Abstract
Background:
Health status of offspring is programmed by maternal diet throughout gestation and lactation. The present study investigates the lasting effects of maternal supplementation with different amounts of soy oil or extra virgin olive oil (EVOO) on weight and biochemical parameters during gestation and lactation of female mice offspring.
Methods:
Eight weeks old female C57BL/6 mice (n=40) were assigned through simple randomization into four isocaloric dietary groups (16% of calories as soy oil (LSO) or EVOO (LOO) and 45% of calories as soy oil (HSO) or EVOO (HOO)) during three weeks of gestation and lactation. After weaning (at 3 weeks), all offspring received a diet containing 16% of calories as soy oil and were sacrificed at 6 weeks. Two-way ANOVA was used to adjust for confounding variables and repeated measures test for weight gain trend. Statistical analyses were performed with the IBM SPSS package.
Results:
At birth and adolescence, the weight of offspring was significantly higher in the soy oil than the olive oil groups (P<0.001 and P<0.001, respectively). Adolescence weight was significantly higher in the offspring born to mothers fed with 16% oil than those with 45% oil (P=0.001). Serum glucose, triglyceride and total cholesterol were significantly higher in the LSO than LOO (P<0.001, P<0.001 and P<0.001), LSO than HSO (P<0.001, P=0.03 and P<0.001), and LOO than HOO (P<0.001, P<0.001 and P<0.001) dietary groups, respectively. Serum triglyceride and total cholesterol were significantly higher in the offspring of HSO than HOO fed mothers (P<0.001 and P<0.001, respectively).
Conclusion:
A maternal diet containing EVOO has better effects on birth weight, as well as weight and serum biochemical parameters in offspring at adolescence.
Keywords: Diet, Body weight, Lipid profile, Glucose, Multiple birth offspring
What’s Known
Studies have shown that a maternal high-fat/high-calorie diet containing omega-6 fatty acids affects the weight and lipid profile in the following generation.
Maternal dietary omega-9 fatty acids have inconclusive results on the weight of the offspring. Although obesity is increasing, no changes have occurred in the amounts of dietary oil.
It is proposed that the type of oil is more important than the amount of oil for weight changes.
What’s New
In an isocaloric diet, extra virgin olive oil (EVOO) (main source of omega-9 fatty acids) consumption during gestation and lactation reduced weight and improved serum biochemical parameters in the female offspring.
Maternal high-fat diet containing low amounts of carbohydrates decreased weight and biochemical parameters in the offspring.
Not only is EVOO a good choice for maternal dietary oil during gestation and lactation, but also the amounts of fat and carbohydrates in the diet are important.
Introduction
Obesity is a major public health problem in developing and developed countries across the world.1 Maternal nutritional status (quality and/or quantity) during the critical periods of life, such as gestation and/or lactation, is the major cause of obesity and predisposes offspring to non-communicable and metabolism related chronic diseases.2,3 Maternal dietary changes during gestation and lactation may cause chronic diseases in adulthood due to maladaptive phenotypic plasticity.4,5 Diet has the main impact on growth and metabolism during the gestation. Nutritional factors such as energy, fatty acids, protein, and micronutrients affect several aspects of fetal growth.6 In addition to energy intake, the macronutrient composition of a diet may play an important role in insulin homeostasis.7 Inadequate distribution of macronutrients, including overconsumption of carbohydrates, fats, or both may lead to lipid disturbances associated with obesity and insulin resistance.7,8
Some studies have investigated the role of maternal calorie rich diet on fetal development.9-11 Obesity and high-fat diet during gestation play pathogenic roles in the development of obese phenotype in offspring.10 The children who are born to obese mothers are likely to be obese than those from lean mothers.11 Increasing evidence suggests that the type and amount of maternal dietary fatty acids have an effect on metabolism.12,13 Only a few studies have assessed the long-term effects of maternal dietary fatty acid changes on offspring,14-17 but no studies have been conducted to assess the long-term effects of different types and amounts of maternal dietary oil with similar energetic values on the offspring at adolescence. Several studies have shown that maternal omega-6 consumption leads to obesity, but the latest review in this context has reported that there are limited studies evaluating the effects of maternal monounsaturated fatty acid (MUFA) intake in offspring until the adolescence.18
The purpose of this study was to compare different amounts of extra virgin olive oil versus soy oil in the maternal isocaloric diet during gestation and lactation on obesity, serum glucose, and lipid profile in female mice offspring at adolescence.
Materials and Methods
Animals, Diets, and Experimental Design
The present study was an interventional animal study. The experimental protocol was approved by the Animal Research Committee of Iran University of Medical Sciences, Tehran, Iran. All institutional and national guidelines for the care and use of laboratory animals were followed. Eight-week-old inbred adult female C57BL/6 mice (21±1.5 g) were obtained from the Razi Vaccine and Serum Research Institute, Tehran, Iran. Each mouse was individually housed at 21-23 °C and controlled humidity (50±5%) under a 12-h artificial light cycle (7 am to 7 pm). To adapt, all animals received AIN93M diet two weeks before the beginning of the study. The AIN93M diet composition (per 1 kg of diet) was 140 g of protein as Casein lactate (Iranian Caseinate Industry, Iran) and 1.8 g as L-Cystein (W326305, Sigma Aldrich, Germany), 40 g of lipid as soy oil or EVOO (Kamzit Company, Iran), 630 g of carbohydrate as corn starch (corn dextrin from corn refinery, Iran) and 100 g as sugar (local confectionery, Iran), 35 g of AIN93G mineral mix (296040002, MP Biomedicals, USA), 10 g of AIN93 vitamin mix (296040201, MP Biomedicals, USA), 2.5 g of choline bitartrate (C1629, Sigma Aldrich, Germany), 0.008 g as tert-butyl hydroquinone (112941, Sigma Aldrich, Germany) and 50 g of fiber (Wheat Bran, Iran).
Each female mouse was mated with one male per cage overnight. After vaginal plug confirmation, mothers were randomly assigned to four different dietary groups (n=10 in each group) as shown in table 1. For the control group, 16% soy oil diet (LSO) was considered. To create an isocaloric diet (same kcal/g); the amount of dietary fiber was increased in the HSO and HOO groups. During the study, all mothers were isocalorically fed with the same kcal/(g body weight) of the group that ate less (pair-fed model).19 In this model, the least amount of diet eaten by the groups is determined and given to the other group of animals housed under identical conditions the following day.
Table 1.
Composition of the experimental diets per 1 kg during the study (AIN93G diet)
| Diets nutrients (g/kg) | (n=10) | |||
|---|---|---|---|---|
| LSO | LOO | HSO | HOO | |
| Casein (g) | 200 | 200 | 200 | 200 |
| Cornstarch (g) | 530 | 530 | 247 | 247 |
| Sucrose (g) | 100 | 100 | 100 | 100 |
| Soy oil (g) | 70 | - | 198 | - |
| EVOO (g) | - | 70 | - | 198 |
| Fiber (g) | 50 | 50 | 204.5 | 204.5 |
| Mineral mix (g) | 35 | 35 | 35 | 35 |
| Vitamin mix (g) | 10 | 10 | 10 | 10 |
| L-cys (g) | 3 | 3 | 3 | 3 |
| Choline bitartrate (g) | 2.5 | 2.5 | 2.5 | 2.5 |
| Tert-butylhydroquinone (g) | 0.008 | 0.008 | 0.008 | 0.008 |
| Energy (Kcal/g) | 3.97 | 3.97 | 3.97 | 3.97 |
| As carbohydrate | 64% | 64% | 35% | 35% |
| As fat | 16% | 16% | 45% | 45% |
| As protein | 20% | 20% | 20% | 20% |
LSO: Low soy oil; LOO: Low olive oil; HSO: High soy oil; HOO: High olive oil; EVOO: Extra virgin olive oil; L-cys: L-Cystein
Mothers were fed by the above-mentioned diets throughout the gestation and lactation periods. After labor, offspring were reduced to 2 pups in each cage through simple randomization and nursed by birth mothers and weaned on day 21. Pups received ad libitum control diet (LSO) after weaning. Animals were weighed weekly on a calibrated balance scale (Marte Scale (EK-3000i, USA)) to record the weight change to the nearest 0.1 gram. Mothers were sacrificed one day after offspring weaning, as well as pups at 6 weeks of life by 40 mg/kg ketamine and 8 mg/kg xylasine. One day after weaning, maternal fasting blood samples were collected in a tube without anticoagulant, but offspring followed the study until adolescence (6 weeks). At 6 weeks, fasting blood samples of the offspring were collected. The samples were centrifuged at 4000 g for 15 minutes and then the serum was separated and stored at -80 °C until the biochemical analyses.
Biochemical Parameters Measurement
For the mothers and offspring, blood levels of total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG) were measured photometrically in an automatic analyzer (Cobas Integra 400, Roche, Mannheim, Germany). Serum glucose was measured by an enzymatic method (Pars Azmoon kit, Pars Azmoon Co., Tehran, Iran) using Liasys auto-analyzer.
Statistical Analysis
For the comparison of continuous variables between groups, one-way ANOVA was performed with post-hoc test and data were expressed by mean±SD (standard deviation). To adjust for potentially confounding variables and to assess the interactions between different variables, two-way ANOVA was used. This test was performed to assess the effect of type and amount of dietary oils on maternal weight gain, biochemical parameters, birth weight of offspring, as well as weight and biochemical parameters at adolescence. Whenever the interaction was significant, the main effect of factors was evaluated in groups. Repeated measures ANOVA test was performed to compare the weight gain trend in the offspring, followed by Scheffe post-hoc test for pair-group comparison. The level of significance was considered at P<0.05. Statistical analyses were performed with the IBM SPSS package (version 20, IBM Corporation).
Results
Effect of Diets on Maternal Parameters
The effects of diets containing different amounts of EVOO and/or soy oil on maternal body weight, serum lipid profile, and glucose were investigated. The results showed that the interaction between type and amount of dietary oil on maternal weight was not significant during the three weeks of gestation and lactation. The main effect of soy oil on maternal weight, adjusted for the amount of dietary oils, was significantly higher than EVOO throughout the three weeks of gestation and lactation (P=0.002, P<0.001, P<0.001, P=0.001, P<0.001, and P<0.001, respectively). Except for the first week of gestation, the main effect of 16% oil fed diets on maternal weight was significantly higher than the 45% oil fed diets (P<0.001, P<0.001, P=0.02, P=0.02, and P=0.01, respectively). As shown in figure 1, at the first week of gestation, maternal weight was significantly higher in the 45% than the 16% oil fed groups (P<0.001). 16% and 45% oil fed mothers
Figure 1.
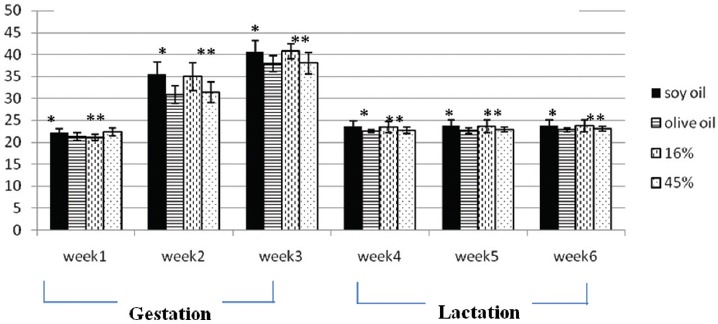
Mean of maternal weight during three weeks of gestation and three weeks of lactation (fed with soy oil and/or olive oil, 16% and/or 45% amounts, n=10 in each group). *Significant difference between soy oil and olive oil fed diets; **Significant difference between the 16% and 45% oil fed mothers.
The interaction between the type and amount of dietary oil on maternal biochemical parameters was not significant after lactation. The main effect of soy oil was significantly higher on serum TC, LDL and glucose levels (P<0.001, P=0.001, and P=0.002, respectively), but it was lower on HDL.C (P=0.023). Serum TG was significantly higher in the 16% oil fed diets (P=0.001), but LDL.C was significantly higher in the 45% oil fed diets (P=0.009) (figure 2).
Figure 2.
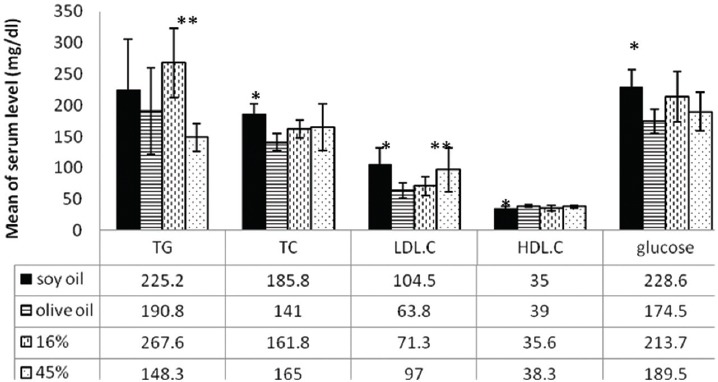
Mean of maternal serum glucose and lipid profile (fed with soy oil and/or olive oil, 16% and/or 45% amounts, n=10 in each group). *Significant difference between the soy oil and olive oil fed diets; **Significant difference between the 16% and 45% oil fed mothers.
Effects of Maternal Diet on Weight Gain Trends
The effects of maternal diet on the trend of weight gain and weight of female offspring at birth and adolescence were investigated. The results showed that the interaction between type and amount of dietary oil on birth weight of female offspring was not significant. The main effect of soy oil on birth weight, adjusted for the amount of dietary oils, was significantly higher than EVOO (1.29±0.2 vs. 1±0.09) (P<0.001), but it was not significantly different between the offspring born to mothers fed with 16% and 45% oil (1.13±0.3 vs. 1.16±0.2) (P=0.63). The interaction between type and amount of dietary oils on weight was not significant at adolescence. The main effect of soy oil on weight was significantly higher than EVOO at adolescence, adjusted for the amount of dietary oil (18.5±1.7 vs. 13.7±1.05) (P<0.001). Adjusting for the type of dietary oils, the main effect of the amount of maternal dietary oil on the weight at adolescence was significantly higher in the low-fat than high-fat diet group (16.8±3.08 vs. 15.4±2.4) (P=0.001).
The trend of weight gain is shown in figure 3. The mean weight gain was significantly increased with respect to time (P<0.001). The interaction between time and groups was significant (P<0.001). In addition, there was a significant difference between the groups (P<0.001). Scheffe post-hoc test showed that all pair-group comparisons for weight gain were significant (P<0.001) except for the offspring born to mothers fed with 16% and 45% olive oil (P=0.79).
Figure 3.
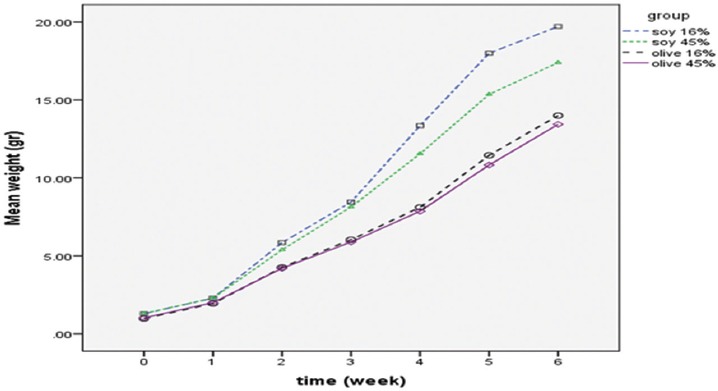
The trend of weight gain in female offspring from birth until adolescence.
Effects of Maternal Diet on Serum Glucose and Lipid Profile in Female Offspring at Adolescence
The results showed that the interaction between the type and amount of dietary oil on serum glucose, TC, and TG of offspring were significant (P<0.001, P<0.001, and P<0.001, respectively). The analysis of these variables is shown in table 2. Serum glucose was significantly higher in the LSO than LOO (P<0.001), LSO than HSO (P<0.001), and LOO than HOO (P<0.001) groups. Serum TC was significantly higher in the LSO than LOO (P<0.001), HSO than HOO (P<0.001), LSO than HSO (P<0.001), and LOO than HOO (P<0.001) groups. Serum TG was significantly higher in the LSO than LOO (P<0.001), HSO than HOO (P<0.001), LSO than HSO (P=0.03), and LOO than HOO (P<0.001) groups. The interaction between the type and amount of dietary oil on serum LDL/HDL ratio was not significant. The mean of serum LDL/HDL ratio was higher in the soy oil than EVOO, adjusted for the amounts of dietary oils (2.8±3.9 vs. 2.2±1.3) (P=0.6). Adjusting for the type of dietary oil, serum LDL/HDL ratio was higher in the 16% than 45% oil diet groups (3.3±3.9 vs. 1.7±0.69) (P=0.11). However, these differences were not significant.
Table 2.
Serum biochemical parameters in female offspring
| Variables Groups | LSO | LOO | HSO | HOO | P value* |
|---|---|---|---|---|---|
| Glucose (mg/dl) | 236±23.5a,c | 188.2±21.2a,d | 218.7±33.1c | 218.7±33.1d | <0.001 |
| TC (mg/dl) | 177.1±61.3a,c | 157.8±42.1a,d | 146.1±16.5b,c | 127±16.7b,d | 0.09 |
| TG | 259±97a,c | 200±80a,d | 176±24b,c | 155±40b,d | 0.02 |
Comparison of four dietary groups evaluated by one-way ANOVA followed by Scheffe post-hoc test;
Significant difference between the LSO and LOO groups;
Significant difference between the HSO and HOO groups;
Significant difference between the LSO and HSO groups;
Significant difference between the LOO and HOO groups;
LSO: Low soy oil diet; LOO: Low olive oil diet; HSO: High soy oil diet; HOO: High olive oil diet; TC: Total cholesterol; TG: Triglyceride; Values are reported as mean±SD (n=10 in each group)
Discussion
Offspring chronic disease susceptibility is determined by maternal dietary status during gestation and/or lactation.20 To the best of our knowledge, this is the first animal study that has investigated the effects of different types and amounts of maternal dietary oil during gestation and lactation in an isocaloric diet; with a focus on EVOO and coupled with offspring post-weaning control diet on weight, fasting glucose, and lipid profile of female offspring at adolescence. Several studies have reported that maternal high-fat diet during gestation and lactation leads to obesity and insulin resistance due to pancreatic remodeling,21,22 but no study to date has examined the effects of type and amount of maternal dietary oil during gestation and lactation in an isocaloric diet on offspring at adolescence.
The present study showed that in isocaloric diet, maternal EVOO consumption during gestation and lactation reduces weight, serum glucose, and lipid profile in the female offspring at adolescence. In addition, the weight, serum glucose and lipid profile were lower in the offspring born to mothers fed with a high-fat than low-fat diet. In this study, we had to increase the fiber content of high-fat diets in order to create an isocaloric diet. Therefore, the best effects of these diets on the variables could be due to the high-fiber content.
The role of dietary fat as the main cause of adult obesity is controversial.23,24 Although the prevalence of obesity is increasing, but no recent changes in the amount of fat consumption have been observed.25 While high-fat intake has been shown to play a role in promoting adipose tissue,26,27 the findings from carefully controlled weight-gain and weight-loss trials, comparing low-, moderate- and high-fat diets, have suggested that the amount of fat in the diet is less important than the type of fat consumed.28,29 A rapidly growing literature indicates that, in Western-style diet, an increase in dietary omega-6 is due to increased consumption of vegetable oil (e.g., soy, sunflower, and corn). Based on the currently available evidence, high levels of n-6 fatty acids in a diet lead to the elevation of blood lipid profiles, insulin resistance, prothrombosis, blood viscosity, vasospasm, and vasoconstriction.25 Recent studies revealed that maternal high-fat diet could modulate the expression of hepatic fatty acid β-oxidation-related genes. Besides, it may affect early lipid metabolism of offspring and metabolic disturbances in adulthood.16 Similar to these findings, we also showed that maternal diet containing low or high amounts of soy oil lead to obesity and lipid profile disturbances in offspring at adolescence in comparison to a diet containing EVOO. Hydroxytyrosol and oleuropein as phenolic components of olive oil affect fasting glucose and lipid serum levels.30,31 Human studies have shown that the administration of a diet supplemented with olive leaf polyphenols (51.1 mg OL, 9.7 mg HT per day) ameliorated insulin action and secretion that regulate glucose homeostasis.19 These results are consistent with our study. Phenolic components of EVOO lead to such benefits.
In a study with high-sucrose and high-fat-sucrose diet, a similar effect on weight gain and adipose deposition has also been found after isocalorically feeding in Wistar rats.32 However, a pair-fed model with high-fat feeding in C57BL/6J mice did not produce statistically significant changes in body weight gain.33 These results indicate that not only the amount of calorie but also the macronutrient composition of a diet may lead to obesity. Inconclusive results are due to different compositions of the diets and the type of oils in all studies. In addition, none of the mentioned pair-fed studies were done during critical periods of life. Our study differs from previous pair-fed studies in terms of the type and amount of dietary oil and the time of intervention.
In the present study, we have evaluated the effect of isocaloric diets with different amounts of carbohydrate-to-fat ratio (1:1, 2:1, or 3:1) on body size and body composition in growing Wistar rats. It is stated that the 3:1 and 2:1 diets increase the weight and progressively decreased body fat composition.19 This is in agreement with our results whereby dietary substitution of fat with carbohydrates led to a higher weight at adolescence in low-fat and high-carbohydrate (LSO and LOO) diets. The weight of offspring in both groups of EVOO fed diet was lower than soy oil diet. Studies have shown that omega-6 fatty acids lead to an increase in fat mass,34 resulting in insulin resistance and elevation in serum lipid profile related to obesity. However, omega-9 fatty acids activate the peroxisome proliferator-activated receptor (PPAR) and reduce the activation of the sterol regulatory element-binding proteins (SREBPs), then inhibit lipogenesis and reduce insulin resistance.35 In another study, a diet containing 7% sunflower oil (n-6 diet), linseed oil (n-3 diet), or soybean oil (n-6/n-3 diet) during pregnancy and lactation were compared.36 Maternal consumption of omega-6 increased inguinal weight relative to body weight and leptin levels in the offspring. These results were in agreement with our findings. The weight of offspring was higher in soy oil than the EVOO fed group.
In a study by Xiaoling Li,37 the effects of high-fat diet (HF) and maternal obesity on the metabolic programming of liver in offspring were assessed. It was shown that maternal high-fat diet leads to suppression of glucose and lipid metabolism-related genes in offspring. The methodological design of his study was different from ours. In that study, maternal obesity and/or high-calorie and high-fat diets were assessed, whereas we compared isocaloric diets with high-fat and/or high-carbohydrate components with different oils. In our study, high-carbohydrate-low-fat diet impaired glucose homeostasis and lipid profile in offspring at adolescence.
Laws et al.38 used maternal diet supplemented with 10% EVOO (rich in MUFA) in the first or second half of gestation in piglets. In their study, MUFA reduced the incidence of low birth weight. In contrast, a study by Priego et al.39 showed that maternal olive oil supplementation decreased body weight gain through an increase in uncoupling protein-1 protein level in brown adipose tissue of offspring at weaning. They proposed that oleic acid prevents the development of obesity and steatosis by stimulating thermogenic capacity. Similar to Priego and colleagues, the results of our study showed that the weight of offspring in EVOO groups was significantly lower than soy oil groups; both at birth and at adolescence. One of the limitations of our study was apparently the amount of fiber in different groups. The fiber content of the low-fat diet was lower than high-fat diet increating isoenergitic values. Therefore, some benefits of high-fat diet versus low-fat diet may be due to fiber content.
Based on our results, a maternal diet containing EVOO has better effects on birth weight as well as weight and serum biochemical parameters of offspring at adolescence. Maternal high-fat diet reduced weight, serum glucose and lipid profile in offspring at adolescence.
One of the main limitations of our study was the fact that weight and lipid profile were measured only at adolescence. Additionally, while periodic measures from birth until aging are suggested, we did not directly measure whether maternal dietary with different types and amounts of oils induced epigenetic changes in metabolism. In future studies, direct assessment of epigenetic changes such as histone modification or DNA methylation will allow us to examine the hypothesis that perinatal programming of the weight and lipid profile occur via epigenetic mechanisms. A free-fed group along with pair-fed groups is recommended in future studies. Since the present study was an animal model investigation, the effects of the diets cannot be directly transposed to humans. Rather, it provides indications of possible effects of such dietary manipulations on the metabolism and may give better insights for future research regarding the underlying mechanisms.
Conclusion
In general, maternal dietary macronutrient components are the most determinant of obesity in the next generation. Offspring of high-fat and low-carbohydrate fed mothers had a lower weight than those with low-fat and high-carbohydrate. A maternal diet containing EVOO decreased weight and improved serum glucose and lipid profile in female offspring at adolescence. This may lead to a decrease in obesity and lipid profile disturbances in adulthood.
Acknowledgment
The authors are very grateful to the colleagues at the Laboratory Animal Reproduction Research Center, Iran University of Medical Sciences, Tehran, Iran. The present article was extracted from the PhD thesis by Seyedeh Neda Mousavi and was financially supported by Iran University of Medical Sciences (grant number 24208).
Conflict of Interest: None declared.
References
- 1.WHO. [Cited 2014 Nov 8];Fact Sheet: Obesity and Overweight [Internet] 2006 Available from: http://www.who.int/mediacentre/factsheets/fs311/en . [Google Scholar]
- 2.Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab. 2007;292:E1702–14. doi: 10.1152/ajpendo.00605.2006. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArdle HJ, Andersen HS, Jones H, Gambling L. Fetal programming: Causes and consequences as revealed by studies of dietary manipulation in rats - A review. Placenta. 2006;27(Suppl A):S56–60. doi: 10.1016/j.placenta.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 6.Mathias PC, Elmhiri G, de Oliveira JC, Delayre-Orthez C, Barella LF, Tófolo LP, et al. Maternal diet, bioactive molecules, and exercising as reprogramming tools of metabolic programming. Eur J Nutr. 2014;53:711–22. doi: 10.1007/s00394-014-0654-7. [DOI] [PubMed] [Google Scholar]
- 7.Axen KV, Dikeakos A, Sclafani A. High dietary fat promotes syndrome X in nonobese rats. J Nutr. 2003;133:2244–9. doi: 10.1093/jn/133.7.2244. [DOI] [PubMed] [Google Scholar]
- 8.Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89–104. doi: 10.1055/s-2001-12932. [DOI] [PubMed] [Google Scholar]
- 9.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: Consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Srinivasan M, Thamadilok S, Patel MS. Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. J Endocrinol. 2009;200:293–300. doi: 10.1677/JOE-08-0429. [DOI] [PubMed] [Google Scholar]
- 11.Whitaker RC. Predicting preschooler obesity at birth: The role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Bernal CL, Rebagliato M, Iñiguez C, Vioque J, Navarrete-Muñoz EM, Murcia M, et al. Diet quality in early pregnancy and its effects on fetal growth outcomes: The infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am J Clin Nutr. 2010;91:1659–66. doi: 10.3945/ajcn.2009.28866. [DOI] [PubMed] [Google Scholar]
- 13.Niculescu MD, Lupu DS, Craciunescu CN. Perinatal manipulation of a-linolenic acid intake induces epigenetic changes in maternal and offspring livers. FASEB J. 2013;27:350–8. doi: 10.1096/fj.12-210724. [DOI] [PubMed] [Google Scholar]
- 14.Sardinha FL, Fernandes FS, Tavares do Carmo MG, Herrera E. Sex-dependent nutritional programming: Fish oil intake during early pregnancy in rats reduces age-dependent insulin resistance in male, but not female, offspring. Am J Physiol Regul Integr Comp Physiol. 2013;304:R313–20. doi: 10.1152/ajpregu.00392.2012. [DOI] [PubMed] [Google Scholar]
- 15.Lauritzen L, Carlson SE. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern Child Nutr. 2011;7(Suppl 2):41–58. doi: 10.1111/j.1740-8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benatti RO, Melo AM, Borges FO, Ignacio-Souza LM, Simino LA, Milanski M, et al. Maternal high-fat diet consumption modulates hepatic lipid metabolism and microRNA-122 (miR-122) and microRNA-370 (miR-370) expression in offspring. Br J Nutr. 2014;111:2112–22. doi: 10.1017/S0007114514000579. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Zhang F, Didelot X, Bruce KD, Cagampang FR, Vatish M, et al. Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genomics. 2009;10:478. doi: 10.1186/1471-2164-10-478. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mennitti LV, Oliveira JL, Morais CA, Estadella D, Oyama LM, Oller do Nascimento CM, et al. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J Nutr Biochem. 2015;26:99–111. doi: 10.1016/j.jnutbio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Gamba CA, Friedman SM, Rodriguez PN, Macri EV, Vacas MI, Lifshitz F. Metabolic status in growing rats fed isocaloric diets with increased carbohydrate-to-fat ratio. Nutrition. 2005;21:249–54. doi: 10.1016/j.nut.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–98. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- 21.Gregorio BM, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Maternal high-fat diet is associated with altered pancreatic remodelling in mice offspring. Eur J Nutr. 2013;52:759–69. doi: 10.1007/s00394-012-0382-9. [DOI] [PubMed] [Google Scholar]
- 22.Borengasser SJ, Kang P, Faske J, Gomez-Acevedo HL, Blackburn ML, Badger TH, et al. High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring. PLoS One. 2014;9:e84209. doi: 10.1371/journal.pone.0084209. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taubes G. Nutrition. The soft science of dietary fat. Science. 2001;291:2536–45. doi: 10.1126/science.291.5513.2536. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC. Dietary fat plays a major role in obesity: No. Obes Rev. 2002;3:59–68. doi: 10.1046/j.1467-789x.2002.00060.x. [DOI] [PubMed] [Google Scholar]
- 25.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560S–9S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 26.Park S, Park NY, Valacchi G, Lim Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediators Inflamm. 2012;2012:984643. doi: 10.1155/2012/984643. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n_3 poly unsaturated fatty acids. Diabetologia. 2006;49:2109–19. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Field AE, Willett WC, Lissner L, Colditz GA. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity (Silver Spring) 2007;15:967–76. doi: 10.1038/oby.2007.616. [DOI] [PubMed] [Google Scholar]
- 30.Cao K, Xu J, Zou X, Li Y, Chen C, Zheng A, et al. Hydroxytyrosol prevents diet-induced metabolic syndromeand attenuates mitochondrial abnormalities in obese mice. Free Radic Biol Med. 2014;67:396–407. doi: 10.1016/j.freeradbiomed.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 31.Jemai H, Fki I, Bouaziz M, Bouallagui Z, El Feki A, Isoda H, et al. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J Agric Food Chem. 2008;56:2630–6. doi: 10.1021/jf072589s. [DOI] [PubMed] [Google Scholar]
- 32.Lomba A, Milagro FI, García-Díaz DF, Marti A, Campión J, Martínez JA. Obesity induced by a pair-fed high fat sucrose diet: Methylation and expression pattern of genes related to energy homeostasis. Lipids Health Dis. 2010;9:60. doi: 10.1186/1476-511X-9-60. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Meijer VE, Le HD, Meisel JA, Akhavan Sharif MR, Pan A, Nosé V, et al. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism. 2010;59:1092–105. doi: 10.1016/j.metabol.2009.11.006. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries PS, Gielen M, Rizopoulos D, Rump P, Godschalk R, Hornstra G, et al. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. Prostaglandins Leukot Essent Fatty Acids. 2014;91:81–5. doi: 10.1016/j.plefa.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Rustan AC, Drevon CA. Fatty acids: Structures and properties. London: Encyclopedia of Life Sciences; 2005. [Google Scholar]
- 36.Korotkova M, Gabrielsson B, Lönn M, Hanson LA, Strandvik B. Leptin levels in rat offspring are modified by the ratio of linoleic to alpha-linolenic acid in the maternal diet. J Lipid Res. 2002;43:1743–9. doi: 10.1203/00006450-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Li X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai) 2013;45:51–60. doi: 10.1093/abbs/gmt142. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laws J, Litten JC, Laws A, Lean IJ, Dodds PF, Clarke L. Effect of type and timing of oil supplements to sows during pregnancy on the growth performance and endocrine profile of low and normal birth weight offspring. Br J Nutr. 2009;101:240–9. doi: 10.1017/S0007114508998469. [DOI] [PubMed] [Google Scholar]
- 39.Priego T, Sánchez J, García AP, Palou A, Picó C. Maternal dietary fat affects milk fatty acid profile and impacts on weight gain and thermogenic capacity of suckling rats. Lipids. 2013;48:481–95. doi: 10.1007/s11745-013-3764-8. [DOI] [PubMed] [Google Scholar]


