Abstract
Health agencies have declared the recent Zika-virus (ZIKV) infection an epidemic and a public health emergency of global concern due to its association with microcephaly and serious neurological disorders. The unavailability of effective drugs, vaccines, and diagnostic tools increases the demand for efficient analytical devices to detect ZIKV infection. However, high costs, longer diagnostic times, and stringent expertise requirements limit the utility of reverse transcriptase-polymerase chain reaction (RT-PCR) methods for rapid diagnostics. Therefore, developing portable, sensitive, selective, and cost-effective sensing systems to detect ZIKV at pM concentrations in biofluids would be a breakthrough in diagnostics and therapeutics. This review highlights the advancements in developing smart sensing strategies to monitor ZIKV progression, with rapid point-of-care (POC) diagnostics as the ultimate aim.
Keywords: Zika-virus infection, electrochemical biosensor, point-of-care sensing systems
Public health implications of Zika virus and the need for Zika diagnostics
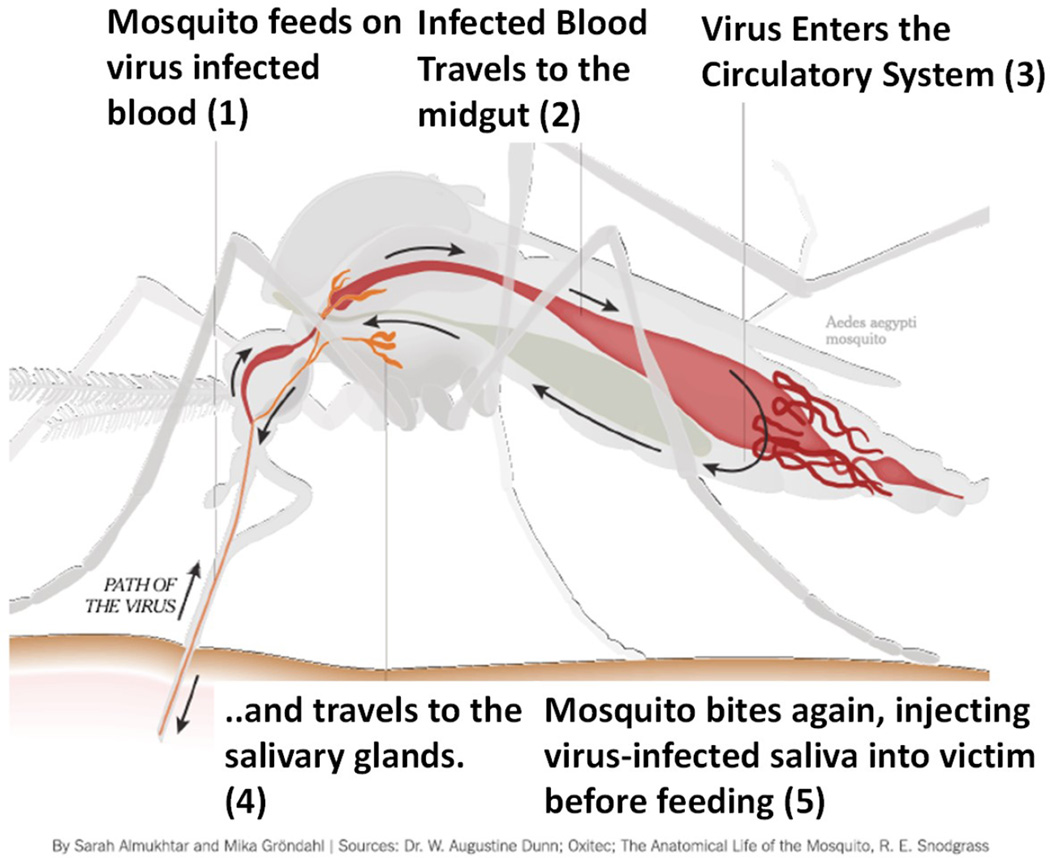
Zika virus (ZIKV, see Glossary) is an infectious disease-causing agent (Box 1) [1, 2] that spreads in humans via the mosquitoes Aedes aegypti and Aedes albopictus. The transmission mode consists of a mosquito biting an infected host, taking in infected blood containing the virus, and further injecting the infectious saliva into the healthy host system. This process ends in a serious infection, resulting in life-threatening pathogenesis and disease progression [1–6]. ZIKV infection has various transmission modes [4, 7–9] and mechanisms including from mother to child; through blood transfusion, bone marrow transplants, or organ transplants; and sexual transmission. [10–12]. Horizontal transmission, i.e., from mother to child during pregnancy, leads to microcephaly in newborns and also other birth defects [8, 9, 13–21], as described in Box 2.
Box 1. Zika Virology.
Zika virus (ZIKV), first isolated in 1947, belongs to the Flavivirus genus within the Flaviviridae family [1–2]. Recently, ZIKV infection has caused an epidemic that has severe adverse health effects and has led to fatality in many cases [1, 3]. Initially, ZIKV was observed to circulate in forests between mosquitoes and non-human primates, but later it was identified among human hosts as well. Since 2007, ZIKV infection has spread across the world, and recently, approximately 1.5 million clinical cases have been documented in Brazil, Latin America, and the USA, especially in Florida [4]. Like other fever-causing flaviviruses, ZIKV infection causes yellow fever and more symptomatic complications, even leading to life-threatening cases [9]. The most common pathway of ZIKV infection in humans is through a bite from an infected female Aedes mosquito.
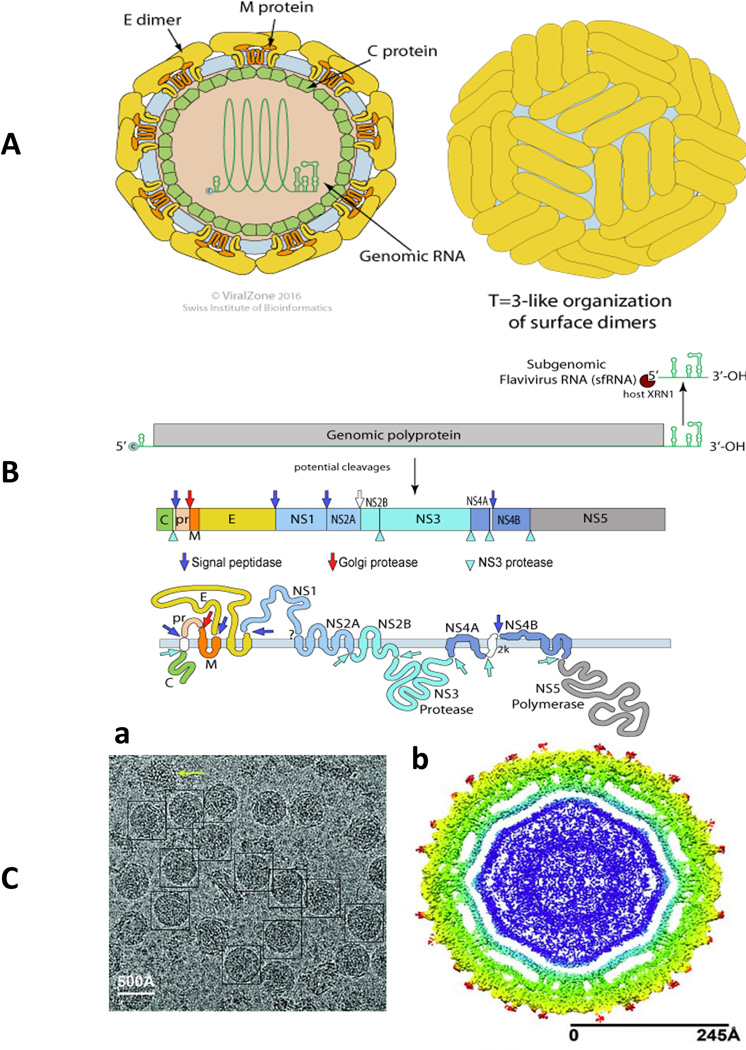
It is very important to understand ZIKV transmission routes, the possible effects of ZIKV infection, the correlation of ZIKV infection with pregnancy, and the link between ZIKV infection and microcephaly. Significant efforts have been made to study the mechanism of ZIKV infection through exploring targets of ZIKV infection along with the effects on cellular proliferation and differentiation [10], sexual transmission [1, 12], the link between infection and pregnancy [13], the effects of infection on birth defects, and the relationship between ZIKV infection and microcephaly. [8, 9, 14, 15]. Garcez et al. used immuno-cytochemistry and electron microscopy studies to examine the effects of ZIKV infection in human neural stem cells. The results of this study confirm that ZIKV targets brain cells and inhibits neurogenesis in the human brain [16]. Exploring the chemical and crystalline structure of ZIKV is important to determine the similarities and differences between ZIKV (Figure I A and B) and other flaviviruses. This information is required for developing novel and effective therapeutics against ZIKV infection. Sirohi et al. obtained a 3.8 Å resolution structure of mature ZIKV using cryo-electron microscopy (Figure I C) [20]. This finding defined the ZIKV structure and compared it to other known flaviviruses.
Figure I, Box 1. Contributions of the Zika virus external morphology and genomic constitution to its pathogenesis. A) Illustration of the ZIKV virion structure and B) genome expression of ZIKV, adapted with permission from http://viralzone.expasy.org/all_by_protein/6756.html. C) The cryo-EM structure of Zika virus at 3.8 Å: (a) cryo-EM image of frozen, hydrated ZIKV, and (b) A cross-section of ZIKV showing the radial density distribution [20].
Box 2. Zika Pathogenesis.
Zika virus infects humans by entering the blood stream through a mosquito bite (Figure II). Considering the seriousness of ZIKV infection in pregnancy and infants, Li et al. investigated how the Asian ZIKV strain SZ01 infects neural progenitor cells (NPCs) in vivo and affects brain development. The model investigated by this group established a link between ZIKV infection and microcephaly, with the potential to further explore the underlying mechanisms and management of ZIKV related pathological effects during brain development [14]. Using a Brazilian ZIKV strain, Cugola et al. described the possible mechanisms of ZIKV infection causing birth defects. This study demonstrated that this specific strain infected fetuses to restrict growth within the uterus, including signs of microcephaly, in mice. Their study model also showed the effects of ZIKV infection on human neurodevelopment [9]. Miner et al. performed similar in vivo studies to explore the effects of ZIKV on pregnancy [15].
Brasil et al. described the effects of acute ZIKV infection in 88 pregnant patients in Rio de Janeiro to explore the clinical manifestations in mothers and its consequences [17]. Based on ultrsonograph data and reverse-transcriptase–polymerase chain reaction (RT-PCR) analysis, 72 women were found positive with acute ZIKV infection within 5 to 30 weeks of gestation. Based on a detailed clinical analysis, the authors concluded that despite mild clinical symptoms, ZIKV infection progression during pregnancy results in serious effects such as fetal death, placental insufficiency, fetal growth restriction, and central nervous system (CNS) injury [17]. Culjat et al. first explored and imaged the clinical progression of an infant with ZIKV embryopathy [18]. The results of this study showed that ZIKV infection reaches the human fetal brain tissue, and with the help of serological studies, the study confirmed that ZIKV follows a route of vertical transmission, therefore classifying it as a congenital infection [18]. Various reports involving serum samples collected from mothers and newborns with ZIKV infection showed that the infants may become infected either by a transplacental transmission route or during delivery [17, 19]. ZIKV seems to have modified itself to suit human hosts and is maintained in a large population via a mosquito-human-mosquito transmission cycle, where nonhuman reservoirs are not mandatory for transmission.
Rossie et al. designed an immuno-competent mouse model to explore the effects of time-dependent ZIKV infection [21]. ZIKV infection in the brain was monitored every day until day 7 post-infection to evaluate the advancement of neurological disease. The authors of this study explained that the progression of ZIKV disease morbidity and mortality is age-dependent.
Figure I. ZIKV infection, Symptoms, and Health Effects. An illustration of how ZIKV infection progresses through a mosquito bite.
The efforts to monitor and control ZIKV infection globally are so far limited and may prove insufficient due to a lack of accountability; lack of organization; and unavailability of drugs, vaccines, and diagnostic tools (see Glossary) to avert infection [22, 23]. The government of the United States has requested that health agencies should spread awareness and understand and develop strategies to manage ZIKV infection in the future. These health agencies promote focusing on the rapid expansion of mosquito control programs, vaccine research, and diagnostic development. Educating health providers, patients, and women who may become pregnant is also very important. There is a pressing need to provide health support to low-income pregnant women and their families in Zika-affected countries. The approach to fighting against Zika should contain support and technical assistance in risk areas, mosquito monitoring, surveillance, and control of transmission. Nabel and Zerhouni suggested that committed and sustained public support, global economic support, well-administrated infrastructure, public-private partnerships, and funding are urgently required for exploring affordable smart medicines and diagnostics tools [23].
In particular, it has become crucial to study the structure of ZIKV [20], to develop specific animal models for understanding the symptoms and pathogenesis of the virus, and to design novel diagnostic kits, vaccine, and drugs to combat ZIKV infection. Recently, a few animal models were developed to study ZIKV pathogenesis and to facilitate vaccine and therapeutic discovery. Such information is useful to develop a platform for testing the efficacy of available antivirals and vaccines [21]. Nevertheless, more animal model studies are needed to demonstrate ZIKV infection mechanisms and progression pathways. Based on these findings, designing and developing novel vaccines and drugs may become easier. In addition to controlling and studying ZIKV infection, its monitoring and early detection has also become important. The next section of this review describes various sensing systems for detecting the viral infection.
The state of the art in detecting ZIKV infection
Various aspects of the impact of ZIKV infection on public health have been studied and analyzed globally [23]. Waggoner et al. described details about the discovery, emergence, and epidemiology of ZIKV, along with its diagnostics. These authors explained various techniques including viral culture, antibody detection, and antigen and RNA detection. These techniques are useful, but they sometimes have shortcomings, including requiring complex instruments or large sample sizes, or their inability to reach disease-affected areas and detect later stages of infection. This report reiterated the need for a smart and efficient ZIKV diagnostic tool for rapid and selective detection of the infection at an early stage. The available ZIKV detection methods, their limitations and challenges, and possible future approaches are discussed below.
RT-PCR
The most commonly available laboratory method to detect ZIKV is reverse transcriptase-polymerase chain reaction (RT-PCR), which can detect ZIKV in biofluids including serum, saliva, urine, and cerebro spinal fluid (CSF)–[24]. Faye et al. proposed a one-step RT-PCR methodology to detect ZIKV in serum [25]. This modified assay targeted ZIKV envelope from ZIKV isolates collected over a 40-year period from patients from African countries. It exhibited a detection limit of 7.7 plaque-forming units (pfu)/reaction in serum. The authors of this study claim that the developed system is rapid but requires validation to be used for clinical diagnosis purposes [25]. The effects of acute ZIKV infection from October 2013 to March 2014 in French Polynesia were explored by Mussoa et al. using RT-PCR [26]. This group selected saliva as a biofluid, which was collected from 1067 samples (855 patients) collected over a period of 6 months, to detect ZIKV RNA. The authors of this study preferred using saliva to blood, especially in young children, due to the difficulties in blood collection. The study found that saliva is a suitable fluid to increase the chances of ZIKV detection (by around 20 %) as compared to blood samples at the early onset of symptoms [26]. In another study, Liuzzi et al. used RT-PCR to detect ZIKV in saliva cultured from patients [27]. The patients were diagnosed with acute infections and had their saliva collected between day 6 and day 29 after the onset of symptoms. These authors concluded that saliva is a suitable sample to explore acute infection but that effort must be taken towards validating and developing novel diagnostics tools for disease management for large-scale epidemiological studies [27].
Gourinat et al. employed RT-PCR to evaluate the kinetics of ZIKV detection [28]. This study was performed with serum and urine samples collected from 6 ZIKV-positive patients, 10 days after the onset of disease. The authors claimed that urine is a useful fluid to confirm ZIKV infection, especially in the case of a high ZIKV level for a long time [28]. De et al. further supported this finding through prolonged detection of ZIKV RNA from urine samples during the ongoing 2016 epidemic in Brazil [29]. The authors used a standard RT-PCR protocol to detect ZIKV RNA from sera and urine collected from patients 2 and 4 days after the onset of symptoms. Their results showed that ZIKV was detected in urine at day 4, but not at day 2. The ZIKV RNA concentration in urine was found to be higher than that detected in serum. This finding was in agreement with previous reports, suggesting that urine can be used as an alternative to serum or plasma to detect ZIKV RNA non-invasively [29].
A single reaction based a real time RT-PCR assay was developed to detect Zika, Chikungunya, and Dengue viruses, referred to as the ZCD assay [30]. The ZCD assay was performed with serum samples from patients, and the results confirmed that only semen tested positive at days 27 and 62 after the onset of febrile illness. This signal was stronger than that observed in serum and urine. This finding is useful to access the prolonged sexual transmission of flavivirus and also for disease monitoring during therapy [30]. The possibility of CSF as a target bio-fluid was explored in two cases of encephalopathy, including seizures and electroencephalogram changes in ZIKV-infected patients [31].
Antibody methods
Despite the above-mentioned diagnostic significance, the RT-PCR technique cannot be recommended to detect ZIKV infection due to its associated complicated procedure, required expensive materials, and long diagnosis time. Alternatively, the enzyme-linked immunosorbent assay (ELISA) [24] has been used to detect immunoglobulin-M (IgM) antibodies against ZIKV. Due to the lack of an advanced ZIKV detection system, only a few laboratories have used these methods due to its poor sensitivity and specificity for ZIKV infection. The major challenges in developing a ZIKV detection system include nonspecific binding, low viral loads, and cross-reactivity of ZIKV antibodies with antibodies against other highly homologous flaviviruses like Dengue [24]. Therefore, developing a smart diagnostics system is essential for sensitive and selective detection of ZIKV at very low concentrations to identify infection at an early stage.
Towards Point-of-care Sensing of ZIKV
The currently available ZIKV sensors are multi-component sensors that require sophisticated components. As of now, no effective facility for ZIKV detection and monitoring is available. Therefore, there is considerable interest in developing a rapid, portable, selective, and sensitive ZIKV detection for point-of-care (POC) applications, which should result in faster diagnostics needed to determine a timely and effective therapeutic intervention.
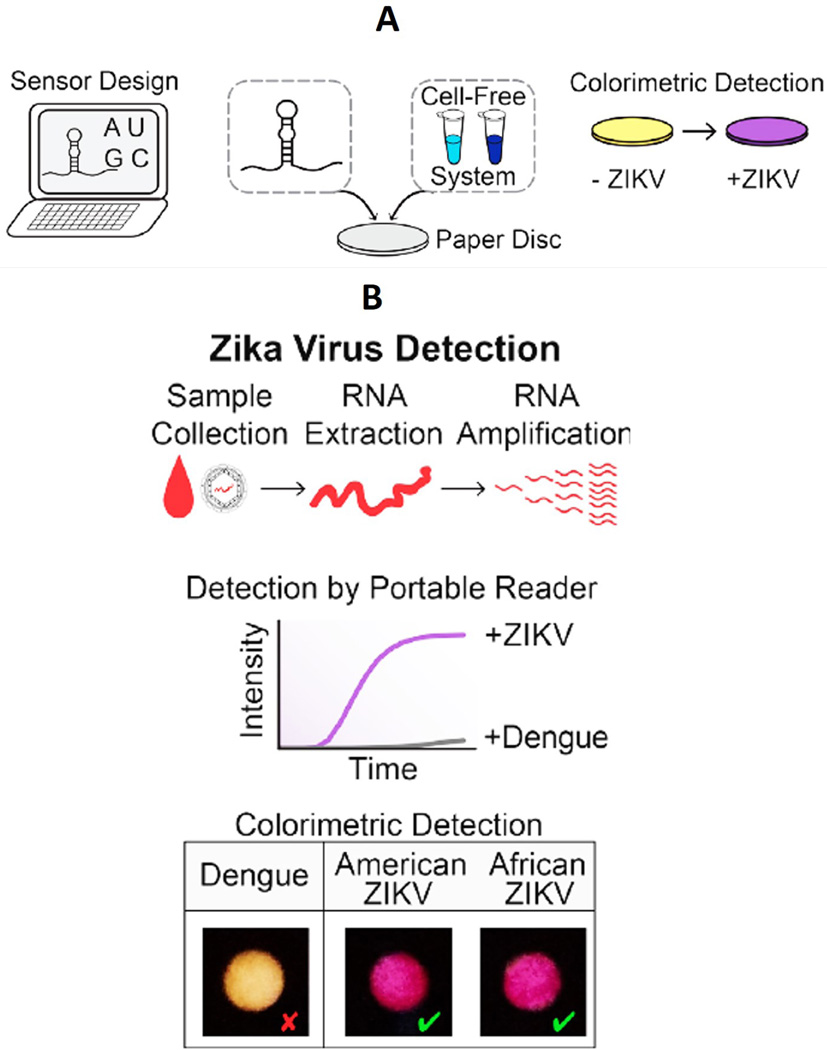
An engineered cell-free paper-based biosensor (Figure 1) for ZIKV detection was recently reported by Pardee et al. [32]. This paper-based optical geno-biosensor is coupled with CRISPR-Cas9 to detect the ZIKV RNA genome. The developed sensing protocol is highly selective due to hybridization between a specifically designed CRISPR-Cas9 system and ZIKV RNA. The authors of this study observed a ZIKV detection limit at femtomolar (fM) concentrations. The selectivity of this developed biosensor was validated by using Dengue virus sequences as a negative control. The use of CRISPR-Cas9 made the sensor highly specific, with the ability to differentiate viral strains with single-base resolution. The authors claimed that the RNA-based method that they developed for ZIKV detection is comparatively rapid and can be used in the field for rapid diagnostics of ZIKV infectious disease [32]. This research was reviewed by Meagher et al., who agreed that engineered paper-based sensors might represent the next generation of sensors for ZIKV detection, but they require considerable optimization for clinical POC application [33].
Figure 1. Workflow of a Paper-Based ZIKV Biosensor Based on CRISPR Technology.
A) Proposed workflow for a CRISPR (clustered regularly interspaced short palindromic repeats) - associated protein-9 nuclease (CRISPR-Cas9) based engineered paper sensor to detect the ZIKV RNA genome, B) illustration of zika/dengue virus sensing according to work flow. Figure adapted with permission from [32].
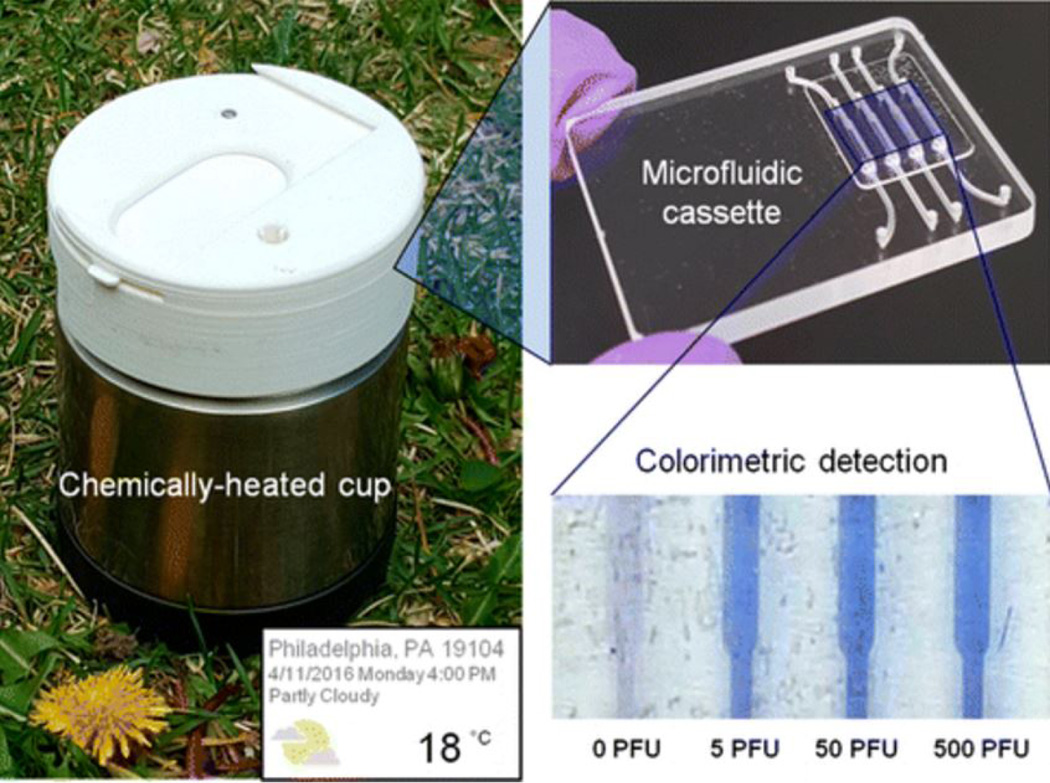
A point-of-care (POC) ZIKV sensing system (Figure 2) based on a highly sensitive reverse transcription-loop mediated isothermal amplification (RT-LAMP) assay was first developed and described by Song et al. [34]. This sensor is composed of a disposable cassette that carries out all of its operations. It contains a chemically heated cup to control the temperature of the system without requiring electrical power. The ZIKV sensing principle is based on detecting a color variation produced in leucocrystal violet dye. The authors of this study examined the color variation by eye, so the developed sensor is an instrument-free POC detection device. This device was sensitive enough to detect 5 pfu in less than 40 minutes in samples including blood, saliva, urine, and semen. This sensor has the potential to be used as an efficient ZIKV diagnostic tool, facilitating detection remotely where medical facilities and clinical instruments are very limited [34].
Figure 2. Instrument Free Detection of ZIKV.
A microfluidic chip was developed and integrated into a miniaturized system to detect ZIKV based on the RT-LAMP method. Figure reprinted with permission from [34].
CIRSPR-Cas9 based paper sensing and the instrument-free RT-LAMP assay can be used as a platform to develop miniaturized POC systems for ZIKV detection and estimation. However, these methods are multi-component colorimetric methods and need to be validated for clinical application. Therefore, there remains an opportunity to develop smart sensing systems capable to perform under the ASSURED (affordable, sensitive, specific, user-friendly, rapid, robust, equipment-free, and deliverable) paradigm [35–37]
To create an ASSURED environment for detecting ZIKV infection, we propose nano-enabled electrochemical immuno-sensing systems. The electrochemical bio-sensing methodology has been developed to detect target analytes in physiological ranges in patients [38–40]. Such developed methodologies can easily be modified with fast, selective, and ultrasensitive detection capabilities to detect different target analytes. To develop an electrochemical biosensor with a desired performance for a targeted disease, state-of-the-art technologies include molecular recognition (including enzymes [41], DNA [42], aptamers [43], antibody [44], and others), nanostructured immobilizing materials (1D, 2D, and 3D nanostructure of organic, inorganic and composites) [45], transduction techniques (miniaturized electronics) [46], fluidic systems to develop BioMEMS [47], novel sensing arrays [48], and advanced flexible substrates [49]. Various nano-enabled biosensors have been developed to detect metabolites, nucleic acids, antigens, pathogens, and targeted biomarkers for diseases diagnostics [50–52]. Over the last few decades, significant advancements have been made in electrochemical bio-sensing technology for measuring virus concentrations to diagnose infectious diseases [53–55].
We suggest that significant efforts should be made towards a) genetic and functional modification of bio-recognition molecules such as DNA, aptamers, CRISPR-Cas9 etc., for sequence-specific detection; b) exploring novel antibodies and enzymes for selectively determining virus concentrations; c) developing sensing arrays of electro-active materials to develop highly sensitive sensing systems; d) designing fluidic structures for automated sampling for precise real-time detection; e) wireless communication for data storage to benefit bio-informatics for disease management; and f) integrating advanced sensing components to develop a miniaturized system for clinical application. These approaches are required to develop a diagnostic tool for ZIKV detection in physiologically relevant ranges.
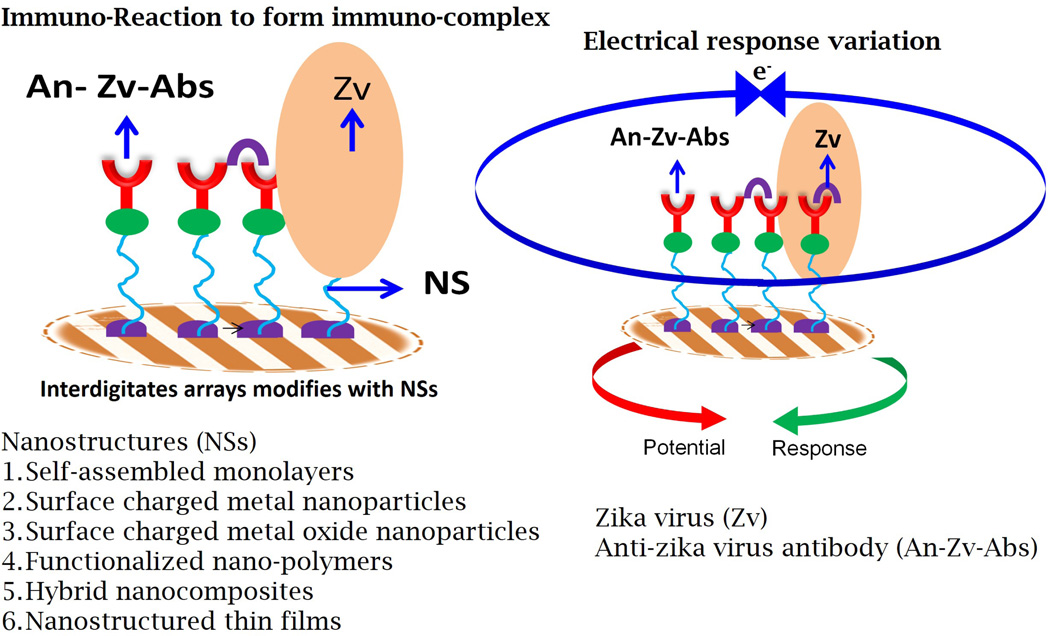
In our proposed sensing system, ZIKV proteins, including the ZIKV envelope protein and non-structural (NS) proteins, could be detected at fM/pM concentrations using their respective specific antibodies (Figure 3, Key Figure). Using nano/micro electrodes [56], nanostructured sensing materials [57], microelectronics, and miniaturized sensing transducers [58–59] to fabricate an electrochemical ZIKV sensor may improve selective screening, monitoring, and detection of ZIKV infection. Current studies in the field of sensing systems available for ZIKV detection remain in an embryonic stage. Therefore, we encourage the development of nano-enabled electrochemical immunosensing technology that can detect ZIKV in infected bio-fluids in patients, mainly pregnant women and infants. Point-of-care ZIKV detection using miniaturized electrochemical nano-sensing systems will facilitate fast personalized health care delivery to patients for better disease management and therapeutics.
Figure 3. Key Figure. Nano-enabled Electrochemical ZIKV Immunosensor.
An interdigitated micro-electrode can be modified with various nanostructures for high loading of ZIKV-specific antibodies to detect ZIKV proteins at pM concentrations using an appropriate electrochemical transduction technique.
Conclusion and Future Prospects
Most of the research conducted in the area of ZIKV diagnostics and therapeutics has claimed that the adverse effects of ZIKV infection are worse than expected [60]. Health agencies have issued global alerts and request accelerating research for developing novel vaccines, drugs, and diagnostics tools. It has been also observed that ZIKV infection at early stages does not necessarily exhibit symptoms but progresses to a severe disease by the time that a patient is diagnosed at a clinic. Therefore, developing a smart sensing system for ZIKV detection at early stages would be useful for better diagnostics.
In spite of significant achievements in exploring a gene-based CRISPR assay and a paper-based colorimetric detection system, the need remains to develop novel portable sensing systems to selectively detect ZIKV at very low levels. These systems are essential to fill the gap between estimating the level of infection and making rapid therapeutics decisions. Efforts must be continuously made toward investigating novel antibodies, edited genes, and assays for detecting ZIKV. Affordable, portable, and reliable sensing systems are already in demand to control ZIKV infection. We propose a specific antibody-based nano-enabled electrochemical immuno-sensing system could be a plausible solution for detecting the ZIKV (at pM/fM concentrations) at early stages, even at the point of care. Such a system would certainly be useful to assess drug or vaccine efficacy and to monitor ZIKV infection during therapy. Furthermore, the information obtained using this next-generation ZIKV sensor would be useful for disease management pertaining to personalized health care.
Outstanding Box.
What is the best strategy to motivate the application of electrochemical ZIKV biosensors in clinics?
How can reasonably priced, disposable electrochemical ZIKV sensing systems be developed?
Which engineering strategies are needed to design sensing components to achieve selective pM/fM detection along with the best approach to integrate nano-enabled sensing components for developing a POC system to detect ZIKV?
How can we use any information gathered from these sensors to evaluate ZIKV infection progression?
How could health agencies establish better methods to analyze sensing data for ZIKV disease management to discover novel drugs and timely therapeutics?
Trends Box.
Zika virus (ZIKV) infection causes severe health disorders, potentially leading to fatality.
There is an urgent need to develop smart sensors to detect ZIKV at the pM level to facilitate early disease detection.
Nano-enabled electrochemical ZIKV sensing may be a promising approach for rapid and selective ZIKV detection.
Miniaturized sensing systems can be developed for the POC application.
The POC detection of ZIKV will enable effective and rapid diagnostics for timely therapeutic intervention.
Acknowledgments
The authors acknowledge grants R01-DA027049, R01-DA034547, R01-DA037838, R01-DA-040537, and R01-DA042706A awarded by National Institutes of Health.
Glossary
- Central nervous system (CNS)
a part of the nervous system made of nerve tissue that controls body activity.
- CIRSPR-Cas9
a genome-editing tool used to edit DNA at a targeted sequence.
- Diagnostic tools
analytical systems utilized to detect specific markers and monitor target diseases.
- Disease management
bioinformatics generated through monitoring disease progression and utilized to determine therapeutics for personalized health care.
- Electrochemical biosensor
an analytical device that converts a biological response to a measurable electrical signal.
- Enzyme-linked immunosorbent assay (ELISA)
an enzyme-based optical immunoassay utilized to detect a target biomarker.
- Point-of-care (POC) systems
miniaturized portable analytical tools for performing on-site diagnostics.
- Reverse transcription-loop mediated isothermal amplification (RT-LAMP)
a technique used for the amplification of RNA.
- Reverse transcriptase-polymerase chain reaction (RT-PCR)
a technique used in molecular biology to detect RNA expression.
- Zika virus
a mosquito-borne virus that causes serious fever and leads to microcephaly and neurological disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Authors declare no conflict of interest
References
- 1.Petersen LR, et al. Zika Virus. N. Engl J. Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 2.Saxena SK, et al. Zika virus outbreak: an overview of the experimental therapeutics and treatment. Virus Dis. 2016;27:111–115. doi: 10.1007/s13337-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shan C, et al. Zika Virus: Diagnosis, Therapeutics, and Vaccine. ACS Infect. Dis. 2016;2:170–172. doi: 10.1021/acsinfecdis.6b00030. [DOI] [PubMed] [Google Scholar]
- 4.Faria NR, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olagnier D, et al. Dengue Virus Immunopathogenesis: Lessons Applicable to the Emergence of Zika Virus. J. Mol. Biol. 2016;428:3429–3448. doi: 10.1016/j.jmb.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Camacho E, et al. Detection of autochthonous Zika virus transmission in Sincelejo, Colombia. Emerging infect. Dis. 2016;22:927. doi: 10.3201/eid2205.160023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamel R, et al. Zika virus: epidemiology, clinical features and host-virus interactions. Microbes Infect. 2016;18:441–449. doi: 10.1016/j.micinf.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Rubin EJ, et al. Zika virus and microcephaly. N. Eng. J. Med. 2016;374:984–985. doi: 10.1056/NEJMe1601862. [DOI] [PubMed] [Google Scholar]
- 9.Cugola FR, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016 doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofer U. Viral Pathogenesis: Tracing the steps of Zika virus. Nat. Rev. Microbiol. 2016;14:401. doi: 10.1038/nrmicro.2016.80. [DOI] [PubMed] [Google Scholar]
- 11.Musso D, et al. Potential sexual transmission of Zika virus. Emerg. Infect. Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogoch II, et al. Anticipating the international spread of Zika virus from Brazil. Lancet (London, England) 2016;387:335–336. doi: 10.1016/S0140-6736(16)00080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mlakar J, et al. Zika virus associated with microcephaly. N. Eng. J. Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 14.Li C, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Miner JJ, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcez PP, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 17.Brasil P, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report. N. Eng. J. Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Culjat M, et al. Clinical and Imaging Findings in an Infant with Zika Embryopathy. Clin. Infect. Dis. 2016 doi: 10.1093/cid/ciw324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mlakar J, et al. Zika Virus Associated with Microcephaly. N. Eng. J. Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 20.Sirohi D, et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi SL, et al. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016;94:1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waggoner JJ, Pinsky BA. Zika Virus: Diagnostics for an Emerging Pandemic Threat. J. Clin. Microbiol. 2016;54:860–867. doi: 10.1128/JCM.00279-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nabel GJ, Zerhouni EA. Once and future epidemics: Zika virus emerging. Sci. Transl. Med. 2016;8:330ed332. doi: 10.1126/scitranslmed.aaf4548. [DOI] [PubMed] [Google Scholar]
- 24.Vorou R. Letter to the editor: diagnostic challenges to be considered regarding Zika virus in the context of the presence of the vector Aedes albopictus in Europe. Euro Surveill. 2016;21:30161. doi: 10.2807/1560-7917.ES.2016.21.10.30161. [DOI] [PubMed] [Google Scholar]
- 25.Faye O, et al. One-step RT-PCR for detection of Zika virus. J. Clin. Virol. 2008;43:96–101. doi: 10.1016/j.jcv.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Musso D, et al. Detection of Zika virus in saliva. J. Clin. Virol. 2015;68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Liuzzi G, et al. Zika virus in saliva-New challenges for prevention of human to human transmission. Eur. J. Intern. Med. 2016;33:e20–e21. doi: 10.1016/j.ejim.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Gourinat AC, et al. Detection of Zika virus in urine. Emerg. Infect. Dis. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de MCR, et al. Prolonged detection of Zika virus RNA in urine samples during the ongoing Zika virus epidemic in Brazil. J. Clin. Virol. 2016;77:69–70. doi: 10.1016/j.jcv.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Waggoner JJ, et al. Single-Reaction Multiplex Reverse Transcription PCR for Detection of Zika, Chikungunya, and Dengue Viruses. Emerg. Infect. Dis. 2016;22:1295–1297. doi: 10.3201/eid2207.160326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roze B, et al. Zika virus detection in cerebrospinal fluid from two patients with encephalopathy, Martinique, February 2016. Euro Surveill. 2016;21:3025. doi: 10.2807/1560-7917.ES.2016.21.16.30205. [DOI] [PubMed] [Google Scholar]
- 32.Pardee K, et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell. 2016;165:1255–1266. doi: 10.1016/j.cell.2016.04.059. [DOI] [PubMed] [Google Scholar]
- 33.Meagher RJ, et al. Engineering Paper-Based Sensors for Zika Virus. Trends. Mol. Med. 2016;22:529–530. doi: 10.1016/j.molmed.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Song J, et al. Instrument-Free Point-of-Care Molecular Detection of Zika Virus. Anal. Chem. 2016;88:7289–7294. doi: 10.1021/acs.analchem.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair M, et al. Getting into the brain: Potential of nanotechnology in the management of NeuroAIDS. Advanced Drug Delivery Reviews. 2016;103:202–217. doi: 10.1016/j.addr.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafiee H, et al. Emerging technologies for point-of-care management of HIV infection. Annual review of medicine. 2015;66:387–405. doi: 10.1146/annurev-med-092112-143017. [DOI] [PubMed] [Google Scholar]
- 37.Kaushik A, et al. Electrochemical monitoring-on-chip (E-MoC) of HIV-infection in presence of cocaine and therapeutics. Biosens. Bioelectron. 86:426–431. doi: 10.1016/j.bios.2016.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaushik A, et al. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014;53:499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 39.Kaushik A, et al. Nano-biosensors to detect beta-amyloid for Alzheimer's disease management. Biosens. Bioelectron. 2016;80:273–287. doi: 10.1016/j.bios.2016.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaushik A, et al. Towards detection and diagnosis of Ebola virus disease at point-of-care. Biosens. Bioelectron. 2016;75:254–272. doi: 10.1016/j.bios.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis. 2006;17:7–14. [Google Scholar]
- 42.Fan C, et al. Electrochemical interrogation of conformational changes as a reagentless method for the sequence-specific detection of DNA. Proc. Natl. Acad. Sci. 2003;100:9134–9137. doi: 10.1073/pnas.1633515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Y, et al. Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew. Chem. 2005;117:5592–5595. doi: 10.1002/anie.200500989. [DOI] [PubMed] [Google Scholar]
- 44.Skottrup PD, et al. Towards on-site pathogen detection using antibody-based sensors. Biosens. Bioelectron. 2008;24:339–348. doi: 10.1016/j.bios.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 45.Solanki PR, et al. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011;3:17–24. [Google Scholar]
- 46.Labib M, et al. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016;116:9001–9090. doi: 10.1021/acs.chemrev.6b00220. [DOI] [PubMed] [Google Scholar]
- 47.Squires T, et al. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005;77:977. [Google Scholar]
- 48.Seker E, et al. The fabrication of low-impedance nanoporous gold multiple-electrode arrays for neural electrophysiology studies. Nanotechnology. 2010;21:125504. doi: 10.1088/0957-4484/21/12/125504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Q, et al. Medium-scale carbon nanotube thin-film integrated circuits on flexible plastic substrates. Nature. 2008;454:495–500. doi: 10.1038/nature07110. [DOI] [PubMed] [Google Scholar]
- 50.Patterson AS, et al. Electrochemical real-time nucleic acid amplification: towards point-of-care quantification of pathogens. Trends Biotechnol. 2013;31:704–712. doi: 10.1016/j.tibtech.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Hsieh K, et al. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc. Chem. Res. 2015;48:911–920. doi: 10.1021/ar500456w. [DOI] [PubMed] [Google Scholar]
- 52.Arugula MA, et al. Biosensors as 21st century technology for detecting genetically modified organisms in food and feed. Anal. Chem. 2013;86:119–129. doi: 10.1021/ac402898j. [DOI] [PubMed] [Google Scholar]
- 53.Moschopoulou G, et al. Engineering of the membrane of fibroblast cells with virus-specific antibodies: A novel biosensor tool for virus detection. Biosens. Bioelectron. 2008;24:1027–1030. doi: 10.1016/j.bios.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 54.Pejcic B, et al. The role of biosensors in the detection of emerging infectious diseases. Analyst. 2006;131:1079–1090. doi: 10.1039/b603402k. [DOI] [PubMed] [Google Scholar]
- 55.Niemz A, et al. Point-of-care nucleic acid testing for infectious diseases. Trends biotechnol. 2011;29:240–250. doi: 10.1016/j.tibtech.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasudev A, et al. An LTCC-based microfluidic system for label-free, electrochemical detection of cortisol. Sens. Actuators, B. 2013;182:139–146. [Google Scholar]
- 57.Kaushik A, et al. Mediator and label free estimation of stress biomarker using electrophoretically deposited Ag@AgO–polyaniline hybrid nanocomposite. Biosens. Bioelectron. 2013;50:35–41. doi: 10.1016/j.bios.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Kaushik A, et al. Electrochemical sensing method for point-of-care cortisol detection in human immunodeficiency virus-infected patients. Int. J. Nanomed. 2015;10:677–685. doi: 10.2147/IJN.S75514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cruz AFD, et al. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014;62:249–254. doi: 10.1016/j.bios.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 60.Vogel G. Experts fear Zika's effects may be even worse than thought. Science. 2016;352:1375–1376. doi: 10.1126/science.352.6292.1375. [DOI] [PubMed] [Google Scholar]