Abstract
Vitamin D has a range of non-skeletal health effects and has been implicated in the response to respiratory infections. The aim of this study was to assess the effect of vitamin D on the response of epithelial cells, neutrophils and macrophages to lipopolysaccharide (LPS) stimulation. BEAS-2B cells (airway epithelial cell line) and primary neutrophils and macrophages isolated from blood samples were cultured and exposed to LPS with and without vitamin D (1,25(OH)2D). The production of IL-6, IL-8, IL-1β and TNF-α of all cells and the phagocytic capacity of neutrophils and macrophages to E. coli were assessed. Vitamin D had no effect on BEAS-2B cells but enhanced the production of IL-8 in neutrophils (p = 0.007) and IL-1β in macrophages (p = 0.007) in response to LPS. Both vitamin D (p = 0.019) and LPS (p < 0.001) reduced the phagocytic capacity of macrophages. These data suggest that the impact of vitamin D on responses to infection are complex and that the net effect will depend on the cells that respond, the key response that is necessary for resolution of infection (cytokine production or phagocytosis) and whether there is pre-existing inflammation.
Vitamin D is a secosteroid hormone which is well-known of its role in mineral and skeletal homeostasis1. A plethora of studies have suggested that vitamin D has a range of roles beyond the regulation of bone metabolism and that it plays a critical role in modulating the immune response; including the response to respiratory infections2. Respiratory tract infections (RTI), which are highly prevalent, are responsible for significant morbidity and mortality, and are associated with the onset, progression and exacerbation of chronic lung diseases3,4.
Epidemiological studies have demonstrated strong associations between serum vitamin D levels and the incidence of RTIs5 and it has been suggested that the seasonal variations in vitamin D levels could explain the increased prevalence of RTIs in winter6, when vitamin D synthesis is low7. However, clinical trials with vitamin D supplementation have reported varying effects of on responses to respiratory infections8,9 such that current evidence linking vitamin D status and RTIs is equivocal.
Airway epithelial cells, as the first defensive barrier in the airway tract, play an important role in orchestrating neutrophil and macrophage recruitment to clear invading pathogens10. Neutrophils and macrophages play an important role in clearing pathogens through their phagocytic capacity by producing oxidants to kill engulfed microorganisms11. It is believed that the local production of 1α, 25-Dihydroxyvitamin D3 (1,25(OH)2D3), the active form of vitamin D, exerts protective effects during infections by upregulating the expression of cathelicidin and β defensin 2 in phagocytes12,13 and epithelial cells14. The clearance of pathogens and apoptotic cells by phagocytosis plays an important role in resolving inflammatory responses, and any impairment of these processes can lead to a chronic inflammatory state15.
While vitamin D is thought to be important in mediating the immune response to infection, as discussed above, the role of vitamin D in modulating cytokines production is unclear. Previous studies have shown conflicting effects (anti-inflammatory and pro-inflammatory) of vitamin D on the production of IL (interleukin) -1β, IL-6 and TNF (tumor necrosis factor) -α in response to a variety of insults16,17,18. IL-1β and TNF-α are essential for the activation of the epithelium and downstream inflammatory responses19. In addition, IL-1β, IL-6, IL-8 and TNF-α are involved in the innate immune responses by neutrophils and macrophages20 and may be influenced by vitamin D16,17,18,21.
Given the inconsistencies in the in vitro and clinical trial data, we aimed to determine the effect of exogenous vitamin D on the inflammatory response in key cells involved in the response to RTIs including epithelial cells, macrophages and neutrophils. In order to achieve this, we examined whether vitamin D modulated the response to bacterial lipopolysaccharide (LPS).
Results
Cytokine production by BEAS-2B cells
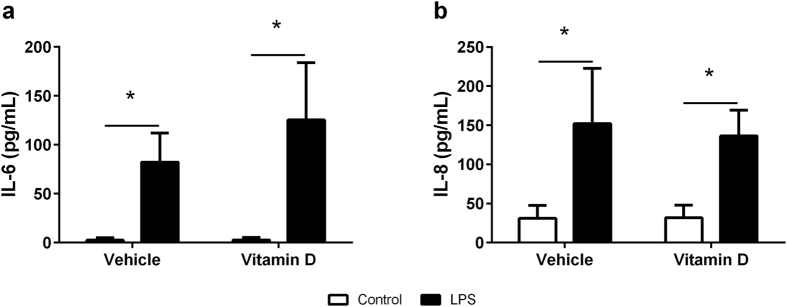
24 hours after exposure to LPS, the production of IL-6 (Fig. 1a, p < 0.001) and IL-8 (Fig. 1b, p < 0.001) was increased in BEAS-2B cells compared to controls. However, the magnitude of the response was not altered by the presence of 1,25(OH)2D3 (IL-6, p = 0.552; IL-8, P = 0.994). IL-1β and TNF-α were not detectable by ELISA in any of the supernatants (data not shown).
Figure 1.
Production of IL-6 (a) and IL-8 (b) in the supernatant of BEAS-2B cells with (black bars) and without (white bars) LPS in the presence of vehicle or vitamin D (1,25(OH)2D3). Data are represented as mean (SD), *indicates p < 0.05, n = 4/group.
Cytokine production by neutrophils
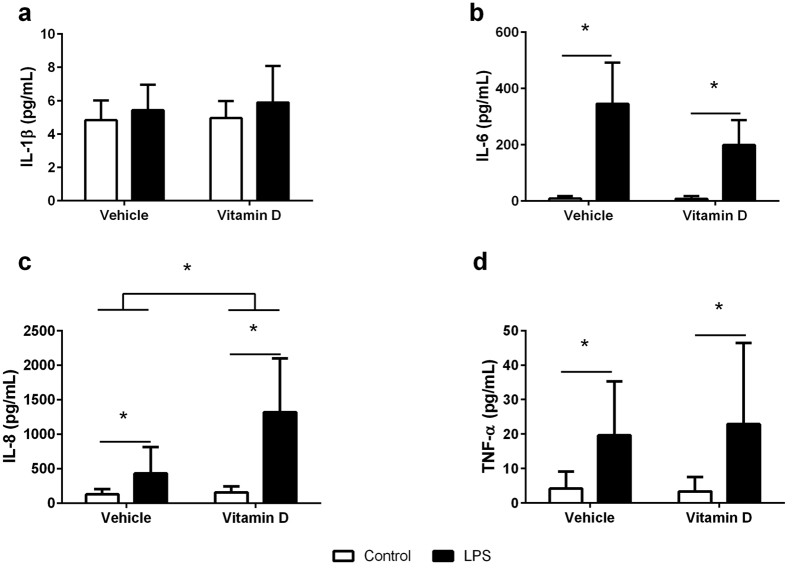
24 hours after exposure to LPS, the production of IL-6 (Fig. 2b, p < 0.001), IL-8 (Fig. 2c, p < 0.001) and TNF-α (Fig. 2d, p = 0.002) was increased in neutrophils compared to controls. Interestingly, while 1,25(OH)2D3 had no effect on the production of IL-6 (p = 0.297) or TNF- α (p = 0.728) by the neutrophils, it increased the IL-8 response (p = 0.007) in both the LPS treated and untreated neutrophils. Neither LPS (p = 0.153) nor 1,25(OH)2D3 had any effect on IL-1β production (Fig. 2a, p = 0.600).
Figure 2.
Production of IL-1β (a), IL-6 (b), IL-8 (c) and TNF-α (d) in the supernatant of neutrophils with (black bars) and without (white bars) LPS in the presence of vehicle or vitamin D (1,25(OH)2D3). Data are represented as mean (SD), *indicates p < 0.05, n = 9/group for IL-1β testing, n = 5/group for IL-6 testing, n = 8/group for IL-8 testing, and n = 9/group for TNF-α testing.
Cytokine production by macrophages
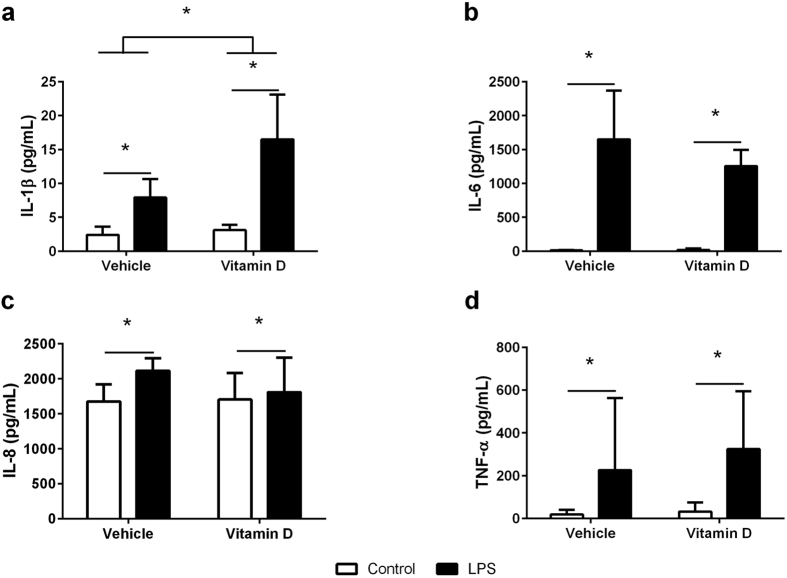
24 hours after exposure to LPS, the production of IL-1β (Fig. 3a, p < 0.001), IL-6 (Fig. 3b, p < 0.001), IL-8 (Fig. 3c, p = 0.049) and TNF-α (Fig. 3d, p < 0.001) was increased in macrophages compared to controls. In contrast to the neutrophils, while 1,25(OH)2D3 had no effect on the production of IL-6 (p = 0.531), IL-8 (p = 0.297) or TNF-α (p = 0.095), it increased the production of IL-1β (p = 0.001) in both the LPS treated and untreated cells.
Figure 3.
Production of IL-1β (a), IL-6 (b), IL-8 (c) and TNF-α (d) in the supernatant of macrophages with (black bars) and without (white bars) LPS in the presence of vehicle or vitamin D (1,25(OH)2D3). Data are represented as mean (SD), *indicates p < 0.05, n = 7/group for IL-1β testing, n = 5/group for IL-6 testing, n = 7/group for IL-8 testing, and n = 8/group for TNF-α testing.
Phagocytic capacity
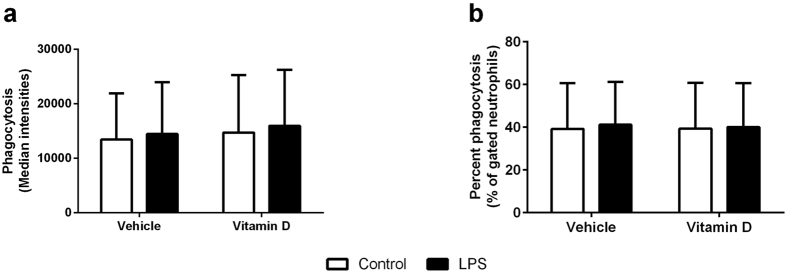
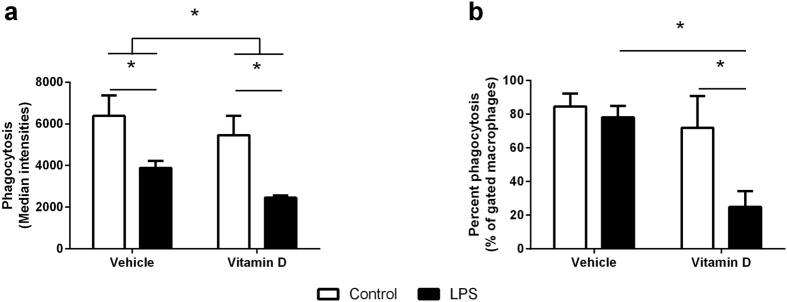
There was no effect of LPS treatment or 1,25(OH)2D3 on total phagocytic capacity (Fig. 4a) (LPS, p = 0.732; 1,25(OH)2D3, p = 0.802) or the percentage of cells that engulfed the E. coli (Fig. 4b) (LPS, p = 0.788; 1,25(OH)2D3, p = 0.936) in neutrophils. In contrast, both LPS (p < 0.001) and 1,25(OH)2D3 (p = 0.019) reduced the phagocytic capacity of macrophages (Fig. 5a). In the case of LPS and 1,25(OH)2D3, in isolation, the effect seemed to be due to the reduced capacity of individual cells because the percentage of cells that phagocytosed E. coli was not changed in response to LPS (p = 0.516) or 1,25(OH)2D3 (p = 0.219) alone (Fig. 5b). However, in combination, LPS and 1,25(OH)2D3 significantly reduced the number of macrophages with phagocytic capacity (Fig. 5b, p < 0.001).
Figure 4.
Phagocytic capacity of neutrophils measured as the total phagocytosis of E. coli (a) or the percentage of cells that phagocytosed E. coli (b) in neutrophils treated with (black bars) or without (white bars) LPS in the presence of vehicle or vitamin D (1,25(OH)2D3). Data are represented as mean (SD), n = 6/group.
Figure 5.
Phagocytic capacity of macrophages measured as the total phagocytosis of E. coli (a) or the percentage of cells that phagocytosed E. coli (b) in macrophages treated with (black bars) or without (white bars) LPS in the presence of vehicle or vitamin D (1,25(OH)2D3). Data are represented as mean (SD), *indicates p < 0.05, n = 3/group.
Discussion
Data on the effect of vitamin D deficiency on responses to respiratory tract infections are conflicting. We aimed to examine the effect of supplemental vitamin D on cytokine production and phagocytic capacity in three cell types that are important in the innate response to respiratory infections; airway epithelial cells, neutrophils and macrophages. We found that 1,25(OH)2D3, the active form of vitamin D, enhanced IL-8 and IL-1β production by neutrophils and macrophages respectively. 1,25(OH)2D3 also decreased the total phagocytic potential of macrophages and, in combination with LPS, reduced the ability of individual macrophages to engulf E. coli. These data suggest that vitamin D has differing effects on the response to individual cell types to a bacterial stimulus. On the one hand the addition of vitamin D enhanced the production of cytokines but reduced the capacity of the macrophages to phagocytose pathogens. This may explain the conflicting associations between vitamin D and respiratory tract infections.
The airway epithelium is the first point of contact for invading pathogens in the respiratory tract. In response to bacterial stimuli, airway epithelial cells produce a range of pro-inflammatory mediators such as IL-6 and IL-822 which act to recruit mononuclear phagocytes23 and neutrophils24. These phagocytes play important roles in responding to, and clearing respiratory infections by phagocytosing and producing reactive oxygen species (ROS) to eliminate pathogens25. As expected, we saw increased production of IL-6 and IL-8 by airway epithelial, BEAS-2B, cells in response to LPS stimulation. These responses were not altered by vitamin D suggesting that vitamin D does not modulate important innate responses by epithelial cells to bacterial infections in the airway.
Macrophages and neutrophils are key cells in generating the innate immune response to invading pathogens. When a pathogen crosses the epithelial barrier and begins to replicate in the tissues of the host, it is immediately recognized by macrophages, that reside in the tissues, which, along with epithelial cells, produce chemokines to recruit large numbers of neutrophils to sites of infection10. Macrophages and neutrophils respond to pathogens, in part, by releasing a plethora of cytokines and chemokines in order to orchestrate the immune response26. Neutrophils have a high phagocytic capacity and, when a pathogen is engulfed, release IL-8, a neutrophil chemoattractant27, which recruits additional neutrophils to site of infection24. Neutrophils also act to eliminate the pathogen by producing an oxidative burst28. As expected, we found that LPS increased the production of IL-8 by neutrophils. Interestingly, 25(OH)2D3 in our study facilitated the production of IL-8 suggesting that vitamin D may boost the capacity of neutrophils to respond to invading pathogens by recruiting additional neutrophils to the site of infection. This is in contrast to other studies on respiratory pathogens including tuberculosis where vitamin D had no effect on neutrophilia29. Similarly, vitamin D has been shown down-regulate IL-8 release in hyper-inflammatory macrophages21. The disparities in these observations suggest that the impact of vitamin D on the production of IL-8 by innate immune cells is dependent on the cell that is producing the cytokine and the infectious stimulus.
Activated macrophages produce a range of inflammatory mediators in response to bacterial infections. Consistent with previous studies30, we found that macrophages increased production of all the cytokines measured, IL-1β, IL-6, IL-8 and TNF-α, in response to LPS stimulation. However, pre-treatment with 1,25(OH)2D3 during macrophage differentiation period facilitated LPS induced IL-1β production but had no effects on the production of IL-6, IL-8 and TNF-α which is in contrast to a recent study which showed that 1,25(OH)2D3 significantly reduced the levels of IL-6 and TNF-α in alveolar macrophages in response to a combination of LPS and IFN-γ31. It is important to note that our study used differentiated PBMCs that were primed with vitamin D during differentiation rather than primary alveolar macrophage, so it is possible that the inconsistencies in these studies reflects different macrophage phenotypes. Again, this suggest that vitamin D can both potentiate and inhibit the inflammatory response under different conditions.
Our study is also in contrast to an early study which suggested that 1,25(OH)2D3 inhibits the proliferation of blood lymphocytes and IL-1 production32, however, recent studies, in line with our data, have found that vitamin D generally boosts infection-stimulated cytokine and chemokine responses; including enhanced IL-1β expression in macrophages18, enhanced macrophage survival and reduced mycobacterial burden by stimulating the anti-mycobacterial capacity of co-cultured lung epithelial cells. IL-1β exerts its protective action against infections by rapidly recruiting neutrophils to inflammatory sites. For example, IL-1 receptor knock-out mice recruit fewer neutrophils in response to influenza infection33. In addition, in vitro studies have shown that IL-1β restricts intracellular Mycobacterium tuberculosis (M. tuberculosis) growth in murine and human macrophages by regulating TNF signalling and caspase-3 activation34. Similar to our observation in neutrophils, these data suggested that vitamin D enhances the capacity of macrophages to respond to bacterial stimuli.
Phagocytosis of pathogens by neutrophils and macrophages is one of their key functions35. While localization of neutrophils to the site of inflammation is crucial for clearance of the infection, which vitamin D appears to enhance through increased IL-8 production, neither LPS nor vitamin D had any impact on the phagocytic capacity of neutrophils when challenged with E. coli. Interestingly, a previous study has clearly demonstrated that vitamin D enhances the phagocytic potential of macrophages that have low phagocytic capacity to live M. tuberculosis36. In contrast, our results have shown that both LPS and 1,25(OH)2D3 independently decreased the phagocytic capacity of individual macrophages to engulf E. coli with a decrease in overall phagocytosis (overall fluorescence) but approximately the same number of cells involved in phagocytosis. Interestingly, LPS and 1,25(OH)2D3 together decreased the number of macrophages that were able to phagocytose E. coli. While there are several differences between these studies that may explain this difference, including the length of prior treatment with vitamin D (2 vs 6 days), the pathogen used, and the length of pathogen challenge (45 minutes vs 3 hours), these observation suggest that vitamin D is a potent modulator of the phagocytic capacity of macrophages. In line with our data, in vivo studies have shown that mice injected with LPS had reduced phagocytic capacity in macrophages that may be due to the fact that the LPS drives macrophages to a more inflammatory, antigen presentation state37. There are numerous receptors involved in bacterial recognition by macrophages38, such that, if the macrophage receptors involved in phagocytosis are reduced, occupied, or internalized by stimuli, the phagocytic capacity can be attenuated39.
Other studies have shown that administration of vitamin D (10−8 M of 1,25(OH)2D3) had no effects on the phagocytic capacity of human alveolar macrophages, but increased the levels of the antimicrobial peptide cathelicidin (LL-37)31. We assessed LL-37 levels in the supernatants from our BEAS-2B cell and macrophage experiments and found that LL-37 levels were not increased in macrophages by pre-stimulation with vitamin D (10−7 M of 1,25(OH)2D3) during differentiation and were undetectable in BEAS-2B cells (Supplementary material; Figure S1). This again highlights the importance of cell phenotype, and protocol, in influencing the cellular response to stimulation with vitamin D. Studies have shown that vitamin D has an effect on the morphology and function of monocyte-derived macrophages40 by inhibiting M1 macrophage activation and promoting M1-M2 phenotype switching41,42. However, our data appear to contradict this and it possible that by priming with vitamin D during differentiation we facilitated M1 phenotype switching with increased the production of IL-1β. These data have important implications for the clearance of pathogens whereby the effect of vitamin D on macrophage phagocytosis and antimicrobial activity seems to be dependent on the type of pathogen, the period of exposure to vitamin D, the presence of pre-existing inflammation, and macrophage phenotype.
In conclusion, vitamin D exerted different effects on different cell types. While there was no effect of vitamin D on LPS induced inflammation in epithelial cells, vitamin D enhanced the production of IL-8 in neutrophils and the production of IL-1β macrophages. Vitamin D had no effect on neutrophils phagocytic capacity but exaggerated the LPS induced decrease in macrophage phagocytic capacity. While acknowledging that we have studied these responses in isolation in vitro, and that these cells have the capacity to modulate 1,25(OH)2D levels in vivo through the upregulation of 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1)43, our data demonstrate the complexity in understanding the effect of vitamin D on responses to infection. In some instances, the net effect of vitamin D on the response to infection may be neutral due to the competing effect of vitamin D on phagocytosis and the production of cytokines that are important in the resolution of infection. This area requires further investigation if we are to understand the complex role of vitamin D in the innate immune response.
Methods
Study subjects
Bronchial epithelial cells (BEAS-2B) were purchased from American Type Culture Collection (ATCC; Manassas, VA). Whole blood samples were collected from normal healthy (no acute or chronic illnesses) volunteers (aged 20–35 years; 5 males and 5 females) to isolate peripheral blood neutrophils and monocytes. The University of Tasmania Research Ethics Committee approved all the studies, and subjects gave written, informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Neutrophil and monocyte isolation
Neutrophils and human peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Lympholyte-poly cell separation media (Cedarlane Labs, Burlington, Canada) composed of sodium diatrizoate and dextran 50044. 30 mL of freshly drawn blood was collected in Vacutainer collection tubes (BD, San Jose, CA) containing 15% K3EDTA solution and then mixed at a ratio of 1:1 (vol/vol) with PBS at room temperature and 15 mL of the mixture was layered with 15 mL of Lympholyte-poly in a 50 mL tube. After centrifugation at 450 g for 35 min at 20 °C, neutrophils and PBMCs buffy coats were collected separately. Neutrophils buffy coats were washed with ice-cold HBSS (without Ca2+/Mg2+) and centrifuged at 450 g for 5 min. The remaining red blood cells were lysed by hypotonic treatment with Red Blood Cell Lysis Buffer (Roche, Basel, Switzerland). This method has been show to yield samples of >95% neutrophils with >95% viability45. PBMCs buffy coats were washed with ice-cold PBS containing 0.1% BSA and 2 mM EDTA and centrifuged at 450 g for 5 min. Monocytes were purified from freshly isolated PBMCs using MACS CD14 microbeads according to the manufacturer’s introductions (Miltenyi Biotec, Bergisch Gladbach, Germany), which are used for positive selection of human monocytes and macrophages from PBMCs46. Briefly, monocytes suspension was passed through the Pre-Separation filter then incubated with CD14 microbeads for 15 min at 4 °C, to allow separation of CD14+ monocytes.
Cytokines
The concentrations of interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor alpha (TNF-α) in the supernatants from the cell culture medium were measured using DuoSet ELISA kits (R&D Systems, Minneapolis, MN). The absorbance was read at 450 nm/570nm using a spectrophotometer (Spectramax M2, Molecular Devices, Sunnyvale, CA).
BEAS-2B inflammatory response
BEAS-2B cells were cultured in bronchial epithelial cell growth medium (BEGM) (Lonza, Walkersville, MD) at 37 °C in a 5% CO2 humidified incubator. BEAS-2B cells were seeded in 12-well plates at a density of 5 × 105 cells/mL with either 0.1% ethanol (vehicle control) or 10−7 M of 1,25(OH)2D3 (Enzo Life Sciences, Farmingdale, NY) for 24 hours. After 24 hours, the culture media was changed, and cells were treated with 10 ng/mL of LPS (Sigma-Aldrich, St.Louis, MO) in the presence of vehicle control or 10−7 M of 1,25(OH)2D3 for 24 hours at which point cell supernatants were collected for enzyme-linked immune sorbent assay (ELISA) assessment of IL-1β, IL-6, IL-8 and TNF-α.
Neutrophil inflammatory response
Isolated neutrophils were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA) supplied with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St.Louis, MO) and 1% Penicillin-Streptomycin (Pen-Strep) (Sigma-Aldrich, St.Louis, MO) at 37 °C in a 5% CO2 humidified incubator. Neutrophils were seeded in 12-well plates at a density of 1 × 106 cells/mL with either 0.1% ethanol (vehicle control) or 10−7 M of 1,25(OH)2D3 for 24 hours prior to treatment with 10 ng/mL of LPS in the presence of vehicle control or 10−7 M of 1,25(OH)2D3 for another 24 hours. Cells suspensions were centrifuged and the supernatant collected for ELISA assessment of IL-1β, IL-6, IL-8 and TNF-α.
Monocyte inflammatory response
The enriched CD14+ monocytes from PBMCs were cultured in RPMI-1640 medium supplied with 10% FBS and 1% Pen-Strep at 37 °C in a 5% CO2 humidified incubator. Monocytes were seeded in 12-well plates at a density of 5 × 105 cells/mL, and differentiated using 100 ng/mL of macrophage colony stimulating factor (M-CSF) (Sigma-Aldrich, St.Louis, MO) in the presence of vehicle control or 10−7 M of 1,25(OH)2D3 for 6 days with fresh 1,25(OH)2D3 added every day30. At day 7, M-CSF containing media was changed, and PBMCs differentiated macrophages were treated with 10 ng/mL of LPS in the presence of vehicle control or 10−7 M of 1,25(OH)2D3 for 24 hours. The supernatants were centrifuged and the supernatant collected for ELISA assessment of IL-1β, IL-6, IL-8 and TNF-α.
Phagocytic capacity of neutrophils and macrophages
Fluorescein conjugated Escherichia coli (K-12 strain) (Thermo Fisher Scientific, Waltham, MA) was used to assess phagocytic capacity. Briefly, after 24 hours of stimulation with LPS, neutrophils or macrophages were incubated with Fluorescein conjugated E. coli at multiplicity of infection (MOI) of 5 for 45 minutes at 37 °C in a 5% CO2 humidified incubator. The reaction was stopped with ice. Cells were collected and washed 3 times with cold PBS, then phagocytes were fixed in 1% paraformaldehyde before been measured by flow cytometer BD FACSCANTO II (BD Biosciences, San Jose, CA). Optimal removal of macrophages required vigorous pipetting. Flow cytometry was performed to estimate the percentage of phagocytes which had engulfed bacteria and quantify the fluorescence intensities of E. coli in engulfed phagocytes. Flow cytometry data were analysed using FCS Express 6 (De Novo Software, Glendale, CA).
Statistical analysis
Group comparisons were made using two-way analysis of variance (Two-way ANOVA) with Holm-Sidak posthoc tests. Data were log-transformed where necessary to satisfy the assumptions of the test (normality and homoscedasticity). Data were analysed in SigmaPlot (Systat, Erkrath, Germany) and reported as mean (SD). P values of <0.05 were considered statistically significant.
Additional Information
How to cite this article: Chen, L. et al. Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide. Sci. Rep. 7, 45172; doi: 10.1038/srep45172 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the support of University of Tasmania – Research Enhancement Grant Scheme (C0022912).
Footnotes
The authors declare no competing financial interests.
Author Contributions L.C. and M.E. carried out the experiments in the study. L.C. and G.R.Z. analysed the data and interpreted the results. L.C. and G.R.Z. conceived the study, and participated in its design. L.C. coordinated and drafted the manuscript. All authors have read and approved the final manuscript.
References
- Chung M. et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 1–420 (2009). [PMC free article] [PubMed] [Google Scholar]
- Borella E., Nesher G., Israeli E. & Shoenfeld Y. Vitamin D: a new anti-infective agent? Ann N Y Acad Sci. 1317, 76–83 (2014). [DOI] [PubMed] [Google Scholar]
- Wilson R. Bacteria, antibiotics and COPD. Eur Respir J. 17, 995–1007 (2001). [DOI] [PubMed] [Google Scholar]
- Papi A. et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 173, 1114–1121 (2006). [DOI] [PubMed] [Google Scholar]
- Ginde A. A., Mansbach J. M. & Camargo C. A. Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 169, 384–390 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. B. Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J. 27, 853 (2008). [DOI] [PubMed] [Google Scholar]
- Berry D. J., Hesketh K., Power C. & Hypponen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 106, 1433–1440 (2011). [DOI] [PubMed] [Google Scholar]
- Yamshchikov A. V., Desai N. S., Blumberg H. M., Ziegler T. R. & Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 15, 438–449 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe D. A., Griffiths C. J. & Martineau A. R. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 136, 321–329 (2013). [DOI] [PubMed] [Google Scholar]
- Janeway C. A. Jr., Travers P., Walport M. & Shlomchik M. J. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; The front line of host defense. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27105/ (2001). [Google Scholar]
- Sibille Y. & Reynolds H. Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 141, 471–501 (1990). [DOI] [PubMed] [Google Scholar]
- Wang T. T. et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 173, 2909–2912 (2004). [DOI] [PubMed] [Google Scholar]
- Gombart A. F., Borregaard N. & Koeffler H. P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 19, 1067–1077 (2005). [DOI] [PubMed] [Google Scholar]
- Yim S., Dhawan P., Ragunath C., Christakos S. & Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros. 6, 403–410 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C. T. The Osler slide, a demonstration of phagocytosis from 1876 Reports of phagocytosis before Metchnikoff’s 1880 paper. Cell Immunol. 240, 1–4 (2006). [DOI] [PubMed] [Google Scholar]
- Almerighi C. et al. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 45, 190–197 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 188, 2127–2135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verway M. et al. Vitamin D induces interleukin-1beta expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 9, e1003407 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. R., Simms B. T., Lupa M. M., Kogan M. S. & Mizgerd J. P. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 175, 7530–7535 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A. Jr., Travers P., Walport M. & Shlomchik M. J. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; Induced innate responses to infection. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27122/ (2001).
- Dauletbaev N. et al. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br J Pharmacol. 172, 4757–4771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C. et al. Differences in LPS-induced activation of bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes (A-549). Scandinavian journal of immunology. 56, 294–302 (2002). [DOI] [PubMed] [Google Scholar]
- Kaplanski G., Marin V., Montero-Julian F., Mantovani A. & Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 24, 25–29 (2003). [DOI] [PubMed] [Google Scholar]
- Gibson P. G. et al. Induced sputum IL-8 gene expression, neutrophil influx and MMP-9 in allergic bronchopulmonary aspergillosis. Eur Respir J. 21, 582–588 (2003). [DOI] [PubMed] [Google Scholar]
- Mittal M., Siddiqui M. R., Tran K., Reddy S. P. & Malik A. B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 20, 1126–1167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. & Stow J. L. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 118, 9–18 (2011). [DOI] [PubMed] [Google Scholar]
- Bazzoni F. et al. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 173, 771–774 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. Activation of the neutrophil respiratory burst oxidase. J Infect Dis 179 Suppl 2, S309–317 (1999). [DOI] [PubMed] [Google Scholar]
- Coussens A. K. et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci USA 109, 15449–15454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarique A. A. et al. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol. 53, 676–688 (2015). [DOI] [PubMed] [Google Scholar]
- Heulens N. et al. 1,25-Dihydroxyvitamin D Modulates Antibacterial and Inflammatory Response in Human Cigarette Smoke-Exposed Macrophages. PLoS One. 11, e0160482 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukas C. D. et al. Inhibition of interleukin-1 production by 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 69, 127–133 (1989). [DOI] [PubMed] [Google Scholar]
- Schmitz N., Kurrer M., Bachmann M. F. & Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 79, 6441–6448 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P. et al. IL-1beta promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol. 190, 4196–4204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T. & Correia-Neves M. Neutrophils and macrophages: the main partners of phagocyte cell systems. Front Immunol. 3, 174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra G., Selvaraj P., Jawahar M. S., Banurekha V. V. & Narayanan P. R. Effect of vitamin D3 on phagocytic potential of macrophages with live Mycobacterium tuberculosis and lymphoproliferative response in pulmonary tuberculosis. J Clin Immunol. 24, 249–257 (2004). [DOI] [PubMed] [Google Scholar]
- de Lima T. M. et al. Phagocytic activity of LPS tolerant macrophages. Mol Immunol. 60, 8–13 (2014). [DOI] [PubMed] [Google Scholar]
- Aderem A. Phagocytosis and the inflammatory response. J Infect Dis. 187 Suppl 2, S340–345 (2003). [DOI] [PubMed] [Google Scholar]
- Samuelsson A., Towers T. L. & Ravetch J. V. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 291, 484–486 (2001). [DOI] [PubMed] [Google Scholar]
- Daigneault M., Preston J. A., Marriott H. M., Whyte M. K. & Dockrell D. H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 5, e8668 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. L., Guo Y. F., Song Z. X. & Zhou M. Vitamin D prevents podocyte injury via regulation of macrophage M1/M2 phenotype in diabetic nephropathy rats. Endocrinology. 155, 4939–4950 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhou M., Guo Y., Song Z. & Liu B. 1,25-Dihydroxyvitamin D(3) Promotes High Glucose-Induced M1 Macrophage Switching to M2 via the VDR-PPARgamma Signaling Pathway. Biomed Res Int. 2015, 157834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyama K. et al. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 277, 1827–1830 (1997). [DOI] [PubMed] [Google Scholar]
- Hirsch G., Lavoie-Lamoureux A., Beauchamp G. & Lavoie J. P. Neutrophils are not less sensitive than other blood leukocytes to the genomic effects of glucocorticoids. PLoS One. 7, e44606 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Siano B. & Diamond S. Neutrophil isolation protocol. J Vis Exp. 17, 745 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreck F. A. et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA 101, 4560–4565 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.