Abstract
Objectives
To describe modes of clinical presentation and echocardiographic, angiographic, and rhythmic features, and prognostic characteristics of left ventricular noncompaction cardiomyopathy (LVNC) in North African adults, through one of the first series in Morocco.
Background
LVNC is a rare congenital disorder, described for the first time by Engberding in 1984. The suspected diagnosis in thromboembolic, hemodynamic, or rhythm events requires both echocardiography and cardiovascular magnetic resonance (CMR). Its therapeutic management is not yet well codified but akin to that proposed for dilated cardiomyopathy.
Patients and design
This study included a retrospective, descriptive, and analytical cohort of 23 cases of cardiomyopathy with LVNC diagnosed in the Noninvasive Explorations Laboratory at the Military Hospital of Rabat, Morocco, between January 2009 and October 2014. The echocardiographic criteria for LVNC include the absence of any coexisting cardiac anomalies. The characteristic appearance of numerous excessively prominent trabeculations and deep intertrabecular recesses and intertrabecular spaces filled by direct blood flow from the ventricular cavity, as visualized on color Doppler imaging with noncompacted/compacted ratio > 2 according to Jenni criteria. Twenty-three adults fulfilled the diagnostic criteria and were followed prospectively.
Results
At diagnosis, the mean age was 47 ± 13 years with a male predominance at 65.2%. Of them, 56.5% had a left bundle branch block and 21.7% were in atrial fibrillation. Left ventricular end-diastolic diameter was 67.7 ± 6.6 mm and ejection fraction was at 27 ± 8%. Apex and/or midventricular segments of both the inferior and lateral wall were involved in more than 80% of patients with an average of 4.8 noncompacted segments. CMR was performed in 12 patients and was decisive for the diagnosis. Major complications were heart failure in 31% of patients, ventricular tachycardia in three patients, and thromboembolic events in one patient. Twenty eight point six percent of patients started a long-term anticoagulant therapy. One patient underwent implantation of a double-room pacemaker. Automated defibrillators were implanted in two patients. There were three deaths: one sudden death and two end-stage heart failure.
Conclusion
LVNC should be looked for at any dilated cardiomyopathy particularly in young patients. It requires a careful echocardiographic examination and sometimes CMR to confirm the diagnosis. It is characterized by severe systolic and diastolic dysfunction that would provide poor prognosis.
Keywords: CMR, Echocardiography, Left ventricular noncompaction, Predictors of mortality
Abbreviations
- CMR
Cardiac Magnetic Resonance
- LVNC
Left Ventricular Non Compaction
- LV
left ventricular
- INR
Index Normalized Ratio
- LVEF
left ventricular ejection fraction
- NC/C
non compacted/compacted
- CRT
Cardiac Resynchronization Therapy
- VT
ventricular tachycardia
- ICD
implantable cardioverter defibrillator
Introduction
Noncompacted myocardium has been categorized as an unclassified cardiomyopathy by the European Society of Cardiology in a published report concerning definitions and classifications of cardiomyopathies [1].
In patients with pulmonary atresia, regression of the embryogenic sinusoids is impaired during ontogenesis by ventricular pressure overload that results in deep recesses that communicate with both the ventricular cavity and the coronary artery system [2], [3], [4]. By contrast, left ventricular noncompaction (LVNC) is characterized by an embryogenic hypothesis characterized by an altered structure of the myocardial wall as a result of intrauterine arrest of compaction of the myocardial fibers and meshwork, an important process in myocardial development in the absence of any coexisting congenital lesion [5], [6]. There is continuity between the LV cavity and the deep intratrabecular recesses that are filled with blood from the ventricular cavity without evidence of communication to the epicardial coronary artery system [7], [8]. Since the original description, there have been only a few publications of case reports and small patient series predominantly describing a pediatric population [9], [10], [11]. Despite an increasing awareness and interest in this anomaly, there is still little knowledge regarding diagnostic criteria, symptoms, and prognosis of this rare congenital disorder categorized as unclassified cardiomyopathy [1]. This study was designed to report clinical, echocardiographic, angiographic, and rhythmic characteristics and long-term outcomes to look for predictive factors of mortality in the largest North African population of adults with LVNC ever described in Morocco.
Methods
Study population
From January 2009 to October 2014, 23 adults (age ⩾ 18 years, 15 men) fulfilled the diagnostic criteria of LVNC and their data were entered into the database of our laboratory. Informed consent was given by all patients that were recruited in two ways: the first comprising patients hospitalized in the cardiology department at the intensive care or the clinical units for heart failure or cardiac rhythm disorder, or patients followed in consultation for LV dysfunction, arrhythmia, or during an exploration of syncope, chest pain, dyspnea, or palpitations.
Data acquisition
Clinical assessment included a detailed medical history using standardized questionnaires. Medical records were reviewed. Sudden death was defined as occurring within 1 hour of the patient’s usual state of health or as an unwitnessed death during sleep [12]. All patients had a 12-lead resting electrocardiogram (ECG), chest radiography, and a complete two-dimensional Doppler echocardiographic exam as described below. Additionally, a 24-hour Holter monitoring was performed in 19 patients. Nonsustained ventricular tachycardia was defined as ventricular tachycardia of more than three premature ventricular contractions lasting up to 30 seconds. A ventricular run of more than 30 seconds was defined as sustained ventricular tachycardia.
Instrumental echocardiography
A transthoracic echocardiogram was performed with Vivid 7 or Vivid E9 (GE Vingmed Ultrasound AS, Horten, Norway). Recorded loops were visualized and analyzed by at least two operators. The echocardiographic diagnostic was retained as proposed by Jenni criteria.
A complete two-dimensional and Doppler echocardiographic examination was performed in all symptomatic patients and in a family screening context performed in almost all retained patients except constraints, according to the recommendations of the American Society of Echocardiography including two-dimensional guided M-mode measurements [13]. Only one case was retained in the family context. LV ejection fraction (LVEF) was calculated using the biplane area SIMPSON method.
The study also considered the number of noncompacted segments and measurement of the noncompacted area/compacted area ratio (NC/C ratio) and associated anomalies and calculated the blood pressure pulmonary systolic.
Cardiovascular magnetic resonance
Cardiovascular magnetic resonance (CMR) was performed in 12 patients with a doubt about the diagnosis at the echocardiography.
Coronary angiography
This was realized for 18 patients.
Statistical analysis
Statistical data were analyzed on Excel then IBM SPSS 20 statistics (IBM Inc., Armonk, NY, USA) and expressed as mean or median ± standard deviation.
Results
Epidemiology data
The mean age was 47 ± 13 years with a male predominance at 65.2% and a sex ratio at 1.8. Fourteen patients (60.8%) had no cardiovascular risk factor. A family history of sudden death was found in one patient.
Clinical data
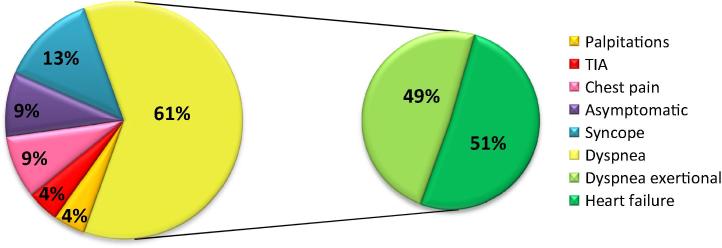
Clinical characteristics of the study population are presented in Fig. 1. The age range at the time of diagnosis was wide (19–72 years). Reasons for referral were: heart failure in 51%, pain chest in 9%, 4% for both palpitations and transient ischemic attack, and syncopal event in 13%. One patient was referred in the context of a family survey; a sister of one patient was screened because of the familial occurrence risk and was diagnosed to have LVNC. Familial occurrence of LVNC was present in one case.
Figure 1.
Clinic symptoms. TIA = transient ischemic attack.
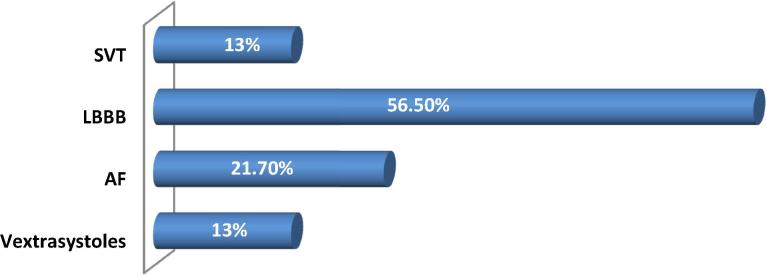
Fifteen patients were followed for another diagnosis within an average of 2.88 years (from 1 month to 21 years) before the LVNC was diagnosed. From the beginning, 91.3% of patients were symptomatic—the diagnosis was fortuitously discovered in only two asymptomatic patients. The ECG was abnormal in all of patients. Fifty six point five percent of patients had left bundle branch block, 21.7% of patients were in atrial fibrillation, and premature ventricular contractions in 13% patients (Fig. 2). No neuromuscular disorders or any other comorbidities were found.
Figure 2.
Electric data. AF = atrial fibrillation; LBBB = left bundle branch block; SVT = supraventricular tachycardia.
Echocardiographic data
Echocardiographic findings at the first presentation are summarized in Table 1. The LV end-diastolic diameter was 67.7 ± 6.6 mm (58; 83) and the ejection fraction was at 27 ± 8% (13; 41). Apex and/or midventricular segments of both the inferior and lateral wall were involved in >80% of patients with an average of 4.8 noncompacted segments (1; 10).
Table 1.
Echocardiographic data.
| Measurements (AVRG ± ET) | Minimum | Maximum | |
|---|---|---|---|
| LVEDD | 67.6 ± 6.6 | 58 | 83 |
| LVEF | 27 ± 8 | 13 | 41 |
| NC segments | 4.8 ± 0.2 | 1 | 10 |
| NC/C ratio | 2.12 ± 0.14 | 2 | 2.4 |
AVRG = average; ET = endothelin; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; NC = noncompacted; NC/C = noncompacted/compacted.
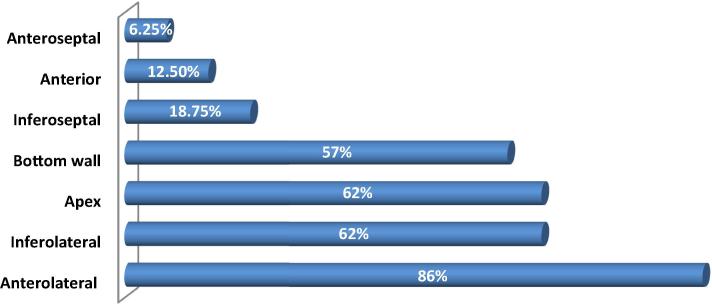
Parts that were of particular concern (according to the 17 segments model) were the anterolateral wall (86%), infero-lateral wall (62%), the apical wall (62%), and the inferior wall (57%). NC/C ratio was 2.12 ± 0.14 (2; 2.4; Fig. 3).
Figure 3.
Various noncompacted cardiac walls (according to the 17 segments model).
Global LV hypokinesia was noted in 69.6% of patients and segmental disorder kinetics in 30.4%. There was a low flow in 17 patients and a restrictive mitral flow in 14 patients. The left ventricle was the site of a spontaneous contrast without visible thrombus in two patients.
Pulmonary arterial hypertension was found in 11 cases (47.8%) with an average of the pulmonary arterial systolic pressure at 49 mmHg. Among the major associated abnormalities, there was an ostium secundum atrial septal defect in two patients without hemodynamic repercussion, tricuspid regurgitation in 18 patients, and minimal to medium mitral regurgitation in all patients. The right ventricular was normal for all patients.
CMR data
Echocardiography was performed for all the patients. It was decisive in 11 cases, but the use of CMR was needed to confirm and retain the diagnosis in 12 patients, taking as diagnosis the threshold of 2.3 in diastole for the thickness NC/C ratio [14].
Coronary was normal in 15 of the 18 patients who had undergone coronary angiography, two had a double vessel lesion and one had a simple vessel lesion.
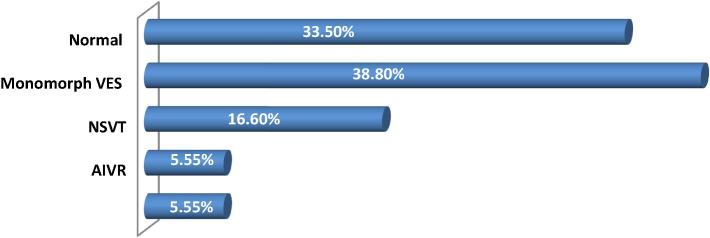
The 24-hour Holter ECG was performed in 18 patients. It showed a monomorphic ventricular extrasystole in seven cases, nonsustained ventricular tachycardia in three, one accelerated idioventricular rhythm, one paroxysmal atrial fibrillation passage, and it was normal in six patients (Fig. 4).
Figure 4.
Holter electrocardiogram data. AIVR = accelerated idioventricular rhythm; NSVT = nonsustained ventricular tachycardia; VES = ventricular extrasystole.
An electrophysiological study was performed in a patient with a history of sudden death in his family and who had a repeated syncope. It concluded at an atrioventricular conduction disorder justifying the final pacing.
Treatment
During the study, the acenocoumarol (international normalized ratio 2–3) was given to 28.6% of patients with LV dysfunction (LVEF < 30%) or atrial fibrillation or a history of thromboembolic episodes. The treatment for heart failure and LV dysfunction was based on current clinical guidelines [15].
A biventricular cardioverter defibrillator was implanted in two patients with a history of sustained ventricular tachycardia. Both patients benefited from the resynchronization therapy. Biventricular pacing systems were implanted in two additional patients with heart failure despite optimal medical treatment.
No patient underwent cardiac transplantation because it is currently an undeveloped therapy in Morocco.
Evolution and mortality
Forty-three point five percent of heart failure patients required hospital admission and 56.5% were stabilized with medications remaining in Class II New York Heart Association (NYHA).
Two died because of end-stage heart failure. Thromboembolic events were represented by an episode of transient ischemic attack observed in one patient.
Three patients sustained a reduced ventricular tachycardia. An implantable cardioverter defibrillator was to be implanted in two patients but one of those patients died (sudden death) from recurrent and refractory sustained ventricular tachycardia before the implanted cardioverter defibrillator.
In addition to the three deaths described above, recently there was a fourth death due to an extracardiac cause which was Lyell’s syndrome.
No patients underwent autopsy during the study.
Characteristics of survivors and nonsurvivors
All nonsurvivors were men (p < 0.001) with LVEF < 30% (p < 0.005). The LV end-diastolic diameter was significantly larger for male nonsurvivors (70 ± 9 mm) and not as much for survivors (61 ± 12 mm; p < 0.005). NYHA Class III/IV (63% vs. 11%; p < 0.005), chronic atrial fibrillation (50% vs. 39%; p < 0.05), bundle branch block (75% vs. 39%; p < 0.045), total number of noncompacted segments more than five (76% vs. 23%; p < 0.001), and NC/C ratio more than 2.2 (89% vs. 14%; p < 0.001; Table 2).
Table 2.
Statistics table.
| Parameters | p in univariate analysis | p in multivariate analysis |
|---|---|---|
| Age | 0.8 | – |
| Male sex | 0.005 | <0.001 |
| Iterative episodes of HF | 0.03 | 0.08 |
| Embolic events | 0.09 | – |
| NYHA Class III/IV | 0.02 | <0.005 |
| Chronic AF | 0.05 | 0.05 |
| LBBB | 0.03 | 0.045 |
| SVT | 0.02 | 0.1 |
| LVEDD >65 mm | 0.03 | <0.005 |
| LVEF <30% | 0.03 | <0.005 |
| NC segments | 0.03 | <0.001 |
| NC/C ratio | 0.02 | <0.001 |
AF = atrial fibrillation; HF = heart failure; LBBB = left bundle branch block; LVEDD = left ventricular end diastolic diameter; LVEF = left ventricular ejection fraction; NC = noncompacted; NC/C = noncompacted/compacted; NYHA = New York Heart Association; SVT = supraventricular tachycardia.
There was no significant difference between survivors and nonsurvivors regarding age at diagnosis, heart failure requiring hospital admissions, and the presence of both embolic events and sustained ventricular tachycardia.
The period of clinical, electrical, and echocardiographic follow-up during the study was set at 2 years. For some patients (collected at the end of the study) monitoring is still ongoing.
During follow-up, the morphology and topography of LVNC did not change, but we have noted a worsening of the LV dysfunction in some patients.
Discussion
We report the largest Moroccan study of adult patients followed for LVNC, including the first formal analysis of prognosis factors. Unlike the previously reported cohorts recruiting inpatients or outpatients and which are generally focused on patients with a symptomatic diagnosis, patients in the present study are identified by an echocardiographic examination recruiting also nonsymptomatic patients. Our data indicate that the presentation spectrum of LVNC can be wider than what was previously thought. Early reports about the disease generally show dramatic cases, whereas subsequent studies broaden progressively the vision of the affected population, extending it to patients with less and less severe disease [16]. The present study confirms and extends this revised vision and provides first outcomes analysis from a single referral center in Morocco.
The noncompaction of the left ventricle can be isolated or associated to other congenital malformations (Ebstein anomaly, complex cyanotic heart disease, etc.). Familial forms are not rare; about 25% of asymptomatic relatives have criteria for LVNC [17]. Eight percent of patients in the French registry reported by Habib et al. [18] were detected as part of a family survey. A number of genes involved have been identified.
A genetic study should be performed in our study in order to support the embryogenic hypothesis and rule out some particular phenotypic characteristics of LVNC which are identified in cases including sickle cell anemia and athletes.
Athletes have trabeculations that do not reach 2:1 ratio for noncompacted and compacted layers. In some patients with myocarditis or pregnancy the criteria for LVNC are reached, but the trabeculations regress after some time. Therefore the embryogenic hypothesis is still controversial [19], [20].
Classification and epidemiology
In 1996, the LVNC was mentioned by the World Health Organization as an unclassified cardiomyopathy. Since 2006, it has been considered by the American Heart Association as a distinct genetic cardiomyopathy [21]. However, for the European Society of Cardiology, the last classification in 2008 still considers it as an unclassified cardiomyopathy. This highlights the nosological uncertainty that persists about this entity [22].
It is caused by an abnormality of the embryogenesis of the endocardium and myocardium in utero, resulting in prominent ventricular trabeculae with deep intertrabecular recesses [21]. And although it is a congenital abnormality, the diagnosis is often established at the onset of complications at adulthood, as in the case of our center which mainly receives a military active population which explains the young age of our series of patients.
Prevalence is still poorly known. In the rare echocardiographic series that involve this disease in adults, the reported prevalence varies from 0.01% to 0.27% [23]. At presentation, the most common reasons for referral are heart failure and uncertain echocardiographic findings.
Clinical presentation
All the major diagnostic pathways commonly encountered in clinical practice were represented in our series of patients. At presentation, the most common reason for referral was heart failure at 51%. Although the fact that the embolic events were a frequent complication in the initial publications, they were the reason for referral of just one patient. This is explained by the early introduction of anticoagulants.
Diagnosis
Diagnosis was delayed in most patients with a delay time range from 1 month to 21 years in our series. Echocardiography is the basic diagnostic tool, but the criteria are not well established. There are currently three echocardiographic definitions: Chin is based on the calculation of an X/Y ratio between the depth of intertrabecular recesses relative to wall thickness, but end-diastolic differentiation between noncompacted and compacted myocardium was often difficult [7]. Therefore, we proposed to identify our patients by the criteria of Jenni et al. [6] which is the most used criteria because they are best suited for the measurement of NC/C ratio ⩾2 at the end-systolic in the left parasternal incidence short axe. Finally, the criteria by Stöllberger et al. [24] for the detection of more than three visible trabeculae on the same cutting plane.
All patients of the cohort have dilated left ventricles and systolic dysfunction. This can be explained by the fact that almost all of our patients were seen at symptomatic stage (Fig. 5).
Figure 5.
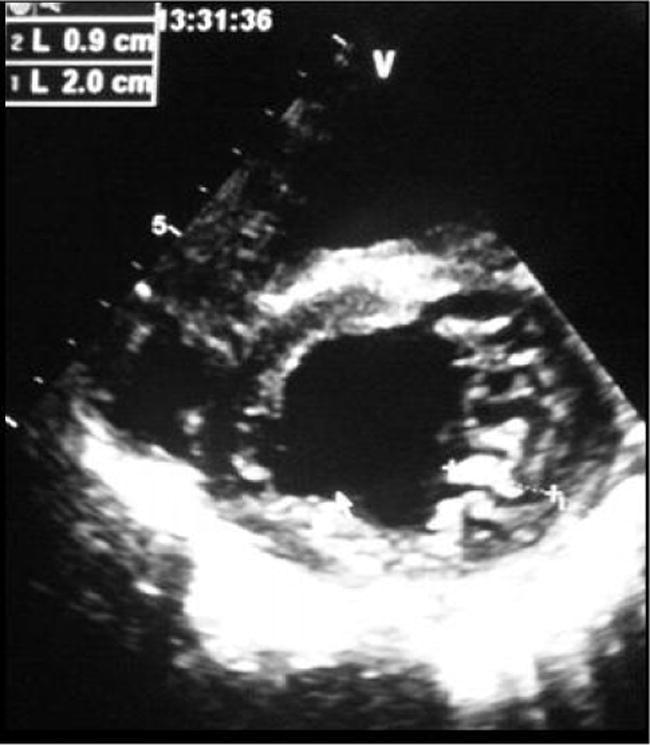
Parasternal short axis showing the trabeculae of the lateral left ventricular wall, with noncompacted/compacted ratio at 2.2.
The echocardiographic diagnosis of this pathology remains difficult, particularly because of the essentially apical localization abnormalities (62% in our series). This explains the delay between the first echocardiographic examination and the diagnosis. The LV systolic function is generally more altered when the amount of noncompacted myocardium is important.
However, it is not unusual to see perfectly normal ejection fractions in patients with LVNC (16% of 105 patients included in the French registry) [18]. Indeed, the correlation study between LVEF and the number of noncompacted segments was performed objectifying a correlation ratio at 0.18 (low correlation). Studies using speckle tracking show early achievement of systolic function even when the LVEF is preserved [25].
Two-dimensional strain also allows to distinguish the noncompaction of the LV and dilated cardiomyopathy, myocarditis, peripartal cardiomyopathy, or hearts of athletes showing a relative preservation of the basal longitudinal deformation with respect to the apex [26], [27].
The CMR presents a number of advantages. It provides high resolution images stamped in apical regions. Petersen et al [14] proposed a diagnosis threshold of thicknesses NC/C ⩾ 2.3 in diastole. Indeed, CMR was performed in 12 patients in our series and was decisive in retaining the diagnosis. The study with gadolinium shows that the importance of delayed enhancement is correlated with the severity of clinical signs and LV dysfunction [28] (Fig. 6).
Figure 6.
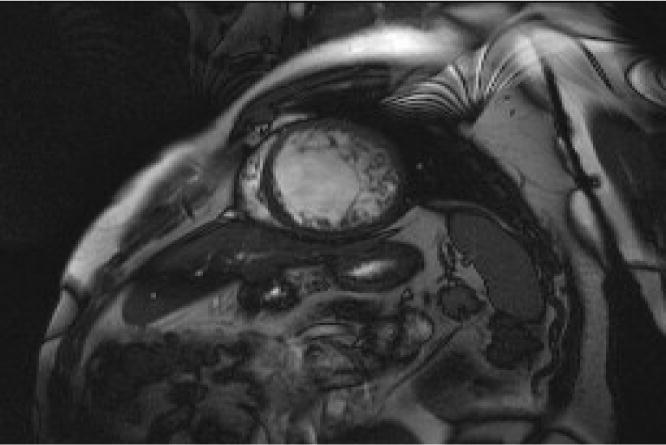
Short axis cine magnetic resonance imaging sequence showing noncompacted lateral wall.
Management
There are currently no specific treatment recommendations for noncompaction of the left ventricle. Symptomatic patients with LV dysfunction should receive the usual treatment of heart failure. Careful rythmological monitoring should be performed (Holter, stress test). The decision of a defibrillator or resynchronization is based on the usual recommendations. The introduction of a systematic anticoagulation is controversial.
Because of the frequent family forms and the theoretical risk of transmission of 50% in autosomal recessive mutation cases, a systematic screening for first-degree relatives should be implemented and allows an earlier detection of asymptomatic patients [24].
Prognosis
The largest current series are still relatively small and do not allow for an accurate assessment of prognosis of this disease. The reported mortality in the original series is high, ranging from 35% to 47% over 42–72 months of follow-up periods, but it may have been overestimated in the French cohort: 11% of patients die during the follow-up (27.6 months). A French study is currently underway to determine the prognostic factors of this disease compared with a population of classic dilated cardiomyopathy.
In our cohort, prognostic factors were identified as mortality predictors. Male sex, the LV end-diastolic diameter reached 70 ± 9 mm with LVEF < 30% for nonsurvivors. NYHA Class III/IV, chronic atrial fibrillation, left bundle branch block, total number of noncompacted segments more than five, and NC/C ratio more than 2.2 were the mortality factors in our series.
Conclusion
This study indicates that LVNC can be associated with a broad spectrum of clinical and pathophysiological findings and strengthens the concept that the overall natural history and prognosis of LVNC may be better than previously thought. In particular, the diagnostic pathway may be an important element of clinical evaluation, with incidental and familial discovery of LVNC being associated with a high probability of a stable course for several years.
Conversely, male patients who have symptoms of heart failure, LV end-diastolic diameter at 65 mm, ejection fraction at 30%, chronic atrial fibrillation, left bundle branch block, total number of noncompacted segments more than five, and NC/C ratio more than 2.2 have an unstable clinical course and a more severe prognosis.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Thiene G, Corrado D, Basso C. Position statement on diagnosis and treatment of cardiomyopathies. Projects and Initiatives European Cardiomyopathy Register 2008.
- 2.Lauer R.M., Fink H.P., Petry E.L., Dunn M.I., Diehl A.M. Angiographic demonstration of intramyocardial sinusoids in pulmonary valve atresia with intact ventricular septum. N Engl J Med. 1964;271:68–72. doi: 10.1056/NEJM196407092710203. [DOI] [PubMed] [Google Scholar]
- 3.Dusek J., Ostadal B., Duskova M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch Pathol. 1975;99:312–317. [PubMed] [Google Scholar]
- 4.Freedom R.M., Patel R.G., Bloom K.R. Congenital absence of the pulmonary valve associated with imperforate membrane type of tricuspid atresia, right ventricular tensor apparatus and intact ventricular septum: a curious developmental complex. Eur J Cardiol. 1979;10:171–196. [PubMed] [Google Scholar]
- 5.Engberding R., Bender F. Echocardiographic detection of persistent myocardial sinusoids. Z Kardiol. 1984;73:786–788. [PubMed] [Google Scholar]
- 6.Jenni R., Goebel N., Tartini R., Schneider J., Arbenz U., Oelz O. Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic and pathologic anatomical findings. Cardiovasc Intervent Radiol. 1986;9:12731. doi: 10.1007/BF02577920. [DOI] [PubMed] [Google Scholar]
- 7.Chin T.K., Perloff J.K., Williams R.G., Jue K., Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 8.Ritter M., Oechslin E., Sutsch G., Attenhofer C., Schneider J., Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72:26–31. doi: 10.4065/72.1.26. [DOI] [PubMed] [Google Scholar]
- 9.Bleyl S.B., Mumford B.R., Brown H.M. Xq28-linked noncompaction of the left ventricular myocardium: prenatal diagnosis and pathologic analysis of affected individuals. Am J Med Genet. 1997;72:257–265. [PubMed] [Google Scholar]
- 10.Shah C.P., Nagi K.S., Thakur R.K., Boughner D.R., Xie B. Spongy left ventricular myocardium in an adult. Tex Heart Inst J. 1998;25:150–151. [PMC free article] [PubMed] [Google Scholar]
- 11.Jenni R., Rojas J., Oechslin E. Isolated noncompaction of the myocardium. N Engl J Med. 1999;340:966–967. doi: 10.1056/NEJM199903253401215. [DOI] [PubMed] [Google Scholar]
- 12.Myerburg R.J., Castellanos A. Cardiac arrest and sudden cardiac death. In: Braunwald E., editor. Heart disease: a textbook of cardiovascular medicine. 4th ed. W.B. Saunders Company; Philadelphia: 1992. pp. 756–789. [Google Scholar]
- 13.Devereux R.B., Alonso D.R., Lutas E.M. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 14.Petersen S.E., Selvanayagam J.B., Wiesmann F. Left ventricular non-compaction. Insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 15.McMurray J.J.V., Adamopoulos S., Anker S.D., Auricchio A., Bohm M., Dickstein K. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 16.Yasukawa K., Terai M., Honda A. Isolated noncompaction of ventricular myocardium associated with fatal ventricular fibrillation. Pediatr Cardiol. 2001;22:512–514. doi: 10.1007/s002460010286. [DOI] [PubMed] [Google Scholar]
- 17.Elliott P., Andersson B., Arbustini E. Classification of the cardiomyopathies: a position statement from the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 18.Habib G., Charron P., Eicher J.C. Isolated left ventricular non-compaction in adults: clinical and echocardiographic features in 105 patients. Results from a French registry. Eur J Heart Fail. 2011;13:177–185. doi: 10.1093/eurjhf/hfq225. [DOI] [PubMed] [Google Scholar]
- 19.Gati S., Papadakis M., Papamichael N.D., Zaidi A., Sheikh N., Reed M. Reversible de novo left ventricular trabeculations in pregnant women: implications for the diagnosis of left ventricular noncompaction in low-risk populations. Circulation. 2014;130:475–483. doi: 10.1161/CIRCULATIONAHA.114.008554. [DOI] [PubMed] [Google Scholar]
- 20.Gati S., Papadakis M., Van Niekerk N., Reed M., Yeghen T., Sharma S. Increased left ventricular trabeculation in individuals with sickle cell anemia: physiology or pathology? Int J Cardiol. 2013;168:1658–1660. doi: 10.1016/j.ijcard.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Maron B.J., Towbin J.A., Thiene G. Contemporary definitions and classification of the cardiomyopathies: an American heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 22.Sedmera D., Pexieder T., Vuillemin M. Developmental patterning of the myocardium. Anat Rec. 2000;2584:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 23.Oechslin E.N., Attenhofer Jost C.H., Rojas J.R., Kaufmann P.A., Jenni R. Long term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36:493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 24.Stöllberger C., Finsterer J., Blazek G. Left ventricular hypertrabeculation, noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol. 2002;90:899–902. doi: 10.1016/s0002-9149(02)02723-6. [DOI] [PubMed] [Google Scholar]
- 25.Bellavia D., Michelena H.I., Martinez M. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular noncompaction. Heart. 2010;96:440–447. doi: 10.1136/hrt.2009.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niemann M., Liu D., Hu K. Echocardiographic quantification of regional deformation helps to distinguish isolated left ventricular noncompaction from dilated cardiomyopathy. Eur J Heart Fail. 2012;14:155–161. doi: 10.1093/eurjhf/hfr164. [DOI] [PubMed] [Google Scholar]
- 27.Bellanger A.R., Miller M.A., Dontireddi U.R. New classification scheme of left ventricular noncompaction and correlation with ventricular performance. Am J Cardiol. 2008;102:92–96. doi: 10.1016/j.amjcard.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 28.Stacey R.B., Andersen M.M., St Clair M. Comparison of systolic and diastolic criteria for isolated LV noncompaction in CMR. JACC Cardiovasc Imaging. 2013;6:931–940. doi: 10.1016/j.jcmg.2013.01.014. [DOI] [PubMed] [Google Scholar]