such mediators play a major factor in septic shock. All in all, our understanding of events that lead to septic shock and cardiac dysfunction have changed dramatically as sensitive and reliable technologies have been developed to measure physiological parameters during development of septic shock. Such developments have dramatically changed our understanding of molecular pathways responsible for development of septic shock.
2. Non-invasive methods to assess heart function in sepsis
There are several non-invasive methods like functional and electrical measurements to assess heart function after onset of sepsis including:
2.1. Echocardiography
Echocardiography is one of the noninvasive and accurate tools used in the intensive care unit (ICU) to assess cardiac function and monitor hemodynamics in patients with sepsis (Guerin and Vieillard-Baron, 2016; Haileselassie et al., 2016). During severe sepsis or septic shock, several mechanisms can lead to hemodynamic failure which is important to be diagnosed quickly to apply appropriate hemodynamic therapy. Echocardiography can provide physicians with this diagnostic possibility, whether or not there is fluid responsiveness, cardiac dysfunction, or persistent vasoplegia (Guerin and Vieillard-Baron, 2016). In the clinical setting of critically ill patients like sepsis echocardiography is used to identify the presence of left ventricular (LV)/right ventricular (RV) failure, optimize fluid replacement, and guide therapies used to alter pulmonary and systemic vascular resistance (Price et al., 2006). Using echocardiography, it is possible to measure cardiac output, intravascular pressures and volumes, systolic and diastolic function of both ventricles, and preload responsiveness of patients suffering from sepsis (De Backer, 2014). There is slower LV filling and aberrant LV relaxation time in humans with sepsis (Jafri et al., 1990; Munt et al., 1998), suggest that impaired compliance may contribute to myocardial depression during sepsis. Myocardial depression during sepsis can perhaps be best described as a global (systolic and diastolic) dysfunction of both the left and right sides of the heart (Antonucci et al., 2014). The main echocardiographic views used to assess hemodynamic status in patients with septic shocked was extensively described in the past showing LV and RV dysfunction (Vieillard-Baron et al., 2003) Cardiac dysfunction occurs in up to 80% of patients with septic shock (Beraud et al., 2014). Echocardiography study on 76 patients admitted to the ICU with septic shock within 72 hours of their admission showed cardiac dysfunction and heart failure. Ejection fraction was abnormal in 35%, wall motion abnormalities were present in 17%, right ventricular was impaired in 31%, diastolic function was abnormal in 53% of patients, and the left atrium was dilated in 42% of patients with diastolic dysfunction (Beraud et al., 2014). These data show the potential usefulness of echocardiography to assess myocardial function in patients with septic shock and to assist their management.
Echocardiography can also be used in murine septic models to study their cardiovascular performance (Alhamdi et al., 2015; Boluyt et al., 2004; Hollenberg et al., 2001; Hoover et al., 2015; Kalbitz et al., 2016; Zanotti-Cavazzoni et al., 2009) We evaluated the cardiac function in our CLP (cecal ligation and puncture) mice model using this technique. All echocardiograms were performed by a registered echocardiographer who was blinded to mouse genotype. Imaging was performed using a Vevo 770 High-Resolution In Vivo Imaging System (Visualsonics Inc., Toronto, ON, Canada) equipped with a RMV-707 30 MHz RMV (Real-Time Visualization) (up to 45MHz) scanhead. LV volumes were measured from the parasternal long axis view at the level of the tips of the leaflets of the mitral valve at end systole (VolS) and end diastole (VolD) and used to calculate stroke volume (SV = VolD – VolS) and ejection fraction (EF % = endocardial SV/endocardial VolD x100). Cardiac output was calculated from stroke volume and heart rate (CO = SV x heart rate). Mitral valve E and A wave inflow velocities were sampled at the tips of the leaflets of the mitral valve from the apical four chamber view. Doppler tissue imaging was performed with acquisition of peak E′ velocity from the lateral (E′la) and septal annulus (E′sa) of the mitral valve imaged from the apical four chamber view. Isovolumic relaxation time (IVRT), from the closure of the aortic valve to the opening of the mitral valve, was measured from the apical five chamber view using Doppler flow imaging. For all experiments heart functions, diastolic and systolic parameters in septic mice were compared with those before inducing the CLP (Kalbitz et al., 2016).
2.2. Electrocardiogram (ECG)
ECG is another non-invasive tool to study heart function after sepsis. Two-thirds of the patients with sepsis and myocardial depression showed ST-elevation myocardial infarction ECG changes. Septic patients showed to develop significant attenuation of QRS complexes as well as increases in QRS duration may be due to altered cardiac excitability, with or without bundle branch block (Madias and Bazaz, 2003; Rich et al., 2002). These ECG changes returned to normal following recovery from septic shock, showing reversible reductions of QRS amplitudes (Rich et al., 2002). Another ECG complication during sepsis is QT prolongation (Tisdale et al., 2013; Varriale and Ramaprasad, 1995). Prolongation of the QT interval was a negative predictive factor in septic patients (Tisdale et al., 2013). ECG from human with sepsis also reported myocardial depression and takotsubo syndrome (TS), which the latter may be the cause of the majority of cases of sepsis-induced myocardial depression (Y-Hassan et al., 2014). Takotsubo, which is a Japanese term, is a pot with a round base and narrow neck used in Japan for trapping octopuses (Tako octopus and Tsubo pot). With increasing recognition of takotsubo syndrome (TS), cases of sepsis-triggered TS have been reported (Y-Hassan et al., 2014).
In septic mice prolongation of the PR, QRS complex (Bustamante et al., 2002; Hoover et al., 2015) and prolongation of QTc intervals was reported in the ECG (Hoover et al., 2015). Details of ECG recordings method in mice was described in some reports (Hoover et al., 2015).
2.3. Evaluating biomarkers in plasma
There are hundreds of biomarkers including C-reactive protein (CRP), procalcitonin (PCT), lipopolysaccharide binding protein (LBP), various cytokines, and cell surface markers which could be potentially used for diagnosis and prognosis in septic patients (Cho and Choi, 2014; Pierrakos and Vincent, 2010; Prucha et al., 2015). Only some of them are used in routine clinical practice because many lack sufficient sensitivity or specificity (Cho and Choi, 2014; Prucha et al., 2015). Among them PCT and CRP protein have been most widely used however neither PCT nor CRP fulfills the role of an ideal biomarker in the diagnostics of sepsis (Pierrakos and Vincent, 2010; Prucha et al., 2015), having limited abilities to distinguish sepsis from other inflammatory conditions or to predict outcome (Henriquez-Camacho and Losa, 2014; Pierrakos and Vincent, 2010). In neonatal sepsis, the diagnostic utility of the following biomarkers seems to be most practical in the early (IL-6, IL-8, TNF, neutrophil CD64), mid (PCT) and late (CRP) phases (Bhandari, 2014).
Recently, usefulness of biomarker-guided antibiotic supervisions was reported. However, the other side of these numerous biomarkers is that no novel single laboratory marker can diagnose, predict, and track the treatment of sepsis (Cho and Choi, 2014).
Some novel markers were introduced during last decade in the setting of sepsis including presepsin (sCD14-ST) (Endo et al., 2012; Masson et al., 2014; Novelli et al., 2013; Shozushima et al., 2011; Ulla et al., 2013; Yaegashi et al., 2005) and soluble triggering receptor expressed on myeloid cells (sTREM-1) (Adib-Conquy et al., 2007; Ferat-Osorio et al., 2008), soluble urokinase plasminogen activator receptor (suPAR) (Eugen-Olsen et al., 2002; Koch et al., 2011), (proadrenomedullin (proADM) (Suberviola et al., 2012) and CD73 (Bellingan et al., 2014; Hasko et al., 2011).
Cardiac Troponin (cTn) is another biomarker which can be detectable in the plasma of critically ill patients like patients with sepsis. The septic patients can have an elevated cTn levels in the plasma in up to 60% of cases (Hamilton et al., 2012) in which its elevated levels is associated with increased mortality risk (Fromm, 2007; Gualandro et al., 2014). Septic patients with elevated cTn levels can have a 2–5 fold increased risk of death, even in the absence of known cardiovascular disease (Mantzouris et al., 2013). Another serum cardiac marker used in sepsis disease is Creatine Kinase (CK) which its high level is associated with higher mortality rate (Oliveira et al., 2008; Zhang et al., 2012). This biochemical parameter improved after different treatment approaches in murine (He et al., 2014; Smith et al., 2016; Zhang et al., 2015) and human (Hua et al., 2012; Zhang et al., 2012) sepsis.
Systemic levels of Pentraxin 3 (PTX3) is also a prognostic value in patients with systemic inflammatory response syndrome (SIRS) or sepsis (Liu et al., 2014).
One of the novel biomarkers newly introduced are extracellular histones which currently draw much attention and are detected in sepsis and some disorders like ischemic reperfusion injuries and trauma. Histones act as damage-associated molecular pattern molecules (DAMP) when they are released into the extracellular space (Chen et al., 2014). Extracellular histones can lead to excessive and overwhelming cell damage and death due to the high cytotoxic and proinflammatory effects, thus contributing to the pathogenesis of sepsis (Xu et al., 2015). Release of histones into the extracellular compartment has been postulated to be a major cause of death during sepsis (Chaput and Zychlinsky, 2009; Ekaney et al., 2014; Nakahara et al., 2013; Xu et al., 2009). More than 2 decades ago there was overwhelming evidence that myocardial performance was depressed in sepsis and in endotoxin shock, the possible role of circulating toxins was suggested to be associated with depressed myocardial performance (Abel, 1989). Circulating histones were reported as major mediators of cardiac injury in patients with sepsis (Alhamdi et al., 2015). We recently showed that extracellular histones appearing in septic plasma play an important role in the cardiomyopathy of CLP mice (Kalbitz et al., 2015). Both C5a receptors (C5aR1 and C5aR2) were required for histone presence (Bosmann et al., 2013; Grailer et al., 2015; Kalbitz et al., 2015) perhaps being released from NETs (neutrophil extracellular traps) (Fattahi et al., 2015). Presence of histones after CLP is linked to disturbances in functional responses of hearts as defined by ECHO/Doppler parameters and this cardiac dysfunction is preserved using antibody against histones (Kalbitz et al., 2015). Moreover, in ECG tracings on perfused mouse hearts with histones or isolated cardiomyocytes incubated with histones we showed harmful effect of histones (Kalbitz et al., 2015). In line, other studies showed antihistone-based treatments (e.g., neutralizing antibodies, activated protein C, and heparin) to have protective effects and have significantly improved the outcomes of mice with sepsis (Xu et al., 2015). Neutralization of Cit H3 also showed survival improvement in septic mice (Li et al., 2014). This data together indicates extracellular histones could serve as potential biomarkers and novel therapeutic targets in sepsis.
3. Roles of complement C5a in septic cardiomyopathy
Complement C5a which is one of the powerful anaphylatoxins generated from the complement system shown to be associated with septic cardiomyopathy (Hoesel et al., 2007b; Kalbitz et al., 2015; Niederbichler et al., 2006). An excessive C5a generation occurs during sepsis (Hoesel et al., 2007b; Niederbichler et al., 2006). In below we explain some of the effects of C5a on cardiomyocytes contributing in cardiac dysfunction.
3.1. C5a causes increased ROS and [Ca2+]i in CMs
Intracellular calcium [Ca2+]i is one of the most important factors for corresponding and efficient contractility in which CM dysfunction after sepsis was linked to the altered calcium transient properties (Ren et al., 2002). In fact, impaired calcium handling in CMs was suggested as one of the underlying mechanisms in septic cardiomyopathy (Maitra et al., 1999).
Reactive oxygen species (ROS) are also increased in the failing heart, has been shown to play an important role in the pathophysiology of cardiac remodeling and reduced LV function. ROS influence contractile function by modifying proteins involved in excitation-contraction link including ryanodine receptor (RyR) to enhance its open probability, the suppression of L-type calcium channel, and oxidative interaction with Ca2+-ATPase in the sarcoplasmic reticulum (SR) to inhibit Ca2+ uptake (Tsutsui et al., 2011; Zima and Blatter, 2006).
We recently showed the effects of C5a on [Ca2+]i homeostasis and electrophysiological functions in single CMs (Kalbitz et al., 2016). We also found ability of rat recombinant C5a to cause increased ROS in isolated rat CMs (Kalbitz et al., 2016). We also found increased [Ca2+]i influx in rat CMs after treating them with C5a in vitro (unpublished data). In line with in vitro data, inducing CLP in wild type (Wt) mice resulted in [Ca2+]i release (unpublished data) and increased ROS (Kalbitz et al., 2016) in mouse CMs. Collectively, the data provide direct evidence of the effects of C5a on the loss of homeostatic control of ROS and [Ca2+]i in CMs during sepsis.
Interestingly, both [Ca2+]i and ROS release require C5a receptors (C5aR1 and C5aR2) (Kalbitz et al., 2016) as both responses were significantly attenuated in the lack of either C5aR1 or C5aR2 especially in C5aR1−/− mice.
3.2. C5a induces defects in contractility and relaxation in CMs
We showed in the past that C5aR1 is expressed in whole heart homogenates of septic rats, perhaps setting the stage for C5a-induced impairment of cardiac function (Riedemann et al., 2002). By evaluating contractility parameters of single cardiomyocytes on the sarcomere level, we were able to find association of the complement system and septic cardiomyopathy (Niederbichler et al., 2006). There was significant reduction in LV pressures in rats after CLP which was in line with earlier reports (Kumar et al., 2001). There was also dramatically impaired contractility in rat cardiomyocytes in vitro after their incubation with 10.0 nM C5a but to a consistently greater degree in cells from CLP rats (Niederbichler et al., 2006). These data correlate with the serum levels of C5a found in septic humans, which have been shown to range between 1.0 and 15.0 nM (Nakae et al., 1994).
C5a can also result in defective action potentials in CMs especially in septic CMs. Using patch-clamp techniques, CMs from rats showed early after depolarizations (EADs) and widened and prolonged action potentials 24 hr after CLP. Addition of C5a (45ng/ml) accentuated this abnormalities and EAD profile. Our very recent data showed exposure of CMs from septic hearts to C5a results in dramatically altered action potentials, with defective values for resting membrane potential, maximal upstroke velocities, prolongation of action potential duration and the appearance of EADs indicating profound prolongation in action potentials as a result of sepsis together with presence of C5a (Kalbitz et al., 2016).
4. ECHO/Doppler defects in heart after onset of sepsis preserved in septic mice lacking C5a receptors
We recently showed sepsis-induced heart dysfunction is preserved in septic mice lacking C5a receptors (C5aR1−/− and C5aR2−/−) (Kalbitz et al., 2016). We studied the cardiovascular performance by echocardiography in septic mice 8 hr after CLP to see if there is any protective effect in septic mice lacking C5a receptors (Kalbitz et al., 2016). Echocardiograms were performed as previously described (Boluyt et al., 2004) according to the recommendations of the American Society of Echocardiography.
Our results showed diastolic function (as measured by IVRT and Doppler tissue imaging) were prolonged 8 hr after CLP induction in Wt but not in C5aR1−/− and C5aR2−/− mice. Tissue Doppler Imaging demonstrated a reduction in annular velocities of the mitral valve at the lateral (E′la) and septal annulus (E′sa) suggesting reduced diastolic function after CLP that is at least partially reversed in the setting of C5aR1 or C5aR2 K.O. Correcting E wave velocities for diastolic function (E/E′ ratio) revealed that CLP resulted in a reduction of LV filling pressures, likely as the result of reduced preload in Wt animals. In C5aR1−/− and C5aR2−/− mice, the E/E′ ratios were similar to sham operated Wt controls, suggesting normalization of ventricular filling pressures which was supported by the assessment of ventricular volumes. Similarly, the significantly decreased end diastolic volumes noted after CLP in Wt mice were less pronounced in the C5aR1−/− and C5aR2−/− mice. These data suggest that CLP mice have reduced cardiac performance that was muted in mice lacking either C5a receptor.
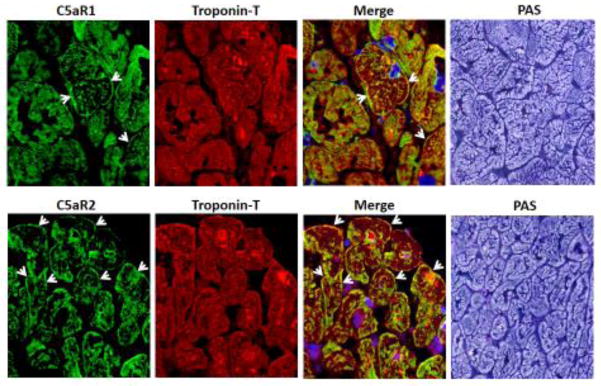
Generally our echo data in Wt CLP mice is in line with earlier reports (Hollenberg et al., 2001; Zanotti-Cavazzoni et al., 2009), but no other echocardiography studies is present looking at the protective effect of lacking C5a receptors. This highlights the importance of the C5a receptors as a potential target for therapeutic purposes in the clinical setting of sepsis. We showed by immunofluorescence staining both C5a receptors are present in human hearts. Fig. 1 shows confocal immunofluorescence using frozen sections of two different human LV samples, confirming the presence of both C5aR1 and C5aR2 proteins in human ventricular wall. As shown in Fig. 1, human myocardium from normal hearts revealed the presence of cytosolic troponin T and both C5a receptors in CMs. Heart-specific troponin T staining (red) was present in CMs and C5a receptors (green staining) were also found in CMs. In the PAS frame, there was clear definition of CMs. The presence of both C5a receptors in human CMs suggests that C5a may interact with the corresponding receptors on CMs during human sepsis. These observations may have therapeutic implications for treatment of humans developing cardiomyopathy during sepsis.
Figure 1. Expression of C5aRs in Human Heart.
Confocal immunofluorescence for C5aR1 and C5aR2 protein, x63. Double-staining with heart-specific troponin T, and for C5aR1 and C5aR2, both x63. Far right, periodic acid-Schiff (PAS) staining of frozen sections from normal human heart tissue, x63.
5. Proteins studies in heart homogenates from CLP mice and protective effect of lacking C5a receptors
5.1. Reduced SERCA2 and NCX protein levels in CLP murine
As stated in section 3.1, one of the mechanisms suggested for sepsis-induced cardiomyopathy is impaired calcium handling in cardiomyocytes. [Ca2+]i is a requirement for contraction and relaxation. During diastole, the [Ca2+]i is maintained at sufficiently low levels to prevent activation of contractile proteins. With each heartbeat, Ca2+ influx via the L-type Ca2+ channel triggers release of Ca2+ from the SR which functions as the storage area for calcium during myocyte relaxation. These two sources combine to elevate [Ca2+]i, which promotes Ca2+ binding to troponin and activation of the contractile process. Contraction is terminated as Ca2+ is pumped back into the SR by the SR Ca2+-ATPase (SERCA) and out of the cell via the sarcolemmal Na+-Ca2+ exchanger (NCX). Modulation of the calcium regulating proteins may have important functional consequences during heart failure, arrhythmias and sepsis. The depressed contractility of the failing heart is thought to involve alterations in myocyte Ca2+ regulation (Piacentino et al., 2003). Impairment of calcium transport mechanisms in the myocardium occurs during sepsis (Dong et al., 2001) SR Ca2+ handling dysfunction also shown to be an early event during endotoxemia (Hassoun et al., 2008). Several studies on rats demonstrated reduced activity of SERCA after CLP (He et al., 2007; Ren et al., 2002; Wu et al., 2004; Wu et al., 2016; Wu et al., 2001; Zhu et al., 2005). In accordance with these findings, SERCA inhibition has been demonstrated in endotoxemia in rats (Comini et al., 2005; Hassoun et al., 2008) and mice (Hobai et al., 2013; Ichinose et al., 2007; Turdi et al., 2012). This SERCA inhibition in endotoxemia was LPS dose dependent (Hobai et al., 2013), further confirming a direct contributing relation. It has also been reported that ATP-dependent calcium uptake by SERCAs is impaired during later stages of endotoxemia and sepsis (Wu et al., 2001; Wu and Liu, 1991). NCX is also shown to be decreased in sepsis (Hobai et al., 2015a; Hobai et al., 2015b).
In line with these data, our recent studies using mRNA expression, Western blot and ELISA methods show decreased levels of these calcium regulatory proteins (SERCA2 and NCX) in LV homogenized mice after CLP (Fig. 2) (Kalbitz et al., 2016). These reductions were dramatically attenuated in the mice lacking either C5aR1 or C5aR2 (Kalbitz et al., 2016), indicating that CLP-induced reductions in these calcium regulatory proteins are C5a receptor-dependent.
Figure 2. Changes in CM Protein Levels for SERCA2 (A), NCX (B) and Na+/K+-ATPase (C) after CLP.
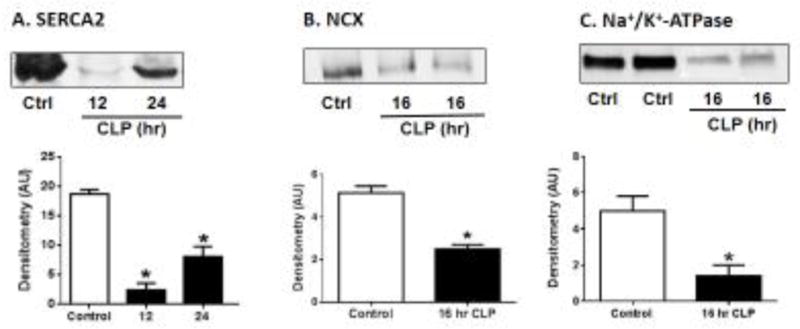
Changes based on Western blots of CM proteins, SERCA2 (A), NCX (B) and Na+/K+-ATPase (C) at indicated time points after CLP. For each bar, n>6 separate samples. *p<0.05
5.2. Reduced enzyme activity and protein levels of Na+/K+-ATPase in CLP mice
In addition to the calcium regulatory proteins we recently measured enzymatic activity of Na+/K+-ATPase in LV homogenates from mice after CLP and found profound reductions in this enzyme activity. In the absence of C5aR1, there was almost no reduction in Na+/K+-ATPase activity after CLP. In C5aR2−/− mice, there appeared to be less protection against the CLP-induced fall in Na+/K+ ATPase, whereas in C5aR1−/− mice the sepsis-induced fall was virtually abolished.
Our Western blot data for measuring Na+/K+-ATPase activity in LV homogenates was consistent with enzymatic activity results showing reductions in Na+/K+-ATPase. We found profound reductions in intensity of LV blots for Na+/K+-ATPase occurred 8 hr after CLP. When we further determined whether C5aR deficiency prevented loss of Na+/K+-ATPase proteins levels in heart homogenates by ELISA, we found significant reduction in heart homogenate proteins in Wt for Na+/K+-ATPase 16 hr after CLP (Fig. 2) while this reduction was attenuated in the absence of C5aR1 or C5aR2 (Kalbitz et al., 2016).
6. Discussion
Figure 3 summarizes our current data, linking C5a and C5a receptors to events that compromise the functional integrity of heart (CMs or LV frozen sections). Several years ago we described that polymicrobial sepsis caused substantial increases in C5aR1 on surfaces of CMs. We further showed that addition of C5a to sham CMs or CMs from septic mice dramatically caused CM dysfunction, involving both contractility and relaxation (Niederbichler et al., 2006). We also demonstrated that blocking antibodies to C5a or blockade or absence of C5aR2 greatly improved survival of septic mice (Huber-Lang et al., 2001b) and reduced evidence of cardiac dysfunction during sepsis based on ECHO/Doppler technology (Kalbitz et al., 2015). We have recently found that sepsis caused both enzymatic and protein reductions in Na+/K+-ATPase in CMs, leading to interference with repolarization of CMs that had undergone depolarization (Kalbitz et al., 2016). In addition, there were reductions in SERCA2 and NCX, involving mRNAs and protein content in CMs from hearts after onset of sepsis. Collectively, changes in these three proteins in CM interfered with the ability to repolarize the cells after CM activation, resulting in defective contractility and relaxation. Reductions in SERCA2 and NCX interfered with clearance of diastolic [Ca2+]i in CMs following their activation (electrical) or during spontaneous systole. During diastole, impairment of SERCA2 or NCX resulted in the inability of CMs to clear diastolic [Ca2+]i, resulting in serious defects in contractility and relaxation (Lompre et al., 1994; Misquitta et al., 1999). During sepsis, there may also be excessive activation of RyR receptors, which would further accentuate the buildup of cytosolic [Ca2+]i during diastole. In the setting of sepsis, the strategy to reduce cardiac dysfunction could include infusion of a neutralizing antibody to C5a or use of one of the new blocking compounds to prevent C5a activation or the functional activity of C5a (Czermak et al., 1999; Flierl et al., 2009; Guo et al., 2000; Guo et al., 2006; Hoesel et al., 2007a; Huber-Lang et al., 2001a; Huber-Lang et al., 2001b; Laudes et al., 2002; Riedemann et al., 2004; Sprong et al., 2003). Since the neutralizing mAb to C5a is safe in normal humans (Hammerschmidt et al., 1980; Stevens et al., 1986) this antibody might become a candidate to intervene in septic individuals entering the phase of septic shock.
Figure 3. Proposed Cascade of Events after CLP Leasing to Septic Heart Dysfunction.

Reductions in key Ca2+ regulatory proteins in CMs and reduction in Na+/K+-ATPase are associated with inability to clear cytosolic Ca2+ after systole and defective action potentials, ultimately leading to the “cardiomyopathy of sepsis”.
7. Conclusions
The early initiating events in sepsis are complement activation with C5a generation which acts directly by engaging its both receptors (C5aR1 and C5aR2) that involve in septic cardiomyopathy. So development of defective cardiac function can be linked to C5a signaling via C5a receptors (summarized in Fig. 3). This figure describes events occurring in the heart after polymicrobial sepsis, resulting in defective action potentials and loss of homeostatic proteins that are crucial for preventing buildup of [Ca2+]i in CMs during diastole. C5a interacts with CM receptors (C5aR1, 2), which results in defective action potentials (associated with faulty repolarization in CMs) as well as the cytosolic buildup of [Ca2+]i in CMs during diastole. These changes cause faulty contractility and relaxation in CMs. Molecular mechanisms linked to these outcomes appear to be related to reduced levels of SERCA2 and NCX and reduced levels of Na+/K+-ATPase. This triad of defects may be key to development of cardiac dysfunction after sepsis.
A neutralizing mAb to C5a might represent an effective strategy for treatment of patients with sepsis. Moreover, presence of extracellular histones occurring after CLP can be linked to appearance of defects in cardiac function during sepsis. Neutralizing histone antibodies or drugs that block histone interactions with CMs, besides neutralizing mAb to C5a, may be potential therapeutic strategies to prevent or ameliorate septic cardiomyopathy.
Highlights.
Cardiomyopathy is a common complication of sepsis, often with a high mortality rate.
Cardiomyopathy has requirements for complement C5a and its receptors.
-
Molecular defects in cardiac dysfunction of sepsis are complement-dependent and develop during early sepsis, with specific defects:
Defective action potentials;
Reduced amounts of SERCA2, NCX and Na+/K+-ATPase;
Development in CMs of increased amounts of ROS and [Ca2+]i which are known to impair contractility and relaxation of cardiomyocytes (CMs).
Extracellular histones formed during sepsis which can lead to excessive damage and death.
Cardiac dysfunction of sepsis disappears after recovery from sepsis, indicating that cardiac dysfunction in sepsis is reversible over time.
Presence of C5a receptors on surfaces of human CMs implies that CMs may respond to complement products, resulting in adverse consequences.
Acknowledgments
We acknowledge support from the Microscopy and Image-analysis Laboratory (University of Michigan Medical School). We thank Sue Scott, Michelle Possley and Robin Kunkel for their excellent assistance in the preparation of this manuscript and figures.
Funding
This work was supported by the National Institutes of Health (grant numbers GM-29507, GM-61656) to PAW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel FL. Myocardial function in sepsis and endotoxin shock. Am J Physiol. 1989;257:R1265–1281. doi: 10.1152/ajpregu.1989.257.6.R1265. [DOI] [PubMed] [Google Scholar]
- Adib-Conquy M, Monchi M, Goulenok C, Laurent I, Thuong M, Cavaillon JM, Adrie C. Increased plasma levels of soluble triggering receptor expressed on myeloid cells 1 and procalcitonin after cardiac surgery and cardiac arrest without infection. Shock. 2007;28:406–410. doi: 10.1097/shk.0b013e3180488154. [DOI] [PubMed] [Google Scholar]
- Alhamdi Y, Abrams ST, Cheng Z, Jing S, Su D, Liu Z, Lane S, Welters I, Wang G, Toh CH. Circulating Histones Are Major Mediators of Cardiac Injury in Patients With Sepsis. Crit Care Med. 2015;43:2094–2103. doi: 10.1097/CCM.0000000000001162. [DOI] [PubMed] [Google Scholar]
- Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29:500–511. doi: 10.1016/j.jcrc.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Atefi G, Zetoune FS, Herron TJ, Jalife J, Bosmann M, Al-Aref R, Sarma JV, Ward PA. Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J. 2011;25:2500–2508. doi: 10.1096/fj.11-183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingan G, Maksimow M, Howell DC, Stotz M, Beale R, Beatty M, Walsh T, Binning A, Davidson A, Kuper M, Shah S, Cooper J, Waris M, Yegutkin GG, Jalkanen J, Salmi M, Piippo I, Jalkanen M, Montgomery H, Jalkanen S. The effect of intravenous interferon-beta-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: an open-label study. Lancet Respir Med. 2014;2:98–107. doi: 10.1016/S2213-2600(13)70259-5. [DOI] [PubMed] [Google Scholar]
- Beraud AS, Guillamet CV, Hammes JL, Meng L, Nicolls MR, Hsu JL. Efficacy of transthoracic echocardiography for diagnosing heart failure in septic shock. Am J Med Sci. 2014;347:295–298. doi: 10.1097/MAJ.0b013e318297d616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V. Effective Biomarkers for Diagnosis of Neonatal Sepsis. J Pediatric Infect Dis Soc. 2014;3:234–245. doi: 10.1093/jpids/piu063. [DOI] [PubMed] [Google Scholar]
- Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol (1985) 2004;96:822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, Standiford TJ, Ward PA. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27:5010–5021. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante JM, Rivarola HW, Fernandez AR, Enders JE, Fretes R, Palma JA, Paglini-Oliva PA. Trypanosoma cruzi reinfections in mice determine the severity of cardiac damage. Int J Parasitol. 2002;32:889–896. doi: 10.1016/s0020-7519(02)00023-1. [DOI] [PubMed] [Google Scholar]
- Chaput C, Zychlinsky A. Sepsis: the dark side of histones. Nat Med. 2009;15:1245–1246. doi: 10.1038/nm1109-1245. [DOI] [PubMed] [Google Scholar]
- Chen R, Kang R, Fan XG, Tang D. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Choi JH. Biomarkers of sepsis. Infect Chemother. 2014;46:1–12. doi: 10.3947/ic.2014.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes GH, Jr, Vucinic M, Weidner MG. Circulatory and metabolic alterations associated with survival or death in peritonitis: clinical analysis of 25 cases. Ann Surg. 1966;163:866–885. doi: 10.1097/00000658-196606000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comini L, Boraso A, Bachetti T, Bernocchi P, Pasini E, Bastianon D, Curello S, Terracciano CM, Ceconi C, Ferrari R. Effects of endotoxic shock on neuronal NOS and calcium transients in rat cardiac myocytes. Pharmacol Res. 2005;51:409–417. doi: 10.1016/j.phrs.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- De Backer D. Ultrasonic evaluation of the heart. Curr Opin Crit Care. 2014;20:309–314. doi: 10.1097/MCC.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Dong LW, Wu LL, Ji Y, Liu MS. Impairment of the ryanodine-sensitive calcium release channels in the cardiac sarcoplasmic reticulum and its underlying mechanism during the hypodynamic phase of sepsis. Shock. 2001;16:33–39. doi: 10.1097/00024382-200116010-00007. [DOI] [PubMed] [Google Scholar]
- Ekaney ML, Otto GP, Sossdorf M, Sponholz C, Boehringer M, Loesche W, Rittirsch D, Wilharm A, Kurzai O, Bauer M, Claus RA. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care. 2014;18:543. doi: 10.1186/s13054-014-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo S, Suzuki Y, Takahashi G, Shozushima T, Ishikura H, Murai A, Nishida T, Irie Y, Miura M, Iguchi H, Fukui Y, Tanaka K, Nojima T, Okamura Y. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother. 2012;18:891–897. doi: 10.1007/s10156-012-0435-2. [DOI] [PubMed] [Google Scholar]
- Eugen-Olsen J, Gustafson P, Sidenius N, Fischer TK, Parner J, Aaby P, Gomes VF, Lisse I. The serum level of soluble urokinase receptor is elevated in tuberculosis patients and predicts mortality during treatment: a community study from Guinea-Bissau. Int J Tuberc Lung Dis. 2002;6:686–692. [PubMed] [Google Scholar]
- Fattahi F, Grailer JJ, Jajou L, Zetoune FS, Andjelkovic AV, Ward PA. Organ distribution of histones after intravenous infusion of FITC histones or after sepsis. Immunol Res. 2015;61:177–186. doi: 10.1007/s12026-015-8628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferat-Osorio E, Esquivel-Callejas N, Wong-Baeza I, Aduna-Vicente R, Arriaga-Pizano L, Sanchez-Fernandez P, Torres-Gonzalez R, Lopez-Macias C, Isibasi A. The increased expression of TREM-1 on monocytes is associated with infectious and noninfectious inflammatory processes. J Surg Res. 2008;150:110–117. doi: 10.1016/j.jss.2007.12.805. [DOI] [PubMed] [Google Scholar]
- Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Rittirsch D, Huber-Lang M, Niederbichler AD, Hoesel LM, Touban BM, Morgan SJ, Smith WR, Ward PA, Ipaktchi K. Inhibition of complement C5a prevents breakdown of the blood-brain barrier and pituitary dysfunction in experimental sepsis. Crit Care. 2009;13:R12. doi: 10.1186/cc7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm RE., Jr Cardiac troponins in the intensive care unit: common causes of increased levels and interpretation. Crit Care Med. 2007;35:584–588. doi: 10.1097/01.CCM.0000254349.10953.BE. [DOI] [PubMed] [Google Scholar]
- Grailer JJ, Fattahi F, Dick RS, Zetoune FS, Ward PA. Cutting edge: critical role for C5aRs in the development of septic lymphopenia in mice. J Immunol. 2015;194:868–872. doi: 10.4049/jimmunol.1401193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualandro DM, Puelacher C, Mueller C. High-sensitivity cardiac troponin in acute conditions. Curr Opin Crit Care. 2014;20:472–477. doi: 10.1097/MCC.0000000000000132. [DOI] [PubMed] [Google Scholar]
- Guerin L, Vieillard-Baron A. The Use of Ultrasound in Caring for Patients with Sepsis. Clin Chest Med. 2016;37:299–307. doi: 10.1016/j.ccm.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Gunnar RM, Loeb HS, Winslow EJ, Blain C, Robinson J. Hemodynamic measurements in bacteremia and septic shock in man. J Infect Dis. 1973;128(Suppl):295–298. doi: 10.1093/infdis/128.supplement_1.s295. [DOI] [PubMed] [Google Scholar]
- Guo RF, Huber-Lang M, Wang X, Sarma V, Padgaonkar VA, Craig RA, Riedemann NC, McClintock SD, Hlaing T, Shi MM, Ward PA. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J Clin Invest. 2000;106:1271–1280. doi: 10.1172/JCI10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RF, Sun L, Gao H, Shi KX, Rittirsch D, Sarma VJ, Zetoune FS, Ward PA. In vivo regulation of neutrophil apoptosis by C5a during sepsis. J Leukoc Biol. 2006;80:1575–1583. doi: 10.1189/jlb.0106065. [DOI] [PubMed] [Google Scholar]
- Haileselassie B, Su E, Pozios I, Fiskum T, Thompson R, Abraham T. Strain Echocardiography Parameters Correlate With Disease Severity in Children and Infants With Sepsis. Pediatr Crit Care Med. 2016;17:383–390. doi: 10.1097/PCC.0000000000000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MA, Toner A, Cecconi M. Troponin in critically ill patients. Minerva Anestesiol. 2012;78:1039–1045. [PubMed] [Google Scholar]
- Hammerschmidt DE, Weaver LJ, Hudson LD, Craddock PR, Jacob HS. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980;1:947–949. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- Hasko G, Csoka B, Koscso B, Chandra R, Pacher P, Thompson LF, Deitch EA, Spolarics Z, Virag L, Gergely P, Rolandelli RH, Nemeth ZH. Ecto-5′-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol. 2011;187:4256–4267. doi: 10.4049/jimmunol.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun SM, Marechal X, Montaigne D, Bouazza Y, Decoster B, Lancel S, Neviere R. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Crit Care Med. 2008;36:2590–2596. doi: 10.1097/CCM.0b013e3181844276. [DOI] [PubMed] [Google Scholar]
- He HB, Yu F, Dai DZ, Dai Y. Down-regulation of FKBP12.6 and SERCA2a contributes to acute heart failure in septic shock and is related to an up-regulated endothelin signalling pathway. J Pharm Pharmacol. 2007;59:977–984. doi: 10.1211/jpp.59.7.0010. [DOI] [PubMed] [Google Scholar]
- He L, Wang B, Yao Y, Su M, Ma H, Jia N. Protective effects of the SEPS1 gene on lipopolysaccharide-induced sepsis. Mol Med Rep. 2014;9:1869–1876. doi: 10.3892/mmr.2014.1991. [DOI] [PubMed] [Google Scholar]
- Henriquez-Camacho C, Losa J. Biomarkers for sepsis. Biomed Res Int. 2014;2014:547818. doi: 10.1155/2014/547818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobai IA, Buys ES, Morse JC, Edgecomb J, Weiss EH, Armoundas AA, Hou X, Khandelwal AR, Siwik DA, Brouckaert P, Cohen RA, Colucci WS. SERCA Cys674 sulphonylation and inhibition of L-type Ca2+ influx contribute to cardiac dysfunction in endotoxemic mice, independent of cGMP synthesis. Am J Physiol Heart Circ Physiol. 2013;305:H1189–1200. doi: 10.1152/ajpheart.00392.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobai IA, Edgecomb J, LaBarge K, Colucci WS. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock. 2015a;43:3–15. doi: 10.1097/SHK.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobai IA, Morse JC, Siwik DA, Colucci WS. Lipopolysaccharide and cytokines inhibit rat cardiomyocyte contractility in vitro. J Surg Res. 2015b;193:888–901. doi: 10.1016/j.jss.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesel LM, Niederbichler AD, Schaefer J, Ipaktchi KR, Gao H, Rittirsch D, Pianko MJ, Vogt PM, Sarma JV, Su GL, Arbabi S, Westfall MV, Wang SC, Hemmila MR, Ward PA. C5a-blockade improves burn-induced cardiac dysfunction. J Immunol. 2007a;178:7902–7910. doi: 10.4049/jimmunol.178.12.7902. [DOI] [PubMed] [Google Scholar]
- Hoesel LM, Niederbichler AD, Ward PA. Complement-related molecular events in sepsis leading to heart failure. Mol Immunol. 2007b;44:95–102. doi: 10.1016/j.molimm.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Dumasius A, Easington C, Colilla SA, Neumann A, Parrillo JE. Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med. 2001;164:891–895. doi: 10.1164/ajrccm.164.5.2010073. [DOI] [PubMed] [Google Scholar]
- Hoover DB, Ozment TR, Wondergem R, Li C, Williams DL. Impaired heart rate regulation and depression of cardiac chronotropic and dromotropic function in polymicrobial sepsis. Shock. 2015;43:185–191. doi: 10.1097/SHK.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Hua SD, Wu ZX, Huang JT, Feng ZC. Clinical features of Candida albicans sepsis in preterm infants: an analysis of 13 cases. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14:728–732. [PubMed] [Google Scholar]
- Huber-Lang M, Sarma VJ, Lu KT, McGuire SR, Padgaonkar VA, Guo RF, Younkin EM, Kunkel RG, Ding J, Erickson R, Curnutte JT, Ward PA. Role of C5a in multiorgan failure during sepsis. J Immunol. 2001a;166:1193–1199. doi: 10.4049/jimmunol.166.2.1193. [DOI] [PubMed] [Google Scholar]
- Huber-Lang MS, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Younkin EM, Laudes IJ, Riedemann NC, Younger JG, Ward PA. Protective effects of anti-C5a peptide antibodies in experimental sepsis. FASEB J. 2001b;15:568–570. doi: 10.1096/fj.00-0653fje. [DOI] [PubMed] [Google Scholar]
- Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res. 2007;100:130–139. doi: 10.1161/01.RES.0000253888.09574.7a. [DOI] [PubMed] [Google Scholar]
- Jafri SM, Lavine S, Field BE, Bahorozian MT, Carlson RW. Left ventricular diastolic function in sepsis. Crit Care Med. 1990;18:709–714. doi: 10.1097/00003246-199007000-00005. [DOI] [PubMed] [Google Scholar]
- Kalbitz M, Fattahi F, Grailer JJ, Jajou L, Malan EA, Zetoune FS, Huber-Lang M, Russell MW, Ward PA. Complement Destabilizes Cardiomyocyte Function in Vivo after Polymicrobial Sepsis and In Vitro. J Immunology. 2016 doi: 10.4049/jimmunol.1600091. Published ahead of print August 12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbitz M, Grailer JJ, Fattahi F, Jajou L, Herron TJ, Campbell KF, Zetoune FS, Bosmann M, Sarma JV, Huber-Lang M, Gebhard F, Loaiza R, Valdivia HH, Jalife J, Russell MW, Ward PA. Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J. 2015;29:2185–2193. doi: 10.1096/fj.14-268730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, Finkel MS. Inflammatory mediators and reversible myocardial dysfunction. J Cell Physiol. 2003;195:1–11. doi: 10.1002/jcp.10213. [DOI] [PubMed] [Google Scholar]
- Koch A, Voigt S, Kruschinski C, Sanson E, Duckers H, Horn A, Yagmur E, Zimmermann H, Trautwein C, Tacke F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. doi: 10.1186/cc10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock: Part I. Clinical manifestation of cardiovascular dysfunction. J Cardiothorac Vasc Anesth. 2001;15:364–376. doi: 10.1053/jcan.2001.22317. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanone S, Mebazaa A, Heymes C, Henin D, Poderoso JJ, Panis Y, Zedda C, Billiar T, Payen D, Aubier M, Boczkowski J. Muscular contractile failure in septic patients: role of the inducible nitric oxide synthase pathway. Am J Respir Crit Care Med. 2000;162:2308–2315. doi: 10.1164/ajrccm.162.6.2001097. [DOI] [PubMed] [Google Scholar]
- Laudes IJ, Chu JC, Sikranth S, Huber-Lang M, Guo RF, Riedemann N, Sarma JV, Schmaier AH, Ward PA. Anti-c5a ameliorates coagulation/fibrinolytic protein changes in a rat model of sepsis. Am J Pathol. 2002;160:1867–1875. doi: 10.1016/S0002-9440(10)61133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Z, Liu B, Zhao T, Chong W, Wang Y, Alam HB. Citrullinated histone H3: a novel target for the treatment of sepsis. Surgery. 2014;156:229–234. doi: 10.1016/j.surg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Qu X, Liu F, Wang C. Pentraxin 3 as a prognostic biomarker in patients with systemic inflammation or infection. Mediators Inflamm. 2014;2014:421429. doi: 10.1155/2014/421429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lompre AM, Anger M, Levitsky D. Sarco(endo)plasmic reticulum calcium pumps in the cardiovascular system: function and gene expression. J Mol Cell Cardiol. 1994;26:1109–1121. doi: 10.1006/jmcc.1994.1130. [DOI] [PubMed] [Google Scholar]
- MacKenzie IM. The haemodynamics of human septic shock. Anaesthesia. 2001;56:130–144. doi: 10.1046/j.1365-2044.2001.01866.x. [DOI] [PubMed] [Google Scholar]
- Madias JE, Bazaz R. On the mechanism of the reduction in the ECG QRS amplitudes in patients with sepsis. Cardiology. 2003;99:166–168. doi: 10.1159/000070674. [DOI] [PubMed] [Google Scholar]
- Maitra SR, Homan CS, Beuhler MC, Thode HC, Jr, Henry M. Alterations in hepatic gluconeogenesis, prostanoid, and intracellular calcium during sepsis. Acad Emerg Med. 1999;6:588–595. doi: 10.1111/j.1553-2712.1999.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Mantzouris T, Gauer R, Mackler L. Clinical Inquiry: Elevated troponin but no CVD: what’s the prognosis? J Fam Pract. 2013;62:585–598. [PubMed] [Google Scholar]
- Marik PE, Varon J. The hemodynamic derangements in sepsis: implications for treatment strategies. Chest. 1998;114:854–860. doi: 10.1378/chest.114.3.854. [DOI] [PubMed] [Google Scholar]
- Masson S, Caironi P, Spanuth E, Thomae R, Panigada M, Sangiorgi G, Fumagalli R, Mauri T, Isgro S, Fanizza C, Romero M, Tognoni G, Latini R, Gattinoni L Investigators AS. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: data from the Albumin Italian Outcome Sepsis trial. Crit Care. 2014;18:R6. doi: 10.1186/cc13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misquitta CM, Mack DP, Grover AK. Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium. 1999;25:277–290. doi: 10.1054/ceca.1999.0032. [DOI] [PubMed] [Google Scholar]
- Munt B, Jue J, Gin K, Fenwick J, Tweeddale M. Diastolic filling in human severe sepsis: an echocardiographic study. Crit Care Med. 1998;26:1829–1833. doi: 10.1097/00003246-199811000-00023. [DOI] [PubMed] [Google Scholar]
- Nakae H, Endo S, Inada K, Takakuwa T, Kasai T, Yoshida M. Serum complement levels and severity of sepsis. Res Commun Chem Pathol Pharmacol. 1994;84:189–195. [PubMed] [Google Scholar]
- Nakahara M, Ito T, Kawahara K, Yamamoto M, Nagasato T, Shrestha B, Yamada S, Miyauchi T, Higuchi K, Takenaka T, Yasuda T, Matsunaga A, Kakihana Y, Hashiguchi T, Kanmura Y, Maruyama I. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS One. 2013;8:e75961. doi: 10.1371/journal.pone.0075961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederbichler AD, Hoesel LM, Westfall MV, Gao H, Ipaktchi KR, Sun L, Zetoune FS, Su GL, Arbabi S, Sarma JV, Wang SC, Hemmila MR, Ward PA. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. J Exp Med. 2006;203:53–61. doi: 10.1084/jem.20051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli G, Morabito V, Ferretti G, Pugliese F, Ruberto F, Venuta F, Poli L, Rossi M, Berloco PB. Pathfast presepsin assay for early diagnosis of bacterial infections in surgical patients: preliminary study. Transplant Proc. 2013;45:2750–2753. doi: 10.1016/j.transproceed.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Oliveira NS, Silva VR, Castelo JS, Elias-Neto J, Pereira FE, Carvalho WB. Serum level of cardiac troponin I in pediatric patients with sepsis or septic shock. Pediatr Crit Care Med. 2008;9:414–417. doi: 10.1097/PCC.0b013e31817e2b33. [DOI] [PubMed] [Google Scholar]
- Packman MI, Rackow EC. Optimum left heart filling pressure during fluid resuscitation of patients with hypovolemic and septic shock. Crit Care Med. 1983;11:165–169. doi: 10.1097/00003246-198303000-00003. [DOI] [PubMed] [Google Scholar]
- Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- Parrillo JE, Burch C, Shelhamer JH, Parker MM, Natanson C, Schuette W. A circulating myocardial depressant substance in humans with septic shock. Septic shock patients with a reduced ejection fraction have a circulating factor that depresses in vitro myocardial cell performance. J Clin Invest. 1985;76:1539–1553. doi: 10.1172/JCI112135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S, Nicol E, Gibson DG, Evans TW. Echocardiography in the critically ill: current and potential roles. Intensive Care Med. 2006;32:48–59. doi: 10.1007/s00134-005-2834-7. [DOI] [PubMed] [Google Scholar]
- Prucha M, Bellingan G, Zazula R. Sepsis biomarkers. Clin Chim Acta. 2015;440:97–103. doi: 10.1016/j.cca.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Rabuel C, Mebazaa A. Septic shock: a heart story since the 1960s. Intensive Care Med. 2006;32:799–807. doi: 10.1007/s00134-006-0142-5. [DOI] [PubMed] [Google Scholar]
- Ren J, Ren BH, Sharma AC. Sepsis-induced depressed contractile function of isolated ventricular myocytes is due to altered calcium transient properties. Shock. 2002;18:285–288. doi: 10.1097/00024382-200209000-00014. [DOI] [PubMed] [Google Scholar]
- Rich MM, McGarvey ML, Teener JW, Frame LH. ECG changes during septic shock. Cardiology. 2002;97:187–196. doi: 10.1159/000063120. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 2004;18:370–372. doi: 10.1096/fj.03-0708fje. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Sarma VJ, Laudes IJ, Huber-Lang M, Warner RL, Albrecht EA, Speyer CL, Ward PA. Expression and function of the C5a receptor in rat alveolar epithelial cells. J Immunol. 2002;168:1919–1925. doi: 10.4049/jimmunol.168.4.1919. [DOI] [PubMed] [Google Scholar]
- Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Dellinger RP. Treatment options for severe sepsis and septic shock. Expert Rev Anti Infect Ther. 2006;4:395–403. doi: 10.1586/14787210.4.3.395. [DOI] [PubMed] [Google Scholar]
- Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011;17:764–769. doi: 10.1007/s10156-011-0254-x. [DOI] [PubMed] [Google Scholar]
- Smith JA, Mayeux PR, Schnellmann RG. Delayed Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Inhibition by Trametinib Attenuates Systemic Inflammatory Responses and Multiple Organ Injury in Murine Sepsis. Crit Care Med. 2016;44:e711–720. doi: 10.1097/CCM.0000000000001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong T, Brandtzaeg P, Fung M, Pharo AM, Hoiby EA, Michaelsen TE, Aase A, van der Meer JW, van Deuren M, Mollnes TE. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood. 2003;102:3702–3710. doi: 10.1182/blood-2003-03-0703. [DOI] [PubMed] [Google Scholar]
- Stevens JH, O’Hanley P, Shapiro JM, Mihm FG, Satoh PS, Collins JA, Raffin TA. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J Clin Invest. 1986;77:1812–1816. doi: 10.1172/JCI112506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suberviola B, Castellanos-Ortega A, Llorca J, Ortiz F, Iglesias D, Prieto B. Prognostic value of proadrenomedullin in severe sepsis and septic shock patients with community-acquired pneumonia. Swiss Med Wkly. 2012;142:w13542. doi: 10.4414/smw.2012.13542. [DOI] [PubMed] [Google Scholar]
- Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, Kovacs RJ. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- Turdi S, Han X, Huff AF, Roe ND, Hu N, Gao F, Ren J. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced myocardial contractile dysfunction: role of autophagy. Free Radic Biol Med. 2012;53:1327–1338. doi: 10.1016/j.freeradbiomed.2012.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, Morello F, Lupia E, Moiraghi C, Mengozzi G, Battista S. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care. 2013;17:R168. doi: 10.1186/cc12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varriale P, Ramaprasad S. Septic cardiomyopathy as a cause of long QT syndrome. J Electrocardiol. 1995;28:327–329. doi: 10.1016/s0022-0736(05)80051-2. [DOI] [PubMed] [Google Scholar]
- Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F. Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med. 2003;168:1270–1276. doi: 10.1164/rccm.200306-816CC. [DOI] [PubMed] [Google Scholar]
- Winslow EJ, Loeb HS, Rahimtoola SH, Kamath S, Gunnar RM. Hemodynamic studies and results of therapy in 50 patients with bacteremic shock. Am J Med. 1973;54:421–432. doi: 10.1016/0002-9343(73)90038-7. [DOI] [PubMed] [Google Scholar]
- Wu G, Yang SL, Hsu C, Yang RC, Hsu HK, Liu N, Yang J, Dong LW, Liu MS. Transcriptional regulation of cardiac sarcoplasmic reticulum calcium-ATPase gene during the progression of sepsis. Shock. 2004;22:46–50. doi: 10.1097/01.shk.0000127685.64611.15. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang J, Gong Y, Li S. Slowed relaxation of diaphragm in septic rats is associated with reduced expression of sarco-endoplasmic reticulum Ca2+-ATPase genes SERCA1 and SERCA 2. Muscle Nerve. 2016 doi: 10.1002/mus.25150. [DOI] [PubMed] [Google Scholar]
- Wu LL, Ji Y, Dong LW, Liu MS. Calcium uptake by sarcoplasmic reticulum is impaired during the hypodynamic phase of sepsis in the rat heart. Shock. 2001;15:49–55. doi: 10.1097/00024382-200115010-00008. [DOI] [PubMed] [Google Scholar]
- Wu LL, Liu MS. Impaired calcium uptake by cardiac sarcoplasmic reticulum and its underlying mechanism in endotoxin shock. Mol Cell Biochem. 1991;108:9–17. doi: 10.1007/BF00239537. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Huang Y, Mao P, Zhang J, Li Y. Sepsis and ARDS: The Dark Side of Histones. Mediators Inflamm. 2015;2015:205054. doi: 10.1155/2015/205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Y-Hassan S, Settergren M, Henareh L. Sepsis-induced myocardial depression and takotsubo syndrome. Acute Card Care. 2014;16:102–109. doi: 10.3109/17482941.2014.920089. [DOI] [PubMed] [Google Scholar]
- Yaegashi Y, Shirakawa K, Sato N, Suzuki Y, Kojika M, Imai S, Takahashi G, Miyata M, Furusako S, Endo S. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother. 2005;11:234–238. doi: 10.1007/s10156-005-0400-4. [DOI] [PubMed] [Google Scholar]
- Zanotti-Cavazzoni SL, Guglielmi M, Parrillo JE, Walker T, Dellinger RP, Hollenberg SM. Fluid resuscitation influences cardiovascular performance and mortality in a murine model of sepsis. Intensive Care Med. 2009;35:748–754. doi: 10.1007/s00134-008-1360-9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong S, Qin Y, Bian X. Protective effect of erythropoietin against myocardial injury in rats with sepsis and its underlying mechanisms. Mol Med Rep. 2015;11:3317–3329. doi: 10.3892/mmr.2015.3155. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang ZC, Dai HW, Yu YH, Yang JD, Hu CB. Usefulness of heart-type fatty acid-binding protein in patients with severe sepsis. J Crit Care. 2012;27:415, e413–418. doi: 10.1016/j.jcrc.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bernecker OY, Manohar NS, Hajjar RJ, Hellman J, Ichinose F, Valdivia HH, Schmidt U. Increased leakage of sarcoplasmic reticulum Ca2+ contributes to abnormal myocyte Ca2+ handling and shortening in sepsis. Crit Care Med. 2005;33:598–604. doi: 10.1097/01.ccm.0000152223.27176.a6. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]