Abstract
Carrots are widely grown and enjoyed around the world. Purple carrots accumulate rich anthocyanins in the taproots, while orange, yellow, and red carrots accumulate rich carotenoids in the taproots. Our previous studies indicated that variation in the activity of regulatory genes may be responsible for variations in anthocyanin production among various carrot cultivars. In this study, an R2R3-type MYB gene, designated as DcMYB6, was isolated from a purple carrot cultivar. In a phylogenetic analysis, DcMYB6 was grouped into an anthocyanin biosynthesis-related MYB clade. Sequence analyses revealed that DcMYB6 contained the conserved bHLH-interaction motif and two atypical motifs of anthocyanin regulators. The expression pattern of DcMYB6 was correlated with anthocyanin production. DcMYB6 transcripts were detected at high levels in three purple carrot cultivars but at much lower levels in six non-purple carrot cultivars. Overexpression of DcMYB6 in Arabidopsis led to enhanced anthocyanin accumulation in both vegetative and reproductive tissues and upregulated transcript levels of all seven tested anthocyanin-related structural genes. Together, these results show that DcMYB6 is involved in regulating anthocyanin biosynthesis in purple carrots. Our results provide new insights into the regulation of anthocyanin synthesis in purple carrot cultivars.
Carrots (Daucus carota L.; 2n = 2x = 18) are an economically important root crop worldwide. The taproot of cultivated carrots exhibits a range of colors including orange, yellow, red, white, and purple. Purple carrots contain anthocyanins, whereas the orange, red, and yellow pigmentation of carrot taproots is due to carotenoids1. White carrots contain very low levels of carotenoids2. Thus, cultivated carrots can be divided into two distinct groups: the anthocyanin or eastern group (Daucus carota ssp. sativus var. atrorubens Alef.) and the carotene or western group (Daucus carota ssp. sativus var. sativus)3.
Anthocyanins are a group of flavonoids that perform various important functions in plants. They provide pigmentation in vegetative and reproductive tissues4,5,6,7,8, enhance cold, drought, and salt tolerance9,10,11, and protect against damage from ultraviolet light, insect herbivory, and pathogen attack12,13. Purple carrots accumulate rich anthocyanins in the fleshy taproot. The role of anthocyanins in carrot taproots is unclear; however, it is conceivable that they could protect the taproot from insect and pathogen attack. Anthocyanins are beneficial to human health14 and are used as natural food colorants in beverages, candies, and ice cream15.
The genetics of anthocyanin biosynthesis have been extensively studied in many plant species. Regulatory genes encoding transcription factors (TFs) control the transcription of structural genes encoding enzymes involved in anthocyanin biosynthesis. In our previous studies, the transcripts of numerous structural genes in the anthocyanin pathway were undetectable or barely detectable in the taproots of non-purple carrots, but were detected at high levels in those of purple carrots. It seems likely that variation in the activity of regulatory genes is the key factor determining anthocyanin production in carrots16.
In many species studied to date, the R2R3-MYB TFs, basic helix-loop-helix (bHLH) TFs, and WD-repeat (WDR) proteins form ‘MBW’ complexes that bind to the promoters of target genes to directly activate the transcription of structural genes in the anthocyanin pathway17. The MYB proteins in this complex are often the key component determining variation in anthocyanin production18,19,20. Anthocyanin-related MYBs have been identified in many plant species, for example, Arabidopsis AtMYB75 (PAP1), AtMYB90 (PAP2), AtMYB113, and AtMYB11421, Vitis vinifera VvMYB1a22, Ipomoea batatas IbMYB18, and Malus × domestica MdMYB10, MdMYB1/MdMYBA19,23,24. Overexpression of genes encoding these MYB TFs in heterologous or homologous plant species leads to enhanced anthocyanin accumulation.
In this study, a gene encoding an R2R3-type MYB, designated as DcMYB6, was isolated from a purple carrot cultivar. The correlation between its expression with anthocyanin production in purple and non-purple carrots was analyzed. The function of DcMYB6 was also analyzed by overexpression in Arabidopsis plants. These results will further our understanding of how anthocyanin synthesis is regulated in carrots.
Results
Sequence analysis of DcMYB6
The amplification products of the genomic DNA sequence and the open reading frame (ORF) sequence of DcMYB6 from the carrot cultivar ‘Deep purple’ are shown in Supplementary Fig. S1. The genomic DNA sequence of DcMYB6 was 1,801 bp long while the ORF sequence of DcMYB6 was 903 bp long, encoding a polypeptide of 300 amino acids (Supplementary Fig. S1A). Alignment analysis of genomic DNA and ORF sequences revealed that the DcMYB6 gene consisted of two introns and three exons (Supplementary Fig. S1B).
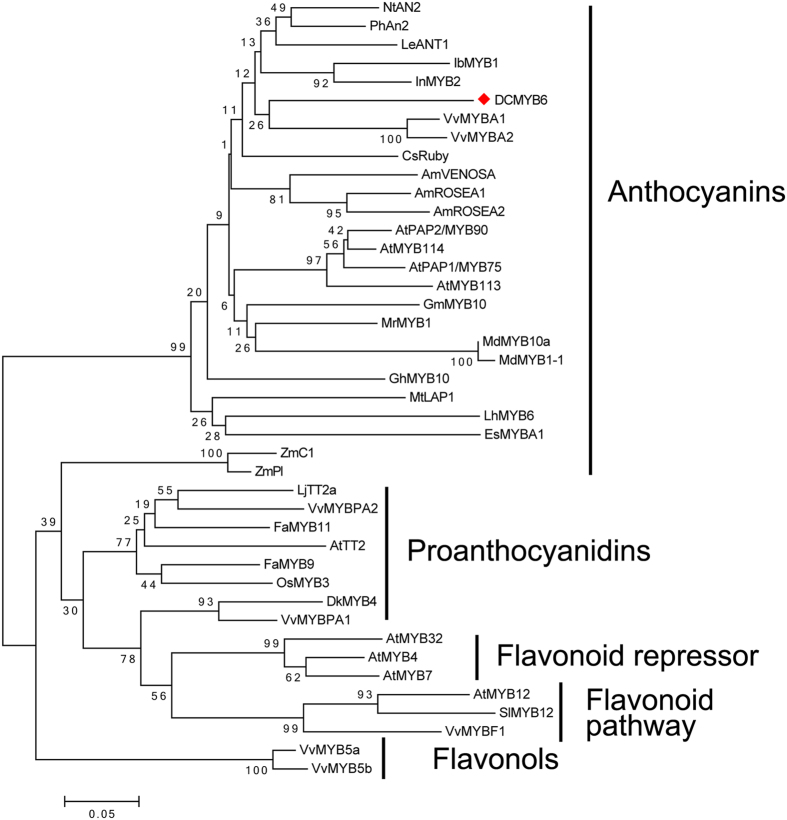
We conducted a phylogenetic analysis with the deduced amino acid sequences of DcMYB6 and other R2R3-MYB TFs involved in the biosynthesis of different secondary metabolites. Supplementary Table S1 lists the GenBank accession numbers of the R2R3-MYBs used to build the phylogenetic tree. In the phylogenetic tree, R2R3-MYB TFs with similar functions clustered together, and DcMYB6 grouped into an anthocyanin biosynthesis-related MYB clade (Fig. 1), which included tobacco (Nicotiana tabacum) NtAN2, petunia (Petunia hybrida) PhAn2, tomato (Lycopersicon esculentum) LeANT1, sweet potato (I. batatas) IbMYB1, morning glory (I. nil) InMYB2, grapevine (V. vinifera) VvMYBA1 and VvMYBA2, blood orange (Citrus sinensis) CsRuby, snapdragon (Antirrhinum majus) AmVENOSA, AmROSEA1, and AmROSEA2, Arabidopsis thaliana AtPAP1, AtPAP2, and AtMYB114, mangosteen (Garcinia mangostana) GmMYB10, Chinese bayberry (Myrica rubra) MrMYB1, apple (Malus × domestica) MdMYB10a and MdMYB1-1, Gerbera hybrida GhMYB10, Medicago truncatula MtLAP1, Lilium hybrid LhMYB6, and Epimedium sagittatum EsMYBA1.
Figure 1. Phylogenetic relationships among DcMYB6 and flavonoid-related R2R3-MYBs from other plant species.
Phylogenetic tree was built using the neighbor-joining method using MEGA 5 software; bootstrap value was set to 1000. Red diamond indicates DcMYB6. Putative functions of all R2R3-MYBs are listed on the right.
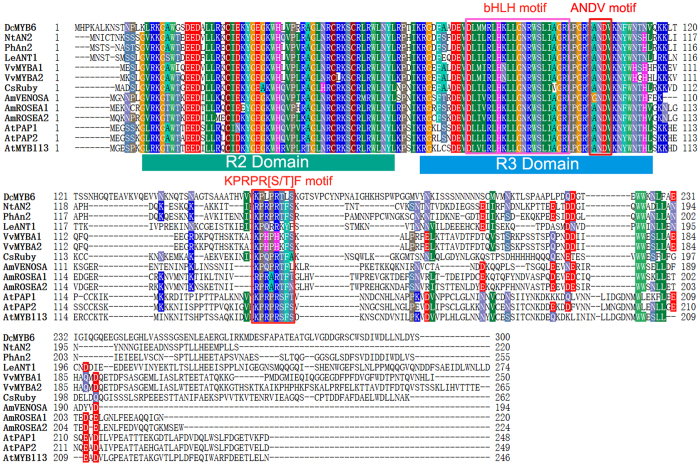
Next, we conducted an alignment analysis of the deduced amino acid sequence of DcMYB6 with those of other MYB TFs related to anthocyanin biosynthesis. Like other MYB TFs, DcMYB6 contained the highly conserved R2R3 domain at the N-terminus (Fig. 2). DcMYB6 showed high sequence homology with other MYB TFs within the R2R3 domain, sharing the highest identity (85%) with LeANT1 and the lowest identity (80%) with AmVENOSA. However, all the MYB TFs showed little homology in the C-terminus sequence to the R2R3 domain. When whole sequences were compared, DcMYB6 shared the highest identity (44%) with PhAn2 and the lowest identity (32%) with AmROSEA1.
Figure 2. Alignment of deduced amino acid sequence of DcMYB6 and R2R3-MYB proteins from other plant species.
Alignment was conducted using BioEdit (Version 7.0.1). Identical amino acid residues are shaded as per color table (threshold for shading was set to 60%). R2 and R3 domains are indicated. Boxes show BHLH, ANDV and KPRPR[S/T]F motifs.
The alignment showed that the [D/E]Lx2[R/K]x3Lx6Lx3 R motif, also known as the bHLH motif25, which is required for the interaction with bHLH proteins, was present in the R3 domain of all the analyzed MYB TFs (Fig. 2). The conserved ANDV motif that has been identified in MYB TFs in the anthocyanin pathway in the Rosaceae26 was also present in all of the analyzed MYB TFs and was modified to [A/G]NDV. Besides these motifs, the MYB TFs contained the motif KPRPR[S/T]F defined by Stracke et al.27, which was modified to [K/R]Pxx[H/R] [K/S/T][F//L/Y], in the C-terminal region.
Quantitative real-time PCR analysis of DcMYB6 in purple and non-purple carrot taproots
At the 90-day-old stage, purple carrot cultivars had accumulated rich anthocyanins whereas anthocyanins were barely detectable in, or absent from non-purple carrot cultivars16. Using specific primer pairs, qRT-PCR analyses were performed to quantify the transcript levels of DcMYB6 in purple and non-purple carrots at this stage. The transcript levels of DcMYB6 in the taproots of three purple carrot cultivars (‘Deep purple’, ‘Purple 68’, and ‘Tianzi2hao’) were approximately 10–229-fold higher than those in the taproots of six non-purple carrot cultivars (‘Kuroda’, ‘Sanhongliucun’, ‘Junchuanhong’, ‘Bejo1719’, ‘Qitouhuang’, and ‘Baiyu’). Among the three purple carrot cultivars, ‘Tianzi2hao’ had the highest transcript level of DcMYB6 and ‘purple 68’ had the lowest. Among the six non-purple carrot cultivars, ‘Baiyu’ had the lowest transcript level of DcMYB6 and ‘Sanhongliucun’ had the highest (Fig. 3).
Figure 3. Transcript profiles of DcMYB6 in 90-day-old taproots of three purple and six non-purple carrot cultivars.
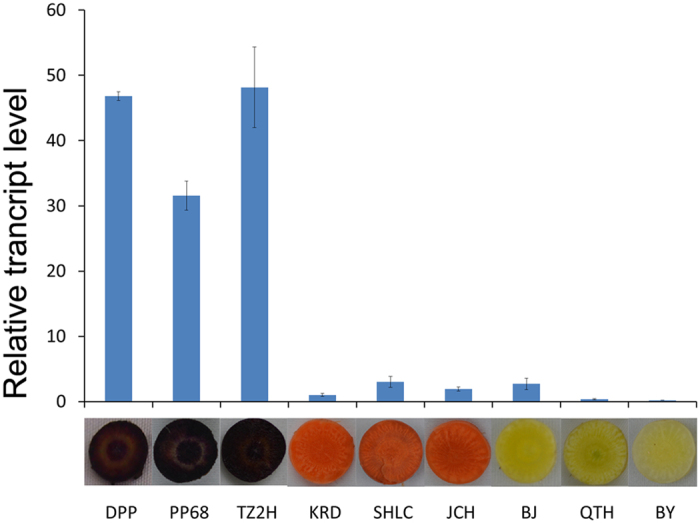
Data represent means of three biological replicates ± SD. Cultivar abbreviations: DPP, Deep purple; PP68, Purple 68; TZ2H, Tianzi2hao; KRD, Kuroda; SHLC, Sanhongliucun; JCH, Junchuanhong; BJ, Bejo1719; QTH, Qitouhuang; BY, Baiyu.
Subcellular localization of DcMYB6 protein
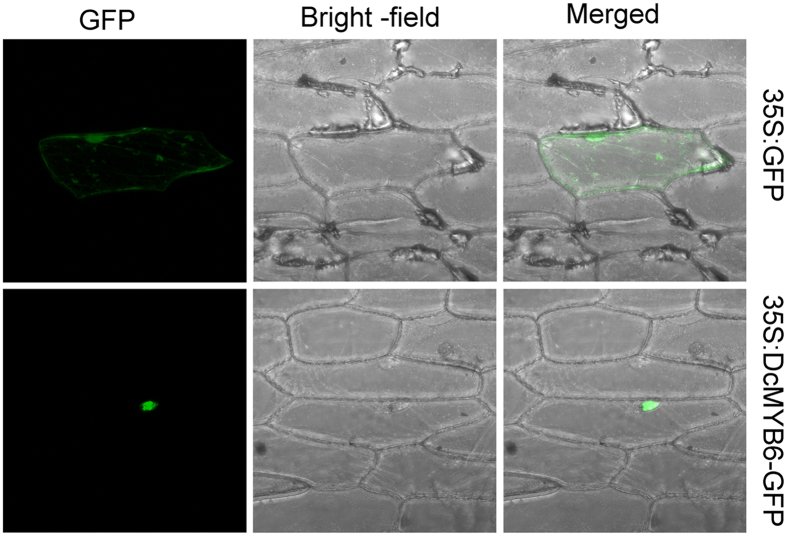
To investigate the subcellular localization of DcMYB6, the DcMYB6 coding sequence was fused in-frame to the 5′ terminus of the gene encoding GFP, and the construct was transiently expressed in onion cells. In onion cells expressing GFP alone, fluorescence was localized in the cytoplasm and nucleus (Fig. 4 up). Onion cells expressing the DcMYB6-GFP fusion protein showed a strong signal in the nucleus (Fig. 4 down).
Figure 4. Subcellular localization of GFP fusions of DcMYB6.
Onion epidermal cells transiently expressing GFP and DcMYB6-GFP under the control of the CaMV 35 S promoter.
Overexpression of DcMYB6 in transgenic Arabidopsis induced anthocyanin production
The DcMYB6 gene driven by the CaMV 35 S promoter was overexpressed in Arabidopsis plants to test its function. Arabidopsis seedlings of three homozygous CaMV 35 S:DcMYB6 transgenic lines (DcMYB6-1, DcMYB6-2, and DcMYB6-3) and one control transgenic line, which were selected on MS agar plates containing hygromycin, showed β-glucuronidase (GUS) activity (Fig. 5A). A PCR product of approximately 900 bp corresponding to the DcMYB6 coding sequence was detected in all three CaMV 35 S:DcMYB6 transgenic Arabidopsis lines analyzed, whereas no such PCR product was amplified from control transgenic plants (Fig. 5B).
Figure 5. Identification of transgenic Arabidopsis plants with histochemical GUS activity and PCR analyses.
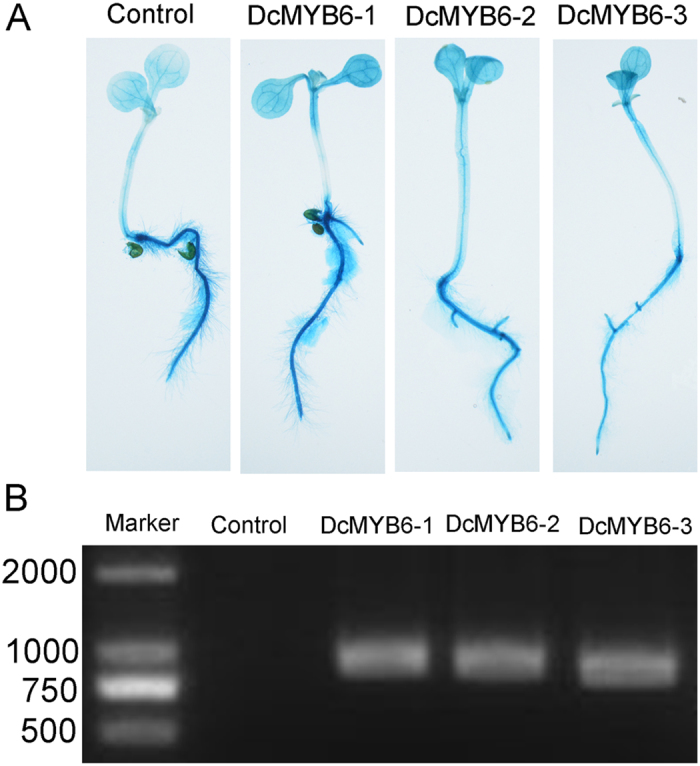
(A) Histochemical GUS activity analysis of transgenic Arabidopsis plants overexpressing empty vector (control) and DcMYB6 (DcMYB6-1, DcMYB6-2, and DcMYB6-3). (B) PCR-amplified DcMYB6 fragments from the same transgenic Arabidopsis plants as above.
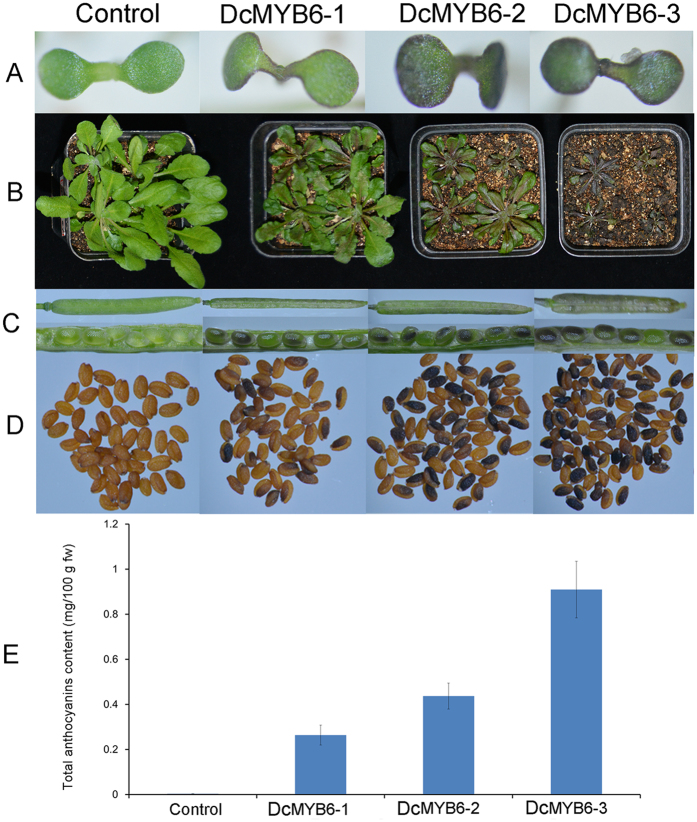
Overexpression of DcMYB6 in Arabidopsis induced anthocyanin accumulation. Compared with control plants, DcMYB6-1, DcMYB6-2, and DcMYB6-3 plants exhibited dark-purple pigments in the leaves, siliques, and immature and mature seed coats (Fig. 6A–D), and delayed growth. The total anthocyanin content in the whole plants of DcMYB6-1, DcMYB6-2, and DcMYB6-3 plants was approximately 66–228-fold higher than that incontrol plants (Fig. 6E). Among the three CaMV 35 S:DcMYB6 transgenic Arabidopsis lines, DcMYB6-1 showed the lowest total anthocyanin content and DcMYB6-3 showed the highest.
Figure 6. Functional analysis of DcMYB6 in Arabidopsis.
(A and B) Ten-day-old and 40-day-old transgenic Arabidopsis plants overexpressing empty vector (control) and DcMYB6 (DcMYB6-1, DcMYB6-2, and DcMYB6-3). (C) Immature capsules and seeds from control and three lines of transgenic Arabidopsis plants overexpressing DcMYB6. (D) Mature seeds from control and three lines of DcMYB6-overexpressing transgenic Arabidopsis plants. (E) Total anthocyanin contents of 40-day-old transgenic Arabidopsis plants overexpressing empty vector (control) and DcMYB6 (DcMYB6-1, DcMYB6-2, and DcMYB6-3).
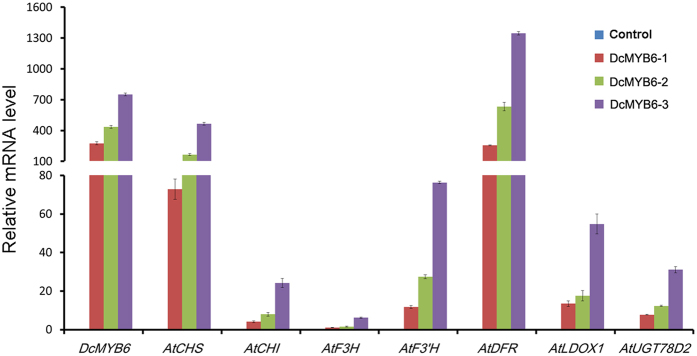
Up-regulation of anthocyanin biosynthetic genes in transgenic Arabidopsis overexpressing DcMYB6
Among the three CaMV 35 S:DcMYB6 transgenic Arabidopsis lines, DcMYB6-1 plants showed the lowest DcMYB6 transcript levels and DcMYB6-3 plants showed the highest (Fig. 7). As expected, DcMYB6 transcripts were undetectable in the control Arabidopsis plants. The results of the qRT-PCR analyses also determined which endogenous anthocyanin pathway structural genes were up-regulated in the transgenic Arabidopsis plants overexpressing DcMYB6. Compared with the control line, the transgenic Arabidopsis plants overexpressing DcMYB6 showed significantly increased transcript levels of AtCHS (chalcone synthase), AtCHI (chalcone isomerase), AtF3H (flavanone 3- hydroxylase), AtF3′H (flavonoid 3′-hydroxylase), AtDFR (dihydroflavonol 4- reductase), AtLDOX (leucoanthocyanidin dioxygenase), and AtUGT78D2 (Fig. 7). Among the three transgenic Arabidopsis lines overexpressing DcMYB6, DcMYB6-1 plants showed the lowest transcript levels of these structural genes and DcMYB6-3 plants showed the highest. Transcripts of these structural genes were undetectable or barely detectable in the control Arabidopsis plants.
Figure 7. Transcript levels of DcMYB6 and seven anthocyanin pathway structural genes in transgenic Arabidopsis plants.
Gene transcript levels in Arabidopsis plants overexpressing empty vector (control) and DcMYB6 (DcMYB6-1, DcMYB6-2, and DcMYB6-3) were detected by quantitative RT-PCR. Data represent means of three biological replicates ± SD.
Discussion
Anthocyanins are water-soluble pigments responsible for purple colors in carrots. In plants, TFs such as MYB, bHLH, and WD40 upregulate the expression of structural genes in the anthocyanin biosynthesis pathway. Two previous studies showed that the expression levels of all anthocyanin pathway structural genes were significantly lower in non-purple carrot cultivars than in purple carrot cultivars, which possibly resulted from the inactivation of regulator genes16,28. In other plant species, many R2R3-MYB TFs are known to control anthocyanin biosynthesis by regulating structural genes in the anthocyanin pathway4,16,17,22,25. However, little is known about the R2R3-MYB TFs involved in regulating the anthocyanin pathway in carrot. A previous study reported that DcMYB3 and DcMYB5 might upregulate the activity of the DcPAL3 promoter29. In the present study, a gene encoding R2R3-MYB, namely DcMYB6, was isolated from ‘Deep purple’, a purple carrot cultivar.
DcMYB6 grouped into the same clade as the MYB TF family of the anthocyanin pathway, and shared high identity with anthocyanin-regulating MYB TFs from other species within the R2R3 domain. DcMYB6 was found to contain the conserved bHLH interaction motif [D/E]Lx2[R/K]x3Lx6Lx3 R in the R3 domain, and an atypical anthocyanin regulator motif KPRPR[S/T]F at the C-terminus. Another conserved motif, [A/G]NDV, which distinguishes anthocyanin and non-anthocyanin MYB TFs in the Rosaceae, was also found in DcMYB6. The presence of these motifs suggested that DcMYB6 may be involved in regulating anthocyanin biosynthesis. In several other plant species, the expression of many R2R3-MYB genes in the anthocyanin pathway is strong correlated with anthocyanin accumulation. For example, MdMYB10 was found to be highly expressed highly in red-fleshed apple and in the colored skin of white-fleshed apple, but was virtually undetectable in the white cortex of white-fleshed apple19. In the present study, DcMYB6 transcript levels corresponded well with anthocyanin pigmentation; there were much higher transcript levels in all three 90-day-old purple carrot taproots than in 90-day-old taproots of the six non-purple carrot cultivars. Therefore, DcMYB6 is probably involved in regulating anthocyanin biosynthesis in purple carrot taproots.
The reason why DcMYB6 transcript levels were much lower in non-purple carrots than in purple carrots is still unknown. In peach (Prunus persica), the heterodimer of BL and PpNAC1 was shown to activate transcription of the anthocyanin-related MYB, PpMYB10.130. In European pear (Pyrus communis), methylation of the PcMYB10 promoter reduced PcMYB10 expression levels and resulted in a peel color change from red to green31. Insertions and deletions in the promoter region have been shown to affect the expression levels of anthocyanin-related MYBs in some species, such as apple (Malus × domestica) and grapevine (V. vinifera)20,22. In future work, we will attempt to establish the reason for the different transcript level of DcMYB6 in purple and non-purple carrots.
In several studies, overexpression of anthocyanin-related MYB TFs in heterologous plant species led to enhanced anthocyanin accumulation32,33. In this study, transgenic Arabidopsis plants overexpressing DcMYB6 exhibited a clearly darker color and accumulated higher levels of anthocyanins in both vegetative and reproductive tissues, compared with those in control Arabidopsis plants. Furthermore, qRT-PCR analyses of the three transgenic Arabidopsis lines with different transcript levels of DcMYB6 and different total anthocyanin levels showed that higher transcript levels of DcMYB6 led to greater anthocyanin accumulation. Also, the transcript levels of all seven tested anthocyanin-related structural genes were much higher in transgenic Arabidopsis plants overexpressing DcMYB6 than in control Arabidopsis plants. Together, these results indicate that DcMYB6 could enhance anthocyanin accumulation in Arabidopsis by upregulating anthocyanin-related structural genes, and suggest that DcMYB6 regulates anthocyanin biosynthesis in purple carrots.
In conclusion, an R2R3-MYB TF, DcMYB6, was isolated from a purple carrot cultivar and was found to be involved in regulating the anthocyanin biosynthetic pathway. The results of this study provide important information on the pigmentation of purple carrots. Other TFs such as bHLH and WD40 that form complexes with MYB proteins and together regulate anthocyanin biosynthesis have not yet been identified in carrots. In future work, we will test whether overexpression of DcMYB6 in non-purple carrot cultivars leads to anthocyanin accumulation.
Methods
Plant materials
Three purple carrot cultivars (‘Deep purple’, ‘Purple 68’, and ‘Tianzi2hao’), three orange carrot cultivars (‘Kuroda’, ‘Sanhongliucun’, and ‘Junchuanhong’), and three yellow carrot cultivars (‘Bejo1719’, ‘Qitouhuang’, and ‘Baiyu’), which are widely cultivated in China, were chosen for this work. Seeds were grown in a controlled artificial climatic chamber under the same conditions as previously described16. Arabidopsis thaliana ecotype Columbia was grown under the same conditions.
RNA and DNA extraction from carrots and cDNA preparation
Total RNA was extracted from taproots of 90-day-old carrot plants using an RNAsimple Total RNA Kit (Tiagen, Beijing, China). First-strand cDNA was synthesized using the PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time; Takara, Dalian, China). cDNA was diluted 20-fold for gene cloning and qRT-PCR analyses. Genomic DNA was isolated from young leaves with a DNAsecure plant kit (Tiangen).
Isolation of genomic DNA and cDNA sequence of DcMYB6
AtPAP1 (AAG42001) was BLASTed against our CarrotDB: a genomic and transcriptomic database for carrot34 and the high-quality carrot genome which spans 421.5 Mb and accounts for ~90% of the estimated genome size (473 Mb)35. Two transcript contigs showing high sequence identity with AtPAP1 and with higher FPKM values in purple carrots than in non-purple carrots were identified in the transcriptomic database of CarrotDB. After assembling these two transcript contigs, an ORF of 903 bp was identified and predicted to be a MYB TF using Pfam (http://pfam.xfam.org/). This MYB TF was designated as DcMYB6 in this study. Two genomic sequence scaffolds (scaffold 016995 and scaffold 029424) that matched the ORF sequence were identified in the genomic database of CarrotDB. However, no genomic sequence matching the ORF sequence was found in the high-quality carrot genome.
The ORF sequence of DcMYB6 was amplified from cDNA produced from 90-day-old ‘Deep purple’ carrot taproots using PrimeSTAR HS DNA polymerase (Takara, Otsu, Japan) with the forward primer (5′-CGCGCGGATCTTCCAGAGATTATGCATCCAAAGGCTTTGAAGAAT-3′) and reverse primer (5′-CACGCCTGCCGTTCGACGATTTTAACTATAATCCAAGTTAAGAAGGTCCC-3′). The ORF sequence was then cloned into the pMD19-T simple vector (Takara, Otsu, Japan) using the ClonExpress II One Step Cloning Kit (Vazyme Biotech Co. Ltd., Nanjing, China) before sequencing (Genscript, Nanjing, China). The same pairs of primers were also used to amplify the genomic clone of DcMYB6 from genomic DNA extracted from carrot leaves. The full-length ORF and DNA sequences of DcMYB6 have been deposited in the GenBank database under the accession numbers KY020445 and KY020446, respectively.
Subcellular localization analysis
The protein-coding region of DcMYB6 was amplified with the forward primer (5′- CACCATCACCATCACGCCATGATGATCAAGAGCACTGGTAATCC-3′) and the reverse primer (5′- CACTAGTACGTCGACCATGGCACTATAGTCCTGGTTGAGAAGATCCC-3′), and was subcloned into the pA7-GFP vector at the Nco I site to create the CaMV 35 S:DcMYB6-GFP fusion construct. This construct and the pA7-GFP empty vector (as control) were both bombarded into onion epidermal cells using a Biolistic PDS-1000 instrument (Bio-Rad, Hercules, CA, USA). After incubation at 25 °C for at least 16 h in the dark, samples were observed under a confocal laser scanning microscope.
Overexpression vector construct preparation and Arabidopsis transformation
The coding sequence of DcMYB6 was amplified with the forward primer (5′-TTTACAATTACCATGGGATCCATGCATCCAAAGGCTTTGAAGAAT-3′) and the reverse primer(5′-ACCGATGATACGAACGAGCTCTTAACTATAATCCAAGTTAAGAAGGTCCC-3′), and then subcloned into the binary vector pCAMBIA-1301 under the control of the CaMV 35 S promoter and the pea rbcSE9 terminator to create the CaMV 35 S:DcMYB6 construct. This construct was introduced into Agrobacterium tumefaciens strain GV3101 by electroporation and then transformed into Arabidopsis using the floral-dip method36. Transgenic Arabidopsis plants carrying the DcMYB6 gene were identified by selection on half-strength Murashige and Skoog (MS) agar plates containing 35 mg/L hygromycin, assaying for GUS activity, and detecting the presence of the transgene by reverse transcription PCR with the forward primer (5′-ATGCATCCAAAGGCTTTGAAGAAT-3′) and the reverse primer (5′-AAGCACAACAAATGGTACAAG-3′), which were designed according to the sequence of DcMYB6 and the pea rbcSE9 terminator, respectively. Three transgenic Arabidopsis lines (DcMYB6-1, DcMYB6-2, and DcMYB6-3) with black leaves were used for further experiments. Arabidopsis plants transformed with the pCAMBIA-1301 empty vector served as controls.
Determination of total anthocyanin content
Total anthocyanins were extracted from 40-day-old transgenic Arabidopsis plants (T3) as described previously37. Total anthocyanin quantities are presented in mg cyanidin 3-O-glycoside equivalents per 100 g fw (mg/100 g fw). Three biological replicates were analyzed for each sample.
Quantitative real-time PCR expression analysis
The mRNA levels of the DcMYB6 gene in 90-day-old carrot taproots and in 40-day-old transgenic Arabidopsis plants were determined by qRT-PCR with the forward primer (5′-GCCATAGGGCACAAGCACTCT-3′) and the reverse primer (5′-GATCCCAATTTCCGCAAACAA-3′). Total RNA was extracted from 40-day-old transgenic Arabidopsis plants and used to synthesize cDNA using the method described above. To determine the transcript levels of anthocyanin pathway structural genes in transgenic Arabidopsis, qRT-PCR assays were performed with the primers listed in Supplementary Table S2. The DcActin1 gene was used as an internal standard in carrot with the same primers as described previously16,38, while the AtActin2 gene was used as an internal standard for normalization in Arabidopsis and was amplified using the primers listed in Supplementary Table S2. Experiments were conducted using three biological replicates for each sample. The relative gene transcript level was calculated with the 2−ΔΔCT method39. To compare DcMYB6 expression patterns among purple and non-purple carrots at the 90-day-old stage, the ΔΔCT was calculated by subtracting ΔCT of ‘Kuroda’ from ΔCT of all carrot cultivars. To compare the transcript levels of DcMYB6 and anthocyanin pathway structural genes among transgenic Arabidopsis plants, the ΔΔCT was calculated by subtracting ΔCT of the AtF3H (flavanone 3-hydroxylase) gene in DcMYB6-1 Arabidopsis plants from the ΔCT of all tested genes in transgenic Arabidopsis plants.
Additional Information
How to cite this article: Xu, Z.-S. et al. A MYB transcription factor, DcMYB6, is involved in regulating anthocyanin biosynthesis in purple carrot taproots. Sci. Rep. 7, 45324; doi: 10.1038/srep45324 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The research was supported by the National Natural Science Foundation of China (31501775) and the Fundamental Research Funds for the Central Universities (KJQN201657).
Footnotes
The authors declare no competing financial interests.
Author Contributions Z. S. X. and A. S. X. initiated and designed the research. Z. S. X., K. F. and F. Q. performed the experiments. Z. S. X. analyzed the data. A. S. X. and W.F. contributed reagents/materials/analysis tools. Z. S. X. wrote the paper. Z. S. X. and A. S. X. revised the paper.
References
- Clotault J. et al. Expression of carotenoid biosynthesis genes during carrot root development. J Exp Bot 59, 3563–3573 (2008). [DOI] [PubMed] [Google Scholar]
- Surles R. L., Weng N., Simon P. W. & Tanumihardjo S. A. Carotenoid profiles and consumer sensory evaluation of specialty carrots (Daucus carota, L.) of various colors. J Agric Food Chem 52, 3417–3421 (2004). [DOI] [PubMed] [Google Scholar]
- Kammerer D., Carle R. & Schieber A. Quantification of anthocyanins in black carrot extracts (Daucus carota ssp sativus var. atrorubens Alef.) and evaluation of their color properties. Eur Food Res Technol 219, 479–486 (2004). [Google Scholar]
- Li P. et al. Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bHLH transcription factor MtTT8. New Phytol 210, 905–921 (2016). [DOI] [PubMed] [Google Scholar]
- Singh R. et al. The oil palm VIRESCENS gene controls fruit colour and encodes a R2R3-MYB. Nat Commun 5, 4106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagne D. et al. An ancient duplication of apple MYB transcription factors is responsible for novel red fruit-flesh phenotypes. Plant Physiol 161, 225–239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimolmangkang S., Han Y., Wei G. & Korban S. S. An apple MYB transcription factor, MdMYB3, is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol 13, 176 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H., Ogasawara F., Sato K., Higo H. & Minobe Y. Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol 143, 1252–1268 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi R. et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77, 367–379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N. U., Park J. I., Jung H. J., Hur Y. & Nou I. S. Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct Integr Genomics 15, 383–394 (2015). [DOI] [PubMed] [Google Scholar]
- Oh J. E., Kim Y. H., Kim J. H., Kwon Y. R. & Lee H. Enhanced level of anthocyanin leads to increased salt tolerance in arabidopsis PAP1-D plants upon sucrose treatment. J Korean Soc Appl Biol Chem 54, 79–88 (2011). [Google Scholar]
- Karageorgou P. & Manetas Y. The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiol 26, 613–621 (2006). [DOI] [PubMed] [Google Scholar]
- Bassolino L. et al. Accumulation of anthocyanins in tomato skin extends shelf life. New Phytol 200, 650–655 (2013). [DOI] [PubMed] [Google Scholar]
- Butelli E. et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26, 1301–1308 (2008). [DOI] [PubMed] [Google Scholar]
- Netzel M. et al. Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins. Innov Food Sci Emerg 8, 365–372 (2007). [Google Scholar]
- Xu Z. S. et al. Transcript profiling of structural genes involved in cyanidin-based anthocyanin biosynthesis between purple and non-purple carrot (Daucus carota L.) cultivars reveals distinct patterns. BMC Plant Biol 14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A. et al. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39, 366–380 (2004). [DOI] [PubMed] [Google Scholar]
- Schwinn K. et al. A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. Plant Cell 18, 831–851 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espley R. V. et al. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49, 414–427 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espley R. V. et al. Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 21, 168–183 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Zhao M., Leavitt J. M. & Lloyd A. M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53, 814–827 (2008). [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Goto-Yamamoto N. & Hirochika H. Retrotransposon-induced mutations in grape skin color. Science 304, 982–982 (2004). [DOI] [PubMed] [Google Scholar]
- Ban Y. et al. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48, 958–970 (2007). [DOI] [PubMed] [Google Scholar]
- Takos A. M. et al. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142, 1216–1232 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann I. M., Heim M. A., Weisshaar B. & Uhrig J. F. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40, 22–34 (2004). [DOI] [PubMed] [Google Scholar]
- Lin-Wang K. et al. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol 10, 50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R., Werber M. & Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Bio 4, 447–456 (2001). [DOI] [PubMed] [Google Scholar]
- Yildiz M. et al. Expression and mapping of anthocyanin biosynthesis genes in carrot. Theor Appl Genet 126, 1689–1702 (2013). [DOI] [PubMed] [Google Scholar]
- Wako T., Kimura S., Chikagawa Y. & Ozeki Y. Characterization of MYB proteins acting as transcriptional regulatory factors for carrot phenylalanine ammonia-lyase gene (DcPAL3). Plant Biotechnol 27, 131–139 (2010). [Google Scholar]
- Zhou H. et al. Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J 82, 105–121 (2015). [DOI] [PubMed] [Google Scholar]
- Wang Z. G. et al. The Methylation of the PcMYB10 Promoter Is Associated with Green-Skinned Sport in Max Red Bartlett Pear. Plant Physiol 162, 885–896 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. J. et al. A R2R3-MYB transcription factor from Epimedium sagittatum regulates the flavonoid biosynthetic pathway. Plos One 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H., Ogasawara F., Sato K., Higo H. & Minobe Y. Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol 143, 1252–1268 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. S., Tan H. W., Wang F., Hou X. L. & Xiong A. S. CarrotDB: a genomic and transcriptomic database for carrot. Database (Oxford) 2014 (bau096), 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorizzo M. et al. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat Genet 48, 657–666 (2016). [DOI] [PubMed] [Google Scholar]
- Clough S. J. & Bent A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- Li Y.-Y. et al. MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol 160, 1011–1022 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. L. et al. Regulation of ascorbic acid biosynthesis and recycling during root development in carrot (Daucus carota L.). Plant Physiol Bioch 94, 10–18 (2015). [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D. & Livak K. J. Analyzing real-time PCR data by the comparative C-T method. Nat Protoc 3, 1101–1108 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.