Abstract
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is seldom recognized clinically in infancy or under the age of 10. We report a case of a 9-year-old girl with ARVC, who presented with signs and symptoms of heart failure and palpitations. Holter monitoring showed frequent premature ventricular beats and echocardiogram revealed dilated and dysfunctional right ventricle with normal tricuspid valve and no evidence of intracardiac shunt. Cardiac magnetic resonance showed classical features of ARVC with both ventricular involvements. After optimization of medical treatment the patient was referred for ICD implantation.
Keywords: ARVC, CMR, Dilated RV
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy, characterized by fibro-fatty replacement of the right ventricular (RV) myocardium. It is associated with cardiac arrhythmias of right ventricular origin, right ventricular dysfunction, heart failure, and sudden cardiac death [1]. Clinically recognized ARVC is unusual in children, probably because the clinical expression of the disease is normally postponed to youth and adulthood. There is now an increased awareness about nonclassical phenotypes of ARVC, including left dominant and biventricular types, and its presence in children.
Here, we report a case of a young girl with ARVC, biventricular involvement, and heart failure.
Case report
A 9-year-old girl presented with signs and symptoms of heart failure and palpitations. Electrocardiography showed sinus rhythm with T wave inversion in chest leads. Morphology and duration of QRS complexes in right precordial leads were normal. There was no delay in activation. Holter monitoring showed frequent ventricular premature beats of left bundle branch block morphology. Echocardiographic examination revealed dilated and dysfunctional right ventricle with normally placed tricuspid valve and no evidence of intracardiac shunt. Holter monitoring showed frequent isolated ventricular ectopies but no evidence of sustained ventricular tachycardia. No information was available about the family history, as the child was adopted by the family from an orphan house. Neither parent was alive and the cause of death was not known.
With the clinical and echocardiographic suspicion of ARVC, the child was referred for cardiac magnetic resonance (CMR), which was done on a Siemens Avanto 1.5 T machine (Siemens, Munich, Germany) with ARVC protocol. Image sequences acquired included: black blood T1-weighted turbo spin echo images with and without fat suppression; steady-state free precession or cine images; and late gadolinium images after gadolinium injection.
RV was dilated (end diastolic volume 132 mL) with dys-synchronous contractility and severely reduced systolic function (ejection fraction 26%). RV free wall was thin looking with evidence of regional/focal dyskinesis (Fig. 1). Left ventricular (LV) volumes were normal with wall motion abnormalities involving the inferolateral wall and apex and mildly reduced systolic function (ejection fraction 44%).
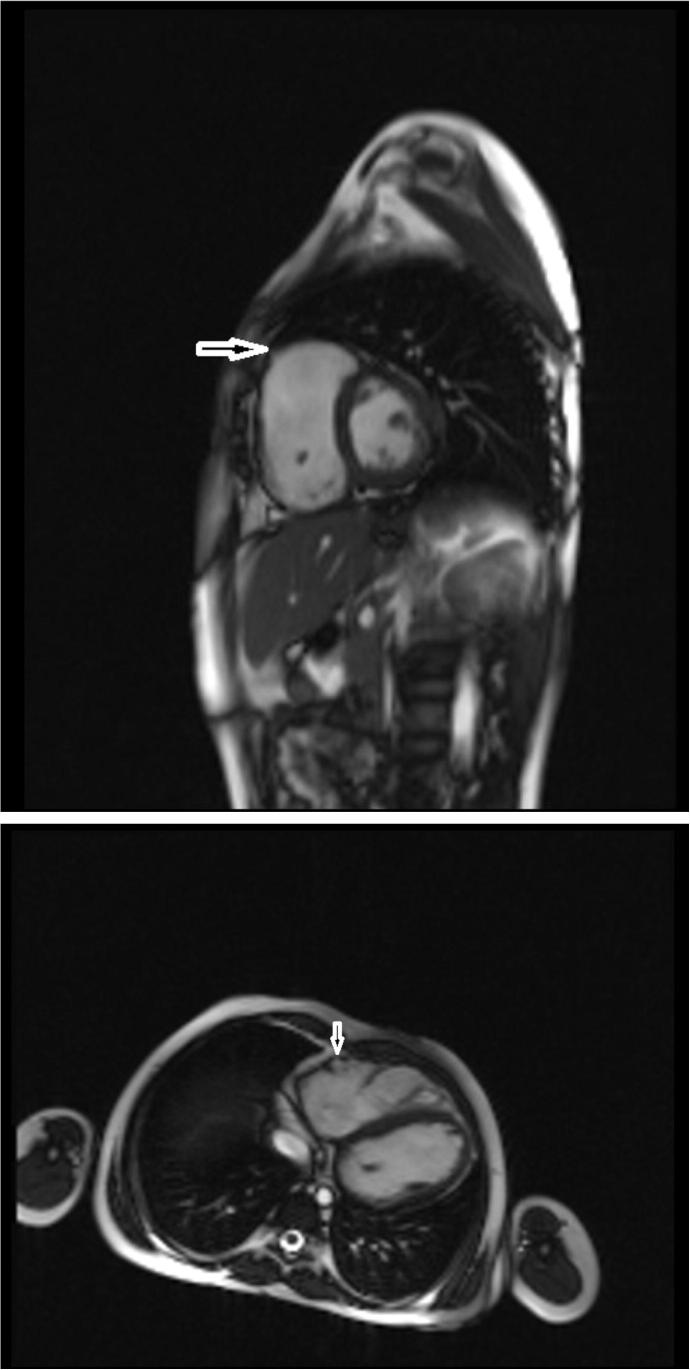
Figure 1.
Steady-state free precession still frame, showing focal aneurysmal areas in the right ventricular free wall, indicated by the arrow.
There was fatty infiltration on the epicardial surface of RV free wall and also fatty replacement of myocardium in the mid to distal lateral wall of the LV (Fig. 2). On late gadolinium images, diffuse hyperenhancement of the RV free wall and RV side of the interventricular septum was noted. There was also hyperenhancement involving the inferolateral segments and apex of the LV (Fig. 3).
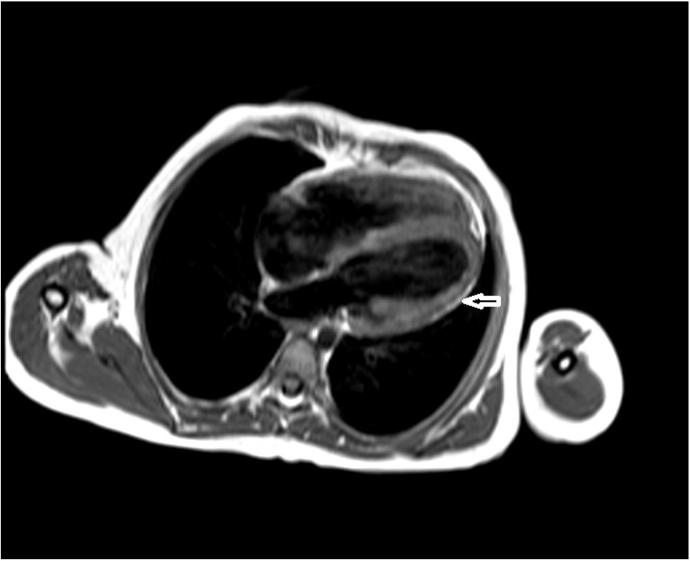
Figure 2.
Turbo spin echo T1 weighted image, showing fatty infiltration of the myocardium, indicated by the arrow.
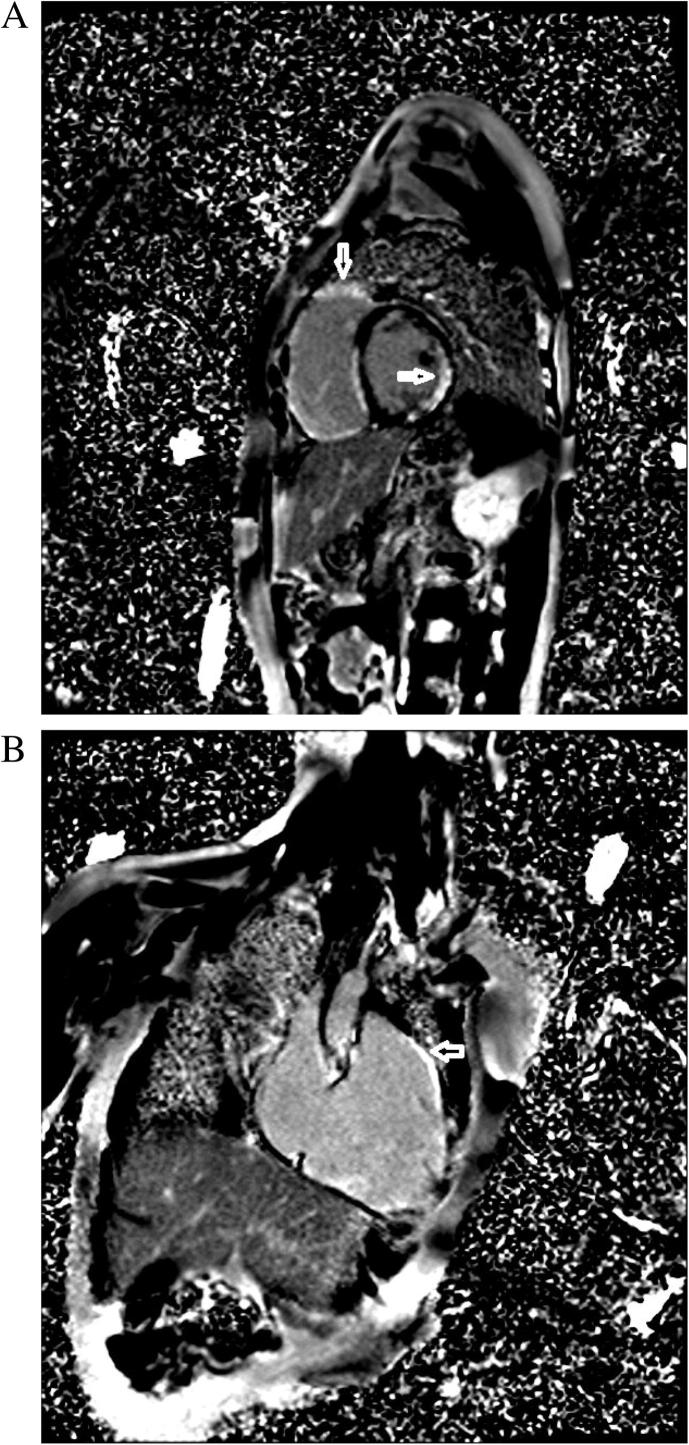
Figure 3.
(A) Delayed enhanced image with gadolinium, showing hyperenhancement in right and left ventricular walls, indicated by the arrow. (B) Delayed enhanced image with gadolinium, showing hyperenhancement in the right ventricular wall, indicated by the arrow.
She was managed medically for heart failure and referred for cardioverter–defibrillator implantation.
Discussion
The prevalence of ARVC is 1 in 2000–5000 individuals [2]. The prevalence of children affected by ARVC in nonselected clinical series usually comprises 5–30% of the series [3]. ARVC is usually diagnosed in the clinical setting at age 20–40 years. The disease is seldom recognized clinically in children under the age of 10 years. In children, it is usually in the concealed phase.
In this instance, however, the presentation of the disease occurred at a very young age with signs of heart failure. This may be due to the extensive nature of the disease and biventricular involvement in this patient.
ARVC may lead to sudden death even in pediatric age and is diagnosed at autopsy. The youngest case on autopsy was reported by Pawel et al. [4] in 1994, involving an Italian child of 7 years. Children who have ARVC may present with ventricular tachycardia. Dungan et al. [5] described three children presenting with ventricular tachycardia at a very young age.
ARVC has also been diagnosed in asymptomatic children who are relatives of identified cases when disease is confirmed either genetically or through clinical testing or biopsy.
Symptomatic children with overt clinical ARVC (i.e., RV structural abnormalities associated with heart failure and ventricular arrhythmias) are rare. Our patient comes into this rare category.
There was a case report from Saudi Arabia of a 12-year-old boy who presented with history of progressive shortness of breath and enlarged heart before developing ventricular arrhythmias [6]. Another 11-year-old Saudi girl presented with multiple ventricular ectopics, tiredness, and palpitations with typical features of ARVC on echocardiogram [7]. To our knowledge, our patient is the youngest of reported cases presenting with overt clinical ARVC.
Echocardiography is usually the first line imaging modality used for ARVC evaluation, as is the case in our patient. However, echocardiography has its limitations in assessing the RV due to its complex geometry.
CMR has now emerged as the imaging modality of choice in ARVC. CMR allows not only the morphological and functional evaluation but also the tissue characterization of the myocardium. RV abnormalities in ARVC have been extensively described in the literature. Our patient had all the classical features of RV involvement, including global reduction in function, enlargement, and focal/regional dyskinesia.
Diagnosis of ARVC was made on the basis of one major and two minor criteria, according to the modified task force criteria for the diagnosis of ARVC [8]. The major criterion was regional RV akinesia or dyskinesia and RV ejection fraction of <40% on CMR. The minor criteria were inverted T waves in chest leads and >500 ventricular premature beats on 24-hour Holter monitoring. Family history was not available in our case as she was an adopted child from an orphan house with no record of the family members. CMR showed evidence of fibro-fatty replacement of the myocardium in our case but this needs to be present on endomyocardial biopsy according to the diagnostic criteria.
The case reported here showed biventricular involvement. LV involvement has been reported in ARVC patients, with the majority having advanced disease. LV involvement in ARVC may manifest as late gadolinium enhancement, often involving the inferior and lateral walls. In our patient, inferolateral, lateral wall, and LV apex were involved. In addition to this constellation of features suggestive of ARVC, LV fatty infiltration is also a prevalent finding in the pathology, often involving the subepicardial lateral wall of LV and resulting in myocardial wall thinning [9]. This patient also exhibited features of fatty infiltration into the subepicardial lateral wall of the LV.
The patient was referred for ICD implantation, which is appropriate according to the international task force consensus statement. A prophylactic ICD implantation is recommended in patients with severe RV dysfunction, who are considered at high risk even in the absence of life-threatening ventricular arrhythmias [10].
In conclusion, this is a unique case of ARVC in a 9-year-old girl with overt clinical ARVC and classical CMR findings.
Acknowledgment
Thanks to the technologist Imran Ahmed for his help.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mehnaz Atiq Ahmed, Email: mehnaz.atiq@aku.edu.
Joseph B. Selvanayagam, Email: joseph.selvanayagam@flinders.edu.au.
References
- 1.Marcus F.I., Fontaine G.H., Guiraudon G., Frank R., Laurenceau J.L., Malergue C. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Sen-Chowdhry S., Morgan R.D., Chambers J.C., McKenna W.J. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu Rev Med. 2010;61:233–253. doi: 10.1146/annurev.med.052208.130419. [DOI] [PubMed] [Google Scholar]
- 3.Nava A., Thiene G., Canciani B., Martini B., Daliento L., Buja G.F. Clinical profile of concealed form of arrhythmogenic right ventricular cardiomyopathy presenting with apparently idiopathic ventricular arrhythmias. Int J Cardiol. 1992;35:195–206. doi: 10.1016/0167-5273(92)90177-5. [DOI] [PubMed] [Google Scholar]
- 4.Pawel B.R., de Chadarévian J.P., Wolk J.H., Donner R.M., Vogel L., Braverman P. Sudden death in childhood due to right ventricular dysplasia: report of two cases. Pediatr Pathol. 1994;14:987–995. doi: 10.3109/15513819409037695. [DOI] [PubMed] [Google Scholar]
- 5.Dungan N.T., Garson A., Jr, Gillette P.C. Arrhythmogenic right ventricular dysplasia: a cause of ventricular tachycardia in children with apparently normal hearts. Am Heart J. 1981;102:745–750. doi: 10.1016/0002-8703(81)90101-0. [DOI] [PubMed] [Google Scholar]
- 6.Al-Jarallah A.S. Arrhythmogenic right ventricular dysplasia: case report and a review of the literature. Ann Saudi Med. 1997;17:350–353. doi: 10.5144/0256-4947.1997.350. [DOI] [PubMed] [Google Scholar]
- 7.Wong A.R., Razak N.A., Al Hadlaq S.M., Al Jarallah A.S. Arrhythmogenic right ventricular cardiomyopathy in an 11-year-old girl and typical echocardiographic features. Pediatr Cardiol. 2008:29427–29430. doi: 10.1007/s00246-007-9082-2. [DOI] [PubMed] [Google Scholar]
- 8.Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Te Riele A.S., James C.A., Philips B., Rastegar N., Bhonsale A., Groeneweg J.A. Mutation-positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced. J Cardiovasc Electrophysiol. 2013;24:1311–1320. doi: 10.1111/jce.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrado D., Wichter T., Link M.S., Hauer R., Marchlinski F., Anastasakis A. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36:3227–3237. doi: 10.1093/eurheartj/ehv162. [DOI] [PMC free article] [PubMed] [Google Scholar]