Abstract
Background:
Cyclosporine A (CsA) is an immunosuppressant with therapeutic indications in various immunological diseases; however, its use is associated with chronic nephropathy. Oxidative stress has a crucial role in CsA-induced nephrotoxicity. The present study evaluates the protective effect of edaravone on CsA-induced chronic nephropathy and investigates its antioxidant and nitric oxide modulating property.
Methods:
Male Sprague-Dawley rats (n=66) were distributed into nine groups, including a control (group 1) (n=7). Eight groups received CsA (15 mg/kg) for 28 days while being treated.
The groups were categorized as:
Group 2: Vehicle (n=10)
Groups 3, 4, and 5: Edaravone (1, 5, and 10 mg/kg) (n=7 each)
Group 6: Diphenyliodonium chloride, a specific endothelial nitric oxide synthase (eNOS) inhibitor (n=7)
Group 7: Aminoguanidine, a specific inducible nitric oxide synthase (iNOS) inhibitor (n=7)
Group 8: Edaravone (10 mg/kg) plus diphenyliodonium chloride (n=7)
Group 9: Edaravone (10 mg/kg) plus aminoguanidine (n=7)
Blood urea nitrogen and serum creatinine levels, malondialdehyde, superoxide dismutase, and glutathione reductase enzyme activities were measured using standard kits. Renal histopathological evaluations and measurements of eNOS and iNOS gene expressions by RT-PCR were also performed. Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test (SPSS software version 18.0).
Results:
Edaravone (10 mg/kg) significantly attenuated CsA-induced oxidative stress, renal dysfunction, and kidney tissue injury. Aminoguanidine improved the renoprotective effect of edaravone. Edaravone reduced the elevated mRNA level of iNOS, but could not alter the level of eNOS mRNA significantly.
Conclusion:
Edaravone protects against CsA-induced chronic nephropathy using antioxidant property and probably through inhibiting iNOS gene expression.
Keywords: Edaravone, Nitric oxide, iNOS, eNOSe, Kidney diseases, Cyclosporine
What’s Known
Edaravone is a potent free radical scavenger.
Edaravone has been used successfully for the treatment of cerebral infarction in Japan since 2001.
Edaravone has been tried successfully in a number of animal models of diseases.
Edaravone may have modulating effects on nitric oxide production.
What’s New
The role of nitric oxide in the renoprotective effect of edaravone in cyclosporine-induced chronic nephropathy was investigated for the first time in this study.
Introduction
Cyclosporine (CsA) is a potent immunosuppressive agent successfully used to prevent organ transplant rejection; however, nephrotoxicity is a serious complication of CsA treatment. CsA induces severe renal vasoconstriction that tends to acute renal impairment often progressing to chronic kidney injury. Acute renal toxicity is characterized by oliguria, a decrease in renal blood flow, and glomerular filtration rate (GFR), which is completely reversible after drug withdrawal and no histological lesion is seen.1 Chronic nephropathy induced by CsA is characterized by irreversible impairment of kidney function and progressive histological lesions, including tubulointerstitial fibrosis, arteriolopathy, and tubular atrophy.2 Although the mechanisms of CsA-induced nephropathy are not fully understood, many studies suggest that oxidative stress is involved in this renal toxicity. CsA increases hypoxia and free radical production in the kidney and some antioxidants such as α-tocopherol, ascorbate, vitamin E, and superoxide dismutase have been shown to diminish CsA-induced nephrotoxicity.3
Nitric oxide is a vasodilating mediator that plays a key role in maintaining vascular tone in the kidney. Nitric oxide is a lipophylic gas, which is produced from its precursor L-arginine by three isoforms of nitric oxide synthase; (i) neuronal NOS (nNOS), which is markedly expressed in the brain, (ii) inducible NOS that is expressed in macrophages after inflammatory stimuli, and (iii) endothelial NOS (eNOS) that is expressed in endothelial cells. All of these three isoforms are present in the kidney.4 CsA has been reported to increase iNOS gene expression.5 It has also been evidenced that the chronic administration of CsA tends to endothelial dysfunction in the endothelium of renal vessels, which is associated with increased eNOS gene expression and leads to the formation of ROS such as superoxide rather than nitric oxide.6 Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is a potent free radical scavenger that is used as a drug for the treatment of cerebral infarction in Japan from 2001 without causing any serious side effects.7 Edaravone is readily distributed throughout the body and its activity is not limited to the brain. Edaravone has been tested in animal models of myocardial injury following ischemia/reperfusion,8 liver injury induced by endotoxins,9 and cisplatin-induced acute kidney injury.10 Edaravone quenches reactive oxygen species (ROS) and protects various types of cells and tissues against damage by ROS.11 Many studies indicate that edaravone in addition to its free radical scavenging activity acts through other mechanisms.12 An in vitro study using primary cell cultures of rat hepatocytes revealed that edaravone directly inhibits the induction of iNOS gene expression in IL-1β-stimulated hepatocytes.13 Edaravone also induced eNOS in the ischemic spinal cord in rabbits and prevented spinal cord damage.14
The purpose of this study was to investigate the possible protective effect of edaravone against chronic nephropathy induced by CsA, and to reveal the role of antioxidant and nitric oxide modulating properties of edaravone in its possible renoprotective effects.
Materials and Methods
Animals
Male Sprague-Dawley rats (n=66), weighing 200 to 250 g bred in the central animal house of Shiraz University of Medical Sciences (Shiraz, Iran) were distributed in standard vivariums, fed with low sodium diet (0.05% sodium, 21 Beyza Company, Shiraz, Iran) and water ad libitum. The animals were housed under standard conditions (12 h light/dark cycle, temperature 20-25 °C, and humidity 25-35%). The experimental protocols were approved by the Institutional Ethical Committee for Care and Use of Animals in Shiraz University of Medical Sciences.
Experimental Design
Chronic nephropathy was induced in rats by daily subcutaneous injection of CsA (Sigma Company, St. Louis, USA) (15 mg/kg) for 28 days.15 Animals were randomized into nine groups of seven to ten animals and treated daily for consecutive 28 days as follows:
Group 1: Treated with olive oil (vehicle for CsA) subcutaneously plus normal saline (vehicle for edaravone) intraperitoneally
Group 2: Received CsA (15 mg/kg) subcutaneously
Groups 3, 4, and 5: Received CsA plus intraperitoneal doses of edaravone (1, 5, and 10 mg/kg bid, respectively) (Sigma Company, St. Louis, USA)
Group 6: Treated with a specific eNOS inhibitor, diphenyliodonium chloride (4 mg/kg) (Sigma Company, St. Louis, USA), in addition to CsA
Group 7: Received CsA plus a specific iNOS inhibitor, aminoguanidine (100 mg/kg) (Sigma Company, St. Louis, USA)
Group 8: Treated with CsA concomitant with edaravone (10 mg/kg bid) and diphenyliodonium chloride (4 mg/kg)
Group 9: Received CsA plus edaravone (10 mg/kg bid) and aminoguanidine (100 mg/kg).
On day 29, animals were anesthetized with intraperitoneal injection of ketamine (40 mg/kg) and xylazine (10 mg/kg) and blood specimens were collected through abdominal aorta. The blood samples were centrifuged; plasma was separated and maintained at -80 °C until biochemical analyses for the determination of blood urea nitrogen and serum creatinine. After scarification of the animals, the left kidney was removed, immediately frozen, and maintained at -80°C for biochemical, enzymatic and gene expression analyses. The right kidney was fixed in 10% formalin solution for histopathological evaluations.
Measurement of Blood Urea Nitrogen (BUN) and Serum Creatinine Levels
Blood urea nitrogen (BUN) and serum creatinine levels were determined by using standard assay kits (Pars Azemoon, Shiraz, Iran).
Measurement of Renal Antioxidant Enzyme Activities
Superoxide dismutase (SOD) and glutathione reductase (GRH) enzyme activities in the kidney homogenate were measured by using standard diagnostic kits (Biorexfars, Shiraz, Iran).
Measurement of Renal Malondialdehyde (MDA) Content
Malondialdehyde (MDA) content was measured in the form of thiobarbituric acid reacting substances. In brief, the reaction mixture consisting of 1.5 ml of 20% acetic acid, 1.5 ml of 0.8% thiobarbituric acid, and 0.2 ml of 8.1% sodium dodecyl sulfate (Sigma Company, St. Louis, USA) was added to 0.2 ml of 10% (w/v) of homogenized tissue supernatant or serial concentrations of the standard solution. The total volume was adjusted to 4.0 ml with distilled water and incubated at 95°C for 60 minutes. After cooling with ice water, 5.0 ml of n-butanol and pyridine solution (15:1 v/v) (Sigma Company, St. Louis, USA) and 1.0 ml of distilled water were added and centrifuged. The supernatant was separated and its UV absorbance was measured at 532 nm. The contents of MDA in the kidney homogenates were obtained according to the standard curve and reported as nm/g tissue.16
RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was extracted from the kidney by phenol-chloroform method using Tri-Pure extraction kit (Roche Applied Science, Penzberg, Germany) according to the manufacturer´s guidelines.17 The purity of the extracted RNA was determined and the concentration of the extracted total RNA was adjusted to 1 µg/ml with DEPC-treated water. The cDNA was synthesized from 1 µg of total RNA using H minus first strand cDNA synthesis kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Primer design was performed using Primer Express software version 2.0 (Applied Biosystems, Foster City, CA, USA). The sequences of the primers used to quantify the eNOS and iNOS genes expression were as follow: eNOS 5’-ATT GGC ATG AGG GAC CTG TG-3’ (forward) and 5’-CCG GGT GTC TAG ATC CAT GC-3’ (reverse); iNOS 5’-AGC TAC GCC TTC AAC ACC AA-3’ (forward) and 5’-CCC AGG CCA AAT ACC GCA TA-3’ (reverse). The sequences of the primers for the determination of β-actin mRNA level were 5’-GCA AAT GCT TCT AGG CGG AG-3’ (forward) and 5’-AAG GGG TGT AAA AAA ACG CAG C-3’ (reverse). Real-time PCR was done with SYBR Premix Ex Taq II (Takara, Shiga, Japan). Thermal cycling and fluorescence detection were performed using an ABI 7500 real-time system (Applied Biosystems, Foster City, CA, USA). Relative changes in gene expression were calculated using the comparative CT method and normalized with the mean Ct of the control gene, β-actin. Real-time PCR amplification was carried out for 40 cycles using the following conditions: denaturation at 95 °C for 15 seconds, annealing and extension at 60 °C for 30 seconds.
Renal Histopathological Evaluations
The right kidney of the rats was removed immediately after sacrificing them and washed with normal saline solution. It was sectioned in blocks and fixed in 10% neutral buffered formalin, dehydrated in graded concentrations of alcohols, embedded in paraffin, and sent for histopathological evaluations. The kidney block was cut into two-micrometer thick sections and stained with hematoxylin and eosin (H&E), Masson´s trichrome, periodic acid-Schiff (PAS), and Jones´ staining.18 The histopathological examination was performed in a blinded manner for tubular atrophy, interstitial fibrosis, chronic inflammatory cell infiltration, and vascular thickening in all of the specimens. At least 10 fields for each kidney slide were evaluated. The severity of kidney tissue damage was scored as follows: (-) for no abnormalities, (+) for mild changes affecting less than 25% of the sample, (++) for moderate changes affecting 25-50% of the sample, and (+++) for severe damage affecting greater than 50% of the sample.
Statistical Analysis
Data were expressed as mean±SEM and analyzed statistically using one-way analysis of variance (ANOVA) followed by Tukey’s test. The data were analyzed using SPSS software version 18.0. P≤0.05 was considered statistically significant.
Results
Effects of Edaravone, Diphenyliodonium Chloride, and Aminoguanidine Treatments on CsA-Induced Chronic Nephropathy
Figure 1 illustrates the effects of edaravone, diphenyliodonium chloride, and aminoguanidine on renal impairment induced by CsA. Administration of CsA for 28 days resulted in severe renal dysfunction in all of the animals and increased BUN and serum creatinine level dramatically compared to the vehicle group (P<0.001). Administration of edaravone (10 mg/kg) significantly attenuated the rise in BUN and serum creatinine level produced by CsA (P<0.001). Administration of diphenyliodonium chloride (4 mg/kg) or aminoguanidine (100 mg/kg) to animals had no significant effect on the elevated BUN and serum creatinine levels induced by CsA. The addition of aminoguanidine (100 mg/kg) to edaravone (10 mg/kg) improved the renoprotective effect of edaravone and reduced BUN and serum creatinine level (P<0.01). On the other hand, addition of diphenyliodonium chloride (4 mg/kg) to edaravone produced no significant change in the effect of edaravone on the level of BUN and serum creatinine (figures 1A and 1B).
Figure 1.
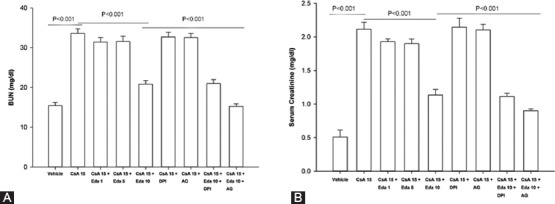
Effect of edaravone (1, 5, 10 mg/kg), diphenyliodonium chloride (4 mg/kg), and aminoguanidine (100 mg/kg) on blood urea nitrogen (A), and serum creatinine (B) levels in CsA-treated rats. Data expressed as mean±SEM (one-way ANOVA followed by Tukey’s test). CsA: Cyclosporine A; Eda: Edaravone; DPI: Diphenyliodonium chloride; AG: Aminoguanidine.
Effect of Edaravone on CsA-Induced Lipid Peroxidation and Alterations in Renal Antioxidant Enzyme Activities
Administration of CsA (15 mg/kg) for 28 days induced a significant increase in MDA level as compared to the vehicle group (P<0.001). Treatment with edaravone (10 mg/kg) significantly lowered the elevated MDA level induced by CsA (P<0.001). Moreover, treatment with CsA led to a significant decrease in superoxide dismutase and glutathione reductase enzyme activities in kidney homogenates (P<0.001), whereas, administration of edaravone (10 mg/kg) preserved the enzyme activities of these enzymes (P<0.001) (table 1).
Table 1.
Effect of edaravone (1, 5, 10 mg/kg) treatment on renal MDA content, and SOD, and GR enzyme activities in chronic CsA (15 mg/kg) treated rats
| Treatment groups | MDA (nM/g of tissue) | SOD (units/mg protein) | GR (units/L) |
|---|---|---|---|
| Vehicle (olive oil) | 61.660±0.667 | 1.591±0.224 | 1400.550±1.617 |
| CsA (15) | 103.285±0.778††† | 0.613±0.017††† | 786.140±1.765††† |
| CsA+edaravone (1) | 103.290±0.680 | 0.651±0.014 | 798.290±1.569 |
| CsA+edaravone (5) | 99.286±0.984* | 0.717±0.032 | 796.140±1.724 |
| CsA+edaravone (10) | 72.286±0.993*** | 1.423±0.059*** | 1115.870±1.404*** |
All values are expressed as mean±SEM (one-way ANOVA followed by Tukey’s test).
P<0.001 as compared to vehicle group;
P<0.05 as compared to CsA-treated group;
P<0.001 as compared to CsA-treated group;
CsA: Cyclosporine A, MDA: Malondialdehyde, SOD: Superoxide dismutase, GR: Glutathione reductase
Effect of Edaravone on CsA-Induced Renal Histopathological Changes
The morphological changes in kidney tissue were graded and summarized in table 2. The vehicle group treated with olive oil was normal and did not show any significant abnormalities (figure 2A) whereas the kidneys of CsA-treated rats showed significant tissue abnormalities in the cortex and outer medulla. The kidney sections showed interstitial chronic inflammatory cells infiltration, tubulointerstitial fibrosis, tubular atrophy, and vascular thickening (figures 2B, 2C, 2D, 2E). By contrast, the kidney sections of rats treated with edaravone (10 mg/kg) showed a near normal morphology (figure 2F).
Table 2.
Effect of edaravone (1, 5, 10 mg/kg), DPI (4 mg/kg), and AG (100 mg/kg) treatment on histological changes of kidney tissue in CsA-treated rats
| Group | Tubular atrophy | Tubulointerstitial fibrosis | Vascular thickening | Chronic inflammatory cells Infiltration |
|---|---|---|---|---|
| Vehicle (olive oil) | - | - | - | - |
| CsA (15) | ++ | ++ | ++ | ++ |
| CsA+edaravone (1) | ++ | ++ | - | ++ |
| CsA+edaravone (5) | ++ | ++ | - | ++ |
| CsA+edaravone (10) | + | + | - | + |
| CsA+DPI (4) | ++ | ++ | - | ++ |
| CsA+AG (100) | ++ | ++ | - | ++ |
| CsA+edaravone (10)+DPI (4) | + | + | - | + |
| CsA+edaravone (10)+AG (100) | + | + | - | + |
None (-), mild (+), moderate (++), severe (+++). CsA: Cyclosporine A; DPI: Diphenyliodonium chloride; AG: Aminoguanidine
Figure 2.
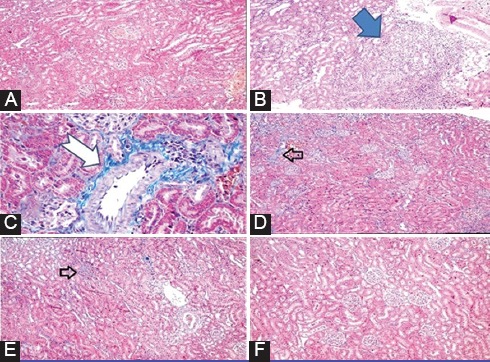
Masson´s trichrome stained sections of rat kidneys: (A) renal cortex of rats treated with vehicle shows no specific pathologic change. (B) Tubular atrophy is seen in the cortex of CsA-treated rats. (C) Vascular thickening is seen in CsA-treated rats. (D) Mild patchy interstitial fibrosis is seen in CsA-treated rats. (E) Mild patchy interstitial inflammatory cells infiltration is seen in CsA-treated rats. (F) Renal cortex of rats treated with edaravone (10 mg/kg) plus CsA shows near normal morphology.
Effect of Edaravone Administration on eNOS and iNOS mRNA Levels
The results of RT-PCR analysis is depicted in figure 3. CsA administration for 28 days elevated the mRNA levels of both eNOS and iNOS enzymes significantly (P<0.001). Treatment with edaravone (10 mg/kg) significantly reduced the mRNA level of iNOS (P<0.001), but it had no significant effect on the level of eNOS mRNA.
Figure 3.
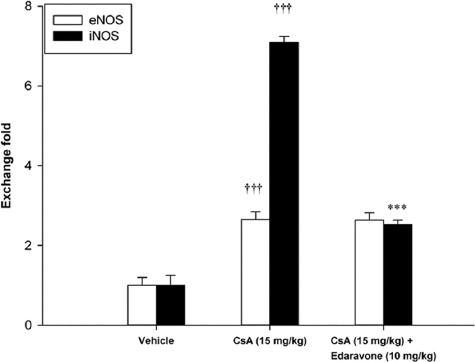
Effect of edaravone (10 mg/kg) on renal eNOS and iNOS mRNA levels in CsA-treated rats. Data expressed as mean±SEM. †††P<0.001 as compared to vehicle group; ***P<0.001 as compared to CsA-treated group; CsA: Cyclosporine.
Discussion
The mechanisms involved in chronic nephropathy induced by CsA have not been fully understood. Several lines of evidence propose that oxidative stress has a main role in the pathogenesis of this type of renal toxicity.19 CsA-induced oxidative stress causes an imbalance in the release of vasoactive substances with predominating vasoconstrictors over vasodilators. This leads to intense renal vasoconstriction that often tends to chronic nephropathy with irreversible histological damage, such as chronic inflammatory cells infiltration, tubulointerstitial fibrosis, vascular thickening of renal vessels, and interstitial atrophy.20 These histological changes are hypothesized to be due to vasoconstrictive effect of CsA that tends the formation of free radicals and renal ischemia/reperfusion injury.21 CsA-induced ROS leads to activation of TGF-β, collagen, and fibronectin genes in mesengial cells that promotes interstitial fibrosis in the kidney.22,23
In the present study, subcutaneous administration of CsA (15 mg/kg) for 28 days resulted in severe renal impairment (disability of kidney to excrete nitrogenous and other waste materials) with a marked increase in blood urea nitrogen (BUN) and serum creatinine levels. This CsA-induced renal impairment was also accompanied with severe oxidative stress as evident by increased renal lipid peroxidation and severely reduced enzyme activities of superoxide dismutase and glutathione reductase. These findings were also supported by histopathological evaluations. The sections showed marked histological changes in the cortex and outer medulla of rats treated with CsA. We also indicated that the administration of edaravone (10 mg/kg) protected against chronic nephropathy of CsA. Edaravone attenuated oxidative stress, prevented the dramatic rise in the levels of BUN and serum creatinine, and improved kidney histology.
Based on our results and regarding the crucial role of oxidative stress in the pathogenesis of CsA-induced chronic nephropathy, it seems that the antioxidant property of edaravone may have a key role in producing potent renoprotective effect.
It has also been evidenced that edaravone in addition to having potent free radical scavenging effect, modulates nitric oxide production.24 Nitric oxide is one of the most important mediators in renal vascular homeostasis.25 Nitric oxide is synthesized from its precursor L-arginine by three NOS isoforms, which are all present in the kidney. Two isoforms are constitutively expressed (eNOS and nNOS) and one isoform is inducible.26 iNOS catalyses the production of large amounts of nitric oxide under pathological conditions.27 Nitric oxide is a free radical, with a half-life of only a few seconds and easily reacts with other reactive oxygen species, mainly superoxide, to form a highly reactive free radical peroxynitrite, which mediates cytotoxic effects. Therefore, nitric oxide produced by iNOS is involved in the inflammatory damage of cells and tissues.28 CsA has been reported to increase iNOS protein expression.5 On the other hand, it has been shown that the administration of edaravone in various animal models of ROS-mediated tissue injury causes the inhibition of iNOS gene expression.29
The results of these studies are in line with ours. The addition of aminoguanidine (a specific iNOS inhibitor) to edaravone enhanced the protective effect of edaravone on renal function; suggesting that edaravone may have inhibiting effect on iNOS gene expression. This idea was supported by real-time PCR analysis. In the current study, we showed that CsA elevated the mRNA level of iNOS in the kidney tissue and edaravone lowered it significantly. Therefore, it seems that edaravone inhibits the destruction of nitric oxide by other free radicals, prevents iNOS expression, and inhibits the subsequent vicious cycle of more inflammation. On the other hand, nitric oxide produced by eNOS is one of the most important mediators in maintaining renal homeostasis.4 A variety of studies demonstrate that chronically treatment with CsA results in endothelial dysfunction30 that was previously believed to be due to decreased production of nitric oxide by endothelium.31 Currently, it has been evidenced that endothelial dysfunction caused by CsA is associated with increased eNOS gene expression in the endothelium of renal vessels, which may cause the formation of ROS such as superoxide rather than nitric oxide.6 Furthermore, it was observed in our study that the renal protection afforded by edaravone was not altered with the addition of diphenyliodonium chloride (a specific eNOS inhibitor) indicating that edaravone may have no significant effect on eNOS gene expression. This idea was confirmed by real-time PCR analysis. In the present study, chronic CsA administration increased eNOS gene expression significantly compared to the vehicle whereas edaravone could not make any significant change in the elevated eNOS gene expression induced by CsA. Moreover, it has been shown that edaravone has improved endothelial vasodilation through restoration of the decreased eNOS gene expression in some animal models of ROS-mediated tissue injury.14 It has been suggested that free radical scavenging by edaravone prevents the decrease of eNOS activity rather than up-regulation of eNOS,32 but this point needs further examination.
The same results for edaravone have been reported in several studies using other animal models of kidney injury caused by ROS.33,34 However, all of the animal studies in this field have investigated the renoprotective effect of edaravone in acute models of kidney injury. To our knowledge, the renoprotective effect of edaravone has not been examined in chronic nephropathy induced by CsA and our study is the first on this topic. However, this study has one limitation. We examined the renoprotective effect of edaravone and its effects on oxidative stress and mRNA levels of eNOS and iNOS in the kidney. This study lacks the measurement of protein levels of these enzymes to confirm the role of nitric oxide modulating effect of edaravone in producing renoprotective properties of this drug.
Conclusion
The results of the present study indicate that the novel free radical scavenger edaravone may act as a renoprotective agent against chronic nephropathy induced by CsA by its antioxidant property and possibly through inhibiting iNOS gene expression.
Acknowledgment
The present article was extracted from the thesis written by Elahe Sattarinezhad and was financially supported by Shiraz University of Medical Sciences (grants number 92-03-04-6547).
Conflict of Interest: None declared.
References
- 1.Busauschina A, Schnuelle P, van der Woude FJ. Cyclosporine nephrotoxicity. Transplant Proc. 2004;36:229S–33S. doi: 10.1016/j.transproceed.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Versluis DJ, Ten Kate FJ, Wenting GJ, Jeekel J, Weimar W. Histological lesions associated with cyclosporin: incidence and reversibility in one year old kidney transplants. J Clin Pathol. 1988;41:498–503. doi: 10.1136/jcp.41.5.498. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parra Cid T, Conejo Garcia JR, Carballo Alvarez F, de Arriba G. Antioxidant nutrients protect against cyclosporine A nephrotoxicity. Toxicology. 2003;189:99–111. doi: 10.1016/S0300-483X(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 4.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. doi: 10.1093/eurheartj/ehr304. 37a-37d. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai H, Nakai H, Suga M, Yuki S, Watanabe T, Saito KI. Effects of a novel free radical scavenger, MCl-186, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J Pharmacol Exp Ther. 1997;281:921–7. doi: 10.1016/s0531-5131(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 6.Minhaz U, Tanaka M, Tsukamoto H, Watanabe K, Koide S, Shohtsu A, et al. Effect of MCI-186 on postischemic reperfusion injury in isolated rat heart. Free Radic Res. 1996;24:361–7. doi: 10.3109/10715769609088034. [DOI] [PubMed] [Google Scholar]
- 7.Kono H, Asakawa M, Fujii H, Maki A, Amemiya H, Yamamoto M, et al. Edaravone, a novel free radical scavenger, prevents liver injury and mortality in rats administered endotoxin. J Pharmacol Exp Ther. 2003;307:74–82. doi: 10.1124/jpet.103.053595. [DOI] [PubMed] [Google Scholar]
- 8.Iguchi T, Nishikawa M, Chang B, Muroya O, Sato EF, Nakatani T, et al. Edaravone inhibits acute renal injury and cyst formation in cisplatin-treated rat kidney. Free Radic Res. 2004;38:333–41. doi: 10.1080/10715760310001646886. [DOI] [PubMed] [Google Scholar]
- 9.Wu TW, Zeng LH, Wu J, Fung KP. MCI-186: further histochemical and biochemical evidence of neuroprotection. Life Sci. 2000;67:2387–92. doi: 10.1016/S0891-5849(99)90974-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XH, Matsuda N, Jesmin S, Sakuraya F, Gando S, Kemmotsu O, et al. Normalization by edaravone, a free radical scavenger, of irradiation-reduced endothelial nitric oxide synthase expression. Eur J Pharmacol. 2003;476:131–7. doi: 10.1016/S0014-2999(03)02151-4. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Kwon AH, Habara K, Yamada M, Kaibori M, Kamiyama Y, et al. Edaravone inhibits the induction of iNOS gene expression at transcriptional and posttranscriptional steps in murine macrophages. Shock. 2008;30:734–9. doi: 10.1097/SHK.0b013e318173ea0b. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi G, Sakurai M, Abe K, Itoyama Y, Tabayashi K. MCI-186 prevents spinal cord damage and affects enzyme levels of nitric oxide synthase and Cu/Zn superoxide dismutase after transient ischemia in rabbits. J Thorac Cardiovasc Surg. 2003;126:1461–6. doi: 10.1016/S0022. [DOI] [PubMed] [Google Scholar]
- 13.Yang HC, Zuo Y, Fogo AB. Models of chronic kidney disease. Drug Discov Today Dis Models. 2010;7:13–9. doi: 10.1016/j.ddmod.2010.08.002. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Dunnill MS. Manual of Histopathological Staining Methods. Journal of Clinical Pathology. 1973;26:728. doi: 10.1136/jcp.26.9.728-c. [ PMC Free Article] [DOI] [Google Scholar]
- 17.Damiano S, Ciarcia R, Montagnaro S, Pagnini U, Garofano T, Capasso G, et al. Prevention of nephrotoxicity induced by cyclosporine-A: role of antioxidants. J Cell Biochem. 2015;116:364–9. doi: 10.1002/jcb.25022. [DOI] [PubMed] [Google Scholar]
- 18.Myers BD, Sibley R, Newton L, Tomlanovich SJ, Boshkos C, Stinson E, et al. The long-term course of cyclosporine-associated chronic nephropathy. Kidney Int. 1988;33:590–600. doi: 10.1038/ki.1988.38. [DOI] [PubMed] [Google Scholar]
- 19.Diederich D, Skopec J, Diederich A, Dai FX. Cyclosporine produces endothelial dysfunction by increased production of superoxide. Hypertension. 1994;23:957–61. doi: 10.1161/01.hyp.23.6.957. [DOI] [PubMed] [Google Scholar]
- 20.Akool el S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, et al. Molecular mechanisms of TGF beta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506. J Immunol. 2008;181:2831–45. doi: 10.4049/jimmunol.181.4.2831. [DOI] [PubMed] [Google Scholar]
- 21.Iglesias-De La Cruz MC, Ruiz-Torres P, Alcami J, Diez-Marques L, Ortega-Velazquez R, Chen S, et al. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int. 2001;59:87–95. doi: 10.1046/j.1523-1755.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, et al. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci. 2013;14:13909–30. doi: 10.3390/ijms140713909. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 2006;187:433–46. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- 24.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/bj3570593. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–53. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994;343:1199–206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- 27.Buffoli B, Pechanova O, Kojsova S, Andriantsitohaina R, Giugno L, Bianchi R, et al. Provinol prevents CsA-induced nephrotoxicity by reducing reactive oxygen species, iNOS, and NF-kB expression. J Histochem Cytochem. 2005;53:1459–68. doi: 10.1369/jhc.5A6623.2005. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hori K, Tsujii M, Iino T, Satonaka H, Uemura T, Akeda K, et al. Protective effect of edaravone for tourniquet-induced ischemia-reperfusion injury on skeletal muscle in murine hindlimb. BMC Musculoskelet Disord. 2013;14:113. doi: 10.1186/1471-2474-14-113. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassila M. Interaction of cyclosporine A and the renin-angiotensin system;new perspectives. Curr Drug Metab. 2002;3:61–71. doi: 10.2174/1389200023337964. [DOI] [PubMed] [Google Scholar]
- 30.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 31.Bouloumie A, Bauersachs J, Linz W, Scholkens BA, Wiemer G, Fleming I, et al. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension. 1997;30:934–41. doi: 10.1161/01.HYP.30.4.934. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita T, Sakamoto K, Yamanishi H, Totani N, Yamamoto J. Effect of a free radical scavenger on nitric oxide release in microvessels. Vascul Pharmacol. 2013;58:134–9. doi: 10.1016/j.vph.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004;65:1714–23. doi: 10.1111/j.1523-1755.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 34.Chiazza F, Chegaev K, Rogazzo M, Cutrin JC, Benetti E, Lazzarato L, et al. A nitric oxide-donor furoxan moiety improves the efficacy of edaravone against early renal dysfunction and injury evoked by ischemia/reperfusion. Oxid Med Cell Longev. 2015;2015:804659. doi: 10.1155/2015/804659. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


