Abstract
Mesenchymal stem cells (MSCs) are gaining relevance for treating equine joint injuries because of their ability to limit inflammation and stimulate regeneration. Because inflammation activates MSC immunoregulatory function, proinflammatory priming could improve MSC efficacy. However, inflammatory molecules present in synovial fluid or added to the culture medium might have deleterious effects on MSCs. Therefore, this study was conducted to investigate the effects of inflammatory synovial fluid and proinflammatory cytokines priming on viability and plasticity of equine MSCs. Equine bone marrow derived MSCs (eBM-MSCs) from three animals were cultured for 72 h in media supplemented with: 20% inflammatory synovial fluid (SF); 50 ng/mL IFN-γ and TNF-α (CK50); and 20 ng/mL IFN-γ and TNF-α (CK20). Proliferation assay and expression of proliferation and apoptosis-related genes showed that SF exposed-eBM-MSCs maintained their viability, whereas the viability of CK primed-eBM-MSCs was significantly impaired. Tri-lineage differentiation assay revealed that exposure to inflammatory synovial fluid did not alter eBM-MSCs differentiation potential; however, eBM-MSCs primed with cytokines did not display osteogenic, adipogenic or chondrogenic phenotype. The inflammatory synovial environment is well tolerated by eBM-MSCs, whereas cytokine priming negatively affects the viability and differentiation abilities of eBM-MSCs, which might limit their in vivo efficacy.
Keywords: horses, mesenchymal stromal cells, joint diseases, proinflammatory cytokines, synovial fluid
Introduction
Articular pathologies such osteoarthritis (OA) are common diseases in both equines and humans, greatly impacting the daily lives of afflicted individuals and acting as a major cause of wastage in the equine industry. Horses have a double role in joint pathologies because they commonly suffer from these diseases [32], and they are considered the most suitable animal model for testing cell based therapies for human joint injuries [3]. Osteoarthritis is a complex condition characterized by damage to the articular cartilage. Inflammation plays an important role in progression of the disease as the secretion of proinflammatory mediators accelerates cartilage degradation. Conventional treatments primarily focus on relieving this inflammation and controlling pain [11]. An ideal therapeutic approach should stop progressive loss of cartilage and stimulate the regeneration of damaged structures. Treatments for equine joint diseases based on the intra-articular (IA) administration of mesenchymal stem cells (MSCs) are gaining importance because of their regenerative role [8]. MSCs show significant potential for cartilage repair, which is attributed to their trophic and differentiation properties, as well as their immunoregulatory ability [21]. The expression of immunoregulatory molecules by MSCs is regulated by proinflammatory cytokines such interferon-γ (IFN-γ), particularly in combination with tumor necrosis factor-α (TNF-α) [29], which suggests that MSC full immunoregulatory function depends on their activation after inflammatory exposure. Therefore, MSC priming with proinflammatory cytokines prior to their use in vivo might enhance their therapeutic efficacy [5].
Joint injuries result in a variable release of different inflammatory molecules into the synovial fluid. Inflammatory synovial fluid may affect the function and characteristics of IA administered MSCs, such as migratory function or differentiation potential [16], or act as inductors of MSC-immune regulation in equine and human species [18,37]. However, the stimulus exerted by synovial fluid could be heterogeneous because variation exists between patients, even within one disease group [18]. Therefore, the combination of definite doses of INFγ with TNF-α leads to a more homogenous stimulus for inducing MSC immunomodulation, which could improve their therapeutic potential. However, the regenerative potential of MSCs through differentiation could be decreased by inflammatory exposure [7,17,20]. In addition, IFN-γ and TNF-α synergistically impair MSC self-renewal, decreasing their effectiveness [38]. To the best of our knowledge, the effects of these cytokines on these properties in equine MSCs have not yet been elucidated.
Because pre-existing joint inflammation may alter the therapeutic efficacy of MSCs when they are IA administered [31], further knowledge of the changes induced in equine MSCs by an inflammatory environment is necessary. Conversely, the use of IFN-γ and TNF-α priming without detriment of viability and plasticity is of major interest to improve cellular therapies. Thus, this study was conducted to assess the possible effects of different inflammatory stimuli on viability and differentiation potential of equine bone marrow derived MSCs (eBM-MSCs) as a step prior for their clinical use in joint injured patients.
Materials and Methods
Experimental design
Equine BM-MSCs (n = 3) were exposed to three different inflammatory conditions and their responses were analyzed: Experiment 1 (SF), 20% allogeneic inflammatory synovial fluid was added to the culture medium to mimic the joint inflammatory environment; Experiment 2 (CK50), IFN-γ (50 ng/mL) and TNF-α (50 ng/mL) were added to the culture medium; and Experiment 3 (CK20), IFN-γ (20 ng/mL) and TNF-α (20 ng/mL) were added to the culture medium. All inflammatory conditions were maintained for 72 h. Subsequently, a proliferation assay was conducted for 7 days. Expression of proliferation and apoptosis related genes was analyzed by real time quantitative polymerase chain reaction (RT-qPCR). Tri-lineage differentiation potential was examined through specific staining and gene expression by RT-qPCR. Triplicate controls were run in each experiment using the same eBM-MSCs (n = 3) cultured with control media.
Animals
Four geldings, named as EQ01, EQ02, EQ03 and EQ04 (weight, 450–500 kg; age, 6–12 years), were used in this study. The horses were patients from the Veterinary Hospital of the University of Zaragoza suffering from varying types of locomotor system injuries, but determined to be in systemic good health based on clinical and hematologic examination. Biological samples (bone marrow [BM] and synovial fluid) were obtained with owner consent and according to local animal welfare regulations.
All procedures were carried out within the Project License 31/11 approved by the in-house Ethic Committee for Animal Experiments of the University of Zaragoza. The care and use of animals were in accordance with the Spanish Policy for Animal Protection RD53/2013, which meets the European Union Directive 2010/63 on the protection of animals used for experimental and other scientific purposes.
Harvesting and characterization of eBM-MSCS
A total of 40 mL of BM from the sternum were collected in heparinized syringes using a 11 g × 101 mm Jamshidi needle (CareFusion, USA) from three donors (EQ01, EQ02 and EQ03). Equine BM-MSCs were isolated using a gradient density separation technique as previously described by our group [27]. The cells were plated and cultured in basal culture medium consisting of low glucose Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 1% glutamine, 1% streptomycin/penicillin and 10% fetal bovine serum (FBS) (all from Sigma-Aldrich, USA) [27]. Cells were expanded until the third passage, then characterized by phenotype determination (flow cytometry and gene expression) and tri-lineage differentiation as previously described [27]. Cells were subsequently frozen in 10% DMSO-90% FBS (Sigma-Aldrich) frozen medium and cryopreserved until experiments started.
Inflammatory synovial fluid collection
Synovial fluid was harvested from EQ04, which presented aseptic synovitis in one tarso-crural joint. Arthrocentesis was performed to reduce the joint effusion and aspirated synovial fluid was collected in heparin-treated tubes (Becton, Dickinson and Company, USA) and used for Experiment 1. Synovial fluid inflammatory status was verified by measuring total protein (g/dL) with a portable optical refractometer (RHB-32 Hand-held Brix Refractometer; Spectrum Technologies, USA), by conducting a cytological examination and determine the concentration of the acute phase proteins (APPs) serum amyloid A (SAA) and haptoglobin (Hp) [2]. Subsequently, synovial fluid was centrifuged at 3,000 × g for 15 min, after which the supernatant was stored at −80℃. All processes were performed under aseptic conditions to prevent culture contamination.
Equine BM-MSCs culture under inflammatory condi
The cytokines used for CK priming were selected based on their described synergy [30]. Synovial fluid and CK inflammatory conditions were determined according to previous reports [29,35,37,39]. The time of exposure was determined to be 72 h [26] for the three conditions. For Experiment 1, inflammatory synovial fluid was added to the basal culture medium at 20% (SF medium). For Experiments 2 and 3, basal media were supplemented with recombinant equine TNF-α and IFN-γ (R&D Systems, USA) by adding 50 ng/mL of each cytokine in Experiment 2 (CK50 medium), whereas medium was supplemented with 20 ng/mL of each cytokine for Experiment 3 (CK20 medium). Basal medium was used in control eBM-MSCs in the three experiments (control medium).
One million eBM-MSCs at third passage from EQ01, EQ02 and EQ03 were thawed at 37℃ and seeded at 5,000 cells/cm2 in basal medium at 37℃ and 5% CO2 until confluence reached 80 to 90%, allowing readjustments of conditions prior to initiating the experiments. SF-, CK50-, CK20- or control-media were then added to the cells. Inflammatory exposure and their controls were carried out in triplicate for each animal in each experiment (SF, CK50, and CK20). Cells were cultured for 72 h in their corresponding media at 37℃ and 5% CO2, after which eBM-MSCs were detached with 0.25% trypsin-EDTA (Sigma-Aldrich), washed three times with PBS (Gibco, USA) to completely remove inflammatory molecules, and then used for the different assays.
Proliferation assay
Cell proliferation after every inflammatory exposure and their controls was evaluated over seven days by MTT proliferation assay as previously described [28]. Viable cell numbers for the different samples were determined using a calibration curve consisting of nine triplicate points of increasing amounts of cells [28]. Basal medium was used as a blank. The cell doubling time for the control and inflammatory-stimulated eBM-MSCs from each experiment was calculated.
Tri-lineage differentiation assay
Control and inflammatory exposed cells were cultured in induction medium (differentiation) and basal medium (control) in triplicate according to each differentiation assay. For osteogenic differentiation, eBM-MSCs from each sample were seeded at 20,000 cells/cm2 in 24-well plates. Osteogenic medium consisted of 10 nmol/L dexamethasone, 10 mmol/L β-glycerophosphate and 100 µmol/L ascorbate-2-phosphate (all from Sigma-Aldrich) supplemented basal medium. After 7 days, typical calcium deposit formation was assessed by Alizarin Red staining. To accomplish this, cells were fixed with 70% ethanol for 1 h at room temperature (RT), stained with 2% Alizarin Red stain (pH 4.6) (Sigma-Aldrich) for 10 min (RT) and washed with PBS.
Equine BM-MSCs from each sample were seeded at 5,000 cells/cm2 in 12-well plates for adipogenic differentiation. Adipogenic medium consisted of 1 µmol/L dexamethasone, 500 µmol/L 3-isobutyl-1-methylxanthine, 200 µmol/L indomethacin and 15% rabbit serum (all from Sigma-Aldrich) supplemented basal medium. After 15 days, typical fat droplets were evaluated by Oil Red O staining. Briefly, cells were fixed with 10% formalin (Sigma-Aldrich) for 15 min at RT, stained with 0.3% Oil Red O (Sigma-Aldrich) (dissolved in 60:40; isopropanol: distilled water) stain for 30 min at 37℃, then washed with distilled water.
Chondrogenic differentiation was conducted in pellet culture. To accomplish this, approximately 300,000 eBM-MSCs from each sample were transferred to conic bottom 15 mL tubes, after which 400 µL of chondrogenic differentiation medium were added and the samples were centrifuged at 300 × g for 5 min to pellet the cells. Chondrogenic medium consisted of 10% FBS, 10 ng/mL TGFβ-3 (R&D Systems), ITS+ premix (Becton, Dickinson and Commpany), 40 µg/mL proline, 50 µg/mL ascorbate-2-phosphate, and 0.1 µmol/L dexamethasone supplemented high glucose DMEM (all from Sigma-Aldrich). After 21 days, chondrogenic differentiation was evaluated by specific staining with Alcian blue staining. Pellets were fixed in 10% formalin, embedded in paraffin and sectioned into 5 µm sections. The sections were then hydrated with increasing gradients of alcohol, stained with Mayer's haematoxylin and 3% Alcian blue, rinsed with distilled water, dehydrated with decreasing amounts of alcohols and mounted.
Real time quantitative polymerase chain reaction (RT-qPCR)
Expression of genes coding for molecules related to proliferation (cyclooxygenase 1 [COX-1] COX-1, Cyclin D2) and apoptosis (BAX, BCL-2, BCL-XL, CASP-8, HSP-27, TNF-α, IFN-γ) was assessed by RT-qPCR to investigate eBM-MSCs viability. A RNAspin Mini RNA Isolation Kit (GE Healthcare, UK) was used to isolate total mRNA from approximately 106 eBM-MSCs from each sample. Genomic DNA was removed using DNAse Turbo (Ambion, USA) and 1.5 µg of mRNA from each sample were retrotranscripted to cDNA with a Superscript Reverse Transcriptase Kit (Life Technologies, USA). mRNA isolation and cDNA retrotranscription from osteogenic and adipogenic differentiation assays were performed using a Cells-to-cDNA II kit (Ambion). mRNA could not be isolated from differentiated CK20-eBM-MSCs or from cells undergoing chondrogenic differentiation because there was not enough sample to perform both staining and mRNA isolation. All processes were conducted according to manufacturer's instructions.
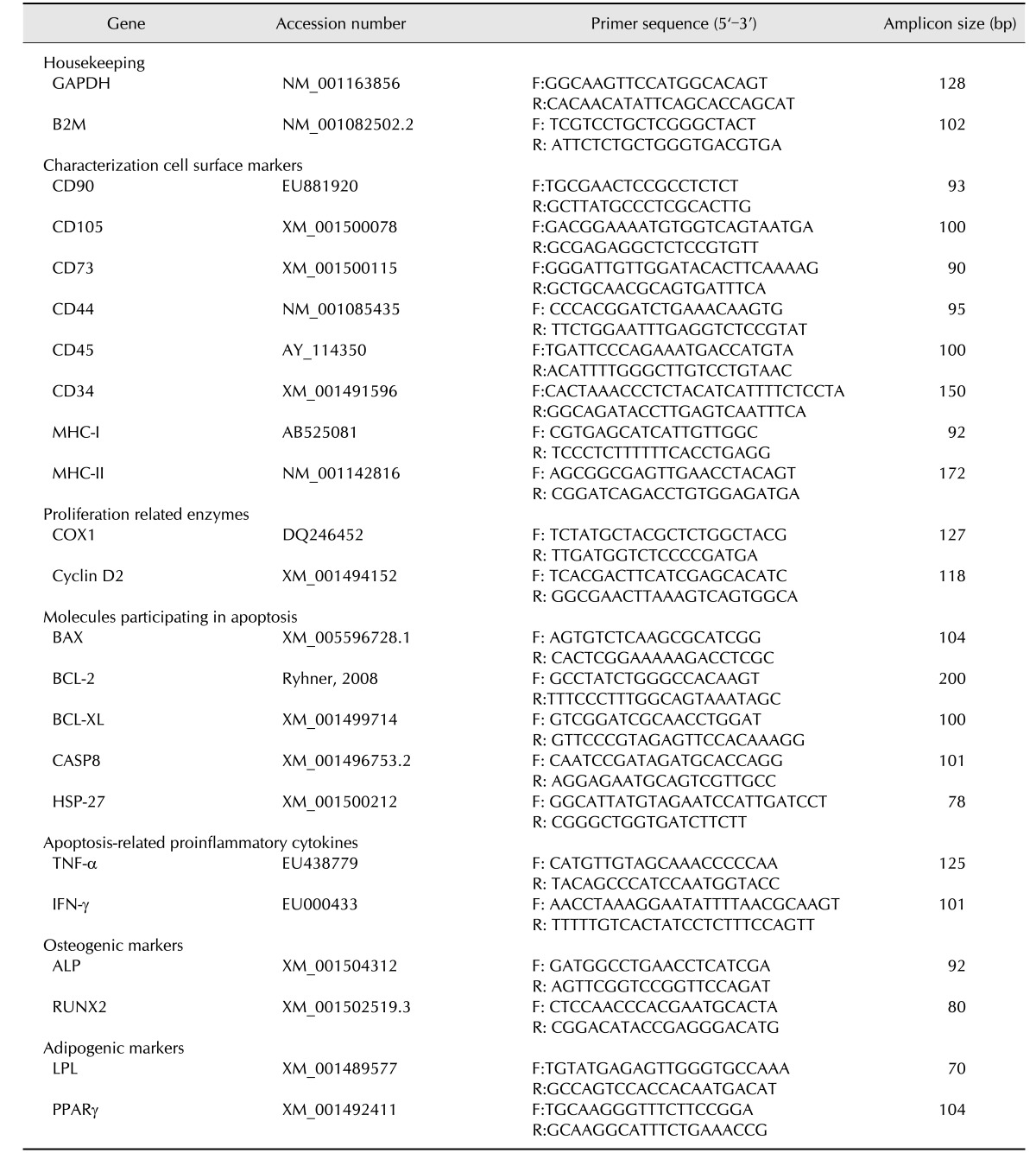
A StepOne Real Time PCR System device (Applied Biosystems, USA) was used to perform and monitor RT-qPCR. All reactions were carried out in a total volume of 10 µL with 2 µL of cDNA as the template and Fast SYBR Green Master Mix (Applied Biosystems). Amplification and analysis were performed as previously described [27]. The expression levels of all genes in each sample were normalized by a normalization factor (NF), which was calculated as the geometric mean of the quantity of two housekeeping genes, GAPDH and B2M [15]. The Primer Express 2.0 software (Applied Biosystems) was used to design primers based on known equine sequences. Information about primers is shown in Table 1.
Table 1. Primers used for gene expression by real time quantitative polymerase chain reaction (RT-qPCR). GenBank accession numbers of the sequences used for primers design. Primers (F: forward and R: reverse) and length of the amplicon in base pairs (bp).
Genes were grouped in agreement with the functions and implications of encoded molecules as follows to facilitate the posterior analysis: housekeeping, cell surface markers, proliferation related enzymes, molecules participating in apoptosis, apoptosis-related proinflammatory cytokines, osteogenic markers and adipogenic markers.
Statistical analysis
Data obtained in this study were subjected to statistical analysis using SPSS 15.0 (SPSS, USA). Proliferation data are presented as the means (n = 3) ± SEM cell count of stimulated and unstimulated eBM-MSCs at each time point (7 days) for each experiment. Differences in mean cell numbers between stimulated and unstimulated eBM-MSCs from each day were analyzed by paired Student's t tests separately for each experiment. RT-qPCR data are reported as the means (n = 3) ± SEM fold increase or decrease of stimulated eBM-MSCs gene expression over unstimulated control eBM-MSC. Differences between eBM-MSCs from every inflammatory condition (SF, CK50 and CK20) and their controls were analyzed by paired Student's t-tests. The significance level was set at p < 0.05 for all analyses.
Results
Isolation and characterization of eBM-MSCS
Cells obtained from EQ01, EQ02 and EQ03 BM aspirates showed plastic-adherent fibroblast-like morphology and were positive for the surface markers CD90, CD105, CD44 and MHC-I and negative for MHC-II by flow cytometry. All samples expressed transcripts for CD105, CD90, CD73, CD44 and MHC-I, but not for CD34 and CD45, and low levels for MHC-II by RT-qPCR. The ability of tri-lineage was confirmed in all cases (data not shown).
Inflammatory synovial fluid harvesting
The average total protein measurement in the harvested synovial fluid was 2.25 g/dL. The SAA concentration was 0.585 × 10−3 g/dL and the Hp concentration was 0.023 g/dL. These data confirmed the inflammatory status of the synovial fluid, and when combined with the cytological examination discarded a septic origin of the joint inflammation.
Effects of inflammatory environment on eBM-MSCs proliferation
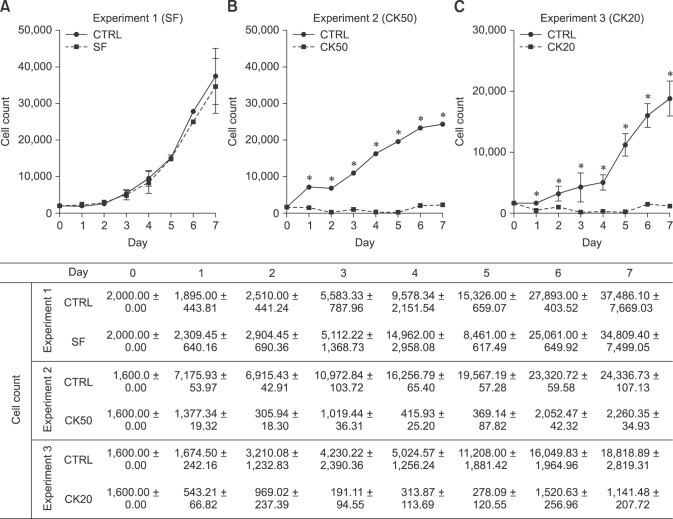
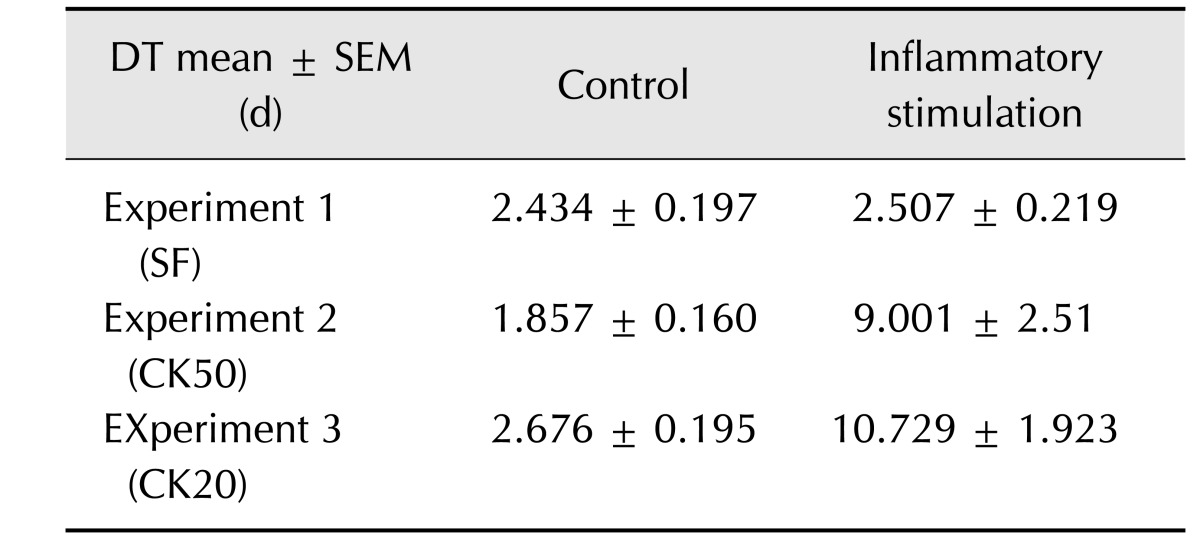
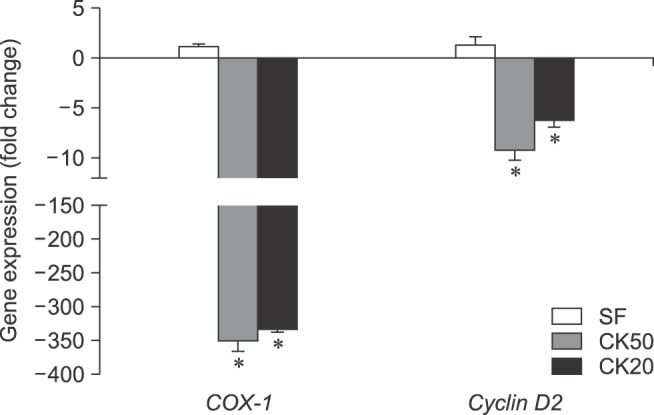
For the MTT assay, the total number of viable cells from every sample was determined by extrapolation from the calibration curve: y = 9 × 10−6 x + 0.0805, r2 = 0,939, where y represents the optical density of the well and x represents the amount of cells. Synovial fluid-exposed eBM-MSCs grew in a similar manner to control cells (panel A in Fig. 1). In both CK50 and CK20 experiments, the number of CK-stimulated cells decreased until the fourth day, then increased slightly until the sixth day, but still remained below the initial level (panels B and C in Fig. 1). Significant differences (p < 0.05) in the number of cells were observed between control and CK-exposed cells in all days under both CK20 and CK50 conditions. As shown in Table 2, the cell doubling times were also similar between control and SF stimulated eBM-MSCs, but increased greatly in both CK50 and CK20-stimulated eBM-MSCs. The proliferation related genes COX-1 and Cyclin D2 were significantly downregulated under the CK50 and CK20 conditions (p < 0.05), and their expression remained similar in the SF experiment (Fig. 2).
Fig. 1. Proliferation related enzymes of control and inflammatory-exposed eBM-MSCs from Experiment 1 (A), 2 (B) and 3 (C) over 7 days. eBM-MSC proliferation potential was unaltered after SF exposure; however, this property was diminished after culturing cells with both CK20 and CK50 media. Proliferation was evaluated by MTT assay. The means ± SEM (n = 3) of the cell count are shown for each experiment at each time-point in both linear graphs (*p < 0.05) and a data table.
Table 2. Cell doubling time (DT) for control and inflammatory-stimulated eBM-MSC.
Results are shown as the mean ± SEM (n=3) of DT for control and inflammatory exposed eBM-MSCs from Experiment 1 (SF), 2 (CK50) and 3 (CK20). Cell doubling time was calculated according to the formula: CD = ln [(Nf/Ni)/ln2] and DT = CT/CD, where DT, cell doubling time; Nf, final number of cells; Ni, initial number of cells; CD, cell doubling number.
Fig. 2. eBM-MSCs expression of proliferation related enzymes is influenced by inflammatory stimulation. Gene expression of COX-1 and CyclinD2 in inflammatory exposed eBM-MSCs is expressed as the mean ± SEM (n = 3) fold increase or decrease compared to the corresponding control from each experiment (*p < 0.05).
Effect of inflammatory environment on apoptosis gene expression of eBM-MSCs
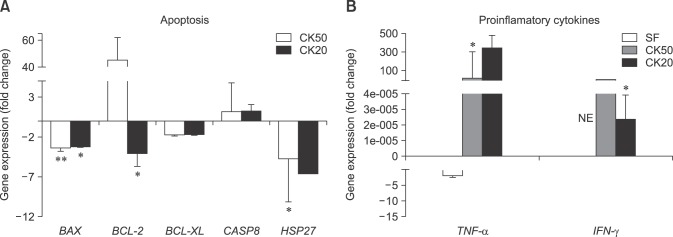
Apoptosis-related gene expression was studied in CK50 and CK20 exposed eBM-MSCs. BAX was significantly downregulated under both CK50 (p < 0.01) and CK20 (p < 0.05) conditions, while BCL-2 was only significantly downregulated under CK20 conditions. CASP8 showed a trend to increase its expression under both CK conditions. The anti-apoptotic factor heat shock protein 27 (HSP27) was significantly downregulated under CK50 conditions (panel A in Fig. 3). TNF-α expression was downregulated and no-expression was found for IFN-γ under SF conditions, whereas significant upregulation of TNF-α (p < 0.05) and IFN-γ (p < 0.05) was observed in CK50 and CK20, respectively (panel B in Fig. 3).
Fig. 3. eBM-MSCs expression of certain genes encoding apoptosis-related molecules can be affected by proinflammatory cytokines. Gene expression of each gene in every experiment is represented as the mean ± SEM (n = 3) fold increase or decrease compared to the corresponding control from each experiment. (A) Expression of genes encoding molecules directly participating in the apoptosis mechanisms BAX, BCL-2, BCL-XL, CASP8, and HSP27. (B) Expression of genes encoding proinflammatory cytokines related to apoptosis TNF-α and IFN-γ. NE, no expression. *p < 0.05 and **p < 0.01.
Effect of inflammatory environment on eBM-MSCs differentiation potential
Osteogenic, adipogenic and chondrogenic differentiation were achieved in control (unstimulated) cells from the three experiments. Spontaneous differentiation was not observed in any of the non-differentiated controls.
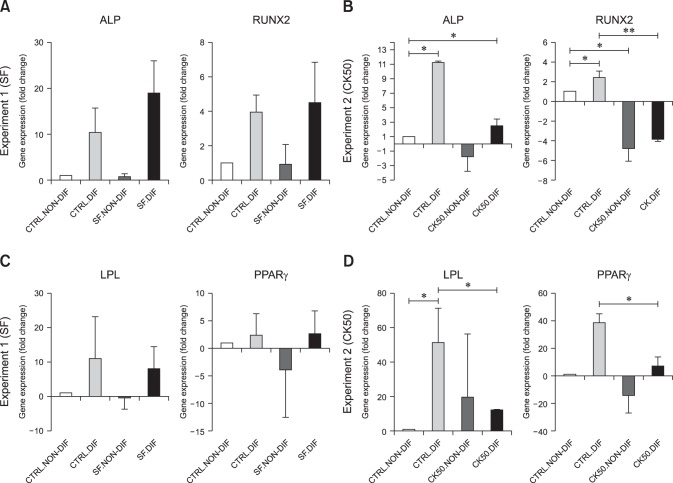
Osteogenic differentiation was confirmed in SF-exposed eBM-MSCs by positive staining of the calcium deposits with Alizarin red. CK-stimulated eBM-MSCs showed irregular morphology, but did not form a calcium-rich matrix (panel A in Fig. 4). Alkaline phosphatase (ALP) and Runt-related transcription factor 2 (RUNX2) gene expression was not significantly modified by SF conditions, but the expression of both genes in SF-exposed eBM-MSCs was slightly higher than in the differentiated control (panel A in Fig. 5). Expression of ALP and RUNX2 was downregulated in CK50-exposed eBM-MSCs compared to the differentiated control; however, this downregulation was only significant for RUNX2 (p < 0.05) (panel B in Fig. 5). ALP increased by 18.93-fold in SF-differentiated cells relative to the control, but only by 2.49 in CK50-differentiated cells. RUNX2 expression in SF-differentiated cells increased by 4.48-fold, whereas a 3.85-fold decrease was observed in CK50-differentiated cells (panels A and B in Fig. 5).
Fig. 4. Staining for osteogenic, adipogenic and chondrogenic differentiation of control and inflammatory-stimulated eBM-MSCs from three experiments. (A) Alizarin red staining of eBM-MSCs cultured for 7 days in osteogenic differentiation medium from control and Experiment 1–3. (B) Oil red O staining of eBM-MSCs cultured for 15 days in adipogenic differentiation medium from control and Experiment 1–3. (C) Haematoxylin and Alcian blue staining of pellets cultured for 21 days in chondrogenic medium from control and Experiment 1–3. Scale bars = 500 µm (A and B), 2 µm (C). 10× (A), 4× (B), 20×(C).
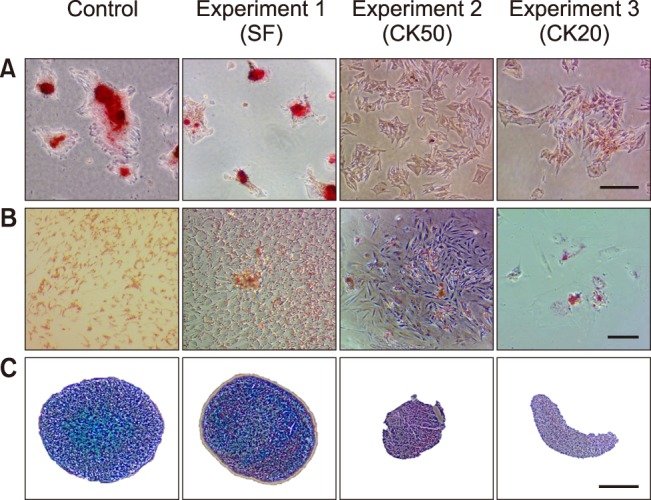
Fig. 5. Effect of inflammatory conditions on gene expression of differentiation markers. Influence of the three inflammatory conditions tested on eBM-MSC expression of genes encoding osteogenesis (ALP and RUNX2) and adipogenesis (LPL and PPARγ) markers. The results are expressed as the mean ± SEM (n = 3) fold increase or decrease of differentiated control cells (CTRL.DIF), non-differentiated SF or CK50 exposed eBM-MSCs (SF.NON-DIF and CK50.NON-DIF) and differentiated SF or CK50 exposed eBM-MSCs (SF.DIF and CK50.DIF) over control unstimulated and non-differentiated cells (CTRL.NON-DIF). The white bar in all graphs (1 ± 0) represents the CTRL.NON-DIF fold change over itself relative to the fold increase or decrease under other conditions. (A and B) Gene expression of osteogenesis markers in cells from Experiment 1 (A) and Experiment 2 (B). (C and D) Gene expression of adipogenesis markers in cells from Experiment 1(C) and Experiment 2 (D).
Oil red O staining revealed lipid droplets in SF-exposed eBM-MSCs undergoing adipogenic induction. Both CK50 and CK20-stimulated cells did not display an adipogenic phenotype (panel B in Fig. 4). Peroxisome proliferator-activated receptor γ (PPARγ) and lipoprotein lipase (LPL) mRNA levels were not significantly modified by SF exposure (panel C in Fig. 5). LPL and PPARγ in CK50- and SF-differentiated cells showed similar increases relative to non-differentiated control cells, but both genes were significantly downregulated in CK50-exposed cells (p <0.05) compared to unstimulated differentiated cells (panel D in Fig. 5).
Synovial fluid-exposed cells maintained their chondrogenic differentiation potential, as indicated by the positive blue staining of the extracellular matrix upon Alcian blue staining. However, neither CK50 nor CK20-exposed eBM-MSCs differentiated into the chondrogenic lineage, and they formed irregular discoid pellets instead of the typical spherical shape (panel C in Fig. 4).
Discussion
Both APPs analyzed in the inflammatory synovial fluid were above the reference ranges [1,14], which is in agreement with the expected changes for these proteins [14]. The inflammatory environment provided by cytokine priming resulted in a remarkable viability impairment of eBM-MSCs, whereas SF conditions did not alter their regular growth. The significant down-regulation of COX1 and CyclinD2, enzymes involved in the regulation of the cell cycle and cell proliferation [33,36], in both CK experiments might be related to the failed proliferation of eBM-MSCs [33,36]. Thus, treatment with both cytokine concentrations appeared to be cytotoxic. To elucidate this phenomenon under CK conditions, the expression of several genes involved in the apoptosis process was investigated. BCL2-Associated X Protein (BAX) is a proapoptotic gene and BCL-2 is an antiapoptotic gene, both of which are involved in the apoptosis mitochondrial pathway [13]. While BAX was downregulated under both CK conditions, BCL-2 expression showed contrasting results between CK50 and CK20 experiments, which suggest that the apoptosis mitochondrial pathway might not be involved. However, the apoptosis death receptor pathway might be involved since CASP8 showed a trend to upregulation [13]. In addition, the downregulation of HSP27, which protects against injury-related MSC apoptosis [34], might be associated with failed cell development and cell differentiation [34]. TNF-α and IFN-γ have a synergic effect in the induction of apoptosis by the death receptor signaling pathway in MSCs [20]. Additionally, TNF-α converts the signaling of the non-apoptotic receptor Fas, which is activated by IFN-γ, into a CASP8 proapoptotic cascade in MSCs [20,38]. Based on these findings, the apoptotic process might be involved in the low viability and plasticity of CK-exposed eBM-MSCs.
Reduction of the tri-lineage differentiation ability of MSCs by proinflammatory cytokines has been described in other species. T-cells mediated secretion of TNF-α and IFN-γ synergistically hamper osteogenesis, chondrogenesis [6,9,23] and adipogenesis [17]. TNF-α or IL-1β exposure significantly reduces the gene expression of osteogenic, adipogenic and chondrogenic markers in murine MSCs [17]. In humans and rats, these cytokines inhibit the expression of the osteoblast differentiation transcription factor, RUNX2 [7,9]. Moreover, TNF-α plays a dual role in osteogenesis. On the one hand, it inhibits the osteoblast differentiation transcription factor RUNX2 [9]. On the other hand, TNF-α can enhance the MSC osteogenic differentiation in a dose-dependent manner, although this effect is avoided in the presence of anti-inflammatory agents, such as dexamethasone, which is added to the osteogenic-induction media [22]. It has been proposed that the inhibition of osteoblast differentiation by TNF-α occurs through the p55 TNF receptor [10]. High secretion of IFN-γ by immune cells has also been shown to prevent osteogenesis in MSCs in allogeneic implants in mice by dramatically reducing the expression of ALP and RUNX2 [6]. Our results agreed with those of previous reports in others species since the transcript levels of ALP and RUNX2 detected were lower in the presence of TNF-α and IFN-γ Adipogenesis could also be impaired by inflammatory conditions because high levels of proinflammatory cytokines, such as TNF-α, lead to a reduction in PPARγ expression by MSCs [4]. In agreement, PPARγ gene expression was down-regulated under our CK conditions, resulting in a commitment of adipogenic differentiation. TNF-α and IFN-γ have also been described as inhibitors of chondrogenesis and collagen formation in rat and mouse MSCs [17,24]. Chondrogenesis has been shown to be hindered through the inhibition of the chondrogenic factor Sox9 caused by these cytokines [23]. According to these reports, our CK conditions also led to a lack of chondrogenic potential in eBM-MSCs. The effects of proinflammatory cytokines on MSC differentiation could vary between species and, to our knowledge, this is the first time that this effect has been studied on equine MSCs. Overall, our results support the findings reported for other species [7,17] demonstrating the inhibitory effect of proinflammatory cytokines on the tri-lineage differentiation of eBM-MSCs.
The natural joint environment could be favorable for chondrogenic differentiation [12,25]. However, when proinflammatory cytokines are present in high amounts, synovial fluid turns into an inflammatory environment, preventing chondrogenesis [19]. Depending on the level of proinflammatory cytokines, inflammatory synovial fluid could be more or less harmful for chondrogenesis and cartilage matrix formation (i.e., rheumatoid arthritis vs. OA) [16]. Under our experimental conditions, tri-lineage differentiation was achieved by SF-exposed eBM-MSCs, suggesting that a moderate inflammatory status of the synovial fluid did not have a significant effect on eBM-MSCs differentiation ability.
The present research provides novel results about eBM-MSCs in inflammatory environments that can contribute to their intra-articular use for therapeutic applications. In summary, an inflammatory synovial environment is satisfactorily tolerated by eBM-MSCs, which maintained their proliferation and differentiation abilities, encouraging the use of cell therapy for joint pathologies. In contrast, cytokine priming tested in this study negatively affected the eBM-MSCs proliferation and differentiation abilities, and appeared to induce apoptosis, possibly compromising their in vivo efficacy and their therapeutic potential.
Acknowledgments
This study was supported by the Ministry of Economy and Competitiveness, Spain (AGL2011-28609) and by the Government of Aragón (Research Group LAGENBIO). Laura Barrachina is funded by a doctoral grant from the Government of Aragón. Ana Rosa Remacha is funded by a doctoral grant (EPIF) from the University of Zaragoza. We thank the horse owners for allowing their animals to be part of this study and the Veterinary Hospital of the University of Zaragoza for facilitating access to equine patients and allowing the use of its installations for collecting samples. We also acknowledge the Department of Biochemistry and Molecular and Cellular Biology of the University of Zaragoza for the help with analyses of acute phase proteins in the synovial fluid.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Basile RC, Ferraz GC, Carvalho MP, Albernaz RM, Araújo RA, Fagliari JJ, Queiroz-Neto A. Physiological concentrations of acute-phase proteins and immunoglobulins in equine synovial fluid. J Equine Vet Sci. 2013;33:201–204. [Google Scholar]
- 2.Casella S, Fazio F, Giannetto C, Giudice E, Piccione G. Influence of transportation on serum concentrations of acute phase proteins in horse. Res Vet Sci. 2012;93:914–917. doi: 10.1016/j.rvsc.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Cellular, Tissue, and Gene Therapies Advisory Committee. Cellular products for joint surface repair. Briefing document of the 38th Meeting of Cellular, Tissue, and Gene Therapies Advisory Committee; 3-4 March 2005; Silver Spring, USA. [Google Scholar]
- 4.Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36:379–386. doi: 10.1007/s10753-012-9557-z. [DOI] [PubMed] [Google Scholar]
- 5.Cuerquis J, Romieu-Mourez R, François M, Routy JP, Young YK, Zhao J, Eliopoulos N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-γ and tumor necrosis factor-α stimulation. Cytotherapy. 2014;16:191–202. doi: 10.1016/j.jcyt.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Dighe AS, Yang S, Madhu V, Balian G, Cui Q. Interferon gamma and T cells inhibit osteogenesis induced by allogeneic mesenchymal stromal cells. J Orthop Res. 2013;31:227–234. doi: 10.1002/jor.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, Hardouin P, Magne D. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84:499–504. doi: 10.1016/j.lfs.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Ferris DJ, Frisbie DD, Kisiday JD, McIlwraith CW, Hague BA, Major MD, Schneider RK, Zubrod CJ, Kawcak CE, Goodrich LR. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg. 2014;43:255–265. doi: 10.1111/j.1532-950X.2014.12100.x. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS, Nanes MS. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2αA) is inhibited by tumor necrosis factor-α. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. Am J Physiol Endocrinol Metab. 2005;288:E1011–E1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- 11.Goodrich LR, Nixon AJ. Medical treatment of osteoarthritis in the horse - a review. Vet J. 2006;171:51–69. doi: 10.1016/j.tvjl.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Hegewald AA, Ringe J, Bartel J, Krüger I, Notter M, Barnewitz D, Kaps C, Sittinger M. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36:431–438. doi: 10.1016/j.tice.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen S, Thomsen MH, Nanni S. Concentrations of serum amyloid A in serum and synovial fluid from healthy horses and horses with joint disease. Am J Vet Res. 2006;67:1738–1742. doi: 10.2460/ajvr.67.10.1738. [DOI] [PubMed] [Google Scholar]
- 15.Kolm G, Klein D, Knapp E, Watanabe K, Walter I. Lactoferrin expression in the horse endometrium: relevance in persisting mating-induced endometritis. Vet Immunol Immunopathol. 2006;114:159–167. doi: 10.1016/j.vetimm.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Krüger JP, Endres M, Neumann K, Stuhlmüller B, Morawietz L, Häupl T, Kaps C. Chondrogenic differentiation of human subchondral progenitor cells is affected by synovial fluid from donors with osteoarthritis or rheumatoid arthritis. J Orthop Surg Res. 2012;7:10. doi: 10.1186/1749-799X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009;17:735–742. doi: 10.1016/j.joca.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Leijs MJC, van Buul GM, Lubberts E, Bos PK, Verhaar JAN, Hoogduijn MJ, van Osch GJVM. Effect of arthritic synovial fluids on the expression of immunomodulatory factors by mesenchymal stem cells: an explorative in vitro study. Front Immunol. 2012;3:231. doi: 10.3389/fimmu.2012.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Xu Y, Chen S, Tan Z, Xiong K, Li Y, Ye Y, Luo ZP, He F, Gong Y. Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic Biol Med. 2014;68:234–246. doi: 10.1016/j.freeradbiomed.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-α on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010;31:1666–1675. doi: 10.1016/j.biomaterials.2009.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-α. J Biol Chem. 2000;275:3687–3692. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- 24.Nanes MS, McKoy WM, Marx SJ. Inhibitory effects of tumor necrosis factor-α and interferon-γ on deoxyribonucleic acid and collagen synthesis by rat osteosarcoma cells (ROS 17/2.8) Endocrinology. 1989;124:339–345. doi: 10.1210/endo-124-1-339. [DOI] [PubMed] [Google Scholar]
- 25.Ogueta S, Muñoz J, Obregon E, Delgado-Baeza E, García-Ruiz JP. Prolactin is a component of the human synovial liquid and modulates the growth and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol Cell Endocrinol. 2002;190:51–63. doi: 10.1016/s0303-7207(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 26.Paterson YZ, Rash N, Garvican ER, Paillot R, Guest DJ. Equine mesenchymal stromal cells and embryo-derived stem cells are immune privileged in vitro. Stem Cell Res Ther. 2014;5:90. doi: 10.1186/scrt479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranera B, Lyahyai J, Romero A, Vázquez FJ, Remacha AR, Bernal ML, Zaragoza P, Rodellar C, Martín-Burriel I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol. 2011;144:147–154. doi: 10.1016/j.vetimm.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Ranera B, Ordovás L, Lyahyai J, Bernal ML, Fernandes F, Remacha AR, Romero A, Vázquez FJ, Osta R, Cons C, Varona L, Zaragoza P, Martín-Burriel I, Rodellar C. Comparative study of equine bone marrow and adipose tissue-derived mesenchymal stromal cells. Equine Vet J. 2012;44:33–42. doi: 10.1111/j.2042-3306.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- 29.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts S, Genever P, McCaskie A, De Bari C. Prospects of stem cell therapy in osteoarthritis. Regen Med. 2011;6:351–366. doi: 10.2217/rme.11.21. [DOI] [PubMed] [Google Scholar]
- 32.Schlueter AE, Orth MW. Equine osteoarthritis: a brief review of the disease and its causes. Equine Comp Exerc Physiol. 2004;1:221–231. [Google Scholar]
- 33.Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 34.Son TW, Yun SP, Yong MS, Seo BN, Ryu JM, Youn HY, Oh YM, Han HJ. Netrin-1 protects hypoxia-induced mitochondrial apoptosis through HSP27 expression via DCC- and integrin α6β4-dependent Akt, GSK-3β, and HSF-1 in mesenchymal stem cells. Cell Death Dis. 2013;4:e563. doi: 10.1038/cddis.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JAN, Bernsen MR, van Osch GJVM. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012;20:1186–1196. doi: 10.1016/j.joca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 37.Vézina Audette R, Lavoie-Lamoureux A, Lavoie JP, Laverty S. Inflammatory stimuli differentially modulate the transcription of paracrine signaling molecules of equine bone marrow multipotent mesenchymal stromal cells. Osteoarthritis Cartilage. 2013;21:1116–1124. doi: 10.1016/j.joca.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Zhao Y, Liu Y, Akiyama K, Chen C, Qu C, Jin Y, Shi S. IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cells. 2013;31:1383–1395. doi: 10.1002/stem.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmermann JA, McDevitt TC. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy. 2014;16:331–345. doi: 10.1016/j.jcyt.2013.09.004. [DOI] [PubMed] [Google Scholar]