Abstract
Background
Prior research has demonstrated inverse associations between maternal prenatal urinary phthalate metabolite concentrations and cognitive development assessed in preschool and school-aged children. While there are a limited number of studies that evaluated these associations during infancy, no study has evaluated whether these associations exist when using the Fagan Test of Infant Intelligence (FTII), which captures novelty preference as a function of visual recognition memory.
Objective
We evaluated associations between phthalate metabolite concentrations in maternal prenatal urine and cognition in infancy using the FTII at 27 weeks and determine if these associations are sex-specific.
Methods
Mono-n-butyl phthalate (MnBP), monobenzyl phthalate (MBzP), monoisobutyl phthalate (MiBP), mono-ethyl phthalate (MEP), mono-3-carboxypropyl phthalate (MCPP) and four di-2-ethylhexyl phthalate metabolites (DEHP) were quantified in urine samples collected from 168 minority women living in urban neighborhoods during their third trimester of pregnancy. The FTII was administered to infants at 27 weeks to measure visual recognition memory and was recorded as the novelty preference score.
Results
There were no associations between prenatal phthalate metabolite concentrations and novelty preference score in the full sample. However, there was evidence of effect modification by infant sex. Sex-stratified models demonstrated that compared to girls in the lowest tertile of MBzP concentrations, girls in tertiles 2 and 3 had, on average, 3.98 and 4.65 points lower novelty preference scores (p-value = 0.04 and 0.03, respectively). The relationship was similar for ΣDEHP, MiBP, and MEP. Effects among boys were inconsistent and generally not significant.
Conclusion
Maternal prenatal exposure to some phthalates was negatively associated with visual recognition memory as measured by the FTII among girls at age 27 weeks.
Keywords: Phthalates, Fagan, infant cognition, visual recognition memory
INTRODUCTION
Phthalates are a class of high production volume chemicals that are widely used as plasticizers and additives for consumer products such as toys and personal care products such as cosmetics, and perfumes (Sathyanarayana, 2008). Several phthalates are endocrine disruptors that act by various mechanisms, including the inhibition of testosterone production and the modulation of thyroid hormone functions (Howdeshell et al., 2008; Sathyanarayana, 2008). In animal studies, some phthalates have been shown to interfere with estrogen synthesis by suppressing aromatase enzyme activity in the brain suggesting that phthalates could affect male and females differently (Andrade, Grande, Talsness, Grote, & Chahoud, 2006). Humans can be exposed to phthalates via oral, dermal and inhalation routes (CDC, 2011). Due to widespread use of products containing phthalates, exposure is ubiquitous (Sathyanarayana, 2008). Phthalates rapidly hydrolyze to monoester metabolites and oxidative metabolites, which are excreted primarily in urine (Heudorf, Mersch-Sundermann, & Angerer, 2007). Urinary concentrations of phthalate metabolites are used as internal dosimeters of exposure because urinary enzymatic activity is negligible. Therefore, the metabolite concentration in urine accurately reflects an individual’s exposure to phthalates as opposed to the external contamination with phthalates during sample collection and processing (Whyatt et al., 2012).
Animal studies have suggested that prenatal phthalate exposure is likely to increase the risk of neurodevelopment impairment (Miodovnik, Edwards, Bellinger, & Hauser, 2014). There are a number of studies demonstrating associations between prenatal exposure to phthalates and adverse cognitive development in school-age children. Among New York City children at 3 years of age, Whyatt et al. (2012) demonstrated an inverse association between prenatal urinary concentrations of mono-n-butyl phthalate (MnBP) and monoisobutyl phthalate (MiBP) and psychomotor development index (PDI) of the Bayley Scales of Infant Development II (BSID) in both boys and girls. MnBP was also inversely associated with mental development index (MDI) of BSID in girls only (Whyatt et al., 2012). Among the same NYC children at age 7 years, Factor-Litvak et al. (2014) found that full-scale IQ as measured by the Wechsler Intelligence Scale for Children (WISC) was inversely associated with prenatal urinary metabolite concentrations of MnBP and MiBP. Additionally, Factor-Litvak et al. (2014) found that associations between MBzP and perceptual reasoning speed, and MiBP and verbal comprehension were stronger among boys than girls (Factor-Litvak et al., 2014). However, studies examining the effects of prenatal phthalate exposures on cognitive development among infants have been limited. Among Korean infants at 6 months, Kim et al. (2011) found that prenatal urinary concentration of mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP) and MnBP were inversely associated with MDI and PDI of the BSID. In sex-stratified analyses, male infants demonstrated a stronger inverse association between phthalate metabolites and developmental indices, whereas female infants exhibited no significant associations (Kim et al., 2011).
The aim of the present study was to evaluate whether effects of prenatal phthalate exposure on cognitive ability are detectable in infants at 6 months of age using the same cohort of NYC children evaluated by Whyatt et al. (2012) and Factor-Litvak et al. (2014). We used the Fagan Test of Infant Intelligence (FTII), which is an instrument that measures visual recognition memory in infants and has been shown to be predictive of IQ in children (McCall & Carriger, 1993). Based on previous reports of sex-specific differences in the effect of prenatal phthalates on cognitive endpoints in older children, we hypothesized that prenatal exposure to the precursors of MnBP, MiBP, monobenzyl phthalate (MBzP), mono-ethyl phthalate (MEP), mono-3-carboxypropyl phthalate (MCPP) and di-2-ethylhexyl phthalate (DEHP) would reduce visual recognition memory as measured by the FTII and that these effects would be sex-specific.
METHODS
Participants
This analysis includes 168 mother-infant pairs enrolled in the Columbia Center for Children’s Environmental Health (CCCEH) longitudinal birth cohort of 727 pregnant women who delivered between 1998 and 2006. The original purpose of this cohort was to assess the effects of air pollutant exposures on birth outcomes and child development (Perera et al., 2002). Subjects included in this study were women who lived in the Washington Heights, Central Harlem and the South Bronx neighborhoods of New York City and self-identified as either Dominican or African-American. Women who were eligible were nonsmoking, aged 18 to 35, registered at the obstetrics and genecology clinics at New York Presbyterian Medical Center and Harlem Hospital by the 20th week of pregnancy, resided in the area for at least one year, and were not diagnosed with diabetes, hypertension, or have known HIV. Mother-infant pairs were included in the present analysis if prenatal phthalate metabolite concentrations were measured in maternal spot urine samples collected during the third trimester of pregnancy and if the infant had completed the Fagan Test of Infant Intelligence (FTII) at 27 weeks. Supplemental Figure 1 schematically demonstrates how the 168 mother-child pairs were selected for this analysis.
Ethics statement
Institutional review boards at the Columbia University Medical Center and the Center for Disease Control and Prevention (CDC) approved the study and the consent procedures. Participating mothers provided written informed consent for themselves and on behalf of their children.
Urine sample collection and phthalate measurements
Spot urine samples were collected during the third trimester of pregnancy. Samples were first stored at −80°C at Columbia University, shipped on dry ice to the CDC and finally stored at −70°C until samples were ready for analysis. Urinary concentrations of MnBP, MBzP, MiBP, MEP, MCPP and four DEHP metabolites (MEHHP, mono-2-ethyl-5-carboxypentyl phthalate (MECPP), MEOHP, and mono(2-ethylhexyl) phthalate (MEHP)) were quantified at CDC using an on-line solid-phase extraction method combined with isotope dilution high-performance liquid chromatography/tandem mass spectrometry as previously described (Kato, Silva, Needham, & Calafat, 2005). We used specific gravity, measured with a handheld refractometer (Atago PAL 10-S, Bellevue, WA), to account for urinary dilution as previously recommended (Hauser, Meeker, Park, Silva, & Calafat, 2004). Intraclass correlation coefficients (ICCs) for the phthalate metabolites in urine samples were calculated to measure reliability. In 48 women from in the CCCEH cohort, urine samples were collected biweekly over 6 to 8 weeks in late pregnancy. There was a total of 135 samples with two to four repeats per woman. ICCs were 0.77 for MBzP, 0.65 for MnBP, and 0.60 for MiBP and ranged from 0.27 to 0.42 for DEHP metabolites after adjusting for specific gravity (Factor-Litvak et al., 2014).
Infant cognitive assessment
The Fagan Test of Infant Intelligence (FTII), which is used to measure infant visual recognition memory, can be administered at 67, 69, 79, and 92 weeks post-conception. The majority (75%) of our participants who were assessed using the FTII were tested using the 67-week version and therefore, in this analysis, we restricted to those tested using this version to eliminate variation by version. The FTII was administered to infants at 67 weeks post-conception (equivalent to 27 weeks after birth for a full-term infant) at the CCCEH by trained examiners. The average post-conception age for this cohort was 66.01 weeks. During the familiarization period, the infant is shown two identical photos. During the novelty phase, the familiar photo is shown with a new photo. The FTII measures the infant’s recognition memory to the familiar photo and the infant’s ability to discriminate between different visual stimuli. Infants will typically dedicate more time to the novel photo than the familiar photo because the novel photo contains more new information than the familiar photo (Fagan & Detterman, 1992). During the test, the infant completes 10 novelty trials in order to compute the novelty preference score, which is the length of fixation time devoted to the novel picture divided by the total fixation time to both the novel and familiar photo, multiplied by 100 (Fagan, 2005). The novelty preference score can be used to identify if an infant is at risk for later cognitive deficits. There are three possible outcomes of the FTII based on the novelty preference score: a novelty preference score that is greater than 54.5 indicates that an infant is at low risk for later cognitive deficits, a score that is greater than 53.1 but less than or equal to 54.5 indicates that an infant is suspected of later cognitive deficits, and a score that is less than or equal to 53.1 indicates that an infant is at risk of later cognitive deficits (Fagan, 2005).
Model covariates
To gather information on potential confounders, a questionnaire was administered to each woman during the third trimester of pregnancy. Trained bi-lingual interviewers administered the detailed questionnaire in homes of the pregnant women to collect information on race/ethnicity, maternal education, maternal marital status, environmental tobacco smoke (ETS) exposure, breastfeeding history and prenatal alcohol consumption. In addition, information was abstracted from infant medical records after delivery to collect information on infant sex, birth weight, and gestation age. Maternal intelligence was measured during the infant’s 6 month visit or a subsequent visit using the Test of Non-Verbal Intelligence (TONI), third edition (Brown, Sherbenou, & Johnson, 1990), which is a 15-minute language-free test that is free of culture bias and provides a stable measurement of general intelligence.
Statistical analysis
For this study, the four DEHP metabolites (MEHHP, MECPP, MEOHP and MEHP) were converted into their molecular weights and summed (ΣDEHP) and the five non-DEHP metabolites (MiBP, MBzP, MnBP, MCPP and MEP) were analyzed separately. Phthalate metabolite concentrations that were below the limit of detection (LOD) were given the value of LOD/√2. Spearman correlations were conducted to determine the correlations between the phthalate metabolite concentrations. To assess the dose response relationship between phthalate metabolites and novelty preference score, each of the phthalate metabolites was categorized into tertiles to describe the relationship between phthalate metabolite and mean novelty preference score. This was conducted for the total sample and also by sex. Because of the apparent nonlinear relationship between phthalates and novelty preference score, multivariable regression analyses were used where novelty preference score generated by the FTII was considered as a continuous variable and phthalate metabolites were treated as tertiles, with tertile 1 as the reference.
Among the 168 dyads included in this study, gestational age, birthweight, prenatal alcohol consumption, ETS exposure, and postnatal age were covariates with missing values (Table 1). One person was missing gestational age and was assigned the mean gestational age of infants included in this analysis (39.05 weeks). Six individuals were missing birthweight; these individuals were assigned the mean birthweight of infants included in this analysis (3332.15 grams). Prenatal alcohol consumption and ETS were missing in five and two individuals, respectively. We coded these individuals as having no alcohol and ETS exposure and found, in sensitivity analyses (not shown) that the results were identical when these individuals were coded as exposed. Three individuals were missing postnatal age and were assigned the mean postnatal age of infants included in this analysis (26.96 weeks). Sensitivity analyses were conducted for observations with no missing data (n=156).
Table 1.
Demographics and covariates of included (n=168) and excluded (n=559) populations
| Included Population | Excluded Population | |||
|---|---|---|---|---|
| Variablea | n | No (%)/Mean (SD) | n | No (%)/Mean (SD) |
| Maternal Characteristics | ||||
| Ethnicity | 168 | 559 | ||
| African American | 60 (35.71) | 194 (34.70) | ||
| Dominican or other Hispanic | 108 (64.29) | 365 (65.30) | ||
| Maternal Education | 168 | 545b | ||
| <High school degree | 52 (30.95) | 205 (36.67) | ||
| ≥High school diploma or GED | 116 (69.05) | 340 (60.82) | ||
| Marital Status | 168 | 553b | ||
| Never married | 110 (65.48) | 363 (64.94) | ||
| Ever Married | 58 (34.52) | 190 (33.99) | ||
| Prenatal Alcohol Consumption | 163c | 540b | ||
| No | 128 (76.19) | 383 (68.523) | ||
| Yes | 35 (20.83) | 157 (28.09) | ||
| Environmental tobacco smoke (ETS) | 166c | 551b | ||
| No | 110 (65.48) | 353 (63.15) | ||
| Yes | 56 (33.33) | 198 (35.42) | ||
| Maternal age at birth (years) | 168 | 24.81 (4.73) | 559 | 25.30 (5.00) |
| Infant Characteristics | ||||
| Sex | 168 | 559 | ||
| Female | 91 (54.17) | 285 (50.98) | ||
| Male | 77 (45.83) | 274 (49.02) | ||
| Gestational age (weeks) | 167c | 39.05 (1.50) | 550b | 39.38 (1.35) |
| Birthweight (grams) | 162c | 3332.15 (487.93) | 540b | 3382.37 (462.76) |
Variables did not differ significantly between included and excluded population (p-value > 0.05)
Of the 559 excluded: 14 were missing race, 6 were missing marital status, 19 were missing alcohol consumption during pregnancy, 8 were missing ETS, 9 were missing gestational age, 19 were missing birthweight.
Of the 168 included: 5 were missing alcohol consumption during pregnancy, 2 were missing ETS, 2 were missing gestational age, 7 were missing birthweight.
Multivariate linear regression models were estimated to analyze the relationship between prenatal phthalate metabolite concentrations and novelty preference score. In these models, dummy variables were created for each phthalate metabolite using the lowest tertile as the reference. In Model 1, infant sex and maternal urinary specific gravity were included as covariates. In addition to the covariates in Model 1, Model 2 included variables that were selected a priori based on previous literature and include maternal education, maternal age at birth, maternal ethnicity, and infant’s postnatal age at testing (Factor-Litvak et al., 2014; Whyatt et al., 2012; Winneke et al., 1998). For Model 3, we considered the inclusion of additional covariates if they changed the regression coefficient for the phthalate metabolite by more than 5% when added to Model 2 one at a time. Using this approach, in addition to the covariates in Model 2, we included ETS exposure in Model 3. We also included infant birthweight and gestational age in Model 3 to determine whether these variables mediated the associations between prenatal phthalate metabolites and novelty preference score. To evaluate if the association of prenatal phthalate exposures and novelty preference score differed by sex of the infant, an interaction term between phthalate metabolite and infant sex was included in the multivariable regression analyses and also examined sex-stratified models. All analyses were conducted using SAS v9.4 or STATA v13.
RESULTS
Maternal demographic characteristics and infant birth characteristics of the 168 mother-infant pairs that are included in this analysis and the 559 mother-infant pairs in the CCCEH cohort that were not included are presented in Table 1. The 168 mother-child pairs did not significantly differ from the remaining subjects in the CCCEH cohort with respect to demographic characteristics (ethnicity, maternal marital status, education level, maternal age at birth), ETS exposure, prenatal alcohol consumption, infant sex, gestational age, and birthweight (all p-values>0.05). Table 2 presents the distribution of the FTII results at 27 weeks of the 168 infants included in this analysis. Among these infants, the average postnatal age was 26.96 (SD= 1.79). The average novelty preference score from the FTII at 27 weeks was 57.77 (SD= 6.18) and 118 (70.24%) infants were identified as low risk, 9 (5.36%) infants that were ‘suspected’ to be at risk, and 41 (24.40%) that are ‘at risk’ for later cognitive deficits based on their FTII scores.
Table 2.
Fagan Test results at 27 weeks (n=168)
| Fagan Outcome Variables | N | No (%)/Mean (SD) | Min | Max |
|---|---|---|---|---|
| Postnatal age (weeks) | 165 | 26.96 (1.77) | 22.29 | 33.71 |
| Novelty Preference Score | 168 | 57.77 (6.18) | 44.28 | 74.92 |
| Fagan Test Result | 168 | |||
| Low Risk (>54.5) | 118 (70.24) | 54.51 | 77.31 | |
| Suspected (≤54.5, >53.1) | 9 (5.36) | 53.27 | 54.44 | |
| At Risk (≤53.1) | 41 (24.40) | 7.82 | 53.04 |
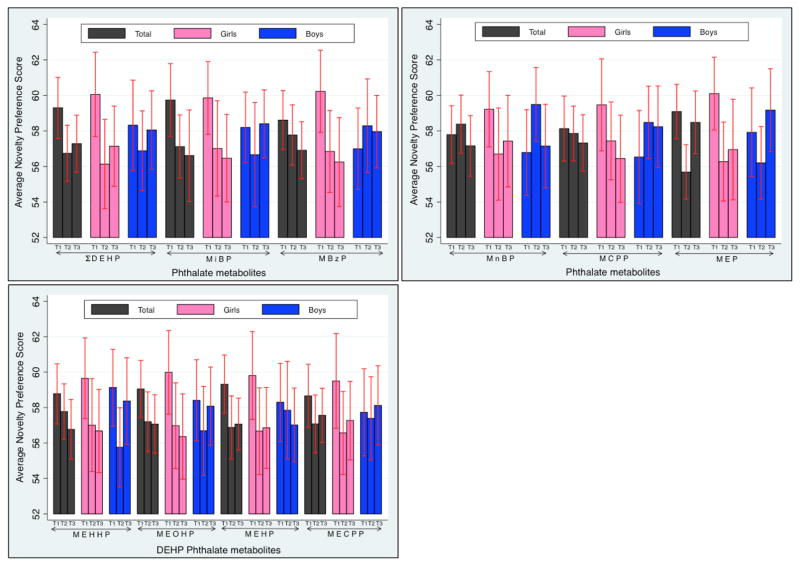
The distribution of the urinary phthalate metabolite concentrations in this sample of children is shown in Table 3. Phthalate metabolites were detected in 96.4–100% of the samples, except 17.9% of the samples had MEHP concentrations <LOD. After adjusting for urine specific gravity, the four DEHP metabolites concentrations were highly correlated (Spearman’s correlation r-values ranged from 0.76 to 0.98) as shown in the shaded blue boxes in Supplemental Table 1. A weaker correlation was observed between DEHP metabolites and non-DEHP metabolites after adjusting for urine specific gravity (r-values ranged from 0.35 to 0.63). The non-DEHP metabolites (MiBP, MBzP, MnBP, MCPP, and MEP) were moderately correlated (r-value range from 0.50 to 0.79). Bivariate associations between tertiles of prenatal phthalate metabolites and novelty preference score are shown in Figure 1. Generally, those in tertile 1 had, on average, higher novelty preference scores than those in tertiles 2 and 3. This pattern was particularly true among girls. The pattern among boys was less consistent.
Table 3.
Distributiona of phthalate metabolites (ng/mL) in maternal spot urine during the third trimester of pregnancy (n=168)
| Phthalate | Phthalate Metabolite | Geo Mean | 95% CI | LOD | % < LOD | Range | 25% | Median | 75% |
|---|---|---|---|---|---|---|---|---|---|
| DEHP | MEHHP | 19.99 | (16.81, 23.78) | 0.70 | 0 | 1.10–349.00 | 10.25 | 20.85 | 40.80 |
| MEOHP | 16.44 | (13.83, 19.56) | 0.70 | 0 | 0.90–240.00 | 8.35 | 17.50 | 37.10 | |
| MEHP | 4.70 | (3.91, 5.65) | 1.20 | 17.86 | <LOD-98.70 | 1.90 | 4.55 | 9.85 | |
| MECPP | 34.07 | (29.15, 39.81) | 0.60 | 0 | 3.00–509.20 | 17.95 | 30.45 | 74.55 | |
| DiBP | MiBP | 6.88 | (5.82, 8.12) | 0.30 | 0.60 | <LOD-374.40 | 4.10 | 7.30 | 14.20 |
| BzBP | MBzP | 12.23 | (10.04, 14.89) | 0.22 | 0 | 0.36–344.20 | 5.48 | 13.07 | 28.51 |
| DnBP | MnBP | 33.45 | (28.29, 39.55) | 0.60 | 0 | 1.20–1110.00 | 17.20 | 33.70 | 70.95 |
| DnOP | MCPPb | 1.96 | (1.68, 2.29) | 0.20 | 3.57 | <LOD-21.00 | 1.10 | 2.05 | 3.90 |
| DEP | MEP | 165.69 | (137.43, 199.77) | 0.53 | 0 | 14.65–6224.80 | 65.05 | 169 | 402 |
concentrations presented are not specific-gravity adjusted
MCPP is also a minor metabolite of DnBP and a non-specific metabolite of various high molecular weight phthalates
Figure 1. Average novelty preference score from lowest to highest tertile of ΣDEHP, MiBP, MBzP, MnBP, MCPP, MEP, and the four DEHP metabolite concentrations (where T1=lowest tertile, T3=highest tertile) sorted by total cohort and by gender.
Error bars represent 95% confidence intervals.
In bivariate analyses shown in Supplemental Table 2, postnatal age was significantly associated with novelty preference score (β=0.54 [95% CI= 0.10, 1.07]). There were no statistically significant bivariate associations between novelty preference score and maternal ethnicity, marital status, ETS, maternal education, prenatal alcohol consumption, maternal age at birth, infant sex, and gestational age. These associations were not different in sex-stratified models.
The adjusted associations between prenatal phthalate metabolite tertile and novelty preference score are presented in Table 4. There were very few significant associations between prenatal phthalate metabolite urinary concentrations and novelty preference score in the full sample. The p-values for the interaction term between infant sex and phthalate metabolites on novelty preference score was less than or equal to p=0.10 for models of MEOHP, MECPP, MBzP, MnBP, and MCPP, suggesting that the effect of prenatal phthalate exposure on novelty preference differed between girls and boys.
Table 4.
Multivariable regression analyses of phthalate metabolites on novelty preference score at 27 weeks (n=168)
| Total Sample | Girls | Boys | sex* metab interact | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | p-val | Beta | 95% CI | p-val | Beta | 95% CI | p-val | p-val | ||
| ΣDEHP | |||||||||||
| Model 1 | T2 v T1 | −2.44 | (−4.93, 0.05) | 0.06 | −4.15 | (−7.932, −0.38) | 0.03* | −0.94 | (−4.30, 2.42) | 0.58 | 0.42 |
| T3 v T1 | −1.80 | (−4.70, 1.10) | 0.22 | −3.21 | (−7.23, 0.81) | 0.12 | 1.09 | (−3.22, 5.40) | 0.62 | 0.13 | |
| Model 2 | T2 v T1 | −2.54 | (−5.07, 0.00) | 0.05* | −4.23 | (−8.13, −0.32) | 0.03* | −1.61 | (−5.05, 1.82) | 0.35 | 0.59 |
| T3 v T1 | −1.76 | (−4.70, 1.18) | 0.24 | −3.23 | (−7.33, 0.87) | 0.12 | 0.78 | (−3.62, 5.18) | 0.73 | 0.14 | |
| MEHHP | |||||||||||
| Model 1 | T2 v T1 | −0.84 | (−3.37, 1.68) | 0.51 | −2.63 | (−6.39, 1.12) | 0.17 | −3.01 | (−6.40, 0.38) | 0.08 | 0.74 |
| T3 v T1 | −1.74 | (−4.62, 1.14) | 0.24 | −2.96 | (−6.96, 1.04) | 0.15 | −0.04 | (−4.16, 4.09) | 0.99 | 0.13 | |
| Model 2 | T2 v T1 | −1.05 | (−3.60, 1.50) | 0.42 | −2.92 | (−6.81, 0.10) | 0.14 | −4.25 | (−7.67, −0.82) | 0.02* | 0.57 |
| T3 v T1 | −1.81 | (−4.71, 1.10) | 0.22 | −3.17 | (−7.23, 0.90) | 0.13 | −0.81 | (−4.98, 3.36) | 0.70 | 0.12 | |
| MEOHP | |||||||||||
| Model 1 | T2 v T1 | −1.71 | (−4.18, 0.76) | 0.17 | −3.15 | (−6.74, 0.45) | 0.09 | −1.23 | (−4.66, 2.20) | 0.48 | 0.70 |
| T3 v T1 | −1.71 | (−4.60, 1.18) | 0.24 | −3.83 | (−7.78, 0.12) | 0.06 | 0.73 | (−3.46, 4.91) | 0.73 | 0.06 | |
| Model 2 | T2 v T1 | −1.80 | (−4.28, 0.678) | 0.15 | −3.09 | (−6.85, 0.68) | 0.11 | −2.27 | (−5.79, 1.25) | 0.20 | 0.82 |
| T3 v T1 | −1.55 | (−4.46, 1.36) | 0.30 | −3.84 | (−7.90, 0.22) | 0.06 | 0.61 | (−3.56, 4.77) | 0.77 | 0.07 | |
| MEHP | |||||||||||
| Model 1 | T2 v T1 | −2.37 | (−4.77, 0.04) | 0.05* | −3.06 | (−6.64, 0.53) | 0.09 | −0.30 | (−3.66, 3.07) | 0.86 | 0.50 |
| T3 v T1 | −2.07 | (−4.87, 0.72) | 0.15 | −2.83 | (−6.66, 1.00) | 0.15 | −0.93 | (−4.86, 3.00) | 0.64 | 0.53 | |
| Model 2 | T2 v T1 | −2.07 | (−4.52, 0.37) | 0.10 | −3.05 | (−6.75, 0.66) | 0.11 | 0.35 | (−3.05, 3.75) | 0.84 | 0.42 |
| T3 v T1 | −1.81 | (−4.64, 1.03) | 0.21 | −2.99 | (−6.99, 1.01) | 0.14 | −0.29 | (−4.29, 3.72) | 0.89 | 0.47 | |
| MECPP | |||||||||||
| Model 1 | T2 v T1 | −1.23 | (−3.67, 1.21) | 0.32 | −2.69 | (−6.31, 0.92) | 0.14 | 0.31 | (−3.04, 3.66) | 0.85 | 0.10 |
| T3 v T1 | −0.31 | (−3.16, 2.54) | 0.83 | −1.90 | (−5.78, 1.98) | 0.33 | 2.25 | (−2.12, 6.61) | 0.31 | 0.16 | |
| Model 2 | T2 v T1 | −1.43 | (−3.92, 1.05) | 0.26 | −2.65 | (−6.36, 1.06) | 0.16 | −0.09 | (−3.58, 3.40) | 0.96 | 0.12 |
| T3 v T1 | −0.31 | (−3.21, 2.60) | 0.84 | −1.93 | (−5.88, 2.03) | 0.34 | 2.36 | (−2.07, 6.80) | 0.29 | 0.15 | |
| MiBP | |||||||||||
| Model 1 | T2 v T1 | −2.07 | (−4.52, 0.37) | 0.10 | −2.78 | (−6.21, 0.65) | 0.11 | −1.00 | (−4.57, 2.57) | 0.58 | 0.43 |
| T3 v T1 | −1.40 | (−4.07, 1.27) | 0.30 | −3.25 | (−7.08, 0.58) | 0.10 | 0.86 | (−2.93, 4.65) | 0.65 | 0.13 | |
| Model 2 | T2 v T1 | −2.32 | (−4.79, 0.14) | 0.06 | −3.12 | (−6.66, 0.42) | 0.08 | −1.16 | (−4.71, 2.39) | 0.52 | 0.35 |
| T3 v T1 | −1.48 | (−4.16, 1.21) | 0.28 | −3.36 | (−7.27, 0.54) | 0.09 | 0.68 | (−3.15, 4.51) | 0.72 | 0.14 | |
| MBzP | |||||||||||
| Model 1 | T2 v T1 | −0.57 | (−3.10, 1.95) | 0.65 | −3.60 | (−7.18, −0.02) | 0.05* | 2.36 | (−1.12, 5.85) | 0.18 | 0.01* |
| T3 v T1 | −1.26 | (−4.03, 1.50) | 0.37 | −4.27 | (−8.12, −0.43) | 0.03* | 2.81 | (−1.26, 6.89) | 0.17 | 0.02* | |
| Model 2 | T2 v T1 | −0.87 | (−3.43, 1.68) | 0.50 | −3.98 | (−7.71, −0.25) | 0.04* | 2.39 | (−1.26, 6.04) | 0.20 | 0.01* |
| T3 v T1 | −1.24 | (−4.10, 1.61) | 0.39 | −4.65 | (−8.79, −0.51) | 0.03* | 2.56 | (−1.59, 6.72) | 0.22 | 0.02* | |
| MnBP | |||||||||||
| Model 1 | T2 v T1 | 1.16 | (−1.33, 3.65) | 0.36 | −2.15 | (−5.81, 1.50) | 0.25 | 3.56 | (0.14, 6.98) | 0.04* | 0.01* |
| T3 v T1 | 0.45 | (−2.46, 3.36) | 0.76 | −1.19 | (−5.29, 2.91) | 0.57 | 2.06 | (−2.07, 6.18) | 0.32 | 0.13 | |
| Model 2 | T2 v T1 | 0.80 | (−1.75, 3.35) | 0.54 | −2.40 | (−6.13, 1.32) | 0.20 | 3.32 | (−0.43, 7.08) | 0.08 | 0.01* |
| T3 v T1 | 0.36 | (−2.56, 3.29) | 0.81 | −1.38 | (−5.57, 2.81) | 0.52 | 2.53 | (−1.75, 6.81) | 0.24 | 0.11 | |
| MCPP | |||||||||||
| Model 1 | T2 v T1 | 0.18 | (−2.28, 2.63) | 0.89 | −2.07 | (−5.75, 1.60) | 0.27 | 2.68 | (−0.59, 5.94) | 0.11 | 0.09 |
| T3 v T1 | 0.18 | (−2.76, 3.11) | 0.91 | −3.11 | (−7.42, 1.21) | 0.16 | 3.48 | (−0.38, 7.34) | 0.08 | 0.04* | |
| Model 2 | T2 v T1 | −0.09 | (−2.55, 2.38) | 0.95 | −2.32 | (−6.12, 1.48) | 0.23 | 2.58 | (−0.69, 5.84) | 0.12 | 0.08 |
| T3 v T1 | 0.26 | (−2.71, 3.23) | 0.86 | −3.25 | (−7.74, 1.24) | 0.15 | 3.09 | (−0.93, 7.12) | 0.13 | 0.05* | |
| MEP | |||||||||||
| Model 1 | T2 v T1 | −3.10 | (−5.56, −0.64) | 0.01* | −4.06 | (−7.80, −0.32) | 0.03* | −1.21 | (−4.44, 2.02) | 0.46 | 0.79 |
| T3 v T1 | −0.14 | (−2.77, 2.50) | 0.92 | −3.44 | (−7.38, 0.51) | 0.09 | 2.26 | (−1.30, 5.82) | 0.21 | 0.27 | |
| Model 2 | T2 v T1 | −3.38 | (−5.93, −0.84) | 0.01* | −4.39 | (−8.29, −0.49) | 0.03* | −0.69 | (−3.94, 2.56) | 0.67 | 0.55 |
| T3 v T1 | −0.35 | (−3.02, 2.32) | 0.79 | −4.04 | (−8.21, 0.14) | 0.06 | 3.08 | (−0.54, 6.70) | 0.09 | 0.11 | |
Model 1 adjusted for maternal specific gravity and infant sex; Model 2 adjusted for covariates in Model 1 plus maternal education, maternal age at birth, maternal ethnicity, and postnatal age;
≤0.05.
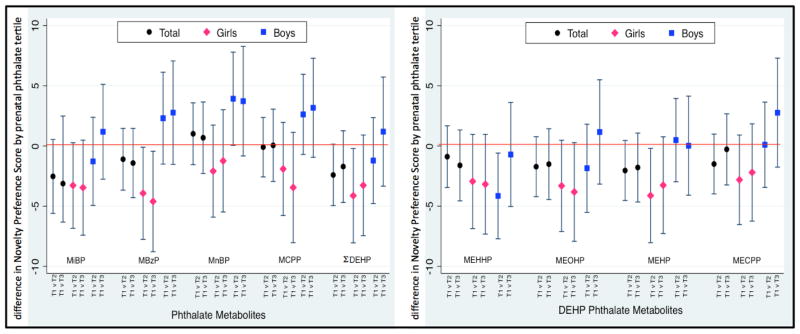
In sex-specific models, there were significant inverse associations between prenatal phthalate metabolite tertile and novelty preference among girls but not boys. The addition of covariates including maternal education, race, maternal age at birth and postnatal age at testing did not appreciably change these associations. The addition of birthweight and gestational age also did not alter the observed associations, indicating that these factors do not mediate the associations between prenatal phthalates and novelty preference (Supplemental Table 3). Among girls, the inverse effects were strongest for MBzP, where those in the second and third tertiles had novelty preference scores that were, on average, 3.98 (95% CI: −7.71, −0.25) and 4.65 (95% CI: −8.79, −0.51) points lower than those in the lowest tertile. As shown in Figure 2, among girls, the magnitude of effect was similar and lower in tertiles 2 and 3 compared to tertile 1 for each of the phthalate metabolites excepting MnBP and MCPP. Among boys, effects were varied and most were not statistically significant. Notably, for three phthalate metabolites--MBzP, MnBP, and MCPP--boys in tertile 2 and 3 had similar and higher novelty preference scores compared to those in tertile 1. In sensitivity analyses, where we included only those infants with no missing data (n=156), the sex-specific associations did not change (Supplemental Table 4).
Figure 2.
Adjusted difference in Novelty Preference Score by phthalate tertile
DISCUSSION
In this analysis, prenatal phthalate exposure was not associated with novelty preference score measured at 27 weeks in the full sample. However, after adjusting for confounders, there were significant inverse associations between phthalate metabolite concentration tertile and novelty preference score measured at 27 weeks among girls, such that girls with higher metabolite concentrations had lower performance. These associations were observed for both DEHP and non-DEHP-related biomarkers.
This study is the first to evaluate the effects of prenatal phthalate exposure on visual recognition memory in infants as measured by the FTII. The premise of the FTII is that individual differences in processing information during infancy are linked to the individual differences in the performance on standard tests of intelligence later in life (Fagan & Detterman, 1992). The tendency of the infant to look longer at a novel target than at a target that was previously seen is one way to measure how an infant processes information (Fagan, 1970). There have been numerous studies that have used the FTII to demonstrate that in the early months of life, average infants are more capable than “at-risk” infants in learning and processing visual information and will therefore achieve a higher novelty preference score. For example, studies that looked at prenatal exposures including cocaine and polychlorinated biphenyls (PCBs) demonstrated inverse associations with novelty preference score (Boucher et al., 2014; Gaultney, Gingras, Martin, & DeBrule, 2005; Winneke et al., 1998). Our analysis suggests that prenatal exposure to some phthalates is negatively associated with novelty preference score in a sex-specific manner.
There are few studies that have examined the effects of prenatal phthalate exposure on infants and among those studies, sex-specific associations have been observed. A cross-sectional study among Korean infants at 6 months found that urinary concentration of DEHP and di-n-butyl phthalate (DnBP) metabolites were negatively associated with MDI and PDI as measured by the BSID, with male infants demonstrating a stronger inverse association than females suggesting effect modification by infant sex (Kim et al., 2011). A study conducted by Yolton et al. (2011) suggested that prenatal exposure to DEHP was associated with non-optimal reflexes in male infants at 26 weeks. However, a study conducted by Engel et al. (2009) found significant negative associations between prenatal exposure to high molecular weight phthalate metabolites (e.g., DEHP metabolites) and orientation score when using the Brazelton Neonatal Behavioral Assessment Scale, which measures neurodevelopment in newborns, among infant girls within five days of delivery.
In our cohort, we previously demonstrated an inverse association between prenatal urinary concentrations of MnBP and MiBP and PDI from the BSID in both boys and girls at age 3 and an inverse association between concentrations of MnBP and MDI of the BSID in girls at age 3 (Whyatt et al., 2012). These findings were corroborated in a study done by Polanska et al. (2014), which found that prenatal exposure to MnBP was inversely associated with child motor development in boys and girls at age 2. Additionally, a study done by Tellez-Rojo et al. (2013) also demonstrated an inverse association between prenatal concentrations of DEHP metabolites and MDI of the BSID-II in girls ages 2 and 3 years. In our cohort, we have further described an inverse association between prenatal urinary metabolite concentrations of MnBP and MiBP and full-scale IQ using the WISC among children at age 7 years (Factor-Litvak et al., 2014). There was some evidence of effect modification by sex, but whether associations were stronger in girls or boys varied by phthalate metabolite and subscale. For example, associations between MnBP and full scale IQ, perceptual reasoning, and working memory were stronger among girls than boys but associations between MnBP and processing speed, between MBzP and perceptual reasoning speed, and between MiBP and verbal comprehension were stronger among boys than girls (Factor-Litvak et al., 2014). When comparing the FTII scores and the MDI and PDI scores of the BSID in our sample, FTII was positively but not significantly associated with BSID outcomes. Our study adds to the growing literature suggesting that prenatal phthalate exposure may relate to neurocognitive effects that are sex-specific in infants and children; however, which sex is more strongly impacted appears to vary by phthalate metabolite, child’s age at assessment, and cognitive endpoint evaluated.
There are a number of mechanisms that may potentially explain the sex-specific associations observed in this study. In animal studies, DEHP has been shown to suppress aromatase P450 enzyme activity in the brain and therefore interferes with estrogen synthesis (Andrade, Grande, Talsness, Grote, & Chahoud, 2006). In epidemiological studies, phthalate metabolites have also been shown to affect thyroid activity in pregnant women and children, with stronger associations found in girls (Boas et al., 2010; Huang, Kuo, Guo, Liao, & Lee, 2007). Although the exact mechanism by which phthalate metabolites interfere with thyroid activity is unknown, there is some suggestion (largely based on in vitro studies) that phthalates may affect the human thyroid receptor (Li et al., 2010), interfere with the binding of the thyroid hormone T3 to the thyroid receptor (Ishihara, Nishiyama, Sugiyama, & Yamauchi, 2003), or modulate the sodium/iodine symporter (NIS) (Breous, Wenzel, & Loos, 2005; Wenzel, Franz, Breous, & Loos, 2005).
There are several limitations when studying the adverse effects of phthalates on infant cognitive development. In addition to the uncertainty of the potential mechanism by which phthalates may elicit sex-specific effects, phthalates possess weak biological activity in vivo as compared to other environmental toxins such as PCBs, lead, and organophosphates, which may therefore explain the inconsistent results within human studies and between human and animal studies (Miodovnik, Edwards, Bellinger, & Hauser, 2014). There are also some limitations that are specific to our study that should be noted. First, our study consisted of 168 mother-infant pairs and this small sample size limits our statistical power, particularly in sex-specific models. It also made it difficult for us to assess whether co-exposures to other environmental chemicals including organophosphates, bisphenol A, and PCBs contribute to the observed effects. Second, spot urine measurements of phthalates metabolites may not accurately reflect long-term exposure because phthalates have elimination half-lives of approximately a few hours (Hoppin, Brock, Davis, & Baird, 2002). Therefore, we cannot rule out the possibility of exposure misclassification. Further, a study done by Gascon et al. (2015) demonstrated that phthalate exposure assessed using multiple urine measurements did not adversely affect children’s cognitive or psychomotor development. However, products containing phthalates are used continuously and we have demonstrated that ICCs from spot urine samples for some phthalate metabolites are fairly high (e.g., 0.77 for MBzP, where we see the strongest associations between exposure and novelty preference scores among girls). Further, any exposure misclassification is likely to be non-differential with respect to FTII score leading to results that are biased towards the null. Third, it is important to interpret the test results of the FTII with some caution. When conducting the FTII, the tester observes and is responsible for recording the infant’s length of visual fixation time by pressing the left or right button of a computer mouse (Fagan, 2005).
Depending on the training of the testers, the testers may not precisely record the length of visual fixation among infants. Even though we were unable to test the consistency of the findings among testers, testers were blinded to exposure status; therefore, it is unlikely that any measurement error was related to phthalate exposure. Lastly, it should be noted that the mother-infant pairs included in this study were African American and Dominican and therefore, the results of this study may not be generalizable to other populations. However, as we reported previously, phthalate metabolite concentrations in our population are slightly higher but overlap with those last reported in a representative sample of U.S. women from the National Health and Nutrition Examination Survey (Whyatt et al., 2012; Factor-Litvak et al. 2014; Zota et al. 2014).
In conclusion, this analysis provides evidence supporting the hypothesis that maternal prenatal urinary phthalate metabolite concentrations are negatively associated with visual recognition memory as measured by the FTII among infant girls. The findings from this study contribute to the growing evidence suggesting that exposures to some phthalates are associated with adverse neurocognitive effects in humans.
HIGHLIGHTS.
This study included 168 mothers who provided prenatal phthalate metabolite concentrations during the third trimester of pregnancy and infants that had completed the Fagan Test of Infant Intelligence at 27 weeks.
There were generally no associations between prenatal phthalate exposure and novelty preference score in the total cohort.
In sex-specific models, there were significant associations between some prenatal phthalate exposure and novelty preference among girls but not boys.
Acknowledgments
FUNDING SOURCES
NIEHS R01 ES013543, R01 ES013543, P01 ES009600, P30 ES009089
We acknowledge the technical assistance of M. Silva, E. Samandar, J. Preau, and J. Tao (Centers for Disease Control and Prevention, Atlanta, GA) in measuring the urinary concentrations of phthalate metabolites.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227(3):185–192. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, Hilsted L, … Main KM. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118(10):1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Jacobson JL, Carter RC, Kaplan-Estrin M, Ayotte P, … Jacobson SW. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect. 2014;122(3):310–316. doi: 10.1289/ehp.1206323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E, Wenzel A, Loos U. The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers. Mol Cell Endocrinol. 2005;244:75–78. doi: 10.1016/j.mce.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou R, Johnson S. Test of Non-verbal Intelligence: A Language-Free Measure of Cognitive Ability. Austin, TX: PRO-ED; 1990. [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; 2011. Retrieved from http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf. [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, Wolff MS. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522–528. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, Whyatt RM. Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years. PLoS One. 2014;9(12):e114003. doi: 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. Memory in the Infant. Journal of Experimental Child Psychology. 1970:9. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- Fagan J. The Fagan Test of Infant Intelligence Manual. 2005. [Google Scholar]
- Fagan J, Detterman D. The Fagan Test of Infant Intelligence: A Technical Summary. Journal of Applied Developmental Psychology. 1992;13:173–193. [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martinez D, Julvez J, … Vrijheid M. Prenatal exposure to phthalates and neuropsychological development during childhood. Int J Hyg Environ Health. 2015;218(6):550–558. doi: 10.1016/j.ijheh.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Gaultney J, Gingras J, Martin M, DeBrule D. Prenatal Cocaine Exposure and Infants’ Preference for Novelty and Distractibility. The Journal of Genetic Psychology. 2005;166(4) doi: 10.3200/GNTP.166.4.385-406. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal Variability of Urinary Phthalate Metabolite Levels in Men of Reproductive Age. Environmental Health Perspectives. 2004;112(17):1734. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210(5):623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hoppin J, Brock J, Davis B, Baird D. Reproducibility of Urinary Phthalate Metabolites in First Morning Urine Samples. Environ Health Perspect. 2002;110(5) doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell K, Wilson V, Furr J, Lambright C, Rider C, Blystone C. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague Dawley rat in a cummulative, dose additive manner. Toxicol Sci. 2008;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22(10):2715–2722. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Nishiyama N, Sugiyama S, Yamauchi K. The effect of endocrine disrupting chemicals on thyroid hormone binding to Japanese quail transthyretin and thyroid hormone receptor. Gen Comp Endocrinol. 2003;134(1):36–43. doi: 10.1016/s0016-6480(03)00197-7. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 Phthalate Metabolites in Urine Using Automated Sample Preparation and On-line Preconcentration/High-Performance Liquid Chromatography/Tandem Mass Spectrometry. Analytical Chemistry. 2005;77(9):2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, … Kim BN. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119(10):1495–1500. doi: 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang D, Zhoug Y, Ma M, Li J, Wang Z. Dibutyl Phthalate Contributes to the Thyroid Receptor Antagonistic Activity in Drinking Water Processes. Environ Sci Technol. 2010;44:6863–6868. doi: 10.1021/es101254c. [DOI] [PubMed] [Google Scholar]
- McCall R, Carriger M. A Meta-Analysis of Infant Habituation and Recognition Memory Performance as Predictors of Later IQ. Child Development. 1993;64(1):57–79. [PubMed] [Google Scholar]
- Miodovnik A, Edwards A, Bellinger DC, Hauser R. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology. 2014;41:112–122. doi: 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Perera F, Rauh V, Tsai W, Kinney P, Camann D, Barr D, … Whyatt R. Effects of Transplacental Exposure to Environmental Pollutants on Birth Outcomes in a Multiethnic Population. Environmental Health Perspectives. 2002;111(2):201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Ligocka D, Sobala W, Hanke W. Phthalate exposure and child development: The Polish Mother and Child Cohort Study. Early Hum Dev. 2014;90(9):477–485. doi: 10.1016/j.earlhumdev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S. Phthalates and Children’s Health. Curr Probl Pediatr Adolesc Health Care. 2008;38:34–49. doi: 10.1016/j.cppeds.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Tellez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, Meeker JD. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ. 2013;461–462:386–390. doi: 10.1016/j.scitotenv.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Franz C, Breous E, Loos U. Modulation of iodide uptake by dialkyl phthalate plasticisers in FRTL-5 rat thyroid follicular cells. Mol Cell Endocrinol. 2005;244(1–2):63–71. doi: 10.1016/j.mce.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, … Factor-Litvak P. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120(2):290–295. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G, Bucholskia A, Heinzowb B, Kramer U, Schmidtc E, Walkowiaka J, … Steingruber H. Developmental neurotoxicity of polychlorinated biphenyls PCBS.: cognitive and psychomotor functions in 7-month old children. Toxicoloy Letters. 1998;102:423–428. doi: 10.1016/s0378-4274(98)00334-8. [DOI] [PubMed] [Google Scholar]
- Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33(5):558–566. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: Findings from the National Health and utrition Examination Survey, 2001–2010. Environ Health Perspect. 2014;122:235–41. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]