Abstract
Terminalia belerica Roxb. fruits have been previously reported against diabetes, ulcer, microbial problems and hepatotoxicity. The present study was aimed to investigate antioxidant and anticancer potential of sequentially fractionated hexane (TBHE), chloroform (TBCE), ethyl acetate (TBEE), butanol (TBBE) and water (TBWE) extracts from the 70% methanolic extract of T. belerica fruits. TBCE, TBEE, TBBE and TBWE showed excellent ROS (reactive oxygen species) and RNS (reactive nitrogen species) scavenging activities which was investigated using 11 different assays for various free radicals. Among 5 fractions, TBHE and TBCE remained nontoxic to any of the malignant cell lines including normal cells (WI-38). TBBE and TBWE inhibited the proliferation of breast (MCF-7), cervical (HeLa) and brain (U87) cancer cells by inducing G2/M arrest while TBEE caused apoptosis. However, these fractions did not inhibit the proliferation of lung (A549) and liver (HepG2) cancer cells. BrdU incorporation study also suggested the efficient anticancer potential of TBEE, TBBE and TBWE. Moreover, TBBE and TBWE treated MCF-7, HeLa and U87 cells showed upregulation of p53 and p21 proteins. Phytochemical analysis reflected the presence of adequate quantities of different phytochemicals. Moreover, HPLC analysis show peaks of purpurin, catechin, tannic acid, reserpine, ellagic acid, methyl gallate, aconitine and rutin in TBBE, TBWE and TBEE. Hence these polar extracts of T. belerica can be used to develop drug against different types of cancer.
Keywords: Free radicals, Anticancer, G2/M arrest, BrdU, HPLC, WI-38
Introduction
Cancer is one of the dreaded diseases in the present global scenario with millions of incidences of affected patients and associated deaths. It is a multistep dysregulation of signaling pathways in living systems. Despite huge developments in diagnostic and therapeutic approaches using chemotherapy, radiation and oncosurgery, along with various remedial treatments, cancer remains a great challenge for clinical therapy, especially cancers of ectodermal (e.g. brain), mesodermal (e.g. breast and cervical) and endodermal (lung and liver) origin. Various new improvements and techniques have been proposed to combat cancer, among which medicinal herbs and plants are also an important strategy. Multistep signaling pathways involved in cellular control, activity and metabolism can be disrupted due to mutagens, carcinogens and various other factors and might lead to cancer. Free radicals like reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated in the body play an important role in achievement of various physiological functions but their imbalance in body cause cellular injury and various clinical disorders like cancer, neuro degeneration and inflammation (Kohen and Nyska 2002). To fight the problem of free radical generation and its associated diseases, natural antioxidant from plants can be considered to act as possible protective agents against various diseases like cancer and might also modify the redox microenvironment to reduce the genetic instability (Su et al. 2013). Not only antioxidants but also there are many chemotherapeutic compounds of plant origin which are effective against many types of cancer. Often natural drugs are preferred to market available anticancer and antitumor drugs like cisplatin which is used against lung and cervical cancer (Giaccone et al. 2004; Buxton et al. 1989) and doxorubicin, used in treatment of liver and breast cancer (Tam 2013; Lin et al. 2012). These drugs often have deleterious side effects (Von Hoff et al. 1979; Christiansen and Autschbach 2006). Hence the search for a better and safer drug is of prime importance from the world of plant and herbal medicines.
Terminalia belerica Roxb., belonging to the Combretaceae family is a large deciduous tree and commonly found in lower hills of South East Asia. Its usage dates back to ancient mythology with another name as ‘Bibhitaki’ and ‘Baheda’ and has been an integral constituent of Triphala with huge importance in Indian and Tibetan traditional medicine (Saroya 2011). Previous studies explained that T. belerica showed pharmacological properties like antioxidant activities (Hazra et al. 2010), antimicrobial properties (Elizabeth 2005) and hepatoprotective property (Kumudhavalli et al. 2010). Various phytochemicals have been identified such as tannins, phenols, belleric acid, bellericoside, thermilignan and ellagic acid (Anand et al. 1997). Previously it was published that 70% methanolic extract of fruits of T. belerica induced apoptosis in lung and breast cancer through regulation of Bax/Bcl-2 (Ghate et al. 2014b).
All these previous findings initiated the idea of sequential fractionation of methanolic extract of T. belerica with different solvents of varying polarity and investigation of their antioxidative and antiproliferative potentials against lung, breast, cervical, liver and brain cancer using A549, MCF-7, HeLa, HepG2 and U87, respectively, as model cell lines.
Materials and methods
Chemicals
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was procured from Roche Diagnostics (Mannheim, Germany) and standard compound, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) from Fluka, Buchs, Switzerland. Potassium persulfate (K2S2O8), ethylene diammine tetraacetic acid (EDTA), 2-deoxy-2-ribose, ascorbic acid, mannitol, trichloroacetic acid (TCA), nitro blue tetrazolium (NBT), reduced nicotinamide adenine dinucleotide (NADH), phenazine methosulfate (PMS), sodium nitroprusside (SNP), 1,10-phenanthroline, sulphanilamide, N-(1-Naphthyl) ethylenediamine dihydrochloride (NED), l-histidine, lipoic acid, sodium pyruvate, quercetin, ferrozine reduced glutathione, bathophenanthroline sulfonate disodium salt and 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) were obtained from Sisco Research Laboratories Pvt. Ltd (Mumbai, India). 2,2-diphenyl-1-picrylhydrazyl (DPPH), and various standards like tannic acid, methyl gallate, rutin, gallic acid, (+) catechin and curcumin were obtained from MP Biomedicals (Illkirch-Graffenstaden, France). HPLC grade acetonitrile, ammonium acetate, hydrogen peroxide, potassium hexacyanoferrate, Folin-ciocalteu reagent, sodium carbonate, mercuric chloride, potassium iodide, anthrone, vanillin, thiourea, 2,4-dinitrophenylhydrazine (DNPH), sodium hypochlorite, aluminum chloride, xylenol orange, butylated hydroxyltoluene (BHT), N,N-dimethyl-4-nitrosoaniline, ammonium iron (II) sulfate hexahydrate [(NH4)2Fe(SO4)2·6H2O], 1-chloro-2,4-dinitrobenzene (CDNB) and Dimethyl-4-aminobenzaldehyde were procured from Merck (Mumbai, India). Catalase, reserpine, streptomycin sulfate and sodium bicarbonate were purchased from HiMedia Laboratories Pvt. Ltd (Mumbai, India). Diethylenetriaminepentaacetic acid (DTPA) was obtained from Spectrochem Pvt. Ltd, Mumbai, India. Thiobarbituric acid (DTPA) was obtained from Spectrochem Pvt. Ltd (Mumbai, India). Thiobarbituric acid (TBA) was obtained from Loba Chemie (Mumbai, India). Evans blue was procured from BDH (Poole, England). Ham F-12, Dulbecco’s Modified Eagle’s Medium (DMEM), antibiotics and Amphotercin-B were obtained from HiMedia Laboratories Pvt. Ltd. d-glucose was procured from Qualigens Fine Chemicals (Mumbai, India). Fetal bovine serum was purchased from HyClone Laboratories, Inc. (Logan, UT, USA. Cell Proliferation Reagent WST-1 and BrdU labeling and detection kit II were procured from Roche Diagnostics.
Collection of plant material and extraction
The fruits of T. belerica were collected from Bishnupur city (23.0679°N, 87.3165°E) of the Bankura district of West Bengal, India and authenticated by the Central Research Institute (Ayurveda), Kolkata, India with specimen no. CRHS 114/08. The fruits of T. belerica were sorted, cleaned and shade-dried for extraction. Finely grinded dried fruits were stirred with 70% methanol in water using magnetic stirrer (100 gm/1000 ml solvent) for 15 h. The mixture was centrifuged at 2850×g and supernatant was collected and the process was repeated with the pellet. The collected supernatants were concentrated together using rotary evaporator and lyophilized to obtain powder of 70% methanolic extract. It was then sequentially extracted with n-hexane, chloroform, ethyl acetate, butanol and water successively to afford hexane (TBHE), chloroform (TBCE), ethyl acetate (TBEE), butanol (TBBE) and water (TBWE) extracts. The dried fractions were stored at −20 °C.
In vitro antioxidant, reactive oxygen (ROS) and reactive nitrogen (RNS) species scavenging activities
The total antioxidant capacities of all five fractions were evaluated by an ABTS·+ radical cation decolorization assay and compared with trolox (standard) using equation y = mx + c where m represents slope of the best fit curve which has been compared and divided by the slope of the standard, trolox to achieve the TEAC value. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay and the reducing powers of the extracts were also estimated (Das et al. 2012). The ROS scavenging capabilities of these fractions were determined by various ROS radical scavenging assays like hydroxyl, superoxide, hypochlorous radical, singlet oxygen and hydrogen peroxide assays by standard procedures. Similarly RNS scavenging activities of these fractions were estimated by nitric oxide and peroxynitrite radical scavenging assays. The fractions were also tested for their iron chelating capacities by a previously published experimental procedure (Hazra et al. 2008).
In vitro anticancer study
Cell lines and culture
Human lung (A549), breast (MCF-7), cervical (HeLa), liver (HepG2), brain (U87) cancer and human lung fibroblast (WI-38) cell lines were purchased from the National Centre for Cell Science (NCCS, Bangalore, India). A549 cells were maintained in Ham’s F-12 medium while the rest of the cell lines were maintained in Dulbecco’s modified Eagle’s medium, each supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin G, 50 μg/ml gentamycin sulphate, 100 μg/ml streptomycin and 2.5 μg/ml amphotericin B. All cell lines were maintained at 37 °C in humidified incubator containing 5% CO2.
WST-1 cytotoxicity assay
Cell viability was quantitated using the WST-1 Cell Proliferation Reagent according to the previously described method (Ghate et al. 2013). In brief, all cells (5 × 103 cells/well) were treated with T. belerica fractions ranging from 0 to 200 µg/ml for 48 h in 96-well culture plate. After treatment, 10 µl of WST-1 cell proliferation reagent was added to each well followed by 3–4 h of incubation at 37 °C. Cell viability was quantified by measuring absorbance at 460 nm using a microplate ELISA reader MULTISKAN EX (Thermo Electron Corporation, Waltham, MA, USA).
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry using the method previously described (Ghate et al. 2013). HeLa, MCF-7 and U87 cells (1 × 106) were treated with bioactive fractions (0–200 µg/ml) for 48 h. After treatment, nuclear DNA of cells was stained with propidium iodide and cell phase distribution was determined on FACS Aria (Becton–Dickinson, Franklin Lakes, NJ, USA) equipped with 488 nm (Blue), 405 nm (Violet) and 640 nm (Red) solid state laser light using BD FACSDiva Software Version 6.0. Total 10,000 events were acquired and data were analyzed using the same software to plot a histogram of DNA content (x-axis, red fluorescence) versus count (y- axis).
Cell proliferation: DNA synthesis in individual cells by BrdU assay
MCF-7, HeLa and U87 cells were plated for 24 h and treated with effective chosen doses of bioactive fractions for 12 h and further labeled with BrdU for observing apoptotic effects and arrest in treated cells following the protocol of the kit. Treated cells incorporated BrdU into their DNA when incubated for short time (1 h). The cells were then fixed and stained with anti-BrdU monoclonal antibody and reincubated with anti-mouse-Ig-alkaline phosphatase. The healthy cells would incorporate BrdU while the treated apoptotic arrested cells would not repair DNA or replicate in order to incorporate BrdU. Finally stained cells were imaged at 200× magnification using a Leica microscope (Wetzlar, Germany).
Western blotting analysis
For elucidation of molecular mechanisms of anticancer activities, MCF-7, HeLa and U87 cells were plated and treated with effective anticancer fractions of T. belerica for 48 h. Post treatment, cells were washed with PBS and lysed using triple detergent cell lysis buffer (50 mM Tris–Cl, pH 8, 150 mM NaCl, 0.02% Sodium azide, 1% triton X-100, 0.1% sodium dodecyl sulphate, 0.5% sodium deoxycholate, 1 μg/ml aprotinin, 100 μg/ml phenyl-methyl-sulfonylfluoride) and lysates were centrifuged at 13,800×g for 20 min at 4 °C. The supernatants were collected and protein concentrations were estimated using Folin-Lowry method. Equal amounts of protein (50 µg for p53 and p21 and 30 µg for β-actin) were used for resolution in 12–15% SDS PAGE. The blots were developed using a protocol previously described (Ghate et al. 2014a). The blots were photographed using imaging system EC3 Chemi HR (UVP, Upland, CA, USA) and the densities of developed blots were analysed using ImageJ 1.45 s software.
Standardization of the fractions
Qualitative and quantitative analysis of the present phytochemicals of the fractions were done following standard methods for carbohydrate, alkaloids, tannins, terpenoids, triterpenoids, anthraquinones, saponins, and glycosides (Harbornen and Baxter 1995; Gokhale et al. 2003). For HPLC analysis, both samples and standards including gallic acid, catechin, reserpine, quercetin and tannic acid were prepared in mobile phase and filtered using 0.45 μm polytetrafluoroethylene (PTFE) filters (Millipore, Billerica, MA, USA). A HPLC-Prominence System RF10AXL (Shimadzu Corp., Kyoto, Japan) equipped with degasser (DGU-20A5), quaternary pump (LC-20AT), auto-sampler (SIL-20A), and detectors of reflective index (RID-10A), fluorescence (RF-10AXL) and diode array (SPD-M20A) were used for analysis. Samples were gradient eluted using mobile phases of acetonitrile and 0.5 mM ammonium acetate in water, at a flow rate of 1 ml/min for 65 min through the column (Z1C-HILIC) that was maintained at 25 °C. The detection was carried out at 254 nm. The injection volume was 20 μl, and the samples and standards were analyzed in triplicates.
Statistical analysis
All spectrophotometric data were represented as the mean ± SD of 6 measurements and cell cycle analysis data was reported as the mean ± SD of 3 measurements. KyPlot version 2.0 beta 15 (32 bit) has been used for statistical analysis. The IC50 values were calculated using the following formula, Y = 100 × A1/(X + A1) where A1 = IC50, Y = response (Y = 100% when X = 0), X = inhibitory concentration.
Results and discussion
More polar extracts of T. belerica prove to be effective antioxidants and potent free radical scavengers
Different solvents yielded extracts of hexane (TBHE 0.31%), chloroform (TBCE 0.27%), ethyl acetate (TBEE 0.81%), butanol (TBBE 3.27%) and water (TBWE 95.33%).
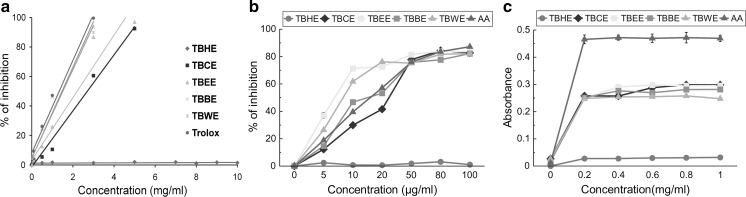
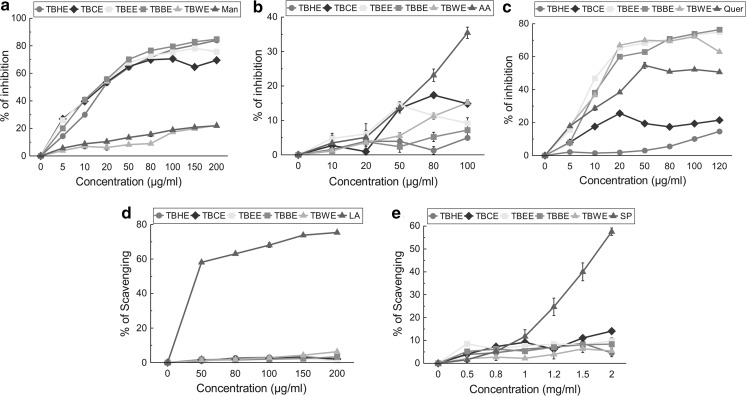
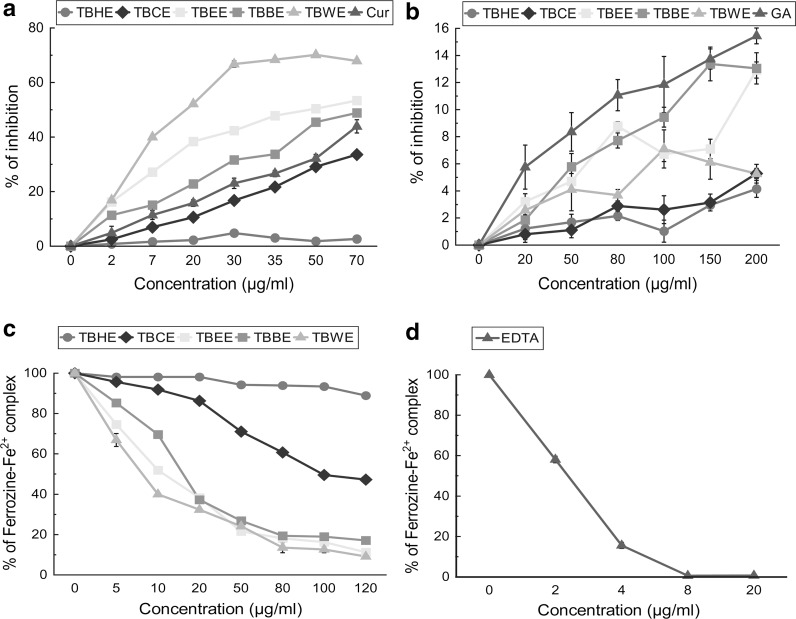
The total antioxidant activity of all fractions was measured and expressed as trolox equivalent antioxidant capacity (TEAC) (Fig. 1a; Table 1). Among all five fractions, TBBE had the highest TEAC of 0.984 ± 0.003 followed by TBWE and TBEE implying high overall antioxidant capacities of polar fractions, comparable to standard Trolox. The equations for the total antioxidant assay of the fractions were as follows: TBHE (y = 0.049x + 1.236), TBCE (y = 18.99x − 1.289), TBEE (y = 21.19x + 3.289), TBBE (y = 30.92x + 4.518), TBWE (y = 29.98x + 4.71) and trolox (y = 31.42x + 8.075) and all fractions follow linear correlation. Their antioxidant efficacies were further tested with scavenging of stable DPPH radical which can be considered to be complementary to the total antioxidant TEAC system (Lissi et al. 1999). TBEE not only showed excellent DPPH radical scavenging activity (IC50 7.11 ± 0.31 µg/ml), compared to standard ascorbic acid, followed closely by TBWE and TBBE (Fig. 1b), but also possessed considerable reducing power property (Fig. 1c). Various ROS are generated in our body like hydroxyl, superoxide, hypochlorous, singlet oxygen and hydrogen peroxide radicals. Hydroxyl radical creates the most detrimental damage to body’s biomolecules especially radicalizing lipid hydroperoxides into proxy and alkoxy radicals, thus generating a chain reaction (Halliwell 1991). At sites of injury and inflammation, hypochlorous radical is generated from oxidation of Cl− ions by neutrophil enzyme, myeloperoxidase, thus inactivating various antioxidant enzymes and vitamins like catalase and ascorbic acid and inducting target cell lysis (Aruoma et al. 1989). Superoxide anion is the initiator radical in formation of ROS like hydrogen peroxide and singlet oxygen in living systems (Stief 2003). Singlet oxygen, generated by UV-radiation and having higher energy than normal oxygen induces hyperoxidation and oxidative cytotoxicity, thereby decreasing the antioxidant activity (Kochevar and Redmond 2000). Hydrogen peroxide although not harmful by itself, stimulates OH− radical generation. Polar fraction TBBE seemed highly effective in scavenging hydroxyl radicals in comparison to mannitol (Fig. 2a) while TBCE > TBEE > TBWE had comparable HOCl scavenging potentials (Fig. 2b). Bioactive polar fractions TBEE, TBBE and TBWE showed high potencies in scavenging superoxide ions and H2O2 while they failed to show good efficacies in neutralizing singlet oxygen (Fig. 2c–e). Nitric oxide is linked to inflammatory condition, tissue toxicity and septic shock during vascular collapse while chronic effect is associated with various cancers and inflammations during juvenile diabetes, multiple sclerosis and ulcerative colitis (Tylor et al. 1997). This condition is aggravated when NO reacts with superoxide anion to form highly reactive peroxynitrite radical. NO was scavenged effectively by TBWE > TBEE > TBBE > curcumin (standard) while peroxynitrite radical was neutralized mostly by the butanol fraction (Fig. 3a, b). Moreover the water extract containing the most polar phytochemicals acted as an effective iron chelator by inhibiting the violet colored Fe2+-ferrozine complex at the highest dose (120 µg/ml) by 91% when compared to standard chelator EDTA (Fig. 3c, d). The IC50 values for all extracts on ROS and RNS scavenging along with iron chelation are shown with the respective standard compounds in Table 1.
Fig. 1.
In vitro antioxidant activities of TBHE, TBCE, TBEE, TBBE and TBWE together with reference compounds. a Total antioxidant activity compared with Trolox, all values are highly significant with p < 0.001 versus control (0 μg/ml), b DPPH radical scavenging compared to ascorbic acid, 20, 50 and 100 µg/ml of TBHE have a significance level of p < 0.05, 10 and 80 µg/ml of TBHE have a significance level of p < 0.01 and the rest of the values are highly significant with p < 0.001. All values are compared to control (0 μg/ml), c reducing power activity compared to ascorbic acid, all values are highly significant at a level of p < 0.001 versus control (0 µg/ml). The results are represented as mean ± S.D. of six parallel measurements. AA ascorbic acid
Table 1.
IC50 values of all fractions of T. belerica for antioxidant and cytotoxicity assays
| TBHE | TBCE | TBEE | TBBE | TBWE | Standard | Values of standard compounds | |
|---|---|---|---|---|---|---|---|
| TEAC value | 0.002 ± 0.001 | 0.604 ± 0.003 | 0.674 ± 0.011 | 0.984 ± 0.003 | 0.954 ± 0.006 | Trolox | 1 |
| IC 50 values of the extracts for free radical scavenging capacity for | |||||||
| Reactive oxygen species (ROS) scavenging assays | |||||||
| DPPH | 4054.95 ± 807.27 | 21.89 ± 0.43 | 7.11 ± 0.31 | 17.20 ± 0.13 | 9.51 ± 0.07 | Ascorbic acid | 5.29 ± 0.28 |
| Hydroxyl (OH−) | 25.93 ± 0.61 | 27.3 ± 0.65 | 21.57 ± 0.54 | 19.42 ± 0.57 | 614.67 ± 18.57 | Mannitol | 571.45 ± 20.12 |
| Hypochlorous acid (HOCl) | 2151.49 ± 400.91 | 451.97 ± 16.83 | 615.88 ± 68.48 | 1382.26 ± 198.44 | 620.69 ± 33.05 | Ascorbic acid | 235.96 ± 5.75 |
| Superoxide (O2 −) | 902.13 ± 52.1 | 322.09 ± 7.37 | 18.77 ± 0.28 | 24.05 ± 0.44 | 21.76 ± 0.54 | Quercetin | 42.06 ± 1.35 |
| Singlet (1O2) | 5684.66 ± 1501.94 | 6121.94 ± 966.73 | 8771.79 ± 2062.24 | 5717.27 ± 976.09 | 3270.87 ± 151.44 | Lipoic acid | 46.15 ± 1.16 |
| Hydrogen peroxide (H2O2) | 20.79 ± 1.35 | 10.29 ± 0.75 | 14.62 ± 1.14 | 17.46 ± 1.62 | 31.05 ± 6.82 | Sodium pyruvate | 3.24 ± 0.3 |
| Reactive nitrogen species (RNS) scavenging assays | |||||||
| Nitric oxide (NO) | 1665.39 ± 202.5 | 134.05 ± 2.23 | 40.44 ± 0.87 | 64.93 ± 0.96 | 16.71 ± 0.23 | Curcumin | 90.82 ± 4.75 |
| Peroxynitritre (ONOO−) | 4784.85 ± 279.08 | 3751.96 ± 145.46 | 1369.81 ± 55.88 | 1092.26 ± 87.02 | 2366.85 ± 306.94 | Gallic acid | 876.24 ± 56.96 |
| Fe chelation | 1094.05 ± 78.26 | 112.40 ± 1.36 | 13.22 ± 0.17 | 18.82 ± 0.61 | 9.49 ± 0.47 | Ethylene di-amine tetra acetic acid | 1.27 ± 0.05 |
| IC 50 values of cytotoxicity of T. belerica fruit fractions against various malignant and normal cell lines | |||||||
| Cell lines | |||||||
| A549 | 2258.78 ± 1157.06 | 1260.5 ± 101.2 | 1478.02 ± 227.52 | 1283.83 ± 115.65 | 1394.33 ± 556.19 | ||
| MCF-7 | 1052.02 ± 188.01 | 204.35 ± 9.91 | 115.90 ± 18.97 | 179.99 ± 0.94 | 122.91 ± 0.58 | ||
| HeLa | 6122.41 ± 1552.33 | 664.79 ± 35.98 | 299.09 ± 16.23 | 389.39 ± 12.74 | 257.56 ± 5.08 | ||
| HepG2 | 1339.93 ± 123.95 | 1157.42 ± 245.31 | 1644.14 ± 287.65 | 1229.53 ± 140.93 | 516.95 ± 66.57 | ||
| U87 | 1208.98 ± 178.86 | 221.97 ± 10.31 | 36.52 ± 1.76 | 20.13 ± 0.93 | 17.27 ± 0.31 | ||
| WI-38 | 1950.72 ± 388.17 | 1805.61 ± 368.25 | 1277.23 ± 15.41 | 494.32 ± 6.87 | 872.07 ± 22.10 | ||
IC50 values of all activities are determined in μg/ml. Data are expressed as mean ± S.D. (n = 6)
Fig. 2.
In vitro reactive oxygen species (ROS) scavenging activities of TBHE, TBCE, TBEE, TBBE and TBWE together with reference compounds. a hydroxyl radical inhibition with reference of mannitol, all values are highly significant with p < 0.001 versus control (0 µg/ml), b hypochlorous radical scavenging compared with standard ascorbic acid, TBHE (80 µg/ml), TBBE (10, 50 µg/ml) have significance value with p < 0.05, TBHE (10, 50 µg/ml), TBCE (10 µg/ml), TBBE (20 µg/ml) and TBWE (10 µg/ml) with a significance level of p < 0.01 and the rest of the values are highly significant with p < 0.001. All values are compared to control (0 μg/ml), c superoxide radical inhibition compared with quercetin, all values are compared to control (0 μg/ml) and are highly significant with p < 0.001 except for TBCE (10 µg/ml) with p < 0.05, d singlet oxygen radical scavenging compared with lipoic acid, TBHE 50 µg/ml value has no significance value, TBHE (150 µg/ml), TBEE (50, 80, 100, 200 µg/ml), TBBE (50, 80 µg/ml) and TBWE (50 µg/ml) have a significance level of p < 0.01, rest of the values are highly significant and all are compared to control (0 µg/ml), e hydrogen peroxide scavenging compared to standard sodium pyruvate, significance: p < 0.05 (TBWE 0.5 mg/ml), significance p < 0.01 (TBHE 2 mg/ml, TBWE 0.8, 1.2 mg/ml) and the rest of the values have a significance level of p < 0.001. All values are compared to control (0 μg/ml). The results are represented as mean ± S.D. of six parallel measurements. Man mannitol, AA ascorbic acid, Quer quercetin, LA Lipoic acid, SP sodium pyruvate
Fig. 3.
In vitro reactive nitrogen species (RNS) scavenging activities and iron chelating capabilities of TBHE, TBCE, TBEE, TBBE and TBWE together with reference compounds. a nitric oxide inhibition compared to standard curcumin, TBHE (2, 7 µg/ml) have a significance level of p < 0.01, rest of the values are highly significant (p < 0.001). All values are compared to control (0 μg/ml), b peroxynitrite radical scavenging compared to standard gallic acid, TBHE (20, 100 µg/ml) has a significance level of p < 0.05, TBCE (20, 50, 100 µg/ml), TBBE (20 µg/ml), TBWE (50 µg/ml) have p value of < 0.01, rest of the values have p value < 0.001. All values are compared to control (0 μg/ml), c iron chelation activity of extracts, all the values are highly significant (p < 0.001 vs control i.e. 0 µg/ml) and d of standard iron chelator EDTA. The results are mean ± S.D. of six parallel measurements. Cur curcumin, GA gallic acid, EDTA Ethylenediamine tetra acetic acid
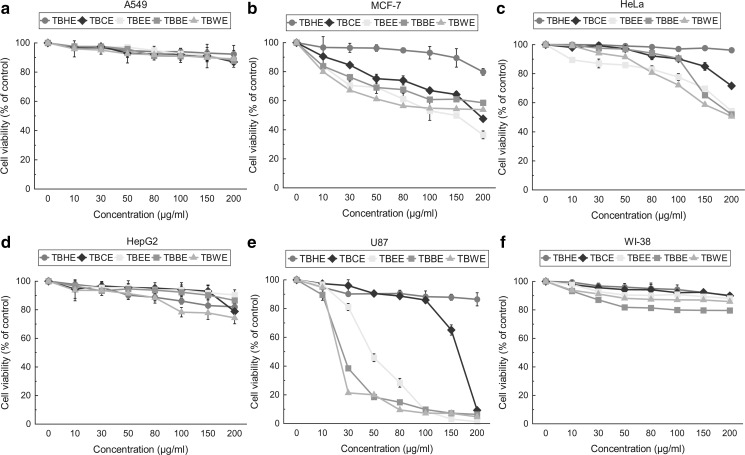
Antioxidant T. belerica fractions selectively inhibit proliferation of MCF-7, HeLa, and U87 cells
All T. belerica fractions were then tested against various malignant cells including non-malignant fibroblast cells and the results were compared to respective untreated cells, i.e. control (0 μg/ml). It was observed that less polar fraction TBEE not only showed most antioxidative effect but also significantly decreased the viability of MCF-7 cells by 50% at 115.90 ± 18.97 µg/ml (Fig. 4b), followed by the polar fractions TBBE and TBWE. HeLa cells (Fig. 4c) were inhibited in a dose dependant manner by TBWE > TBEE > TBBE while TBWE showed highest cytotoxicity towards glioblastoma U87 (Fig. 4e) with an IC50 value of 17.27 ± 0.31 µg/ml, followed by TBBE and TBEE (Table 1). However, A549 (Fig. 4a) and HepG2 (Fig. 4d) cells were not inhibited by TBEE, TBBE or TBWE. TBHE and TBCE did not show cytotoxicity towards any of the cancer cells (IC50s > 500 µg/ml). These fractions also did not show cytoxicity towards normal WI-38 fibroblast cells (Fig. 4f). Hence bioactive specific cytotoxic fractions were to be further checked for their cell cycle effects on MCF7, HeLa and U87 cancer cells.
Fig. 4.
Dose dependant effects of TBHE, TBCE, TBEE, TBBE and TBWE on cytotoxicity and proliferation of various cell lines a A549, p-value < 0.05 values—TBHE (10, 150, 200 µg/ml), TBCE (10 µg/ml), TBEE (10, 30 µg/ml), TBWE (50 µg/ml), p-value < 0.01 values—TBHE (50, 80, 100 µg/ml), TBCE (30, 50, 150 µg/ml), TBEE (50, 80, 100, 150 µg/ml), TBBE (10, 30 µg/ml) and TBWE (30, 80, 100, 200 µg/ml), rest values have p-value < 0.001. All values are compared to control (0 μg/ml), b MCF-7, TBHE (10 µg/ml) value is non-significant, TBHE (30, 100 µg/ml) have p-value < 0.05, TBHE (50, 150 µg/ml) have p-value < 0.01 and rest other values are highly significant. All values are compared to control (0 μg/ml), c HeLa, TBHE and TBCE (10, 30, 50 µg/ml) has p-value < 0.05, rest values have p-value < 0.001. All values are compared to control (0 μg/ml), d HepG2, TBHE (10, 30 µg/ml), TBCE 10 µg/ml have p-value > 0.05, TBCE (30, 80, 100, 150 µg/ml), TBEE (10, 30, 50, 150 µg/ml) have p-value < 0.05, TBHE (50 µg/ml), TBEE (100, 200 µg/ml), TBBE (10, 50, 80 µg/ml), TBWE (30, 50 µg/ml) have p-value < 0.01, rest values have p-value < 0.001. All values are compared to control (0 μg/ml), e U87 cancer cells, TBCE and TBEE (10 µg/ml) has p-value < 0.05, TBHE (100, 200 µg/ml), TBCE and TBBE (10 µg/ml) have p-value < 0.01, rest values are highly significant. All values are compared to control (0 µg/ml) and f WI-38 normal fibroblast cell, TBHE and TBCE (10, 30, 50, 80 µg/ml), TBEE (10 µg/ml) have p-value < 0.05, TBHE (100 µg/ml) has p-value < 0.01, rest of the values have p-value < 0.001, All values are compared to control (0 μg/ml). Cells were treated with doses ranging from 0 to 200 μg/ml for 48 h. The cell proliferation and viability were determined with WST-1 cell proliferation reagent and results were expressed as cell viability (% of control). All results are expressed as mean ± S.D. of six replicates
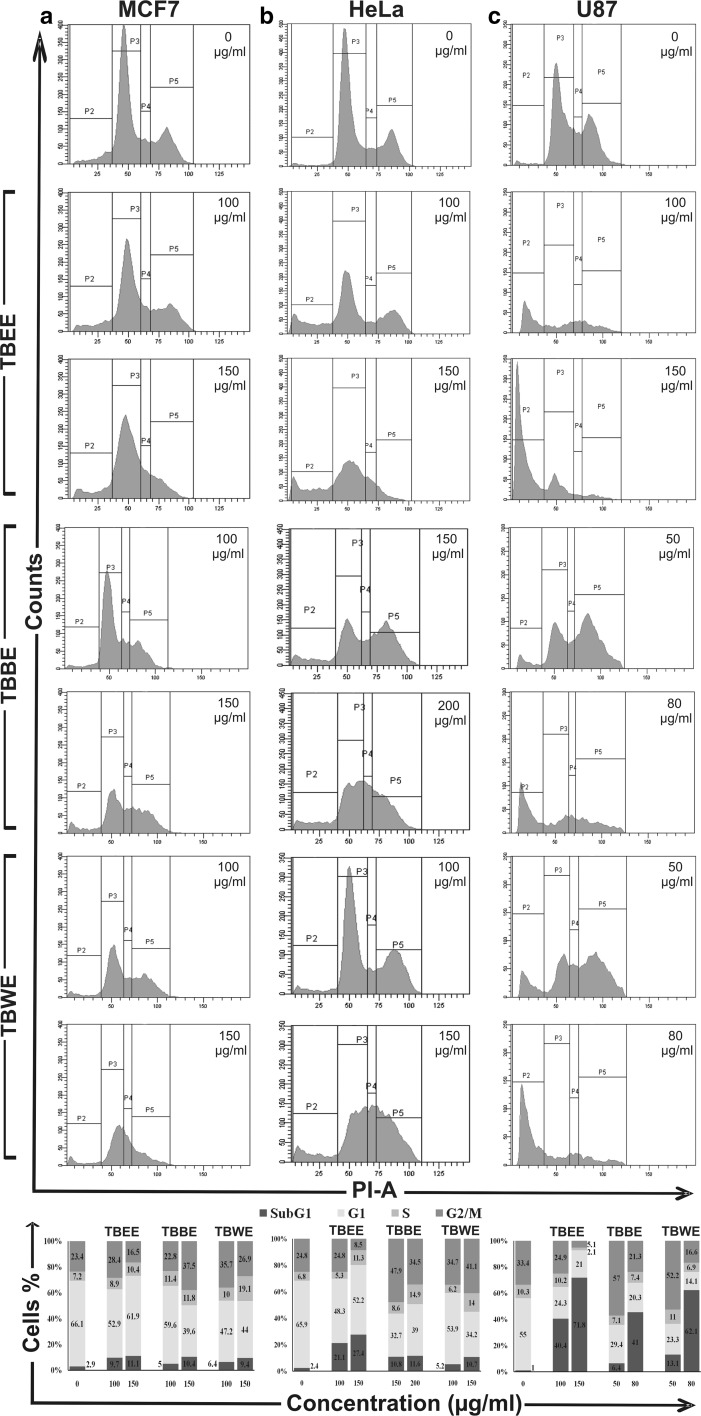
G2/M arrest in MCF-7, HeLa and U87 cells by T. belerica fractions
Flow cytometry was performed to determine whether the cytotoxicity induced by the T. belerica fractions was either due to apoptosis or cell cycle arrest. Different concentrations of bioactive cytotoxic fractions of T. belerica (TBEE, TBBE, TBWE) were applied to MCF-7, HeLa and U87 cells to evaluate the effects on cell cycle distribution. On analysis of MCF-7 cell cycle, TBBE showed induction of G2/M arrest at 100 and 150 µg/ml doses showing an increase in G2/M cell population from 23.4 to 37.5% and consecutive decrease in G1 population from 66.1% (control) to 39.6%, at a higher dose. Moreover the most polar fraction TBWE also caused similar effect on breast cancer cells while TBEE had not such a profound effect (Fig. 5a). The TBWE fraction caused G2/M arrest in HeLa at concentrations of 100 and 150 µg/ml (Fig. 5b). After 48 h treatment with TBWE, the cell population at G2 phase increased significantly from 24.8% (0 µg/ml) to 34.7% (100 µg/ml) and 41.1% (150 µg/ml) along with increase of S phase by 2.06 fold at higher dose. There was also a significant decrease in the G1 population from 65.9% in untreated cells to 34.2% in higher dose of TBWE. Similar effect was noticed on treatment with TBBE at 150 µg/ml, showing decrease in G1 phase and increase in both S and G2/M phases. Unlike the previous fractions, the lesser polar fraction of ethyl acetate did not cause arrest but led to apoptotic cell death with increase in sub G1 population from 2.4 to 27.4% i.e. an increase over tenfold apoptotic death for HeLa at 150 µg/ml.
Fig. 5.
Flow cytometric analysis of cell cycle distribution of cells treated with TBEE, TBBE and TBWE with graphical representation of percentage of cell population in different phases for a MCF-7, b HeLa and c U87 cells. MCF-7 cells were treated with 100 and 150 µg/ml of TBEE, TBBE and TBWE. HeLa cells were treated with 100 and 150 µg/ml of TBEE, 150 and 200 µg/ml of TBBE and 100 and 150 µg/ml of TBWE. U87 cells were treated with 100 and 150 µg/ml of TBEE and 50 and 80 µg/ml of TBBE and TBWE
Often mechanisms involved in G2 arrest are the result of check point modulation but at times it may be due to mitosis (M) phase effect (Tyagi et al. 2002). Cell cycle arrest at G2/M is normally initiated by p53 up-regulation due to DNA damage which in turn inhibits cdc2-cyclin B complex. These in turn trigger dephosphorylation of cyclin dependant kinase (cdk) leading to cell cycle arrest (Lukas et al. 2004). Previously many studies have been done using active fractions and their phytoconstituents leading to G2/M arrest (Ghate et al. 2013). Similar cell cycle arrest has also been reported for chemotherapeutic drugs like doxorubicin (O’Loughlin et al. 2000).
In case of glioblastoma (U87) cells, TBEE showed apoptosis with increase in sub G1 population from 1% in untreated cells to 40.4 and 71.8% in 100 and 150 µg/ml, respectively, and decrease in G1 and G2/M populations (Fig. 5c). TBBE however caused G2/M arrest in U87 cells at lower dose (50 µg/ml) but increased apoptotic sub G1 population to 41% at 80 µg/ml. Similar effect was also observed when U87 cells were treated with TBWE. Cell cycle arrest by anticancer drugs is generally associated with cytotoxicity and anti-proliferative effect in cancer cells (Chao et al. 2004). Sub-G1 population accumulates mainly due to nuclear DNA fragmentation and is also an indicator of apoptotic cells (Han and Park 2009).
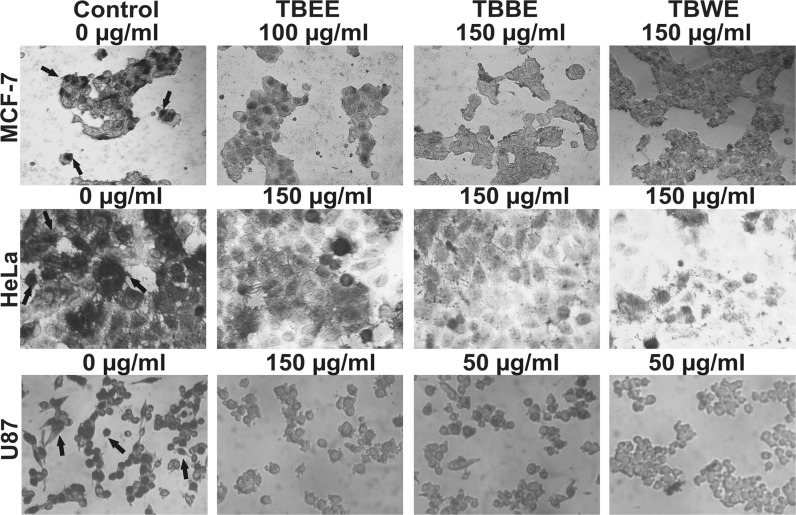
BrdU incorporation and cellular morphology
Anti-BrdU antibody stained TB fractions treated cells were imaged and represented in Fig. 6. Untreated MCF-7 cells showed incorporation of BrdU into replicating healthy nuclei (dark black patch inside the cells indicated by black arrows). After TBBE (150 µg/ml) and TBWE (150 µg/ml) treatment, BrdU failed to incorporate into nuclei indicating the inhibition in proliferation of cells; these observations corroborated with the FACS data which showed G2/M arrest. TBEE on the other hand allowed little BrdU uptake at 100 µg/ml leading to partial number of cells stained. Similar effect was also observed when HeLa cells were treated with TBBE and TBWE (150 µg/ml). On contrary, TBEE failed to arrest HeLa cells which led to nuclei visualization though lesser in number as compared to control. In untreated U87 cells, BrdU incorporation was highly evident from their nuclear stain and healthy morphology denoting normal growth. TBEE at 150 µg/ml caused huge apoptosis as evident by round shaped shrunken morphology of the unstained cells. TBBE and TBWE at 50 µg/ml, however, led to reduced cellular BrdU uptakes as cells were arrested at G2/M phase leading to further apoptosis. Hence these cells showed shrunken rounded unstained morphology.
Fig. 6.
Photomicrograph of cancer cells having incorporated BrDU on treatment with TBEE, TBBE and TBWE. MCF-7 cells were treated with TBEE (100 µg/ml), TBBE (150 µg/ml) and TBWE (150 µg/ml), HeLa cells were treated with 150 µg/ml of TBEE, TBBE and TBWE and U87 cells were treated with TBEE (150 µg/ml), TBBE (50 µg/ml) and TBWE (50 µg/ml). Control cells showed dark black patches inside the cells (BrdU incorporation into healthy cells) while arrested cells showed a reduced number of stained nuclei and shrunken morphology. Black arrows indicate proliferative cells with BrdU incorporated into the nucleus
Due to treatment arrested cells met the fate of apoptosis at a later stage and BrdU incorporation into such cells were rarely demonstrated during physiological programmed cellular death. Cells showing a uniform BrdU label in their nuclei were scored as replicating healthy cells while cells with scattered BrdU foci and limited area of BrdU uptake in the nuclei (not well imaged) were differentiated as DNA repairing cells, normal to post G2/M arrest. Hence arrested cells remained 90% BrdU negative since DNA repairing mechanisms led to discrete BrdU foci which are difficult to be imaged through light microscopy. Cells after treatment with TBBE and TBWE were treated with BrdU supplemented medium for an hour during which cell repair machinery probably targets to revive the cellular DNA repair mechanism (Kao et al. 2001). Thus BrdU incorporation occurs less in arrested cells leaving their nuclei undetected. Further studies are hence required to conclude clearly the arrest and apoptotic states with investigation into ATR, p53, p21 and H2AX phosphorylation (Fragkos et al. 2009).
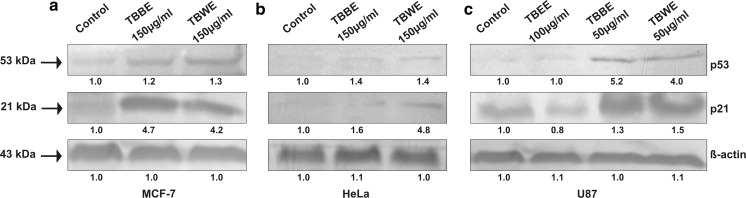
TBBE and TBWE treated MCF-7, HeLa and U87 cells show upregulation of p53 and p21
p53 is a tumour suppressor phosphoprotein with a nuclear DNA binding domain which normally exists as a tetramer to bind specific DNA sequences. It is widely known that the p53 plays a pivotal tumour suppressive role by two mechanisms- cell cycle arrest and apoptosis (Levine 1997). These effects are manifested through upregulation of various effectors, predominantly p21 (also known as WAF1/Cip1) (Roninson 2002). Here the antiproliferative effects of the extracts are emphasized at cell cycle arrest (G2/M) and finally apoptosis of various cancer cells. So the hypothesis of involvement of p53 and p21 in effecting G2/M arrest and cell death in cancer cells may be a result of the T. belerica extracts. Although residual wild type activity of p53 is required for malignancy maintenance, yet tilting the balance in favour of p53 might reestablish its tumour suppressing function.
On treatment of MCF-7 cells with both TBBE and TBWE at 150 µg/ml for 48 h, it was observed that p53 upregulation took place with further upregulation of its effector molecule, p21 when compared to control cells (Fig. 7a). Similarly after treatment of HeLa cells with butanol and water extracts (150 µg/ml), enhanced expression of p53 along with p21 were observed at the protein level (Fig. 7b). Previously it has been shown that upregulation of p53 took place due to DNA damage and replicative errors, thereby arresting cells at the G2/M check point and activation of target gene, p21 (Agarwal et al. 1998; Lakin and Jackson 1999). p21 also has a significant role in DNA repair process after cell cycle arrest (Moldovan et al. 2007). Moreover presence of functional p21 indicates that apoptosis occured in extract treated cancer cells through p53 dependant apoptotic pathway by activating p21. Here we observed that p53 and p21 levels increased on treatment with butanol and water extracts of T. belerica. Hence compounds present in these extracts might be responsible for DNA damage and p53 upregulation with cell cycle arrest at G2/M phase followed by p53–p21 mediated apoptosis of MCF-7 and HeLa cells by probable upregulation of pro-apoptotic protein Bax and other tumor necrosis factor related death receptors (Kang et al. 1999). Since TBEE did not show profound G2/M arrest or remarkable apoptotic effect against breast cancer MCF-7 cells and cervical cancer HeLa cells, it has not been tested for p53 and p21 status.
Fig. 7.
Immunoblot analysis of tumour suppressor proteins p53 and p21 in cell lysates of a MCF-7 on treatment with TBBE (150 µg/ml) and TBWE (150 µg/ml), b HeLa on treatment with TBBE (150 µg/ml) and TBWE (150 µg/ml) and c U87 when treated with TBEE (100 µg/ml), TBBE (50 µg/ml) and TBWE (50 µg/ml). β-actin (43 kDa) served as loading control. Relative densities of the blots are mentioned below to express the expression intensities
However the effect was different in case of U87 glioma cells when treated with ethyl acetate extract (100 µg/ml) of T. belerica (TBEE). Both p53 and p21 protein levels did not increase when compared with untreated cells (Fig. 7c) although both p53 and p21 upregulation was observed in U87 cells when treated with TBBE and TBWE (50 µg/ml). Phytocompounds in TBEE might be responsible for causing apoptosis in the cells independent of p53 pathway while TBBE and TBWE caused p53-dependent cell cycle arrest at G2/M and apoptosis.
Phytochemical analysis and HPLC standardization of the extracts
Qualitative tests for five major classes of phytochemicals namely terpenoids, triterpenoids, saponins, anthraquinones and glycosides were performed in order to assess their levels in the five extracts of T. belerica (Table 2). TBCE, TBEE, TBBE and TBWE were seen to be positive for terpenoids while the non-polar and lesser polar fractions TBHE, TBCE and TBEE showed presence of triterpenoids. The chloroform fraction and polar fractions afterwards denoted the presence of anthraquinones and saponins whereas glycosides were detected only in most polar fractions like TBBE and TBWE.
Table 2.
Phytochemical analysis of all fractions of T. belerica fruit
| Fractions | Test | Phytochemicals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phen | Flav | Carb | Tan | Alka | Asc | Ter | Triter | Anth | Sap | Gly | ||
| TBHE | Qualitative | − | + | + | − | + | + | − | + | − | − | − |
| Quantitative | − | 2.95 ± 0.27 | 4.35 ± 0.62 | − | 24.52 ± 2.7 | 4.51 ± 0.18 | ND | ND | ND | ND | ND | |
| TBCE | Qualitative | + | + | + | + | + | + | + | + | + | + | − |
| Quantitative | 113.70 ± 0.62 | 9.35 ± 0.19 | 8.91 ± 0.56 | 0.40 ± 0.22 | 92.12 ± 0.18 | 6.0 ± 0.28 | ND | ND | ND | ND | ND | |
| TBEE | Qualitative | + | + | + | + | + | + | + | + | + | + | − |
| Quantitative | 117.82 ± 1.09 | 11.65 ± 1.68 | 11.81 ± 0.23 | 0.20 ± 0.06 | 91.49 ± 0.38 | 5.3 ± 0.40 | ND | ND | ND | ND | ND | |
| TBBE | Qualitative | + | + | + | + | + | + | + | − | + | + | + |
| Quantitative | 117.23 ± 0.84 | 13.49 ± 0.16 | 14.06 ± 0.25 | 0.34 ± 0.07 | 94.27 ± 0.50 | 0.52 ± 0.13 | ND | ND | ND | ND | ND | |
| TBWE | Qualitative | + | + | + | + | + | + | + | − | + | + | + |
| Quantitative | 115.58 ± 0.94 | 13.55 ± 0.99 | 15.54 ± 0.17 | 0.29 ± 0.21 | 90.71 ± 0.34 | 1.36 ± 0.31 | ND | ND | ND | ND | ND | |
Total phenolics (mg/100 mg extract gallic acid equivalent), Total flavonoids (mg/100 mg extract quercetin equivalent), Carbohydrate (mg/100 mg extract glucose equivalent), Tannin (mg/100 mg extract catechin equivalent). Alkaloid (mg/100 mg extract reserpine equivalent), Ascorbic acid (mg/100 mg extract L-ascorbic acid equivalent)
Phen, Phenol; Flav, Flavonoid; Carbo, Carbohydrate; Tan., Tannin; Alka, Alkaloid; Asc, Ascorbic acid; Ter, Terpenoids; Triter, Triterpenoids; Anth, Anthraquinones; Sap, Saponin; Gly, Glycoside; +, presence of the phytoconstituent; −, absence of the phytoconstituent; ND, not determined
Further analyses were aimed to quantify important classes of phytochemicals like phenols, flavonoids, carbohydrates, tannins, alkaloids and ascorbic acid. It was observed that bioactivities of the tested fractions correlated well with the phytochemical contents (Table 2). Tannins, phenolic acids, flavonoids and alkaloids are the important ingredients to prevent against oxidative stress and decrease the activity of cholinesterase and xanthine oxidase and also alleviate mucus secretion in airway glands (Yildirim et al. 2001). They also formed an important constituent of Ayurveda, Siddha, folk and Chinese traditional medicines. Furthermore, phenols are vital plant constituents due to their reducing as well as metal chelation abilities. Moreover their high radicals scavenging activities are due to the presence of hydroxyl groups (Cook and Samman 1996). Flavonoids show their antioxidant action through scavenging or chelating process. Alkaloids have been used in medicine for centuries and one of their common biological properties is their cytotoxicity (Rice-Evans et al. 1995). Phenols, flavonoids, tannins and alkaloids contents were estimated much higher in polar fractions like TBEE, TBBE and TBWE. Thus presence of these important phytochemicals in the polar fractions might play an important role in establishing phytochemical content- function relationship, explaining their antioxidant and antiproliferative activity through various mechanisms like cell cycle arrest and apoptosis.
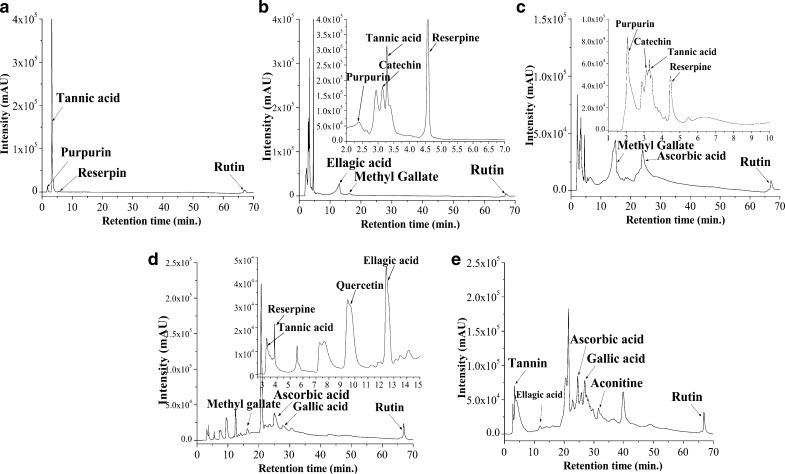
Identification of possible bioactive compounds by HPLC
Various peaks with different retention times (RT) were observed on analyzing the chromatograms and comparing them with standard compounds, run in same condition. TBHE showed (Fig. 8a) very few peaks of compounds like tannins, purpurin and a minor peak of reserpine which explains its low antioxidant activity. TBCE (Fig. 8b) being more polar than TBHE had peaks of purpurin, catechin, tannic acid, reserpine, ellagic acid, methyl gallate and rutin at RTs of 2.42, 3.14, 3.28, 4.58, 12.96, 16.16 and 67.11 min, respectively. TBEE also showed presence of identical compounds as compared to TBCE in addition to an ascorbic acid peak at 24.05 min (Fig. 8c). Highly bioactive polar fraction TBBE (Fig. 8d) showed the presence of all the above mentioned compounds but at higher intensities and amount while TBWE (Fig. 8e) showed the peaks for tannins, ellagic acid, ascorbic acid, gallic acid, aconitine and rutin. From the chromatogram analysis it was very much evident that TBEE, TBBE and TBWE contained the maximum number of compounds while TBCE chromatogram also revealed the presence of quite a few compounds thus correlating with their antioxidant potential.
Fig. 8.
HPLC chromatograms of a TBHE, b TBCE, c TBEE, d TBBE and e TBWE. Peaks are marked with arrows to signify the compound retention times. Inset shows expanded view of chromatogram with retention time of minutes
Conclusion
Polar fractions of T. belerica were seen to act as potent in vitro reactive oxygen and nitrogen species scavengers. Moreover butanol (TBBE) and water (TBWE) fractions were observed to be selectively cytotoxic towards breast cancer (MCF-7), cervical cancer (HeLa) and glioblastoma (U87) cell lines. These fractions arrested growth of MCF-7, HeLa and U87 cells at G2/M phase while ethyl acetate fraction (TBEE) caused apoptosis to check the growth of these cancer cells but none showed toxicity towards normal fibroblast cells (WI-38). These observations were further confirmed with imaging where arrested cells did not incorporate BrdU in DNA as compared to control cells. Moreover, butanol and water extracts of T. belerica upregulated p53 and p21 tumour suppressor proteins in MCF-7, HeLa and U87 cells which corroborated with G2/M arrest and apoptosis. Hence our findings suggest T. belerica polar fractions (TBEE, TBBE, TBWE) as potent antioxidant and anticancer extracts which can be selectively used as a remedy against various types of cancer, probably due to the presence of various identified bioactive compounds.
Acknowledgements
The authors are thankful to Mr. Ranjit Kumar Das, Mr. Pradip Kumar Mallik and Mr. Ranjan Dutta for their technical assistance.
Compliance with ethical standards
Conflict of interest
Authors declared no conflict of interest.
Footnotes
Tapasree Basu and Sourav Panja have contributed equally to this work.
References
- Agarwal ML, Agarwal A, Taylor WR, et al. A p53-dependent S-phase checkpoint helps to protect cells from DNA damage in response to starvation for pyrimidine nucleotides. Proc Natl Acad Sci USA. 1998;95:14775–14778. doi: 10.1073/pnas.95.25.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KK, Singh B, Saxena AK, et al. 3, 4, 5-trihydroxy benzoic acid (Gallic acid), the hepatoprotective principle in the fruits of Terminalia bellerica bioassay guided activity. Pharmacol Res. 1997;36:315–321. doi: 10.1006/phrs.1997.0236. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Hoey BM, et al. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Rad Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-X. [DOI] [PubMed] [Google Scholar]
- Buxton EJ, Meanwell CA, Hilton C, et al. Combination bleomycin, ifosfamide, and cisplatin chemotherapy in cervical cancer. J Natl Cancer Inst. 1989;81:359–361. doi: 10.1093/jnci/81.5.359. [DOI] [PubMed] [Google Scholar]
- Chao JI, Kuo PC, Hsu TS. Down-regulation of survivin in nitric oxide induced cell growth inhibition and apoptosis of the human lung carcinoma cells. J Biol Chem. 2004;279:20267–20276. doi: 10.1074/jbc.M312381200. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Autschbach R. Doxorubicin in experimental and clinical heart failure. Eur J Cardiothorac Surg. 2006;30:611–616. doi: 10.1016/j.ejcts.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Cook NC, Samman S. Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. Nutr Biochem. 1996;7:66–76. doi: 10.1016/0955-2863(95)00168-9. [DOI] [Google Scholar]
- Das A, Chaudhuri D, Mandal N, et al. Study of antioxidant and reactive oxygen species scavenging activity of the edible tuber of “greater yam” (Dioscorea alata L.) from north-east India. Asian J Pharm Clin Res. 2012;5:74–84. [Google Scholar]
- Elizabeth KM. Antimicrobial activity of Terminalia bellerica. Indian J Clin Biochem. 2005;20:150–153. doi: 10.1007/BF02867416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkos M, Jurvansuu J, Beard P. H2AX is required for cell cycle arrest via the p53/p21 pathway. Mol Cell Biol. 2009;29:2828–2840. doi: 10.1128/MCB.01830-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghate NB, Chaudhuri D, Sarkar R, et al. An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. PLoS ONE. 2013;8:e82293. doi: 10.1371/journal.pone.0082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghate NB, Hazra B, Sarkar R, et al. In vitro anticancer activity of Spondias pinnata bark on human lung and breast carcinoma. Cytotechnology. 2014;66:209–218. doi: 10.1007/s10616-013-9553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghate NB, Hazra B, Sarkar R, et al. Alteration of Bax/Bcl-2 ratio contributes to Terminalia belerica induced apoptosis in human lung and breast carcinoma. In Vitro Cell Dev Biol Anim. 2014;50:527–537. doi: 10.1007/s11626-013-9726-x. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non–small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol. 2004;2:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Gokhale SB, Kokate CK, Purohit AP. Pharmacognosy. Pune: Nirali Prakashan; 2003. [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991;91:S14–S22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Han YH, Park WH. Growth inhibition in antimycin A treated-lung cancer Calu-6 cells via inducing a G1 phase arrest and apoptosis. Lung Cancer. 2009;65:150–160. doi: 10.1016/j.lungcan.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Harbornen JB, Baxter H. Phytochemical dictionary: a handbook of bioactive compounds from plants. London: Taylor and Francis; 1995. [Google Scholar]
- Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 2008;8:63. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra B, Sarkar R, Biswas S, et al. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement Altern Med. 2010;10:20. doi: 10.1186/1472-6882-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KH, Kim WH, Choi KH. p21 promotes ceramide-induced apoptosis and antagonizes the antideath effect of Bcl-2 in human hepatocarcinoma cells. Exp Cell Res. 1999;253:403–412. doi: 10.1006/excr.1999.4644. [DOI] [PubMed] [Google Scholar]
- Kao GD, McKenna WG, Yen TJ. Detection of repair activity during the DNA damage-induced G2 delay in human cancer cells. Oncogene. 2001;20:3486–3496. doi: 10.1038/sj.onc.1204445. [DOI] [PubMed] [Google Scholar]
- Kochevar EI, Redmond WR. Photosensitized production of singlet oxygen. Methods Enzymol. 2000;319:20–28. doi: 10.1016/S0076-6879(00)19004-4. [DOI] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Kumudhavalli MV, Vyas M, Jayakar B. Phytochemical and pharmacological evaluation of the plant fruit of Terminalia belerica Roxb. Int J Pharm Life Sci. 2010;1:1–11. [Google Scholar]
- Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene. 1999;18:7644–7655. doi: 10.1038/sj.onc.1203015. [DOI] [PubMed] [Google Scholar]
- Levine A. p53 the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Liu YS, Yeh HH, et al. Selfassembled poly (e-caprolactone)-g-chondroitin sulfate copolymers as an intracellular doxorubicin delivery carrier against lung cancer cells. Int J Nanomed. 2012;7:4169–4183. doi: 10.2147/IJN.S33602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissi EA, Modak B, Torres R, et al. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of the ABTS and DPPH methods. Free Rad Res. 1999;30:471–477. doi: 10.1080/10715769900300511. [DOI] [PubMed] [Google Scholar]
- Lukas J, Lukas C, Bartek J. Mammalian cell cycle checkpoints: signaling pathways and their organization in space and time. DNA Repair. 2004;3:997–1007. doi: 10.1016/j.dnarep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- O’Loughlin C, Heenan M, Coyle S, et al. Altered cell cycle response of drug-resistant lung carcinoma cells to doxorubicin. Eur J Can. 2000;36:1149–1160. doi: 10.1016/S0959-8049(00)00071-X. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Bolwell PG, et al. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Rad Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- Roninson IB. Oncogenic functions of tumour suppressor p21Waf1/Cip1/Sdi1: association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 2002;179:1–14. doi: 10.1016/S0304-3835(01)00847-3. [DOI] [PubMed] [Google Scholar]
- Saroya AS. Herbalism phytochemistry and ethanopharmacology. Enfield: Science Publishers; 2011. pp. 357–361. [Google Scholar]
- Stief TW. The physiology and pharmacology of single oxygen. Med Hypotheses. 2003;60:567–572. doi: 10.1016/S0306-9877(03)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Cheng Y, Liu M, et al. Comparision of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. PLoS ONE. 2013;8:e54505. doi: 10.1371/journal.pone.0054505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam K. The roles of doxorubicin in hepatocellular carcinoma. ADMET DMPK. 2013;1:29–44. [Google Scholar]
- Tyagi AK, Singh RP, Agarwal C, et al. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin Can Res. 2002;8:3512–3519. [PubMed] [Google Scholar]
- Tylor BS, Kion YM, Wang QI, et al. Nitric oxide down-regulates hepatocyte-inducible nitric oxide synthase gene expression. Arch Surg. 1997;132:1177–1183. doi: 10.1001/archsurg.1997.01430350027005. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]