Abstract
The back skin of C57BL/6 mice was exposed to a single 400 mJ/cm2 dose of ultraviolet B (UVB), and parameters of hypothalamic-pituitary-adrenal (HPA) axis in relation to immune activity were tested after 30–90 min following irradiation. Levels of brain and/or plasma corticotropin releasing hormone (CRH), β-endorphin, ACTH and corticosterone (CORT) were enhanced by UVB. Hypophysectomy had no effect on UVB-induced increases of CORT. Mitogen induced IFNγ production by splenocytes from UVB-treated mice was inhibited at 30, 90 min and after 24 h. UVB also led to inhibition of IL-10 production indicating an immunosuppressive effect on both Th1 and Th2 cytokines. Conditioned media from splenocytes isolated from UVB-treated animals had no effect on IFNγ production in cultured normal splenocytes, however IFNγ increased with conditioned media from sham-irradiated animals. Sera from UVB-treated mice suppressed T cell mitogen-induced IFNγ production as compared to sera from sham-treated mice. IFNγ production was inhibited in splenocytes isolated from UVB-treated animals with intact pituitary, while stimulated in splenocytes from UVB-treated hypophysectomised mice. Thus, cutaneous exposure to UVB rapidly stimulates systemic CRH, ACTH, β-endorphin, and CORT production accompanied by rapid immunosuppressive effects in splenocytes that appear to be independent of the HPA axis.
Graphical Abstract
INTRODUCTION
Skin covers and protects the body from the external environment, while maintaining internal homeostasis (1–4). Keratinocytes and melanocytes, representing the major component of the epidermis, have a common neuroectodermal origin and, therefore, are the source of neuroendocrine factors, which are either capable of entering the blood circulation and/or activating ascending neuronal pathways (5, 6). Specifically, skin expresses all elements of the hypothalamic-pituitary-adrenal (HPA) axis (7). This local “equivalent” of the HPA axis follows the same activatory pattern as the central HPA (8, 1). The noxious stimuli encoded as stressors sensitize skin cells and/or enhance cutaneous production or release of corticotropin releasing hormone (CRH) from nerve endings (8, 9). This stressocortin, upon binding to a specific receptor (CRH-R1), triggers the release of ACTH, and increases expression of proopiomelanocortin (POMC), which undergoes enzymatic cleavage yielding several important neuropeptides that in addition to ACTH include β-endorphin (β-END) and α-melanocyte stimulating hormone (αMSH) (8, 10–12). Pituitary ACTH reaches the adrenal cortex, via blood, and induces release of glucocorticosteroids (GC) with stimulation of glucosteroidogenesis from cholesterol with involvement of steroidogenic acute regulatory protein (StAR) and steroid 11β-hydroxylase (CYP11B1) which are encoded by StAR and Cyp11b1 genes, respectively (13, 14). In addition, peripheral organs such as skin can produce regulatory GC (15, 16) including cortisol (COR) in humans (15, 17–22) and corticosterone (CORT) in rodents (23, 24). This cutaneous steroidogenesis can also proceed from cholesterol (25, 26).
Recent evidence showed that ultraviolet B (UVB), when applied to the skin, triggers both local - cutaneous (20, 23) and central HPA axis (27) with the global consequences manifested by elevated levels of CRH, urocortin, ACTH, β-END, and CORT in plasma and brain (27) in mice. Thus, UVB-stimulated CRH in the paraventricular nucleus (PVN) is followed by pituitary release of ACTH and increases in CORT plasma levels, with the latter effects requiring an intact pituitary (27). Similar activation of the central hypothalamic-pituitary (HP) axis leading to increased levels of α-MSH in the serum has been reported after exposure of the eye to UVB energy (28, 29). Therefore, it has been proposed that UVB-related activation of either the central HP and/or HPA axis, through overlapping but distinct mechanisms, operates in order to maintain peripheral neuroimmune homeostasis (30). Most recently, we have also shown that UVB can also activate the POMC signaling system in the arcuate nucleus (Arc) of the hypothalamus (31). These findings emphasize the central role of the brain in coordination and translation of UVB signals into the systemic responses (30).
The skin with subcutis represents the largest organ in the body that is endowed with neuroendocrine activities that regulate local and global homeostasis [reviewed in (1, 6, 32)]. UVB is a major cutaneous stressor that, on the one hand, induces multiple skin pathologies including cancer (33–35), while on the other is necessary for vitamin D production (36, 37), activation of local neurohormones (1, 8) and stimulation of melanin pigmentation (38, 39, 4, 34, 40). UVB has also local and systemic immunosuppressive effects, the mechanisms of which are still being investigated (41–43, 40, 44, 45). The above information [as well as strong evidence for reciprocal communication cross-talk between skin, brain, endocrine and immune systems (1)] prompted us to test the effects of a single exposure of back skin to the UVB on the systemic neuroendocrine parameters and immune functions.
MATERIALS AND METHODS
Animals
All procedures involving mouse tissue collection were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center (UTHSC) and were adherent to the Animal Welfare Act and Regulations. Regular (n=24) and hypophysectomised (hypox, n=24) six week old C57BL/6 females from Taconic Biosciences, Inc. were maintained at the UTHSC animal facility under specific pathogen-free conditions for a week to avoid transportation stress. The animals had free access to standard laboratory chow and water and were maintained on a 12:12 light/dark cycle with room temperature ranged from 20° to 24° C. At the age of 7–8 weeks, when the hair cycle was in telogen phase (46), and a day before irradiation, all backs of mice were shaved with animal clippers. The back skin of the mice was then irradiated with UVB wavelength (under isoflurane anesthesia) with their eyes covered by aluminum foil to prevent any signal transmission through the optic nerve (27) or sham-irradiated (as a control). All animals were sacrificed under deep anesthesia in the early morning (around 7 am) to control for their circadian rhythm, and then plasma, skin, brain, adrenals and spleen were collected for further examination.
UV radiation
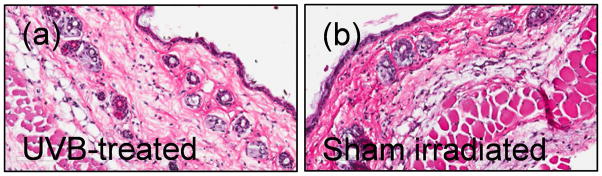
Details regarding specific lamp set up, wavelength, and dosimetry are provided in (47, 48, 27, 49). After brief isoflurane anesthesia, mice were irradiated with a single dose of 400 mJ/cm2 of UVB (290–320 nm), which corresponds to a 4.1 Standard Erythema Dose (SED) and left for 30, 60, 90 min, and 12, 24 h survival time. One SED is equivalent to an erythema effective radiant exposure of 100 J/m2 (MKS) or 0.01 J/cm2 (CGS) (48). Kodacel filter (Kodacel™, Eastman Kodak, Rochester, NY), which acts as a cut-off filter of UVC allowing sole transmission of wavelengths longer than 290 nm, was applied. A preliminary study showed that smaller doses of UVB [25, 50, and 100 mJ/cm2 (27, 31)] or shorter time (15 min, not shown) were not effective in stimulating the central neuroendocrine responses in mice. Furthermore, as previously reported (27), 400 mJ/cm2 of UVB does not damage cutaneous structures that would be manifested as detectable epidermal necrosis, marked keratinocyte apoptosis, or acute inflammatory responses (Fig. 1). The UVB irradiation was performed exclusively during early mornings when the activity of rodent HPA axis is the lowest (the opposite to humans) based on a circadian rhythm (50).
Figure 1.
Single 400 mJ/cm2 dose of UVB light does not lead to visible damage in murine skin. No detectable epidermal necrosis, keratinocyte apoptosis, or acute inflammatory responses were noticed in UVB- treated (a) or sham-irradiated (b) skin of C57Bl/6. H&E staining, magnification 100x.
Experiments with lymphocytes isolated from spleen
The sham- (control) or UVB-treated animals were sacrificed 30 min, 90 min, 12 h, and 24 h following the irradiation, spleens were collected and processed as described (51). Briefly, lymphocytes (splenocytes) were collected after erythrocyte lysis by hypotonic shock and set up at 4×106 cells/mL in triplicate of 48-well tissue culture plates (Corning®Costar® Life Sciences, Tewksbury, MA) in RPMI 1640 medium supplemented with 9% FCS, antibiotics, and Glutamax (Thermo Fisher Sci., Waltham, MA). T cells were stimulated with 0.2 μg/mL hamster anti-mouse CD3e monoclonal antibody [(MoAb), BD Pharmingen, San Jose, CA] or PBS as vehicle (control). Plates were cultured for 48 h at 37°C in a humidified incubator with atmosphere containing 5% CO2. Next, supernatants were harvested and subjected to ELISA for measurement of IFNγ and IL-10.
Effects of cultured splenocyte supernatants from UVB- and sham-treated mice on IFNγ production by anti-CD3e T cell mitogen-stimulated splenocytes from normal untreated mice
Ninety minutes after brief UVB exposure, mice were sacrificed, spleens were isolated, and cultured as described above with-or-without hamster anti-mouse anti-CD3e MoAb (BD Biosciences-Pharmingen, San Jose, CA) (0.2 μg/ml in 500 μl RPMI 1640 medium supplemented with 1% FCS, antibiotics, and Glutamax in 48-well tissue culture plates). 48 h later, supernatants from each of the sham-treated mice were pooled together and from the UVB-treated mice were pooled together. To prepared cultures of normal C57BL/6 splenocytes at a concentration of 2×106 cells/wells of RPMI 1640 medium supplemented with 1% FCS, antibiotics and Glutamax and to triplicate wells was added 0%, 3.125%, 6.25%, 12.5%, or 25% (volume:volume) of either the above-described, anti-CD3e-stimulated cultures of splenocytes from sham-treated or UVB-treated mice. After 24 h incubation at 37°C, 5% CO2 humidified atmosphere, 0.2μg/ml hamster anti-mouse CD3e was added to each culture well and incubation continued for 48 additional hrs. The cultured supernatants were then harvested and levels of IFNγ determined by a commercial ELISA test (R&D Systems, Minneapolis, MN).
Effect of serum from UVB- and sham-treated mice on IFNγ production by anti-CD3e T cell mitogen-stimulated splenocytes from normal untreated mice
After 90 min, sham- (control) (n=5) and UVB-treated (n=6) of C57BL/6, as described above, were sacrificed and sera collected and pooled together regarding treatment, and added at a final concentration of 0.1% by volume to triplicate wells of 48 well tissue culture plates containing per well 2×106 splenocytes from untreated normal C57BL/6 mice in 450 μl RPMI 1640 medium containing 1% FCS and supplemented with Glutamax and antibiotics. After 2 h incubation, 0.2 μg/ml hamster anti-mouse CD3e was added to 3 wells containing 0.1% serum from sham-treated mice and to 3 wells containing 0.1% serum from UVB-treated mice. As a control, PBS was added to three wells. The final volume of all wells was 500 μl and then plates were incubated for 48 h. Cultured supernatants were then harvested and analyzed for levels of IFNγ by ELISA kit (R&D Systems, Minneapolis, MN). Repeated measures ANOVA (SigmaStat, San Jose, CA) was used to analyze for differences in IFNγ concentration in cultures supplemented with different percentages of splenocyte-cultured supernatants or mouse sera from sham-treated vs. UVB-treated mice. P values ≤0.05 were considered statistically significant.
Quantitative real time RT-PCR (qRT-PCR)
The adrenals were dissected out, and total RNA was extracted with TRIZOL® (Invitrogen, Carlsbad, CA) as described (31). One microgram of RNA was reverse transcribed into cDNA with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Primers for murine Star (NM_011485.4; F: ttgggcatactcaacaacca, R: acttcgtccccgttctcc) and Cyp11b1 exon 2/3 (NM_000497.3; F: aggtggacagcctgcatc, R: ccattcaggcccattcag) were used for qRT-PCR amplification. The PCR reactions were performed in triplicate with KAPA SYBR® Fast Master Mix (Kapa Biosystems, Inc. Woburn, MA) and data were collected on a Light Cycler 480 from Roche. The amount of amplified product for each gene was compared to that of the reference gene (β-actin) using a comparative ΔΔCT method and presented as a fold change ± SD.
Enzyme-linked immunosorbent assays (ELISA) of CRH, ACTH, β-END and CORT
Blood, following centrifugation (1,600 g, 5 min, 4°C) yielded plasma samples which were diluted in a ratio 1:10 with 0.9% NaCl and processed with commercially available kits to measure CRH (#FEK-019-06, Phoenix Pharm., Burlingame, CA), ACTH (#21-ACTHU-E01, Alpco Immunoassays, Salem, NH), β-END (#S-1245, Peninsula Lab., San Carlos, CA), and CORT (ADI-900-097, Enzo Life Sci., Plymouth Meeting, PA), according to the manufacturer’s directions [details are in (27, 31)]. Brain was removed and hypothalamic area was dissected out with the use of Zivic brain slider (27) according to the http://mouse.brain-map.org/ and homogenized using the homogenizer (Polytron PT-MR2100, Swiss) in ice-cold T-PER® buffer (Thermo Sci., Rockford, IL) supplemented with protease inhibitor cocktail (10 μl/1mL; Sigma, St. Louis, MO), and then centrifuged at 12,000 g for 25 min at 4°C. The supernatants were collected with the following protein concentration calculated with BCA assay (Thermo Sci., Rockford, IL) and adjusted to the same value (20μg/μL). For CRH examination, the peptide extraction was performed with equilibrated SEP-COLUMN containing 200 mg of C18 (#RK-SEPCOL-1, Phoenix Pharmaceuticals Inc., Burlingame, CA) according to the manufacturer’s recommendations. Eluent was dried with N2 and extracted peptide reconstituted in ELISA buffer. All assays were run in triplicate, the OD’s were read with a spectrometer SpectraMax M2 and the concentrations were calculated from the 4 parametric standard curve with SoftMax Pro software (Molecular Devices, Sunnyvale, CA) and presented either in pg/mL, ng/mL, or recalculated as pg/1μg of total proteins for brain tissue. Results are expressed as mean±SEM in pg/mL per treatment group and analyzed for statistical significance with ANOVA with exact P value provided (GraphPad Software, San Diego, CA).
Histological examination
Skin was fixed in buffered (pH=7.4) paraformaldehyde and processed into paraffin blocks. Ten μm sections were deparafinized and stained with hematoxylin-eosin (H&E). The slides were scanned with Aperio ImageScope (Leica Biosystems Inc., Buffalo Grove, IL) analyzed, and pictures processed with ImageJ (NIH).
Statistical analyses
The data are presented as means ± SD and are analyzed using Prism 4.00 (GraphPad Software, San Diego, CA) with Student’s t-test or ANOVA (SigmaStat, San Jose, CA). Statistically significant differences are denoted by *, **, or ***, where P is considered statistically significant at <0.05, <0.005, or <0.001; respectively.
RESULTS
Previously we have documented the induction of the central HPA axis (27) or paraventricular POMC signaling (31) 12 or 24 h after single exposure to 400 mJ/cm2 of UVB to shaved back murine skin. In the present study, we have evaluated systemic body responses to the same UVB protocol but after shorter, 30–90 min, time following irradiation.
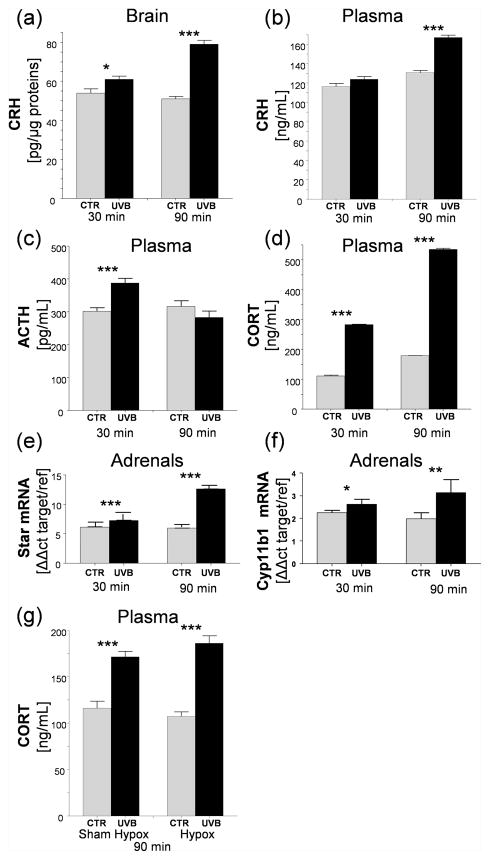
Thus, exposure of the skin to 400mJ/cm2 of UVB enhanced significantly hypothalamic CRH levels by 12% and 55%, after 30 and 90 min, respectively, compared to sham-irradiated animals (Fig. 2a). Significant increases in plasma CRH were noted only after 90 min following UVB challenge (Fig. 2b), while ACTH levels increased transiently after 30 min with a negligible decrease after 90 min of exposure (Fig. 2c). The plasma levels of CORT were significantly elevated by 155% at 30 min and 200% stimulation at 90 min after UVB radiation in comparison to control (Fig. 2d). Increases in plasma CORT levels were accompanied by enhanced expression of Star and Cyp11b1 genes in adrenal cortex with stronger stimulation seen at 90 min vs. 30 min after irradiation (Fig. 2e, 2f).
Figure 2.
UVB induces rapid increases in gene expression, neuropeptide and CORT production in murine brain, adrenals and plasma. CRH levels in brain (a) and plasma (b); levels of ACTH (c) and CORT (d) in plasma, evaluated with ELISA. The Star (e) and Cyp11b1 (f) gene expression in murine adrenals; qRT-PCR data is presented as fold change based on ΔΔ method with β-actin used as housekeeping gene. Plasma CORT levels increases after UVB radiation in hypox and shame-hypox mice (g). Data presented as means ± SD were analyzed using Student’s t-test: * P<0.05, ** P<0.01, and *** P<0.001.
To test a possible involvement of pituitary in plasma CORT responses, we used hypophysectomised (hypox) and control (sham-hypox) mice. Both hypox and control animals showed increased plasma CORT concentrations at 90 min after UVB exposure indicating extra-pituitary signal involvement in such stimulations (Fig. 2g).
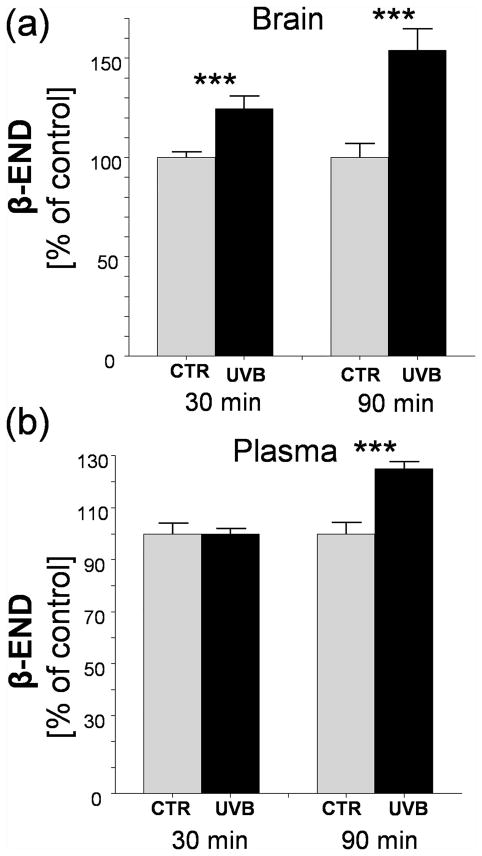
UVB also enhanced β-END levels in the brain (hypothalamus) after 30 and 90 min (maximal stimulation) with plasma levels increasing significantly only after 90 min of exposure (Fig. 3a, 3b).
Figure 3.
Cutaneous UVB enhances brain (a) and plasma (b) β-endorphin levels. Data presented as means ± SD were analyzed using Student’s t-test: *** P<0.001.
Next, we have tested the UVB effect on immune activity of lymphocytes isolated from spleens of mice, the skin of which was exposed to a single dose of UVB (400 mJ/cm2). In the first experiment, spleens were collected 30 min, 90 min, 12 h, and 24 h after UVB exposure, splenocytes isolated, and stimulated with T cell specific anti-CD3e MoAb for 48 h after which IFNγ levels were measured by ELISA. As shown in Table 1, a significant inhibition in IFNγ production by anti-CD3e-stimulated splenocytes after UVB treatment compared to control cells was seen 90 min following exposure. This inhibitory effect persisted in splenocytes isolated 12 h and 24 h after UVB treatment. Next, we tested production of IFNγ and IL-10 in anti-CD3e-stimulated splenocytes isolated from mice 90 min after UVB exposure and found significant reduction of levels of both cytokines in splenocyte cultures derived from UVB-treated vs. control group (Table 2). Next, we tested whether supernatants derived from cultures of anti-CD3e stimulated splenocytes isolated from UVB-treated and control animals can affect IFNγ production. As shown in Table 3, supernatants from cultures of anti-CD3e-stimulated splenocytes obtained from UVB-treated mice had no significant effect on IFNγ production by normal cultured splenocytes, which was in contrast to supernatants from sham-irradiated animals, which activated IFNγ production in a dose-dependent manner. We also tested the effect of serum isolated from control and UVB-treated animals on IFNγ production. As shown in Table 4, normal mouse splenocytes treated with 0.1% serum derived from UVB-radiated animals exhibited lack of IFNγ stimulation after exposure to anti-CD3e MoAb T cell mitogen, which contrasted with significant increases of IFNγ levels from cultures of normal splenocytes to which were added 0.1% serum from sham-treated, non-irradiated animals (Table 4). Splenocytes not stimulated with anti-CD3e contained very low levels of IFNγ (ranging from 4–12 pg/ml) (data not shown).
Table 1.
Cutaneous exposure to UVB light suppresses splenocyte T cell mitogen (Anti-CD3e MoAb)-induced IFNγ production in C57BL/6 in time dependent manner.#
| Time after treatment | IFNγ concentration | |
|---|---|---|
|
| ||
| Sham-treated | UVB-treated | |
| pg/mL | ||
|
|
||
| 30 min | 119 ± 45 | 285 ± 98 |
| 90 min | 2,470 ± 362 | 518 ± 97* |
| 12 h | 357 ± 130 | 102 ± 30** |
| 24 h | 360 ± 51 | 191 ± 37** |
Splenocytes from UVB-treated or sham-treated mice were harvested 30 min, 90 min, 12 h, or 24 h later and cultured with or without anti-CD3e MoAb for 48 h after which splenocyte culture supernatants were analyzed for levels of IFNγ as described in the Materials and Methods section. Data presented as Mean ± SEM (n=5-6 mice per time point after UVB treatment per sham- or UVB-treated groups;
P<0.05,
P<0.01 by Student’s t-test.
Table 2.
UVB radiation suppresses generation of Th1 (IFNγ) and Th2 (IL-10) cytokine production by cultured splenocytes stimulated in vitro by mitogen (Anti-CD3e MoAb) 90 min after radiation.#
| Treatment of mice | IFNγ concentration | |
|---|---|---|
|
| ||
| IFNγ | IL-10 | |
| pg/mL | ||
| Sham (control) | 2,470 ± 362 | 70 ± 11 |
| UVB (400 mJ/cm2) | 300 ± 94** | 30 ± 12* |
Splenocytes from UVB- or Sham-treated mice (90 min later) were stimulated for 48 h with anti-CD3e MoAb and harvested supernatants were analyzed for levels of IFNγ and IL-10 as described in the Materials and Methods section. Data presented as mean ± SEM (n=6–7),
P<0.01, or
P<0.05 by Student’s t-test.
Table 3.
Supernatants from splenocytes isolated from UVB-treated animals have no effect, while those from Sham-irradiated animals stimulate IFNγ production in normal mouse splenocytes.#
| Final Supernatant Concentration | Splenocyte Supernatant (Sham-treated) | Splenocyte Supernatant (UVB-treated) | Statistical Analysis |
|---|---|---|---|
|
| |||
| % | IFNγ, pg/mL | P value | |
| 0 | 669 ± 20 | 570 ± 47 | 0.221 |
| 3.125 | 863 ± 72 | 636 ± 48 | 0.026 |
| 6.25 | 852 ± 39 | 574 ± 28 | 0.046 |
| 12.5 | 1,091 ± 49 | 684 ± 39 | 0.011 |
| 25 | 1,466 ± 13 | 789 ± 36 | 0.004 |
Supernatants from splenocytes stimulated by anti-CD3e MoAb for 48 h from 5 sham- and 5 UVB-treated mice (90 min survival time) were pooled together and added at the % indicated to the cultures of normal mouse spleens stimulated for 48 h with anti-CD3e MoAb for 48 h. Levels of IFNγ quantitated as described in detail in the Materials and Methods section. Data presented as Mean ± SEM of triplicate cultures; exact P values evaluated with ANOVA (SigmaStat, San Jose, CA).
Table 4.
Serum-derived factors from UVB-treated animals inhibit IFNγ production by mouse splenocytes cultured with (Anti-CD3e MoAb).#
| Splenocytes incubated with 0.1% serum | IFNγ, pg/mL |
|---|---|
| Sham-treated mice | 64 ± 0.9 |
| UVB-treated mice | 8.1 ± 0.9* |
Sera from 6 UVB-treated and 5 Sham-treated mice were harvested 90 min after irradiation and pooled together (condition-dependent) and added for 2 h at 0.1% by volume to cultures of normal mouse splenocytes treated either with PBS or anti-CD3e MoAb in triplicates and incubated for 48 additional hours. IFNγ levels in harvested supernatants were analyzed ELISA as described in the Materials and Methods section. Statistical differences were evaluated with Student’s t-test, where
P<0.05.
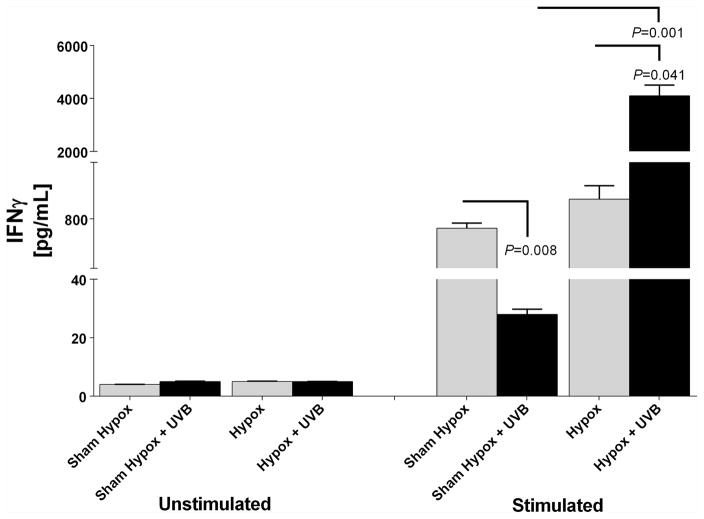
To test whether hypophysectomy can change immune responses secondary to UVB exposure, we isolated splenocytes from either hypox- or shame-hypox animals 90 min after UVB or shame exposure and tested their ability to produce IFNγ with and without stimulation of T cells with anti-CD3e MoAb (Fig. 4). Splenocytes isolated from both mouse groups showed similar and very low IFNγ production when incubated with PBS (vehicle) vs increased cytokine levels in cells treated with anti-CD3e MoAb. There was a significant decrease in IFNγ production in splenocytes isolated from UVB-treated animals with intact pituitary and sustained production of CORT, consistent with data presented in Tables 1–3. In contrast, UVB had an opposite effect in hypox mice (without pituitary, lacking the HPA axis-derived CORT production) e.g., mitogen significantly (P<0.041) increased production of IFNγ by splenocytes (Fig. 4). There was no statistical difference in IFNγ production by anti-CD3e MoAb stimulated splenocytes isolated from unirradiated hypox and intact (sham-hypox) animals.
Figure 4.
Hypophysectomy reverses UVB-induced suppression of splenocyte mitogen-induced IFNγ production. Results are expressed as means ± SEM in pg/mL per treatment group and statistically analyzed with Anova with exact P values provided (GraphPad Prism 4).
DISCUSSION
In this study we show for the first time, that a single dose of UVB applied exclusively to the skin rapidly (30–90 min after exposure) enhances brain and/or plasma concentrations of CRH, β-END, ACTH, and CORT. The stimulation of CORT was independent of the pituitary (seen also in hypox mice) and was associated with an enhanced expression of Cyp11b1 and Star mRNA in adrenals. This single dose UVB exposure also led to rapid (already at 90 min after exposure) and significant suppression of T-cell mitogen-stimulated immune activity of lymphocytes isolated from spleen of exposed animals. This effect was reversed by hypophysectomy where UVB exposure enhances IFNγ production in mitogen-activated splenocytes compared to control.
Our previous study demonstrated that plasma CORT was elevated 12 h and 24 h after UVB treatment which was the result of the activation of the central HPA axis, and required an intact pituitary since in hypophysectomized animals the effect was abolished (27). Similarly, significant activation of all elements of the cutaneous (local) HPA axis by UVB was also seen 12 h and 24 h in human skin (20) and 6 h, 12 h, and 24 h in mouse skin (23) after exposure. In addition, activation of the POMC signaling system in the Arc of the hypothalamus was also observed 12 h and 24 h following irradiation (31). Therefore, the stimulation of CORT 30 and 90 min after UVB that was accompanied by increased levels of CRH, β-END, and ACTH was an unexpected finding and requires explanation. Similar response to UVB by hypox animals and mice with intact pituitary, both show similar increases of plasma CORT levels and demonstrate that this stimulation follows a pathway independent of the HPA axis. Thus, the described in brain (hypothalamus) and/or plasma increases in CRH, β-END, and ACTH are dissociated from the classical HPA axis and represent separate activities specific for hypothalamus (CRH and β-END) and serum (CRH, β-END, and ACTH) organized along HP axis (hypothalamic CRH → pituitary ACTH). Clarification of these signaling pathways operating either in the brain or endocrine levels will require extensive in vivo studies. However, the rapid process seen in this study suggests neuronal signaling in conveying afferent information to the brain (hypothalamus) with descending transmission to peripheral organs including activation of the adrenal cortex to synthesize and release CORT and probably other mediators. Observed stimulatory action triggered by UVB was manifested by the increased production of CRH in the hypothalamus, as early as 30–90 min after radiation. Likewise, an rapid production of POMC-derived ACTH and β-END with rapid increases in CORT plasma levels indicates that the rapid activation of paraventricular nucleus of hypothalamus takes place via neuronal activation rather than via endocrine/immune mediators released by the skin. This proposition is supported by bidirectional communication between the skin and brain in regulation of body homeostasis in reaction to external noxious environmental factors (52, 32, 1, 8). Although detailed routing of such activation requires extensive in vivo studies, we speculate that activation of adrenocortical CORT production is secondary to neural stimulation. Since the hypothalamus governs both HPA axis (11, 10, 13) and sympathetic nervous system activity (53, 13), there is a high probability for mutual activation of the above mentioned centers by UVB light, with CRH perhaps playing a crucial role. Adrenals, unlike other organs, receive direct “preganglionic” cholinergic inputs from hypothalamus, mediolateral spinal cord (54) and are the so-called “modified sympathetic ganglia”. Based on this present study and previous studies (27, 31), we propose that UVB light leads to activation of several brain areas, including preganglionic sympathetic neurons localized in the hypothalamus (55) with subsequent activation of corticosteroidogenic activity of adrenal cortex as an alternative to the HPA-led activation.
Although systemic immunosuppressive effects of UVB are well documented (41, 43, 42, 44), the mechanisms of such UVB activity are not fully understood. They include production of immunosuppressive cytokines and neuropeptides, reprogramming of immune competent cells, and vitamin D dependent and independent suppression of adaptive immunity (43, 42, 44, 40, 1). Thus, our finding on UVB immunosuppression was expected. However, the rapidity (90 min) with which it occurred was unexpected. The immunosuppressive effect in the internal immune organ (spleen) affected both Th1 (IFNγ) and Th2 (IL-10) cytokines, which persisted after 12 h or 24 h after irradiation. While the latter effects can be explained either by activation of the central HPA axis (27) or mechanisms extensively discussed by others (43, 42, 44, 40, 1), the rapid induction of immune suppression cannot be explained by activation of HPA, because an opposite effect (immunostimulation, Figure 4) was seen in hypox animals exposed to UVB. An alternative explanation could be triggering by UVB of neural stimuli transmitted from the skin up to spinal cord and brain with direct neurotransmission to the spleen through the sympathetic system (56). This hypothesis appears to be supported by sympathetic innervation of spleen in rodents (57, 58), aggravation of immune responses by sympathectomy with 6-OHDA (59), neuronal origin of immunosuppression in chronic (60) or acute (61, 62) stress and expression of receptors for neurotransmitters on lymphocytes (63, 64). However, a supplemental role of serum factors in the rapid UVB-induced immunosuppression is indicated by the inhibition of splenocyte immune activity by serum collected from UVB-treated animals. This effect could again be secondary to CORT production/release induced by neural impulse sent to the adrenal cortex or activation of the adrenal medulla to produce catecholamines (55). Neuroanatomical study with the use of retrograde tracers provided the evidence that spleen in mice and rat receives exclusively sympathetic innervation with no sensory inputs either from vagal nerve or dorsal root ganglia (DRG) (57, 58), which would be consistent with the hypothesis that UVB triggers central coordination of sympathetic inhibition of immune activity in the spleen. Other serum-derived factors would include β-END and α-MSH, of which serum levels are stimulated by UVB, and which show immunosuppressive properties (65–68). Clarification of these possible routes of UVB-induced immunosuppression will require challenging and extensive experimental effort. The nature of the triggering stressor (UVB in this case) determines the body stress-response manifested as a rapid action mediated through what is called “HPA cross-sensitization” with immune and behavioral consequences (69).
In summary, we provided evidence that UVB light can rapidly activate neuroendocrine effects leading to increases in plasma levels of neuropeptides and CORT that is pituitary independent, leading to dramatic immunosuppression.
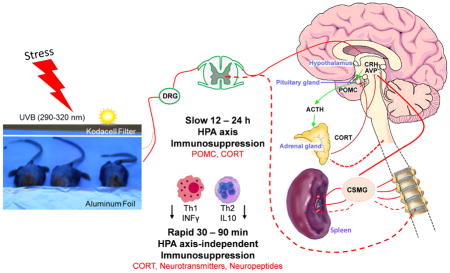
Possible neuro-endocrine pathways involved in splenic immunosuppressive action evoked by exposure of murine skin to ultraviolet B (UVB) radiation.
UVB-induced afferent neural signals activate the central HPA axis resulting in pituitary POMC-derived ACTH and adrenal corticosterone release to plasma. Slower immunosuppressive action, takes 12 – 24 h (upper part).
UVB-induced afferent neuronal signals affect CNS and directly activate adrenal gland (CORT, neurotransmitters) and spleen (neurotransmitters, neuropeptides). Rapid immunosuppression, 30 – 90 min (lower part).
Acknowledgments
The excellent technical assistance by Patricia Wheller in performing splenocyte isolation and culture and ELISA assays for IFNγ and IL-10 is acknowledged. This work was supported by grants from the National Science Foundation (# IOS-0918934) and National Institutes of Health (2R01AR052190-A6, R21AR066505-01A1 and 1R01AR056666-01A2) to AS.
Footnotes
This article is part of a Special Issue – descriptive phrase will be added during proofing stage.
AUTHOR CONTRIBUTION
CS performed experiments, contributed to the study design, analysis, interpretation, and writing of this manuscript. AS was involved in the study design, analysis, interpretation, writing of this manuscript, and contributed to the experiments. AP performed, designed, and analyzed experiments on splenocytes and was involved in writing of the manuscript
References
- 1.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias PM. Structure and function of the stratum corneum extracellular matrix. The Journal of investigative dermatology. 2012;132:2131–2133. doi: 10.1038/jid.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Man MQ, Lin TK, Santiago JL, Celli A, Zhong L, Huang ZM, Roelandt T, Hupe M, Sundberg JP, Silva KA, Crumrine D, Martin-Ezquerra G, Trullas C, Sun R, Wakefield JS, Wei ML, Feingold KR, Mauro TM, Elias PM. Basis for enhanced barrier function of pigmented skin. The Journal of investigative dermatology. 2014;134:2399–2407. doi: 10.1038/jid.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 5.Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. The Journal of clinical investigation. 2007;117:3166–3169. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiological reviews. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 12.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 13.Chrousos GP. Stress and disorders of the stress system. Nature reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 14.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: Implications for local immune functions. The Journal of steroid biochemistry and molecular biology. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015;103:72–88. doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 18.Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. The Journal of investigative dermatology. 2006;126:1177–1178. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 20.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. The Journal of biological chemistry. 2011;286:10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 23.Skobowiat C, Nejati R, Lu L, Williams RW, Slominski AT. Genetic variation of the cutaneous HPA axis: an analysis of UVB-induced differential responses. Gene. 2013;530:1–7. doi: 10.1016/j.gene.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta. 2000;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 25.Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. The Journal of steroid biochemistry and molecular biology. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, CTR A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. European journal of biochemistry/FEBS. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skobowiat C, Slominski AT. UVB Activates Hypothalamic-Pituitary-Adrenal Axis in C57BL/6 Mice. The Journal of investigative dermatology. 2015;135:1638–1648. doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiramoto K, Yamate Y, Kobayashi H, Ishii M, Sato EF, Inoue M. Ultraviolet B irradiation of the mouse eye induces pigmentation of the skin more strongly than does stress loading, by increasing the levels of prohormone convertase 2 and alpha-melanocyte-stimulating hormone. Clinical and experimental dermatology. 2013;38:71–76. doi: 10.1111/j.1365-2230.2012.04439.x. [DOI] [PubMed] [Google Scholar]
- 29.Hiramoto K, Yanagihara N, Sato EF, Inoue M. Ultraviolet B irradiation of the eye activates a nitric oxide-dependent hypothalamopituitary proopiomelanocortin pathway and modulates functions of alpha-melanocyte-stimulating hormone-responsive cells. The Journal of investigative dermatology. 2003;120:123–127. doi: 10.1046/j.1523-1747.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- 30.Slominski AT. Ultraviolet radiation (UVR) activates central neuro-endocrine-immune system. Photodermatol Photoimmunol Photomed. 2015;31:121–123. doi: 10.1111/phpp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skobowiat C, Slominski AT. Ultraviolet B stimulates proopiomelanocortin signalling in the arcuate nucleus of the hypothalamus in mice. Exp Dermatol. 2016;25:120–123. doi: 10.1111/exd.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 33.Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, Zjawiony J, Tuckey RC. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med Chem. 2014;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powers JG, Patel NA, Powers EM, Mayer JE, Stricklin GP, Geller AC. Skin Cancer Risk Factors and Preventative Behaviors among United States Military Veterans Deployed to Iraq and Afghanistan. The Journal of investigative dermatology. 2015;135:2871–2873. doi: 10.1038/jid.2015.238. [DOI] [PubMed] [Google Scholar]
- 36.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 37.Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 38.Pawelek JM, Chakraborty AK, Osber MP, Orlow SJ, Min KK, Rosenzweig KE, Bolognia JL. Molecular cascades in UV-induced melanogenesis: a central role for melanotropins? Pigment Cell Res. 1992;5:348–356. doi: 10.1111/j.1600-0749.1992.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 39.Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(Suppl 2):8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becklund BR, Severson KS, Vang SV, DeLuca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6418–6423. doi: 10.1073/pnas.1001119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer research. 1994;54:6102–6105. [PubMed] [Google Scholar]
- 42.Schwarz A, Navid F, Sparwasser T, Clausen BE, Schwarz T. In vivo reprogramming of UV radiation-induced regulatory T-cell migration to inhibit the elicitation of contact hypersensitivity. The Journal of allergy and clinical immunology. 2011;128:826–833. doi: 10.1016/j.jaci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochemistry and photobiology. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz A, Navid F, Sparwasser T, Clausen BE, Schwarz T. 1,25-dihydroxyvitamin D exerts similar immunosuppressive effects as UVR but is dispensable for local UVR-induced immunosuppression. The Journal of investigative dermatology. 2012;132:2762–2769. doi: 10.1038/jid.2012.238. [DOI] [PubMed] [Google Scholar]
- 45.Legat FJ, Wolf P. Cutaneous sensory nerves: mediators of phototherapeutic effects? Front Biosci (Landmark Ed) 2009;14:4921–4931. doi: 10.2741/3577. [DOI] [PubMed] [Google Scholar]
- 46.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Huang Y, Li F, Zheng S, Epstein PN. FVB mouse genotype confers susceptibility to OVE26 diabetic albuminuria. Am J Physiol Renal Physiol. 2010;299:F487–494. doi: 10.1152/ajprenal.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.International Commission on Illumination (CIE) International Standard ISO 17166:1999(E) - CIE 007/E:1998, Erythema Reference Action Spectrum and Standard Erythema Dose. International Organization for Standardization (ISO); Geneva, Switzerland: 1999. [Google Scholar]
- 49.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 51.Myers LK, Kang AH, Postlethwaite AE, Rosloniec EF, Morham SG, Shlopov BV, Goorha S, Ballou LR. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000;43:2687–2693. doi: 10.1002/1529-0131(200012)43:12<2687::AID-ANR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets. 2014;13:177–190. doi: 10.2174/1871528113666140522104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Llewellyn-Smith IJ. Anatomy of synaptic circuits controlling the activity of sympathetic preganglionic neurons. J Chem Neuroanat. 2009;38:231–239. doi: 10.1016/j.jchemneu.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Sato A. Neural mechanisms of somatic sensory regulation of catecholamine secretion from the adrenal gland. Adv Biophys. 1987;23:39–80. doi: 10.1016/0065-227x(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 55.Nance DM, V, Sanders M. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 57.Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 58.Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 59.Harle P, Mobius D, Carr DJ, Scholmerich J, Straub RH. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 2005;52:1305–1313. doi: 10.1002/art.20987. [DOI] [PubMed] [Google Scholar]
- 60.Cao L, Hudson CA, Lawrence DA. Acute cold/restraint stress inhibits host resistance to Listeria monocytogenes via beta1-adrenergic receptors. Brain Behav Immun. 2003;17:121–133. doi: 10.1016/s0889-1591(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 61.Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chavan SS, Tracey KJ. Regulating innate immunity with dopamine and electroacupuncture. Nat Med. 2014;20:239–241. doi: 10.1038/nm.3501. [DOI] [PubMed] [Google Scholar]
- 63.Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol. 2001;166:232–240. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- 64.Blalock JE, Smith EM. Conceptual development of the immune system as a sixth sense. Brain Behav Immun. 2007;21:23–33. doi: 10.1016/j.bbi.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Blalock JE. Proopiomelanocortin and the immune-neuroendocrine connection. Ann N Y Acad Sci. 1999;885:161–172. doi: 10.1111/j.1749-6632.1999.tb08673.x. [DOI] [PubMed] [Google Scholar]
- 66.Catania A, Cutuli M, Garofalo L, Carlin A, Airaghi L, Barcellini W, Lipton JM. The neuropeptide alpha-MSH in host defense. Ann N Y Acad Sci. 2000;917:227–231. doi: 10.1111/j.1749-6632.2000.tb05387.x. [DOI] [PubMed] [Google Scholar]
- 67.Lipton JM, Catania A, Ichiyama T. Marshaling the Anti-Inflammatory Influence of the Neuroimmunomodulator alpha-MSH. News Physiol Sci. 2000;15:192–195. doi: 10.1152/physiologyonline.2000.15.4.192. [DOI] [PubMed] [Google Scholar]
- 68.Luger TA, Paus R, Slominski A, Lipton J. The proopiomelanocortin system in cutaneous neuroimmunomodulation. An introductory overview. Ann N Y Acad Sci. 1999;885:1–476. doi: 10.1111/j.1749-6632.1999.tb08661.x. [DOI] [PubMed] [Google Scholar]
- 69.Belda X, Fuentes S, Daviu N, Nadal R, Armario A. Stress-induced sensitization: the hypothalamic-pituitary-adrenal axis and beyond. Stress. 2015;18:269–279. doi: 10.3109/10253890.2015.1067678. [DOI] [PubMed] [Google Scholar]