Abstract
Cancer associated fibroblasts (CAFs) comprise the majority of the tumor bulk of pancreatic adenocarcinomas (PDACs). Current efforts to eradicate these tumors focus predominantly on targeting the proliferation of rapidly growing cancer epithelial cells. We know that this is largely ineffective with resistance arising in most tumors following exposure to chemotherapy. Despite the long-standing recognition of the prominence of CAFs in PDAC, the effect of chemotherapy on CAFs and how they may contribute to drug resistance in neighboring cancer cells is not well characterized. Here we show that CAFs exposed to chemotherapy play an active role in regulating the survival and proliferation of cancer cells. We found that CAFs are intrinsically resistant to gemcitabine, the chemotherapeutic standard of care for PDAC. Further, CAFs exposed to gemcitabine significantly increase the release of extracellular vesicles called exosomes. These exosomes increased chemoresistance-inducing factor, Snail, in recipient epithelial cells and promote proliferation and drug resistance. Finally, treatment of gemcitabine-exposed CAFs with an inhibitor of exosome release, GW4869, significantly reduces survival in co-cultured epithelial cells, signifying an important role of CAF exosomes in chemotherapeutic drug resistance. Collectively, these findings show the potential for exosome inhibitors as treatment options alongside chemotherapy for overcoming PDAC chemoresistance.
Keywords: pancreatic, cancer, exosomes, chemoresistance, stroma, fibroblasts
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) has a dismal 5-year survival rate of less than 7% (1). PDAC is currently the fourth leading cause of cancer-related deaths in the United States and is predicted to become the second leading cause of cancer-related deaths by 2030 (2). The poor prognosis of the disease is associated with late detection, aggressive tumor biology, and poor response to available therapies. Gemcitabine, a nucleoside analog, is the standard chemotherapeutic agent for adjuvant therapy of resectable PDAC and a commonly used agent in other treatment settings, including neoadjuvant treatment of borderline resectable PDAC and palliative treatment of metastatic PDAC. Despite gemcitabine being one of the most commonly used, single chemotherapeutic agents in pancreatic cancer, response rates are poor. The vast majority of patients (74%) receiving adjuvant gemcitabine eventually show tumor recurrence (3). This dismal prognosis shows an urgent need for greater understanding of the complete biology of PDAC in order to develop effective therapeutic strategies.
Current therapies focus predominantly on targeting the proliferation of the rapidly growing epithelial cancer cells. However, many cells types, including supporting cells called fibroblasts, contribute to the microenvironment surrounding cancer cells. Remarkably, the majority of the tumor bulk of PDACs consists of fibroblasts (4). Fibroblasts may inhibit or foster tumor development (5). They were previously believed to serve merely a passive role in PDAC drug resistance, impeding drug delivery by physically blocking cytotoxic chemotherapeutics from reaching their target epithelial cells (6, 7). This led to the development of fibroblast-depleting therapies. Unfortunately, these therapies showed either very small increases in survival (8) or more aggressive tumors (9). These results show a need to better understand how fibroblasts react to chemotherapy and how they may contribute to drug resistance, instead of merely depleting fibroblasts, in order to devise effective treatment strategies.
Recent studies have shown that exosomes, secreted membrane vesicles that range in size from 30–100 nm in diameter (10, 11), released from fibroblasts have been found to increase invasive behavior (12) and therapy resistance pathways (13) in breast cancer cells. Exosomes contain mRNA, DNA, proteins, and are enriched with miRNA (14). Several studies have shown that exosomal-derived miRNAs promote metastases (15) and enhance endothelial cell migration (16). Yet no studies have examined the effect(s) of exosomes derived from fibroblasts exposed to chemotherapy.
In this study, we show that cancer-associated fibroblasts (CAFs) are innately chemoresistant and play an active role in regulating the chemoresistance of cancer cells. CAFs exposed to gemcitabine dramatically increase the release of exosomes that increased cell proliferation and survival in recipient epithelial cancer cells. Mechanistically, we demonstrated that the expression of Snail (SNAI1) as well as the Snail target, microRNA-146a, was increased in the exosomes of gemcitabine-treated CAFs. Furthermore, suppressing CAF exosome release in vitro reduced Snail expression in co-cultured epithelial cancer cells and reduced survival of drug-resistant cancer cells, suggesting that blocking exosome communication may be a promising new therapeutic strategy for patients receiving gemcitabine-based treatment regimens.
RESULTS
Pancreatic Fibroblasts are Innately Chemoresistant
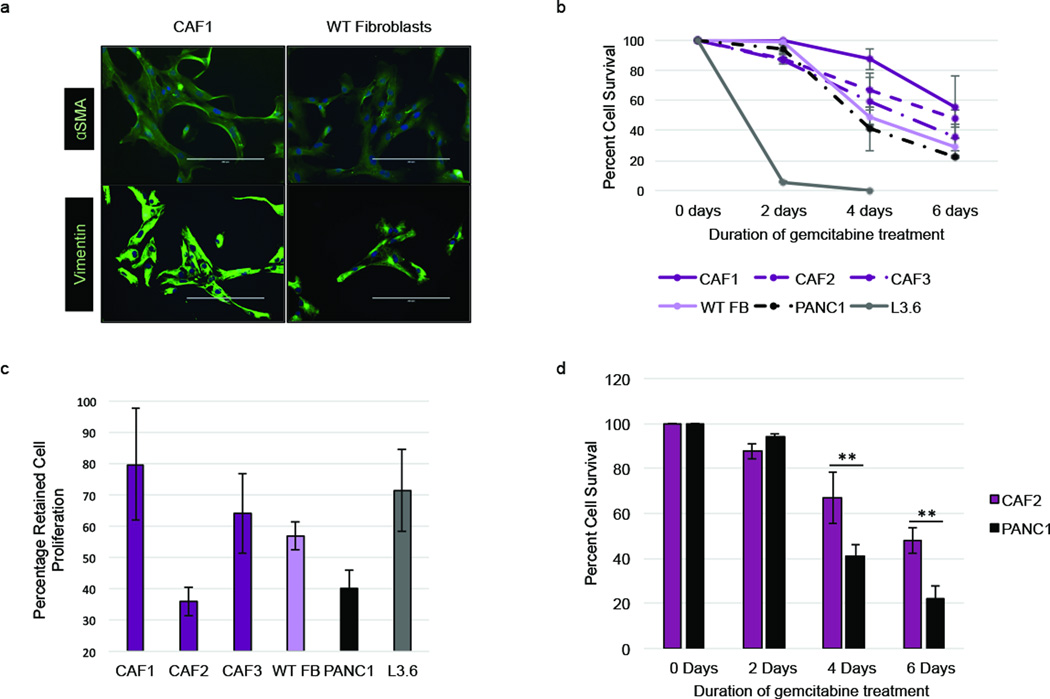
We first compared the innate drug resistance of cancer-associated fibroblast (CAF) cell lines created from patient-derived tumor samples with that of epithelial cancer cell lines. Patient-derived fibroblasts were grown out of tumor samples obtained from patients who had undergone surgical resection. The CAFs displayed an elongated, mesenchymal morphology, and stained positively for fibroblast markers vimentin and α-SMA (17) (Figure 1a). Sequencing revealed no KRAS mutation, indicating that these CAF cell lines were truly of fibroblast origin (Supplementary Figure S1). CAFs and normal fibroblasts had greater survival rates than chemoresistant epithelial cells (PANC1) and chemosensitive epithelial cells (L3.6) when treated with the same dosage of the chemotherapeutic agent, gemcitabine (GEM) (Figure 1b). Having shown that CAFs are resistant to GEM, we next assessed if the increased survival of CAFs exposed to GEM could be a result of CAFs undergoing senescence and not incorporating the drug. Therefore, we analyzed cell proliferation of GEM-treated CAFs and epithelial cells. The most chemoresistant CAF cell line, CAF1, also retained the most proliferation during GEM treatment, while the second leading resistant CAF cell line, CAF2, showed dramatically decreased proliferation (Figure 1c). To further elucidate the role of proliferation on chemoresistance, we compared the survival rate of CAFs and epithelial cells with similar proliferation rates (CAF2 and PANC1 cell lines, respectively). Although CAF2 and PANC1 cells both demonstrate a relatively low proliferation rate following exposure to GEM, CAF2 cells still showed more than a 2-fold higher cell survival rate compared to PANC1 cells following GEM treatment (Figure 1d). Taken together, these data demonstrate that fibroblasts have an innate resistance to GEM instead of a growth-dependent resistance mechanism.
Figure 1.
Pancreatic fibroblasts are innately chemoresistant. (a) Immunofluorescence stain for αSMA and vimentin of cancer-associated fibroblasts (CAF1) and wild-type (WT) fibroblasts. (b) Cells were treated with 1µM gemcitabine for 2–6 days and live and dead cells were counted to obtain percent cell survival. (c) Cells were treated with 1µM gemcitabine for 2 days or left untreated and total cells were counted to obtain percentage of proliferation retention during GEM treatment. (d) Percent cell survival of CAFs (CAF2) and epithelial cells (PANC1) with similar proliferation retention rate over 6 days 1µM gemcitabine treatment. **p-value<0.01
Pancreatic CAF-Conditioned Media Increases Proliferation and Survival of Epithelial Cancer Cells
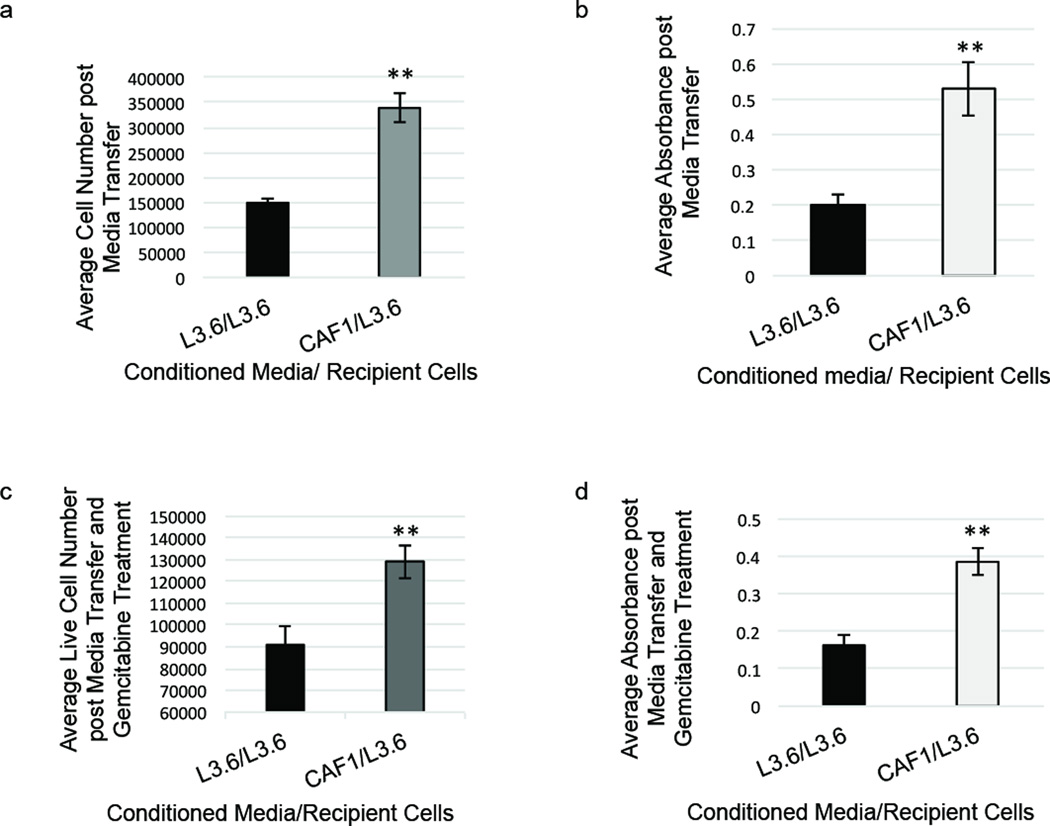
Considering the important role of cell extrinsic factors on cell growth and survival, we next assessed whether factors secreted by the innately chemoresistant fibroblasts could affect proliferation and survival of epithelial cancer cells. We first determined the effect CAF-conditioned media had on the proliferation of chemosensitive L3.6 cells. An equivalent number of L3.6 or CAF cells were plated and incubated in DMEM for 24 hours. Conditioned cell media from either the L3.6 or the CAF cells was then transferred onto recipient L3.6 cells each day for six days. CAF-conditioned media increased proliferation of L3.6 cells by more than 50% compared to L3.6-conditioned media (Figure 2a–b). Having demonstrated that media from GEM-resistant CAF cells could increase the proliferation of GEM-sensitive L3.6 cells, we next assessed if this effect was CAF specific or if GEM-resistant epithelial cancer cells could also elicit this change in proliferation. We observed that conditioned media from the chemoresistant PANC1 epithelial cancer cell line did not elicit a significant increase in proliferation (Supplementary Figure S2). Next, we determined if CAF-conditioned media also affected the chemoresistance of epithelial cells. L3.6 cells were grown in either L3.6 or CAF cell-conditioned media for 6 days then treated with 100nM gemcitabine for 3 days, and cell survival was assessed. We observed that L3.6 cells grown in CAF-conditioned media and subsequently treated with gemcitabine showed a significant increase in cell survival compared to L3.6 cells grown in L3.6 conditioned media (Figure 2c–d). Taken together, these data show that CAF-secreted factors affect proliferation and drug resistance of epithelial cancer cells.
Figure 2.
Pancreatic CAF1-conditioned media increases proliferation and survival of epithelial cells. (a) L3.6 cells were grown in CAF1-conditioned media or L3.6-conditioned media for 8 days and total cells were counted. (b) Cell proliferation assay (MTT assay) was performed after 8 days L3.6 cell growth in conditioned media. (c) L3.6 cells were grown in cell-conditioned media for 6 days then treated with 100nM gemcitabine for 3 days, and live cells were counted. (d) Cell proliferation assay (MTT) was performed after 6 days L3.6 cell growth in conditioned media and 3 days of 100nM gemcitabine treatment. **p-value<0.01
Gemcitabine Increases CAF Exosome Secretion
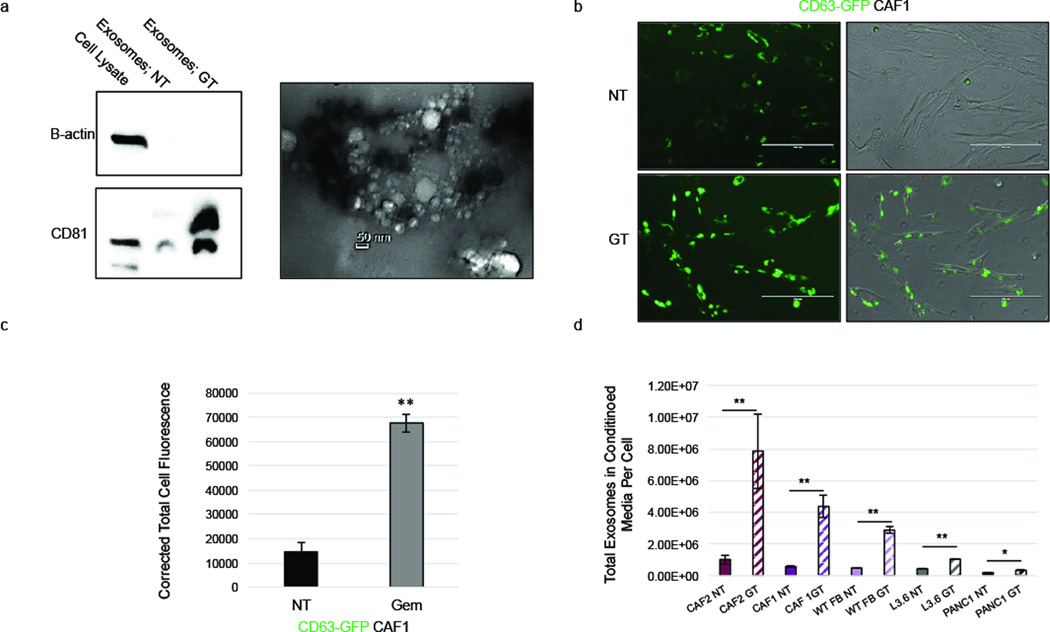
Exosomes are cell-derived vesicles that can serve as important intercellular regulators of many oncogenic properties (18). Given that CAF-conditioned media regulates proliferation and drug resistance of epithelial cancer cells, we asked whether CAF-derived exosomes might contribute to this effect. First, exosomes were purified from the conditioned media of CAF cells and their size and structure was confirmed using transmission electron microscopy as well as particle size analysis. Particles ranged from ~20nm to ~80nm in diameter, indicative of exosomes (19), and immunoblotting of purified exosomes revealed expression of CD81, a known exosome marker (20) (Figure 3a; Supplementary Figure S3). We next assessed the effect gemcitabine treatment had on exosome release in CAFs. Samples obtained from the media of gemcitabine-treated CAFs displayed a more intense CD81 band by immunoblotting than untreated CAFs, suggesting a greater number of exosomes were present in the media of GEM treated CAFs (Figure 3a). To investigate this further, CAFs were transduced with a lentiviral vector that allowed the visualization of exosomes by labeling CD63, a known exosome marker (20), with Green Fluorescent Protein (GFP). Upon gemcitabine treatment, CAFs displayed increased levels of GFP expression (Figure 3b–c), suggesting that GEM treatment caused augmented levels of CD63 expression, potentially from increased exosome production. To confirm this possibility, conditioned media was collected from untreated and gemcitabine treated CAF cell lines and epithelial pancreatic cancer cell lines, and the number of exosomes was quantified. While all cell lines increased exosome secretion upon gemcitabine treatment, pancreatic CAFs and wild type fibroblasts displayed the largest increase in exosome release, significantly increasing the amount of exosomes found in the media more than 7-fold following gemcitabine treatment (Figure 3d). Having shown that gemcitabine caused increased exosome release from CAF cell lines, we next assessed if this effect was observed in CAFs treated with nab-paclitaxel, an agent that is now commonly used along with gemcitabine (8). CAFs treated with nab-paclitaxel also showed a significant increase in released exosomes compared to untreated controls, but to a lesser extent than the release observed following gemcitabine treatment (Supplementary Figure S4). These data reveal that CAFs increase exosome secretion during chemotherapy treatment.
Figure 3.
Gemcitabine increases CAF exosome secretion. (a) CAF1s were left untreated (NT) or treated with 1µM gem (GT) for 4 days. Exosomes were isolated from conditioned cell media, and protein lysates were used to perform a western blot for CD81 and beta-actin (left). Isolated exosomes were examined for size and structure via transmission electron microscopy (right). (b) CAF1 cells transduced with a GFP-CD63 lentivirus (GFP-CD63-CAF1s) were treated with 1uM gemcitabine (GT) or left untreated (NT) and fluorescence was analyzed via microscopy. (c) Total corrected cell fluorescence of GFP-CD63-CAF1 cells was quantified using ImageJ. (d) Cells were treated with 1µM gemcitabine (Fibroblasts and PANC1), 10nM gemcitabine (L3.6), or left untreated for 4 days (NT), and exosomes were collected and quantified. Scale bar, 200 µm. *p-value<0.05; **p-value<0.01
CAF Exosomes Increase Proliferation and Survival of Cancer Epithelial Cells
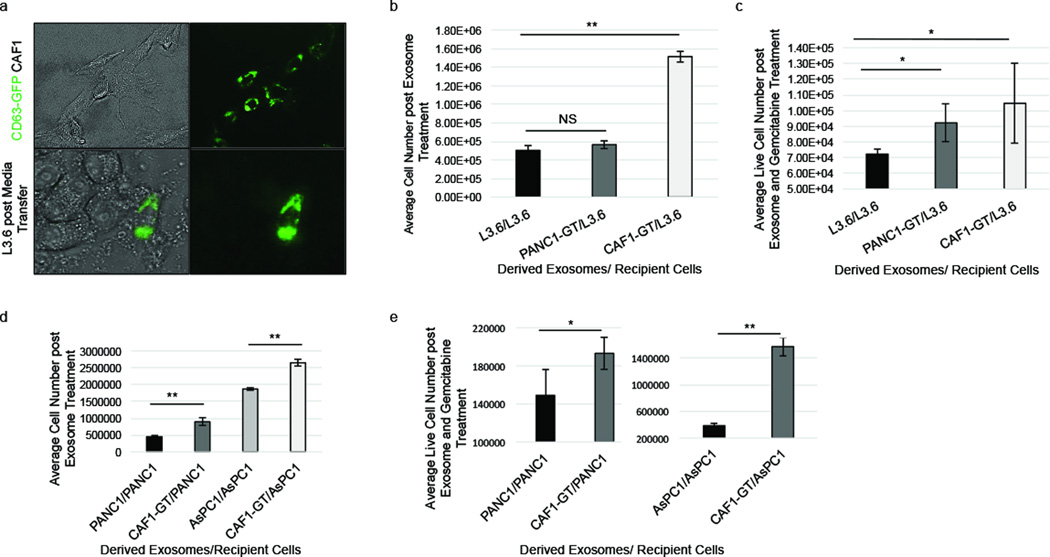
To determine if these exosomes released by gemcitabine-treated CAFs could affect epithelial cell behavior we first tested if epithelial cells could naturally take up CAF-derived exosomes. We collected media from CAFs transduced with a GFP-CD63 lentivirus (GFP-CD63-CAFs). This media was incubated on L3.6 cells for 48 hours, and the cells were imaged. We observed that L3.6 cells, as well as several other PDAC cell lines, cultured with media from GFP-CD63-CAFs were positive for GFP-expression, indicative of exosome uptake (21) (Figure 4a; Supplementary Figure S5).
Figure 4.
GT-CAF exosomes increase cell number and survival of epithelial cells. (a) L3.6 cells were treated with GFP-CD63-CAF1 conditioned media for 48 hours and exosome uptake was visualized. (b) L3.6 cells were treated with L3.6, GT-PANC1, or GT-CAF1 exosomes for 6 days and total cells were counted. (c) L3.6 cells were treated with L3.6, GT-PANC1 or GT-CAF1 exosomes for 6 days and 1µM GEM for 3 days, and live cells were counted. (d) PANC1 cells were treated with PANC1 or GT-CAF1 exosomes for 6 days. AsPC1 cells were treated with AsPC1 or GT-CAF1 exosomes for 6 days. Total cells were counted. (e) PANC1 cells were treated with PANC1 or GT-CAF1 exosomes for 6 days, and AsPC1 cells were treated with AsPC1 or GT-CAF1 exosomes for 6 days. All cells were then treated with 1µM GEM for 3 days, and live cells were counted. *p-value<0.05; **p-value<0.01
Having confirmed that epithelial cells could uptake CAF-derived exosomes, we next studied the effect gemcitabine-treated CAF exosomes have on cell behavior in recipient cells. Equivalent numbers of CAFs were plated in exosome free media and then treated with PBS or 1µM gemcitabine for the same duration of time. Exosomes were then isolated from the CAF cell conditioned media and added to the epithelial cancer cell lines. Exosomes derived from gemcitabine-treated (GT) CAFs significantly increased epithelial cell proliferation and chemoresistance more than exosomes derived from untreated CAFs (Supplementary Figure S6). Therefore, we further assessed the role of GT-CAF-derived exosomes in regulating epithelial cell proliferation and chemoresistance in both chemosensitive and chemoresistant PDAC cell lines. GT-CAF exosomes increased both proliferation (Figure 4b) and survival (Figure 4c) of chemosensitive epithelial L3.6 cells. In contrast, exosomes from GT-chemoresistant-epithelial-PANC1 cell exosomes did not elicit the same response (Figure 4b–c). Further, GT-CAF exosomes also increased proliferation and survival of chemoresistant PANC1 and AsPC1 cells (Figure 4d–e). Thus, these data indicate that exosomes released by gemcitabine-treated CAFs are capable of increasing proliferation and survival in recipient epithelial cells.
Pancreatic CAFs Alter Snail and miR-146a Expression during Gemcitabine Treatment
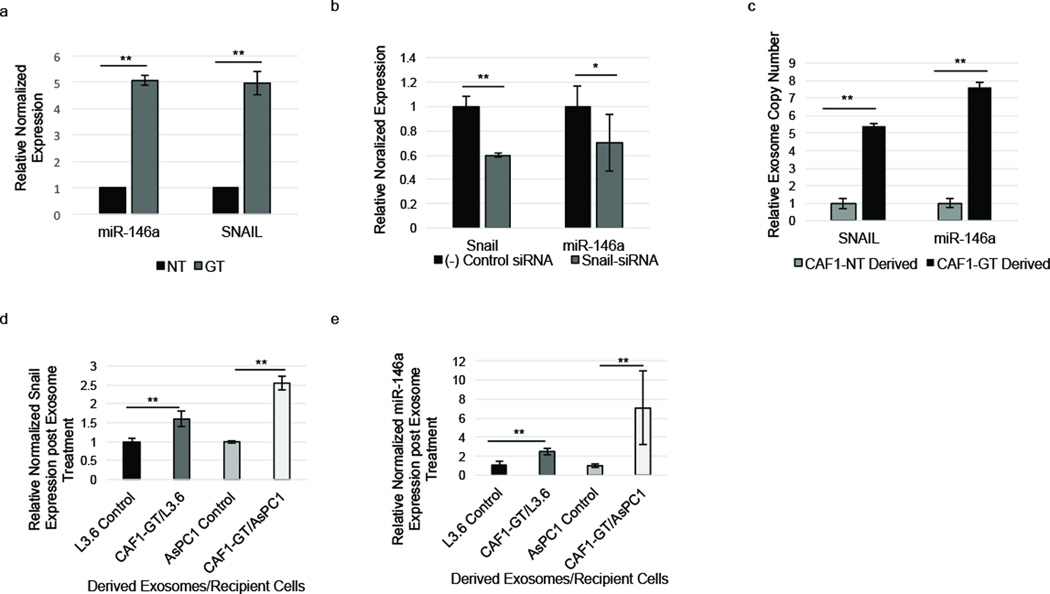
Prompted by these results, we next sought to investigate the molecular mechanism responsible for the ability of exosomes derived from gemcitabine-treated CAFs to increase proliferation and survival in recipient epithelial cells. To elucidate this, we first sought to determine how pancreatic fibroblasts are innately chemoresistant. CAFs were treated with gemcitabine, and the expression levels of miRNAs were analyzed via miRNA-Seq. We observed that fibroblasts showed a marked increase in microRNA-146a (miR-146a) expression following gemcitabine treatment (Supplementary Table S1). To further investigate this, we utilized RT-PCR to determine the expression of miR-146a in CAFs treated with gemcitabine compared to untreated CAFs. Gemcitabine-treated CAFs demonstrated significantly higher expression of miR-146a compared to untreated CAFs (Figure 5a). miR-146a is directly regulated by the promoter binding transcription factor, Snail, which promotes chemoresistance, EMT, and metastasis (22). Therefore, we investigated if gemcitabine treatment of CAFs caused increased expression of Snail. Indeed, gemcitabine exposure also increased Snail expression in CAFs (Figure 5a). Furthermore, knockdown of Snail expression using siRNA caused downregulation of miR-146a expression in CAFs (Figure 5b). These data establish a gemcitabine-induced upregulation of the Snail pathway in CAFs.
Figure 5.
Pancreatic fibroblasts upregulate and secrete miR-146a and Snail during gemcitabine treatment. (a) RT-PCR. miR-146a and Snail levels were altered in CAF1s during 1 µM GEM treatment (GT) (3 days) compared to untreated control (NT). (b) RT-PCR. CAF1s were treated with Snail-siRNA, and Snail and miR-146a expression was measured compared to negative siRNA control treated CAFs. (c) Exosomes from untreated and 1µM GEM-treated CAF1s were isolated and Snail mRNA and miR-146a within CAF1 exosomes was quantified via RT-PCR using relative Ct values. (d–e) L3.6 cells were treated with GT-CAF1 exosomes for 6 days (GT-CAF1/L3.6) or left untreated (L3.6 control). AsPC1 cells were treated with GT-CAF1 exosomes for 6 days (GT-CAF1/AsPC1) or left untreated (AsPC1 control). Snail (d) and miR-146a (e) levels were quantified in recipient cells via RT-PCR. *p-value<0.05; **p-value<0.01
Gemcitabine Increases the Secretion of Both miR-146a And Snail through Pancreatic CAF Exosomes
Since GEM upregulates Snail and miR-146a expression in PDAC CAFs and GT-CAF exosomes increase proliferation and survival in epithelial cells, we next investigated if Snail and miR-146a could be delivered to cells through GT-CAF exosomes. Since exosomes contain both microRNAs and mRNA (14) we first tested if both miR-146a and Snail mRNA were increased in GT-CAF-derived exosomes. We observed that gemcitabine treatment increased the amount of both miR-146a and Snail mRNA in CAF-derived exosomes (Figure 5c). Furthermore, the addition of GT-CAF exosomes to epithelial cell media also increased the levels of Snail mRNA (Figure 5d) and miR-146a (Figure 5e) within recipient chemosensitive and chemoresistant epithelial cells. Thus, our data reveal that exosomes produced by gemcitabine-treated CAFs augment Snail and miR-146a levels in epithelial cells.
Blocking CAF Exosome Secretion Reduces PDAC Cell Survival
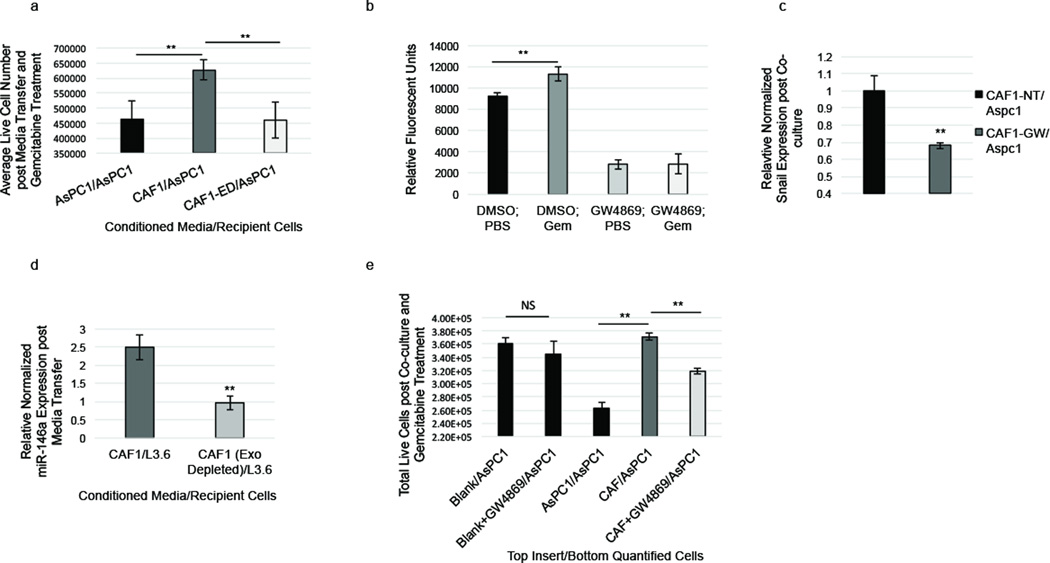
Having shown that chemotherapy treatment of CAFs causes the increased secretion of chemoresistance-inducing exosomes, and increased cell survival, we next assessed whether blocking exosome secretion would sensitize PDAC cells to gemcitabine. First, we assessed whether physically removing exosomes from CAF-condition media would affect its ability to increase cell survival in epithelial cells following exposure to gemcitabine. AsPC1 cells were cultured in CAF-conditioned and exosome-depleted CAF-conditioned media. Exosome-depleted conditioned media was spun down at 120,000× g to pellet exosomes without depleting the media of proteins. We observed that culturing AsPC1 cells in CAF-conditioned media, compared to AsPC1-conditioned media, led to greater survival rate during GEM treatment; however, this survival rate was significantly reduced when exosomes in CAF-conditioned media were removed (Figure 6a). Having shown that physically removing exosomes from CAF-conditioned media reduced its ability to increase chemoresistance, we next sought to determine if a compound that pharmacologically blocks exosome secretion would elicit the same effect. Therefore, the drug, GW4869, which blocks exosome secretion (23), was utilized to block CAF exosome release in vitro and determine its impact on epithelial cell survival. First, we determined if GW4869 could successfully block exosome secretion in CAFs. We found that GW4869 decreased CAF exosome secretion by ~70% in vitro in both untreated and gemcitabine treated CAFs (Figure 6b). Furthermore, we found that depletion of exosomes from CAF-conditioned media, using GW4869 treatment or centrifugation, significantly reduced expression of both Snail (Figure 6c) and miR-146a (Figure 6d) in recipient epithelial cells receiving the CAF-conditioned media. Next, we utilized co-culture studies to assess if GW4869 treatment of CAFs would affect cell survival in recipient epithelial cells. CAFs were plated on permeable inserts above chemoresistant or chemosensitive epithelial cells. While cells co-cultured with CAFs showed a significantly increased survival rate following exposure to gemcitabine, blocking CAF exosome secretion using GW4869 treatment significantly reduced this survival benefit in multiple cell lines (Figure 6c; Supplementary Figure S7).
Figure 6.
Inhibition of CAF exosome signaling suppresses chemoresistance. (a) AsPC1 cells were grown for 5 days in AsPC1-conditioned media (AsPC1/AsPC1), CAF1-conditioned media (CAF1/AsPC1) or CAF1-conditioned media depleted of exosomes (CAF1-ED/AsPC1) and then treated with 1µM GEM for 3 days and live cells were counted. (b) CAF1s were treated with 20µm GW4869 or DMSO along with 1µM gemcitabine or PBS for 3 days. Exosomes in media were collected, dyed with CFSE, and quantified. (c) AsPC1 cells were co-cultured with AsPC1 cells, CAFs, GW4869-treated CAF1s, DMEM alone (Blank/AsPC1), or GW4869 in DMEM (Blank+GW4869) for 6 days then treated during co-culture with 1µM gemcitabine for 3 days. Live co-cultured AsPC1 cells at the bottom of the plate were counted. (d) Snail expression was measured via RT-PCR in AsPC1 cells co-cultured with untreated CAFs (CAF-NT/AsPC1) or GW4869-treated CAFs (CAF-GW/AsPC1). (e) L3.6 cells were cultured in CAF1-conditioned (CAF1/L3.6) media or CAF1-conditioned media depleted of exosomes (CAF1-ED/L3.6). miR-146a expression was measured via RT-PCR. **p-value<0.01
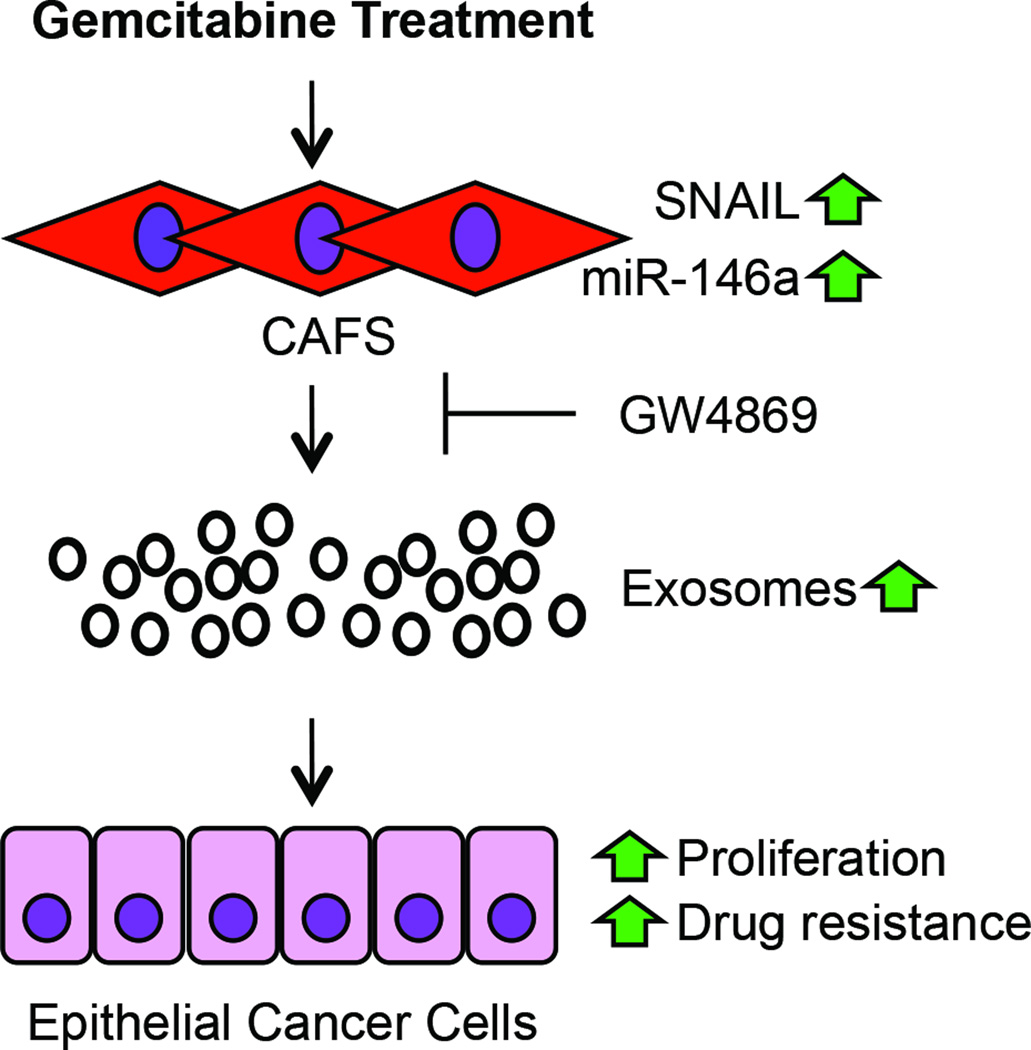
Lastly, we utilized a subcutaneous model to determine the effect of blocking exosome secretion with GW4869 on tumor growth in vivo. CAFs and AsPC1 cells were subcutaneously implanted into NOD/SCID mice and treated with PBS (control), gemcitabine, or GW4869 alongside gemcitabine (100mg/kg body weight) twice weekly for two weeks. Tumors of control mice and mice treated with gemcitabine steadily increased in size over time, while tumors of mice given combination therapy (GW4869 and gemcitabine) remained relatively the same size, displaying significantly reduced growth rate 10 days after treatment compared to control (Supplementary Figure S8). Taken together, these data suggest that GT-CAF derived exosomes are a key regulator of PDAC cell chemoresistance; however, blocking exosome release may circumvent increased chemoresistance caused by exosome-mediated signaling (Figure 7).
Figure 7.
Schematic overview of CAF exosome signaling during gemcitabine treatment. Gemcitabine treatment leads to upregulation of Snail and miR-146a as well as exosome secretion in CAFs that could lead to increased cell proliferation, tumor growth, and chemoresistance of adjacent cancer epithelial cells. GW4869 suppresses exosome release and therefore exosomal transfer of Snail and miR-146a.
DISCUSSION
In this study, we show that exosomes from CAFs exposed to chemotherapy are critical regulators of epithelial cancer cell proliferation and survival. We first established that CAFs are innately resistant to gemcitabine. Next, we determined that CAF-conditioned media supported epithelial cell growth and survival during gemcitabine treatment. More importantly, we demonstrate that gemcitabine-treated CAFs prolifically secrete exosomes that contain chemoresistance-promoting factors like mRNA and microRNA to recipient epithelial cells. miR-146a levels were highly increased in CAFs during gemcitabine treatment and found within these exosomes, along with mRNA of its upstream transcription factor, Snail. PDAC epithelial cells treated with gemcitabine-treated CAF exosomes displayed increased levels of Snail mRNA, increased proliferation, and increased chemoresistance. Finally, reduction of exosome release suppressed the chemoresistance promoting abilities of CAF cells.
Gemcitabine is the standard of care for adjuvant therapy of resectable PDAC and is still one of the most commonly used chemotherapeutic agents used in combination therapy. However, 74% of patients receiving adjuvant gemcitabine eventually relapse (3). Moreover, gemcitabine has already proven to be a paradoxical drug as it not only promotes Snail expression, but also triggers NFκB activation, CXCR4 expression, induction of reactive oxygen species, upregulation of cancer-stem cell markers and AKT activity. Together, these undesired side effects lead to increased chemoresistance and cell motility (24–28). We show a previously unknown tumor-promoting side effect of gemcitabine treatment. In our study we observe that gemcitabine treatment causes CAFs to greatly increase the secretion of chemoresistance-promoting exosomes. This evidence suggests that gemcitabine may promote chemoresistance through both cell intrinsic and cell extrinsic mechanisms. Gemcitabine was previously shown to upregulate expression of the transcription factor Snail in PDAC cancer cells (29). Inhibiting Snail suppresses tumor growth, metastasis, and chemoresistance in many cancers, including PDAC (29, 30). Our data, showing gemcitabine increases Snail expression in pancreatic CAFs, provides new evidence that Snail plays a role in mediating chemoresistance in pancreatic cancer fibroblasts in addition to pancreatic cancer epithelial cells. Further, our data showing that gemcitabine induces hypersecretion of Snail mRNA through exosomes is a previously unknown potential mechanism of cell-extrinsic chemoresistance.
Recent studies show oncogenic exosomes can promote invasion (12), deliver oncogenic DNA to normal cells (31), increase drug resistance of cancer cells (32, 33), and can prime distant organs for metastasis (34–36). Recent data show that that exosomes from cancer-associated fibroblast play a role in promoting chemoresistance in colorectal and breast cancer cells (32, 33). In our study, we show new evidence that gemcitabine treatment of cancer-associated fibroblasts causes increased release of chemoresistance promoting exosomes. Based on evidence that the tumor bulk in PDAC is comprised mostly of fibroblasts (4), it is possible that the role of CAF-derived exosomes may be even more important in pancreatic cancer than other cancers. Our data supports the exploration of using exosome secretion inhibitors in combination with currently approved therapeutic regimens to combat chemoresistance. GW4869 was shown to greatly diminish CAF exosome secretion as well as alleviate chemoresistance in co-cultured cancer cells in vitro and contributed to suppressed tumor growth in vivo. Further in vivo studies are needed to fully explore the potential benefit of using GW4869 to increase efficacy of combination treatments. This would include testing GW4869 and various chemotherapy combinations on autochthonous PDAC mouse models and monitoring tumor growth and survival.
In conclusion, we show that fibroblasts exposed to chemotherapy play an active role in promoting proliferation and chemoresistance of cancer cells through exosome signaling. The role of exosomes in PDAC chemoresistance is an area that requires much elucidation. Currently available therapeutic regimens may have greater efficacy when treatments designed to inhibit exosome secretion are utilized.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Commercial pancreatic cancer epithelial cell lines were purchased from ATCC. Dr. Timothy Donahue (University of California, Los Angeles) provided L3.6pl cells (37). Fibroblast cell lines were generously gifted by Dr. Melissa Fishel of IU Simon Cancer Research Center. Briefly, patient-derived PDAC tumor tissue was minced into 1–3mm fragments, trypsinized for 30 minutes, washed in DMEM with 10% FBS, plated in a petri dish with DMEM containing 10% FBS, and fibroblasts were allowed to grow out of tumor fragments for 2–3 weeks. Cells were infected and immortalized with hTERT by the Hanenberg Lab (IUPUI). Cells were authenticated by IDEXX RADIL™ and were found to be mycoplasma free and did not genetically match any cell line in the DSMZ database. Cells used in experiments ranged from passage 2–10. Fibroblast nomenclature was reduced for purposes of simplicity with “CAF1” referring to UH1301-63 cells, “CAF2”referring to UH1303-02 cells, and “CAF3” referring to UH1303-49 cells. SC00A5 cells are wild-type pancreatic fibroblasts purchased from VitroBioPharma. Cells were grown in culture according to standard procedures and protocols with DMEM (Sigma) or RPMI (Sigma) supplemented with 10% FBS (RMBI) and 1% Pen-Strep (Life Technologies). L3.6 and fibroblast cells were grown in DMEM with 10% FBS and 1% Pen-Strep. Cells were tested throughout studies for mycoplasma using the MycoAlert™ kit (Lonza).
Lentivirus Transduction
CD63-GFP labeled fibroblasts were created using the pCT-CD63-GFP (pCMV, Exosome/Secretory, CD63 Tetraspanin Tag, plasmid DNA) HIV lentiviral vector purchased from System Biosciences. Briefly, 50,000 cells were plated per well of a 6-well plate and lentivirus was added 24 hours later utilizing an MOI of 20. Cells were left undisturbed for 48 hours then moved to a flask and treated with puromycin (1:1000) until all remaining cells were fluorescent and thereafter cultured normally.
Exosome Collection, Detection, and Quantification
Collection
Cells were grown in exosome-free media. Media from cells was collected, cells were washed with 3ml PBS, and PBS was collected. Media and PBS together was spun down at 1,200 RPM for 5 minutes, supernatant was spun down at 16,500× g for 20 minutes, and exosomes in supernatant were isolated with the ExoQuick-TC™ system according to System Bioscience’s protocol: https://www.systembio.com/downloads/Manual_ExoTC_WEB.pdf. Exosome pellets were resuspended PBS.
Transmission Electron Microscopy
Exosomal protein was measured via a BCA assay, and 10µg exosomes were placed on copper grid. Exosomes were wicked off to create a thin layer prior to addition of a thin layer of 2% uranyl acetate in water. Grids were allowed to dry overnight, and TEM performed the next day
Particle Size Analysis
Exosomes were resuspended in PBS and particle sizes were measured using the Beckman Coulture® Delsa™ Nano S Particle Analyzer which uses the Brownian motion of particles and dynamic light scattering to measure particle diameters.
Western Blot
Exosomes were isolated with ExoQuick-TC™ from 20ml of cell-conditioned media and protein blotting was performed according to the manufacture’s protocol and standard procedures: https://www.systembio.com/downloads/Manual_ExoTC_WEB.pdf.
Quantification
Exosomes were quantified using light transmission spectroscopy, as previously described (38) measuring number of particles 30–150nm in diameter. For relative quantification exosome pellets were resuspended in 200µl PBS and incubated with 10µM CFSE at 37°C for 2 hours. Relative fluorescent units were measured via the Molecular Devices SpectraMax® M3 Multi-Mode Microplate Reader. Total corrected cell fluorescence used to quantify CD63-GFP labeled cells was obtained using ImageJ, wherein corrected total cell fluorescence=integrated density-(area of fluorescent cells × mean of background fluorescence)
In Vitro Exosome Transfer, Media Transfer, and Co-culture
Exosome Treatment
Cells were plated at 500,000 cells/flask in exosome-free media and were either treated with 1µM gemcitabine or PBS for 4 days. Exosome pellets were collected with ExoQuickTC (see protocol link above) and resuspended in 400µl PBS. Recipient cells were plated in 6-well plates and treated with new media and10µl exosomes per day for 6 days.
Media Transfer
Both donor and recipient cells were plated at 60,000 cells per well in 6 well plates, the donor media was spun down at 1,200 RPM for 5 minutes, and the supernatant was transferred to recipient cells each day for 6 days. Cells were then treated with gemcitabine or PBS for 3 days prior to cell quantification. In studies with AsPC1 cells, CAFs and AsPC1 cells were plated at one million cells cells/flask in exosome free media and conditioned media was collected and spun down at either 16,500× g for 20 minutes or 16,500× g for 20 minutes as well as 120,000× g for 70 minutes to deplete the media of exosomes without depletion of proteins. Cells were grown in conditioned media for four days, treating with new conditioned media each day, then treated with 1µM gemcitabine for three days while in conditioned media prior to quantification of live cells.
Co-culture
Cells were plated in a 12-well plate and on 12-well Transwell® polyester permeable supports (Corning) with 0.4µm pore size. Cells were co-cultured for 3–6 days while cells on the permeable support were treated with GW4869 (20µM) or DMSO, and then cells below the support were treated with gemcitabine (100nM–1µM) for 3 days prior to quantification of live cells. The number of cells plated per well and concentration of gemcitabine used was dependent upon the previously determined chemosensitivity of the cell lines (39) and cell proliferation rate. Cells on permeable supports were treated with 20µM GW4869 or DMSO once every 3 days. Permeable supports of the control group received either media alone or media + GW4869. Epithelial cells treated with CD63-GFP-labelled CAF-conditioned media were incubated in new conditioned media each day for two days prior to washing the cells and imaging. CD63-GFP CAFs were grown in phenol red free media for 24 hours, media was spun at 16,500× g for 20 minutes, and supernatant was placed onto recipient cells.
RT-PCR and RNA Collection
RNA was collected using TRIzol® according to standard protocol: https://tools.thermofisher.com/content/sfs/manuals/trizol_reagent.pdf. RT-PCR was performed according to the QIAGEN provided protocol. QIAGEN SYBR® Green QuantiFast RT-PCR kit and protocol was utilized for quantification of mRNA, and QIAGEN miScript II RT Kit and protocol along with QIAGEN miScript SYBR® Green PCR Kit and protocol was utilized for quantification of miRNA with the Bio-Rad CFX Connect™ Real-Time PCR Detection System. miRNA and mRNA primer assays from QIAGEN were utilized. mRNA was normalized to GAPDH and miRNA was normalized to RNU6. Exosomal RNA was collected with the ExoQuick-TC™ reagent and TRIzol® RNA isolation method. Exosome pellets were incubated in TRIzol® for 1–2 hours prior to the isolation procedure. See exosome and RNA collection methods above.
Western Blot
Protocol was carried out as previously described (40). E-cadherin antibody was used at 1:1000 dilution and HRP-conjugated secondary used at 1:3000. See supplementary table S2 for antibodies used.
Statistical Analyses
Five biological replicated were utilized in MTT assays. Six biological replicates were used in mouse studies. All other studies utilized three biological replicates. RT-PCR experiments also utilized three technical replicates. Sample sizes were determined to ensure adequate power to detect a pre-specified effect size when applicable based on previously generated data. Data are presented as the mean ± standard deviation. Statistical significance was calculated via Microsoft Excel using a Student t test (one-sided) or ANOVA as appropriate. Data generated displayed normal distribution with similar variances, and analysis was performed assuming equal variances. * Denotes p-value<0.05 **denotes p-value<0.01
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Robert Stahelin of the Indiana University School of Medicine for generously allowing us to use his particle analyzer and ultracentrifuge, Dr. Niranjan Awasthi of the Indiana University School of Medicine–South Bend for helpful discussions, and Dr. Steven Ruggiero of the University of Notre Dame for allowing us to perform light transmission spectroscopy in his lab. This research was funded by the Indiana CTSI and Harper Cancer Research Institute of Notre Dame.
Financial Information: R. Hill was awarded financial support from the Walther Cancer Foundation and the Joseph D. Boyle Memorial Fund. Work by M.L. Fishel was supported by grants from the National Institutes of Health, NCI CA167291, with additional support from the Biomedical Research Grant and in part by Jeff Gordon Children’s Foundation.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nature reviews. 2010;7(3):163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 4.Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Lohr JM, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121(4):699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 5.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. Journal of cellular biochemistry. 2007;101(4):887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 6.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68(3):918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York NY. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer cell. 2014;25(6):735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 11.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Boelens Mirjam C, Wu Tony J, Nabet Barzin Y, Xu B, Qiu Y, Yoon T, et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell. 159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. Journal of Extracellular Vesicles. 2014;3 doi: 10.3402/jev.v3.23743. 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 17.Kadera BE, Li L, Toste PA, Wu N, Adams C, Dawson DW, et al. MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated fibroblasts promotes metastasis. PloS one. 2013;8(8):e71978. doi: 10.1371/journal.pone.0071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braicu C, Tomuleasa C, Monroig P, Cucuianu A, Berindan-Neagoe I, Calin GA. Exosomes as divine messengers: are they the Hermes of modern molecular oncology? Cell death and differentiation. 2015;22(1):34–45. doi: 10.1038/cdd.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernyshev VS, Rachamadugu R, Tseng YH, Belnap DM, Jia Y, Branch KJ, et al. Size and shape characterization of hydrated and desiccated exosomes. Anal Bioanal Chem. 2015;407(12):3285–3301. doi: 10.1007/s00216-015-8535-3. [DOI] [PubMed] [Google Scholar]
- 20.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. International immunology. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 21.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. The Journal of biological chemistry. 2013;288(24):17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang WL, Jiang JK, Yang SH, Huang TS, Lan HY, Teng HW, et al. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nature cell biology. 2014;16(3):268–280. doi: 10.1038/ncb2910. [DOI] [PubMed] [Google Scholar]
- 23.Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochimica et biophysica acta. 2015;1852(11):2362–2371. doi: 10.1016/j.bbadis.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora S, Bhardwaj A, Singh S, Srivastava SK, McClellan S, Nirodi CS, et al. An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor kappaB- and hypoxia-inducible factor 1alpha-mediated up-regulation of CXCR4. The Journal of biological chemistry. 2013;288(29):21197–21207. doi: 10.1074/jbc.M113.484576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69(13):5575–5583. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- 26.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, et al. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22(21):3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, et al. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65(19):9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 28.Quint, Tonigold, Di F, Montalbano, Lingelbach, Rückert, et al. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. International journal of oncology. 2012;41(6):2093–2102. doi: 10.3892/ijo.2012.1648. [DOI] [PubMed] [Google Scholar]
- 29.Namba T, Kodama R, Moritomo S, Hoshino T, Mizushima T. Zidovudine, an anti-viral drug, resensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine by inhibition of the Akt-GSK3beta-Snail pathway. Cell death & disease. 2015;6:e1795. doi: 10.1038/cddis.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufhold S, Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention. Journal of experimental & clinical cancer research : CR. 2014;33:62. doi: 10.1186/s13046-014-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee TH, Chennakrishnaiah S, Audemard E, Montermini L, Meehan B, Rak J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochemical and biophysical research communications. 2014;451(2):295–301. doi: 10.1016/j.bbrc.2014.07.109. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Yan C, Mu L, Huang K, Li X, Tao D, et al. Fibroblast-Derived Exosomes Contribute to Chemoresistance through Priming Cancer Stem Cells in Colorectal Cancer. PloS one. 2015;10(5):e0125625. doi: 10.1371/journal.pone.0125625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boelens Mirjam C, Wu Tony J, Nabet Barzin Y, Xu Bihui, Qiu Yu, Yoon Taewon, et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray K. Pancreatic cancer: Pancreatic cancer exosomes prime the liver for metastasis. Nature reviews. 2015;12(7):371. doi: 10.1038/nrgastro.2015.93. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arensman MD, Kovochich AN, Kulikauskas RM, Lay AR, Yang PT, Li X, et al. WNT7B mediates autocrine Wnt/beta-catenin signaling and anchorage-independent growth in pancreatic adenocarcinoma. Oncogene. 2014;33(7):899–908. doi: 10.1038/onc.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun N, Johnson J, Stack MS, Szajko J, Sander C, Rebuyon R, et al. Nanoparticle analysis of cancer cells by light transmission spectroscopy. Analytical biochemistry. 2015;484:58–65. doi: 10.1016/j.ab.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69(14):5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gifford JB, Huang W, Zeleniak AE, Hindoyan A, Wu H, Donahue TR, et al. Expression Of GRP78, Master Regulator Of The Unfolded Protein Response, Increases Chemoresistance In Pancreatic Ductal Adenocarcinoma. Molecular cancer therapeutics. 2016 doi: 10.1158/1535-7163.MCT-15-0774. [DOI] [PubMed] [Google Scholar]
- 41.Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer's disease. Neurobiology of aging. 2014;35(8):1792–1800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.