Abstract
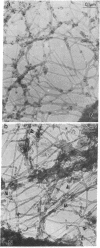
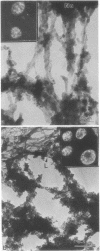
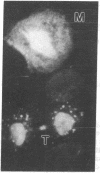
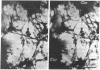
We describe two methods for staining resinless thin sections with antibodies and gold-conjugated second antibodies. Immunolocalization of specific proteins is a powerful tool for cell structure studies but current techniques do not develop its full potential. Immunofluorescence provides only low-resolution localization, whereas conventional thin-section electron microscopy images and immunostains only the section surface. Resinless sections of extracted cell structures offer a simple and effective means of immuno-electron microscopy. Without embedding plastic or soluble proteins, the cell cytostructure produces high-contrast, three-dimensional images. Resinless sections of detergent-extracted cells are prepared by embedding in diethylene glycol distearate, sectioning, and removing diethylene glycol distearate before microscopy. In the first method of immunostaining, extracted cells were fixed and stained with antibodies before embedment, sectioning, removal of the embedding resin, and critical point drying. In the postembedment method, the sample was embedded and sectioned, the diethylene glycol distearate was removed, and the sample was rehydrated before antibody staining. With these techniques, specific proteins were localized with high resolution throughout the entire section. Stereoscopic micrographs of resinless sections revealed the precise localization of specific cytoskeleton and nuclear matrix proteins in three dimensions with unprecedented clarity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond M., Somlyo A. V. Dense bodies and actin polarity in vertebrate smooth muscle. J Cell Biol. 1982 Nov;95(2 Pt 1):403–413. doi: 10.1083/jcb.95.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Krochmalnic G., Penman S. A new method of preparing embeddment-free sections for transmission electron microscopy: applications to the cytoskeletal framework and other three-dimensional networks. J Cell Biol. 1984 May;98(5):1878–1885. doi: 10.1083/jcb.98.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. H., Kargacin G., Craig R., Fogarty K., Fay F. S. Molecular structure and organization of filaments in single, skinned smooth muscle cells. Prog Clin Biol Res. 1987;245:1–25. [PubMed] [Google Scholar]
- De Mey J., Moeremans M., Geuens G., Nuydens R., De Brabander M. High resolution light and electron microscopic localization of tubulin with the IGS (immuno gold staining) method. Cell Biol Int Rep. 1981 Sep;5(9):889–899. doi: 10.1016/0309-1651(81)90204-6. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Taylor G. M. An immunocolloid method for the electron microscope. Immunochemistry. 1971 Nov;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Penman S. Nuclear matrix proteins reflect cell type of origin in cultured human cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):121–125. doi: 10.1073/pnas.85.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Dutton A. H., Tokuyasu K. T., Singer S. J. Immunoelectron microscope studies of membrane-microfilament interactions: distributions of alpha-actinin, tropomyosin, and vinculin in intestinal epithelial brush border and chicken gizzard smooth muscle cells. J Cell Biol. 1981 Dec;91(3 Pt 1):614–628. doi: 10.1083/jcb.91.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Yerna M. J., Schloss J. A. Localization and organization of microfilaments and related proteins in normal and virus-transformed cells. J Supramol Struct. 1976;5(2):155–183. doi: 10.1002/jss.400050206. [DOI] [PubMed] [Google Scholar]
- Ip W., Fischman D. A. High resolution scanning electron microscopy of isolated and in situ cytoskeletal elements. J Cell Biol. 1979 Oct;83(1):249–254. doi: 10.1083/jcb.83.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuma Y., Marceau N., Ohta M., French S. W. Cytokeratin intermediate filaments of rat hepatocytes: different cytoskeletal domains and their three-dimensional structure. Hepatology. 1988 May-Jun;8(3):559–568. doi: 10.1002/hep.1840080321. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K. R. The cytomatrix: a short history of its study. J Cell Biol. 1984 Jul;99(1 Pt 2):3s–12s. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E. L., Stolinski C., Hughes-Jones N. C. An antiglobulin reagent labelled with colloidal gold for use in electron microscopy. Immunochemistry. 1974 Aug;11(8):521–522. doi: 10.1016/0019-2791(74)90162-1. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Franzini-Armstrong C. New views of smooth muscle structure using freezing, deep-etching and rotary shadowing. Experientia. 1985 Jul 15;41(7):841–856. doi: 10.1007/BF01970000. [DOI] [PubMed] [Google Scholar]
- Stanley M. A., Browne H. M., Appleby M., Minson A. C. Properties of a non-tumorigenic human cervical keratinocyte cell line. Int J Cancer. 1989 Apr 15;43(4):672–676. doi: 10.1002/ijc.2910430422. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Ishikawa H. Association of actin and 10 nm filaments with the dense body in smooth muscle cells of the chicken gizzard. Cell Tissue Res. 1983;229(2):233–242. doi: 10.1007/BF00214972. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J. The application of polyethylene glycol (PEG) to electron microscopy. J Cell Biol. 1980 Aug;86(2):675–661. doi: 10.1083/jcb.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]